Introduction
Sann-Joong-Kuey-Jian-Tang (SJKJT), a Traditional
Chinese Medicinal preparation, has been prescribed as a
complementary medicine for patients with solid tumors in Taiwan. It
consists of 17 species of medicinal herbs: Curcuma
aeruginosa Roxb., Laminaria japonica Aresch,
Bupleurum scorzoneri folium Willd (Bupleurum chinense
DC), Coptis chinensis Franch, Paeonia lactiflora
Pall, Pueraria lobata Ohwi, Trichosanthes
cucumeroides Maxim, Phellodendron amurense Rupr.,
Glycyrrhiza uralensis Fisch, Sparganium stoloniferum
Buch., Anemarrhena asphodeloides Bunge, Angelica
sinensis Diels, Cimicifuga heracleifolia Komar,
Scutellaria baicalensis Georgi, Gentiana scabra
Bunge, Platycodon grandiflorus and Forsythia suspensa
Vahl. It has been shown that SJKJT does not exert significant toxic
effects on certain types of normal cells (1). A previous study by our group
demonstrated that SJKJT increased the protein expression levels of
tumor necrosis factor-α (TNF-α), caspase-8 and caspase-3 in colo
205 colon cancer cells, thereby inducing apoptosis in vivo
and in vitro (2). It was
also shown that SJKJT inhibited the proliferation of Hep-G2
hepatocellular carcinoma cells by increasing the expression of
TNF-α, caspase-8, caspase-3 and B-cell lymphoma 2
(Bcl-2)-associated X (Bax) and decreasing that of translationally
controlled tumor protein (TCTP) and myeloid cell leukemia 1 protein
(Mcl-1) in vitro (3).
Delayed diagnosis and a poor response to current forms of
chemotherapy render pancreatic cancer a challenging malignancy to
treat and the fourth leading cause of cancer-associated mortality
in the USA (4,5). These statistics indicate that the
development of novel therapeutic agents is required. Mujumdar et
al (6) demonstrated that
triptolide kills pancreatic cancer cells via two different
mechanisms. It induces caspase-dependent apoptotic death in human
BxPC-3 pancreatic cancer cells and caspase-independent autophagic
death in metastatic cell lines. Traditional Chinese Medicinal herbs
are increasingly recognized as having the potential for development
as alternative therapies for a number of human malignant tumors
(7). Previous studies have also
shown that SJKJT inhibits the proliferation of human BxPC-3
pancreatic cancer cells by decreasing the expression of TCTP and
Mcl-1 expression and increasing that of TNF-α and Bax in
vitro (8). SJKJT is a
potential chemotherapeutic agent for use in pancreatic cancer. The
anticancer effects of SJKJT on human pancreatic cancer have not yet
been fully elucidated, and therefore, further studies are required
to investigate the mechanisms of action underlying its effects.
Autophagy, derived from auto (self) and phagy (to eat), refers to
self-digestion as a means of providing an alternative energy
source. When cellular stress leads to continuous or excessive
induction of autophagy, cell death may ensue. Thus, cell survival
or cell death depends on the duration and severity of the insult
(9). It has been shown that
autophagy is activated in pancreatic cancer cells and is correlated
with a poor patient outcome (10).
It has also been demonstrated that pancreatic cancer requires
autophagy for tumor growth (11).
Previous studies have shown that SJKJT inhibits the proliferation
of human colo 205 colon cancer cells by increasing the expression
of the microtubule-associated protein II light chain 3 (LC3-II)
protein in vitro (12). The
present study focused on the mechanisms underlying the induction of
autophagy by SJKJT in human BxPC-3 pancreatic cancer cells.
