Introduction
Prostate cancer (PCa) is the commonest type of
cancer in males, and which was expected to account for 26%
(220,800) of cases of cancer in males in the United States in 2015.
PCa was expected to account for 9% (27,540) of all cancer-related
mortality in males and continues to be the second leading cause of
cancer-associated mortality in this group (1).
Common risk factors for the development of cancer,
including obesity and smoking, have only a weak association with
PCa. In addition to age, ethnicity and family history, DNA
methylation is an epigenetic event that exhibits an association
with increased incidence of PCa (2,3). DNA
methylation affects cell function by altering gene expression, and
refers to the covalent addition of a methyl group, catalyzed by DNA
methyltransferases (DNMTs), to the 5-carbon of cytosine in a CpG
dinucleotide. The majority of CpG islands are unmethylated and
associated with genes capable of active transcription in normal
cells. By contrast, numerous CpG islands exhibit aberrant
hypermethylation, and consequent gene inactivation, in cancer cells
(4). A number of inactivated
genes, including glutathione-S-transferase-P1 (GSTP1) and
adenomatous polyposis coli (APC) encode proteins that act as tumor
suppressors. Silencing of these genes is associated with tumor
initiation, development and progression (5). Hypermethylation of these genes is
associated with PCa (6).
Reliance on a single molecular marker may have
limitations in the diagnosis of PCa. Therefore, it may be
advantageous to develop multiple sensitive and specific molecular
markers to be used simultaneously (7,8). The
aim of the present study was to examine the methylation of GSTP1
and APC, in order to determine whether GSTP1 and APC methylation
may be associated with PCa and, if so, whether they are clinically
significant markers of disease. The potential use of
hypermethylated genes as biomarkers to detect PCa are discussed in
the present study.
Materials and methods
Human tissue samples
The present study was approved by the ethics
committee of Zhengzhou University (Zhengzhou, China), and written
informed consent was obtained from all of the patients. The
clinical and pathological data was obtained for 56 PCa tissue
samples, collected from the Urology department of the First
Affiliated Hospital of Zhengzhou University (Zhengzhou, China)
between January 2010 and December 2012. The samples consisted of 4
PCa radical prostatectomy tissue specimens, 19 sections of
transurethral resection tissue and 33 biopsy specimens. The samples
were obtained from patients aged between 50 and 93 years, with a
median age of 77 years. According to the standard Gleason grading
system for PCa, 9 samples were identified as well-differentiated
adenocarcinoma, 17 samples were moderately-differentiated
adenocarcinoma and 30 samples were poorly-differentiated
adenocarcinoma. Based on the American Jewett-Whitmore-Prout system,
4 samples were stage A, 8 samples were stage B, 11 samples were
stage C and 33 samples were stage D. A total of 10 further samples
were identified as benign prostatic hyperplasia (BPH). Patients
with BPH underwent open surgical resection and tissue specimens
obtained from these individuals were used as controls. These
patients were aged between 55 and 80 years, with a median age of 68
years. Patients had not been previously treated with radiotherapy,
chemotherapy or other treatments for cancer.
Immunohistochemistry
Tissue samples from the PCa and BPH specimens were
investigated using immunohistochemistry. Deparaffinized sections
were stained using hematoxylin and eosin (H&E). For
immunostaining of paraffin-embedded sections, the slides were
deparaffinized in xylene (Beijing Chemical Reagent Company,
Beijing, China) and rehydrated in a graded alcohol series.
Endogenous biotin was blocked using the SP-9000 kit (ZSGB-Bio,
Beijing, China). Immunostaining was performed with three
antibodies: Anti-DNMT1 mouse monoclonal antibody (cat. no. ab54759;
Abcam, Cambridge, UK; 1:500); anti-GSTP1 mouse monoclonal antibody
(cat. no. sc-376013; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA; 1:500) and anti-APC rabbit polyclonal antibody (cat. no.
sc-20987; Santa Cruz Biotechnology, Inc.; 1:500). Incubation with
the primary antibodies was performed in a humidified chamber at
37°C for 1 h. The goat anti-rabbit immunoglobulin G secondary
antibody (cat. no. SP9000; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China; 1:5,000) was then added
and incubated for 30 min at room temperature. The slides were
washed between steps with Tris-buffered saline. Immunoreactions
were visualized via a streptavidin-biotin complex, using a
3,3′-diaminobenzidine chromogenic kit (ZSGB-Bio). The specimens
were subsequently counter-stained with hematoxylin. The omission of
primary antibodies served as a negative control.