Materials and methods
Cells and reagents
The BxPC-3 human pancreatic cancer cell line (BCRC
number: 60283) was obtained from the American Type Culture
Collection (ATCC no. CRL-1687; Passage no. 9; mycoplasmic,
negative; Manassas, VA, USA). The crude extract of SJKJT was
obtained from Chuang Song Zong Pharmaceutical Co., Ltd. (Ligang
Plant, Taiwan). Fetal bovine serum (FBS) and glutamine were
obtained from Gibco BRL (Invitrogen Life Technologies, Grand
Island, NY, USA). MTT, sodium deoxycholate, leupeptin, Triton
X-100, Tris-HCl, ribonuclease-A, sodium pyruvate,
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES),
dimethyl sulfoxide (DMSO) and RPMI-1640 were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Potassium phosphates and
Tris-EDTA (TE) buffer were purchased from Merck Millipore
(Darmstadt, Germany). BioMax film was obtained from Kodak
(Rochester, NY, USA). The following antibodies were used:
Monoclonal insulin-like growth factor 1 receptor (IGFR; no. 3018,
MW 95 kDa), monoclonal protein kinase B (AKT; no. 3063S, MW 60
kDa), monoclonal mammalian target of rapamycin (mTOR; no. 2983, MW
289 kDa), polyclonal beclin-1 (no. 3738, MW: 60 kDa) and polyclonal
LC3-II (no. 3775, MW 14, 16 kDa) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA); monoclonal Caspase-3 (Novus,
Lot: NB500-210), monoclonal autophagocytosis associated protein
(Atg) 7 (GTX63486, MW: 78 kDa), monoclonal Atg 3 (GTX63041, MW: 40
kDa), monoclonal Atg5-Atg12 (GTX62601, MW: 55 kDa); mouse
anti-β-actin. The antibodies were diluted in 5% nonfat dry milk, 1X
Tris-buffered saline and 0.1% Tween-20 (Sigma-Aldrich) at 4°C with
gentle shaking, overnight. Penicillin-streptomycin was obtained
from Sigma-Aldrich.
Cell culture
The BxPC-3 cells were maintained in RPMI-1640 medium
containing 10% FBS, 1% penicillin/streptomycin (10,000 U/ml
penicillin, 10 mg/ml streptomycin) at 37°C in a humidified
atmosphere containing 5% CO2.
Cytotoxicity of SJKJT in BxPC-3
cells
The BxPC-3 cells were plated in 96-well plates at a
density of 1×104 cells per well for 16–20 h and then
treated with various concentrations of SJKJT (0, 0.1, 0.2, 0.4,
0.6, 0.8 and 1 mg/ml) for different durations (24, 48 and 72 h).
Subsequently, the cells were incubated with 1 mg/ml of MTT in fresh
RPMI medium for 2 h. The surviving cells were measured at 590 nm
using a microplate reader. The relative percentage of cell
viability was calculated by dividing the absorbance of treated
cells by that of the control in each experiment using the following
formula: Proliferation rate
(%)=(ODtest-ODblank)x100, where
ODtest and ODblank are the optical density of
the test substances and the blank control, respectively.
Western blot analysis
The effects of SJKJT on the protein expression of
mTOR, beclin-1, Atg7, Atg3, Atg5-12 and LC3-II in BxPC-3 cells were
assessed using western blot analysis. BxPC-3 cells were treated
with various concentrations (0, 0.3, 0.6 and 1.2 mg/ml) of SJKJT
for 48 or 72 h and the protein levels of mTOR, beclin-1, Atg7,
Atg3, Atg5-12 and LC3-II were evaluated. BxPC-3 cells were also
treated with SJKJT (0.6 mg/ml) for different durations (0, 12, 24,
36, 48 and 72 h) and the protein levels of mTOR, beclin-1, Atg7,
Atg3, Atg5-12 and LC3-II were evaluated by western blot
analysis.