RNA isolation and semi-quantitative
reverse transcription-polymerase chain reaction (RT-PCR)
analysis
Total RNA was extracted from PCa and BPH specimens
using TRIzol reagent (Gibco-BRL, Gaithersburg, MD, USA). cDNA was
synthesized from 1 μg of RNA using the Thermoscript reverse
transcriptase system (Fermentas, Burlington, ON, Canada). DNMT1
mRNA was amplified The following primer sequences were used:
Forward 5′-CTA CCA GGG AGA AGG ACA GG-3′ and reverse: 5′-CTC ACA
GAC GCC ACA TCG-3′ for DNMT1, forward: 5′-GCC CTA CAC CGT GGT CTA
TT-3′ and reverse: 5′-GAC GCA GGA TGG TAT TGG A-3′ for GSTP1,
forward: 5′-CCA ACA AGG CTA CGC TAT-3′ and reverse: 5′-TCT GCT CGC
CAA GAC AAA-3′ for APC and forward: 5′-AGG CAT TGT GAT GGA CTC
CG-3′ and reverse: 5′-AGT GAT GAC CTG GCC GTC AG-3′ for β-actin.
β-actin was used as an internal control. The primer pair amplified
a 152 base pair (bp) fragment for DNMT1, a 209 bp fragment for
GSTP1, a 127 bp fragment for APC and a 301 bp fragment for β-actin.
PCR was performed in a thermal cycler (GeneAmp PCR system 9700;
Applied Biosystems, Foster City, CA, USA) for 35 cycles, consisting
of denaturation at 94°C for 30 sec, annealing at 65°C for 45 sec
(β-actin) or 54°C for 30 sec (DNMT1, GSTP1 and APC), and extension
at 72°C for 90 sec, followed by a final 5 min extension at 94°C.
The reaction products were loaded onto 1.5% agarose gels containing
ethidium bromide and visualized under Biospectrum 600 imaging
system (UVP, Upland, CA, USA). The band intensities of the PCR
products were analyzed using the UVP VisionWorks LS 6.6a system
(UVP) and are expressed as the mean ± standard deviation.
Methylation-specific PCR (MS-PCR)
Genomic DNA samples from PCa and BPH tissue were
isolated using a DNA extraction kit (Axygen Biotechnology,
Hangzhou, China). Genomic DNA (1 μg) was treated with sodium
bisulfite using the CpGenome™ DNA modification kit (Epigentek,
Brooklyn, NY, USA). MS-PCR was performed in the thermal cycler
(GeneAmp PCR system 9700; Applied Biosystems). For GSTP1, the
following primer sequences were used: Forward: 5′-GAT GTT TGG GGT
GTA GTG GTT GTT-3′ and reverse: 5′-CCA CCC CAA TAC TAA ATC ACA
ACA-3′ for unmethylated GSTP1 DNA and forward: 5′-TTC GGG GTG TAG
CGG TCG TC-3′ and reverse: 5′-GCC CCA ATA CTA AAT CAC GAC G-3′ for
methylated GSTP1 DNA. PCR amplification of the GSTP1 gene was
conducted as follows: 94°C for 5 min, followed by denaturation at
94°C for 30 sec, annealing at 58°C for 45 sec and extension at 72°C
for 90 sec, followed by a final 5 min extension at 94°C for 35
cycles. The primers amplified a 97(u)/93(m)-bp fragment,
respectively. For APC, the following primers were used: Forward:
5′-GTG TTT TAT TGT GGA GTG TGG GTT-3′ and reverse: 5′-AAC CAA TCA
ACA AAC TCC CAA CAA-3′ for unmethylated APC DNA and forward: 5′-TAT
TGC GGA GTG CGG GTC-3′ and reverse: 5′-TCG ACG AAC TCC CGA CGA-3′
for methylated APC DNA. PCR amplification of the APC gene was
conducted as follows: 94°C for 5 min, followed by denaturation at
94°°C for 30 sec, annealing at 59°C for 45 sec and extension at 72
for 90 sec, followed by a final 5 min extension at 94°C for 35
cycles. The primers amplified a 111(u)/98(m)-bp fragment,
respectively. The reaction products were loaded onto 2% agarose
gels containing ethidium bromide (Beijing Chemical Reagent Company)
and visualized under Biospectrum 600 imaging system (UVP). The band
intensities of the PCR products were analyzed using UVP VisionWorks
LS 6.6a (UVP) and are expressed as the mean ± standard
deviation.
Statistical analysis
Data was analyzed statistically using the SPSS 17.0
package (SPSS, Inc., Chicago, IL, USA). The results are expressed
as the mean ± standard deviation. The χ2 test and
Spearman’s rank correlation coefficient analysis were used to
assess the univariate association between the correlation of the
expression of DNMT1, GSTP1 and APC, and the methylation status of
GSTP1 and APC and the clinical significance of this marker.
P<0.05 was considered to indicate a statistically significant
difference.