Following treatment with SJKJT, cells were lysed in
ice-cold whole cell extract buffer containing protease inhibitors
(BioVision, Inc., Milpitas, CA, USA). The lysate was agitated for
30 min at 4°C and centrifuged at 7,267 × g for 10 min. Protein
concentration was measured using a bicinchoninic acid protein assay
kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Equal
quantities of protein were subjected to electrophoresis using 12%
SDS-PAGE. To ensure equal protein loading and transfer, proteins
were then transferred onto polyvinylidene difluoride membranes and
the membranes were blocked overnight at 4°C using blocking buffer
(5% non-fat dried milk in solution containing 50 mM Tris/HCl, pH
8.0; 2 mM CaCl2; 80 mM sodium chloride; 0.05% Tween 20;
and 0.02% sodium azide). Membranes were then incubated for 2 h at
25°C with the primary antibodies listed above, followed by
anti-rabbit or anti-mouse immunoglobulin G-horseradish
peroxidase-conjugated secondary antibodies. The membranes were
washed three times for 10 min with washing solution. Finally, the
protein bands were visualized on an X-ray film using the
K-12045-D50 WesternBright™ enhanced chemiluminescence kit
(Advansta, Inc., Menlo Park, CA, USA) and quantified using Image J
software, version 1.44 (National Institute of Health, Bethesda, MD,
USA) The reference proteins GAPDH and β-actin were used as internal
control.
Statistical analysis
Data are presented as the mean ± standard deviation.
Student’s t-test was used to analyze statistical significance.
P<0.05 was considered indicate a statistically significant
difference.
Results
Cytotoxicity of SJKJT to BxPC-3
cells
The results showed that SJKJT inhibits the
proliferation of BxPC-3 cells. When cultured with various
concentrations of SJKJT (0, 0.1, 0.2, 0.4, 0.6, 0.8 and 1 mg/ml)
for 24, 48 and 72 h, the percentages of viable cells relative to
those of the control were 95.91±5.23, 91.05±5.72, 79.69±7.08,
77.18±4.33, 64.35±4.52 and 60.03±4.16% at 24 h; 78.56±8.72,
72.59±6.48, 62.83±4.53, 52.17±3.98, 44.49±4.31 and 37.48±4.64% at
48 h; and 65.54±4.74, 56.92±2.52, 40.94±4.41, 31.10±4.98,
26.51±4.20 and 34.34±4.76% at 72 h, respectively (Fig. 1). The results demonstrated that
SJKJT inhibited the proliferation of human pancreatic cancer BxPC-3
cells in a time- and dose-dependent manner.
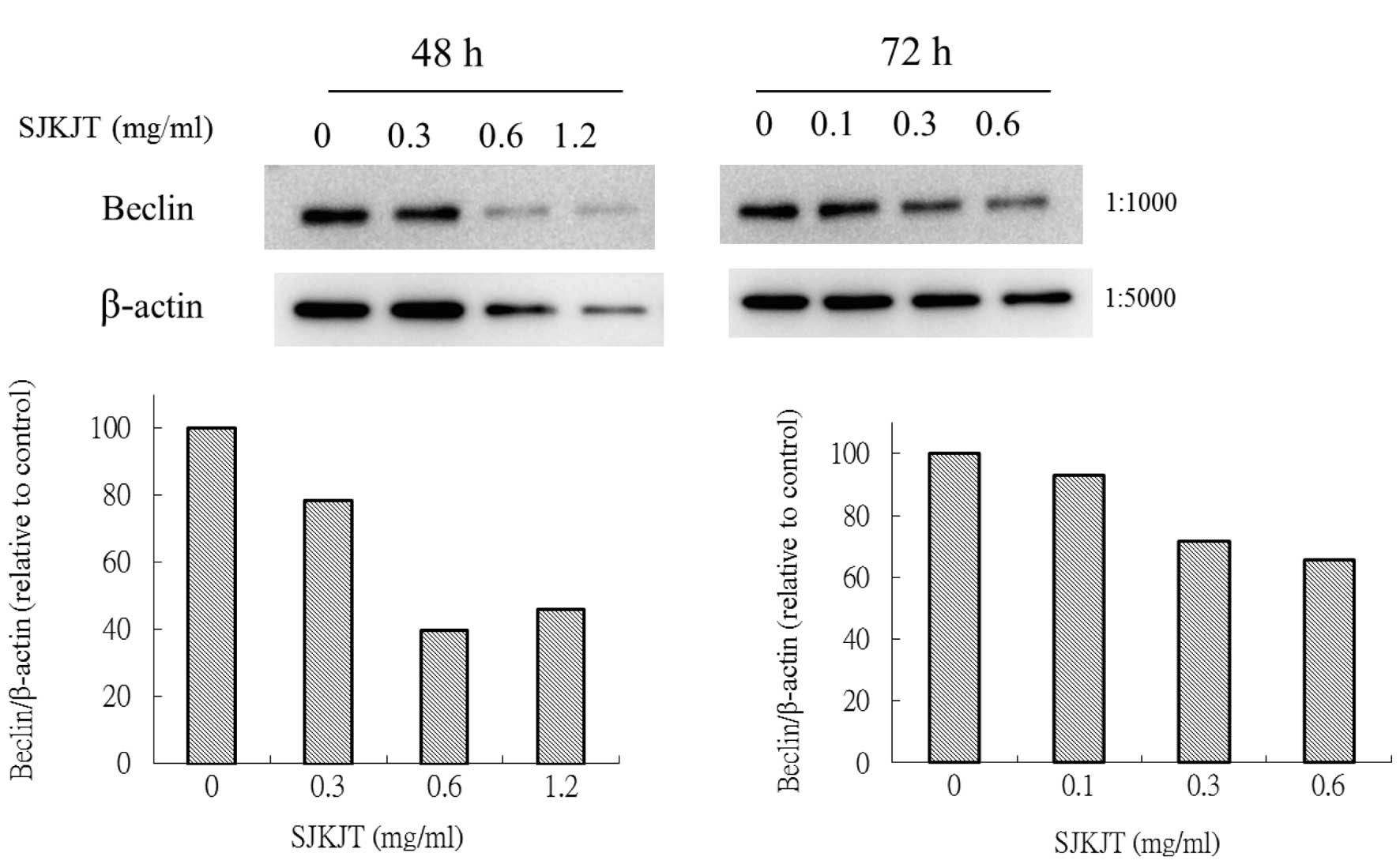
Expression of mTOR, beclin-1, Atg7, Atg3,
Atg5-12 and LC3-II proteins in BxPC-3 cells treated with SJKJT
BxPC-3 cells were treated with various
concentrations (0, 0.3, 0.6 and 1.2 mg/ml) of SJKJT for 48 or 72 h
and the expression of beclin-1, Atg7, Atg3, Atg5-12 and LC3-II
proteins was evaluated using western blot analysis. The results
showed that when BxPC-3 cells were treated with various
concentrations of SJKJT for 48 or 72 h, the expression levels of
the mTOR (Fig. 2), beclin-1
(Fig. 3), Atg7 (Fig. 4A), Atg3 (Fig. 4B) and Atg5-12 (Fig. 4C) proteins were decreased.
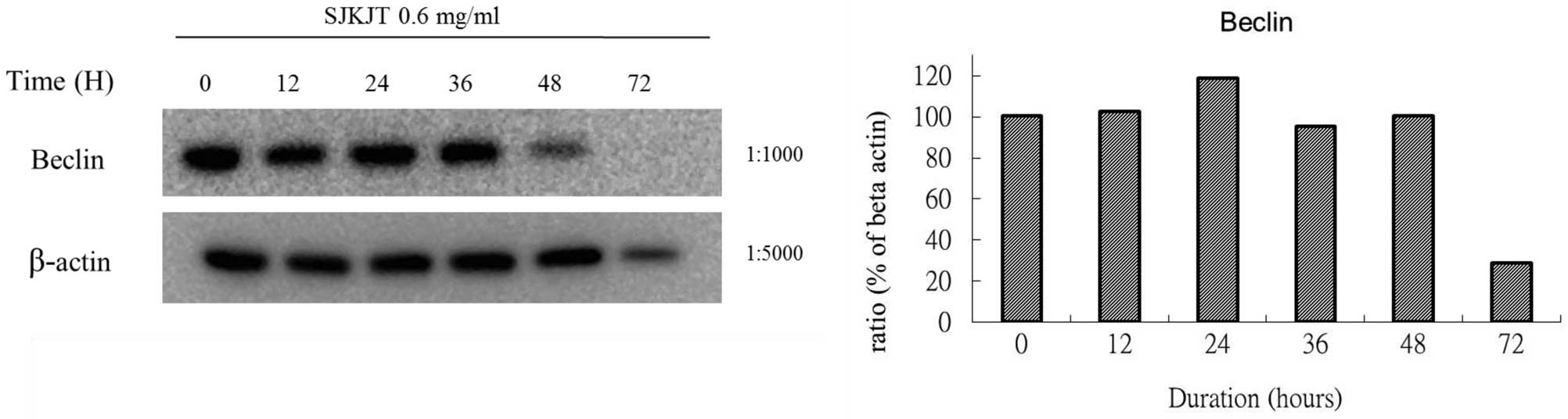
BxPC-3 cells were also treated with SJKJT (0.6
mg/ml) for different durations (0, 12, 24, 36, 48 and 72 h) and the
expression levels of mTOR, beclin-1, Atg7, Atg3, Atg5-12 and LC3-II
proteins were evaluated by western blotting. The results obtained
using different reference proteins (GAPDH or β-actin) provided
similar results. The results demonstrated that when BxPC-3 cells
were treated with SJKJT for different durations, the expressions of
the beclin-1 (Figs. 5 and 8), Atg7 (Figs. 6A and 9A), Atg3 (Figs. 6B and 9B) and Atg5-12 (Figs. 6C and 9C) proteins were increased during the
first 24 h, but decreased at 48 and 72 h. By contrast, the
expression of the LC3-II protein increased in a time-dependent
manner (Figs. 7 and 10).
 | Figure 6Protein expression of (A) Atg7, (B)
Atg3 and (C) Atg5-Atg12 in BxPC-3 cells. BxPC-3 cells were treated
with SJKJT (0.6 mg/ml) for different durations (0, 12, 24, 36, 48
and 72 h). Expression of the proteins was evaluated by western blot
analysis. When BxPC-3 cells were treated with SJKJT for different
durations, the expression of the Atg7, Atg3 and Atg5-Atg12 proteins
increased during the first 24 h, but that of Atg3 and Atg5-Atg12
decreased at 48 to 72 h. Atg, autophagocytosis associated protein;
SJKJT, Sann-Joong-Kuey-Jian-Tang. |
 | Figure 9Protein expression of Atg7, Atg3 and
Atg5-Atg12 in BxPC-3 cells. BxPC-3 cells were treated with SJKJT
(0.6 mg/ml) for different durations (0, 12, 24, 36, 48 and 72 h).
Expression of these proteins was evaluated by western blot
analysis. The reference protein GAPDH was used as internal control.
(A) When BxPC-3 cells were treated with SJKJT for different
durations the expression of the Atg7 protein increased during the
first 12 to 48 h, but decreased at 72 h. (B) The expression of the
Atg3 protein increased during the first 12 to 36 h, but decreased
at 48 to 72 h. (C) The expression of the Atg5-Atg12 protein
increased during the first 12 to 36 h, but decreased at 48 to 72 h.
Atg, autophagocytosis-associated protein; SJKJT,
Sann-Joong-Kuey-Jian-Tang. |
Discussion
Autophagy is one of forms of programmed cell death
(type II cell death) (13). LC3-II
levels may be used as a marker of autophagy and monitored using
western blot analysis (14–15).
When cellular stress leads to a continuous or excessive induction
of autophagy, cell death may ensue (9). It has been shown that autophagy is
activated in pancreatic cancer cells and is correlated with poor
patient outcome (10). Yang et
al (11) also demonstrated
that pancreatic cancers require autophagy for tumor growth. The
results of the present study showed that SJKJT inhibited the
proliferation of BxPC-3 cells in a time- and dose-dependent manner
in vitro. The results also demonstrated that when BxPC-3
cells were treated with various concentrations of SJKJT for 48 or
72 h, the expression levels of the mTOR protein were decreased.
When BxPC-3 cells were treated with SJKJT for varying durations,
the expression of the LC3-II protein was increased in a time- and
dose-dependent manner. Autophagy has been proposed as a novel
target for anticancer therapy (16). A possible molecular mechanism
underlying the inhibition of BxPC-3 human pancreatic cancer cells
by SJKJT may proceed via reduction in the expression of mTOR,
thereby leading to activation of autophagy in vitro. To the
best of our knowledge, the present study was the first to
demonstrate that SJKJT decreased the protein expression of mTOR but
increased that of LC3-II, which was associated with the inhibition
of BxPC-3 human pancreatic carcinoma cells. The therapeutic
potential of SJKJT in human pancreatic cancer requires further
investigation.
Acknowledgements
This study was supported by the Changhua Christian
Hospital (grant no. 100-CCH-ICO-06-1).
References
|
1
|
Yang CH and Craise LM: Development of
human epithelial cell systems for radiation risk assessment. Adv
Space Res. 14:115–120. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng CY, Lin YH and Su CC:
Sann-Joong-Kuey-Jian-Tang up-regulates the protein expression of
Fas and TNF-α in colo 205 cells in vivo and in vitro. Mol Med Rep.
3:63–67. 2010.PubMed/NCBI
|
|
3
|
Chen YL, Yan MY, Chien SY, Kuo SJ, Chen
DR, Cheng CY and Su CC: Sann-Joong-Kuey-Jian-Tang inhibits
hepatocellular carcinoma Hep-G2 cell proliferation by increasing
TNF-α, Caspase-8, Caspase-3 and Bax but by decreasing TCTP and
Mcl-1 expression in vitro. Mol Med Rep. 7:1487–1493.
2013.PubMed/NCBI
|
|
4
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mujumdar N, Mackenzie TN, Dudeja V, et al:
Triptolide induces cell death in pancreatic cancer cells by
apoptotic and autophagic pathways. Gastroenterology. 139:598–608.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verhoef MJ, Balneaves LG, Boon HS and
Vroegindewey A: Reasons for and characteristics associated with
complementary and alternative medicine use among adult cancer
patients: a systematic review. Integr Cancer Ther. 4:274–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chien SY, Kuo SJ, Chen DR and Su CC:
Sann-Joong-Kuey-Jian-Tang decreases the protein expression of Mcl-1
and TCTP and increases that of TNF-α and Bax in BxPC-3 pancreatic
carcinoma cells. Int J Mol Med. 32:85–92. 2013.PubMed/NCBI
|
|
9
|
Dalby KN, Tekedereli I, Lopez-Berestein G
and Ozpolat B: Targeting the prodeath and prosurvival functions of
autophagy as novel therapeutic strategies in cancer. Autophagy.
6:322–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujii S, Mitsunaga S, Yamazaki M, et al:
Autophagy is activated in pancreatic cancer cells and correlates
with poor patient outcome. Cancer Sci. 99:1813–1819.
2008.PubMed/NCBI
|
|
11
|
Yang S, Wang X, Contino G, et al:
Pancreatic cancers require autophagy for tumor growth. Genes Dev.
25:717–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng CY, Lin YH and Su CC:
Sann-Joong-Kuey-Jian-Tang increases the protein expression of
microtubule-associated protein II light chain 3 in human colon
cancer colo 205 cells. Mol Med Rep. 2:707–711. 2009.PubMed/NCBI
|
|
13
|
Clarke PG: Developmental cell death:
morphological diversity and multiple mechanisms. Anat Embryol
(Berl). 181:195–213. 1990. View Article : Google Scholar
|
|
14
|
Yang YP, Liang ZQ, Gu ZL and Qin ZH:
Molecular mechanism and regulation of autophagy. Acta Pharmacol
Sin. 26:1421–1434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Konado Y and Kondo S: Autophagy and cancer
therapy. Autophagy. 2:85–90. 2006. View Article : Google Scholar
|
|
16
|
Moretti L, Yang ES, Kim KW and Lu B:
Autophagy signaling in cancer and its potential as novel target to
improve anticancer therapy. Drug Resist Updat. 10:135–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|