Results
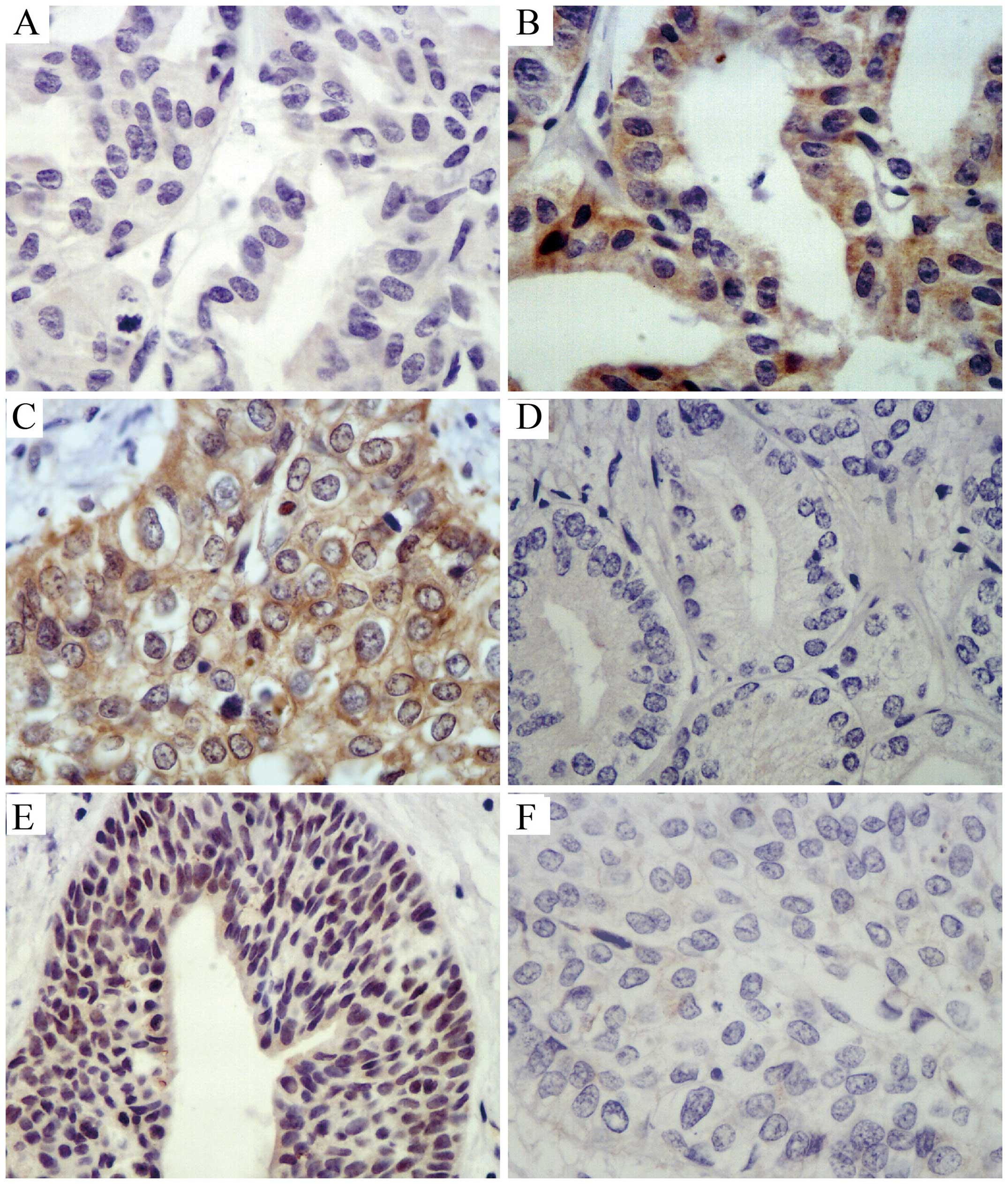
Immunohistochemical analysis of the
expression of DNMT1, GSTP1 and APC in PCa and BPH tissues
DNMT1 expression in the majority of cases of PCa was
characterized histopathologically by yellow to brown nuclear
staining, partial staining of the cytoplasm, with a focal or
diffuse distribution to the staining. GSTP1 was expressed
throughout the basal cell cytoplasm and exhibited partial nuclear
staining in BPH tissues, with minimal staining in the nucleus and
cytoplasm of cells from cancerous tissue. The APC protein was
expressed predominantly in the cytoplasm in BPH tissues, and was
negatively expressed in PCa tissues (Fig. 1, Table
I).
 | Table IExpression of DNMT1, GSTP1, APC
protein in prostate cancer and benign prostatic hyperplasia, as
determined by immunohistochemistry. |
Table I
Expression of DNMT1, GSTP1, APC
protein in prostate cancer and benign prostatic hyperplasia, as
determined by immunohistochemistry.
| Tissue | n | DNMT1 | GSTP1 | APC |
|---|
| BPH | 10 | 3 (30.0) | 9 (90.0) | 7 (70.0) |
| Well-differentiated
PCa | 9 | 5 (55.6) | 4 (44.4) | 5 (55.6) |
|
Moderately-differentiated PCa | 17 | 12 (70.6) | 5 (29.4) | 8 (47.6) |
| Poorly-differentiated
PCa | 30 | 26 (86.7)a | 4 (13.3)b | 7 (23.3)c |
Association of the expression of DNMT1,
GSTP1 and APC in PCa and BPH
DNMT1 expression and GSTP1, APC expression was
negatively correlated in PCa and BPH (rs=−0.891,
P<0.0001). Furthermore, DNMT1 expression and APC expression was
negatively correlated in PCa and BPH (rs=−0.721, P<0.0001; data
not shown).
Expression of DNMT1, GSTP1 and APC mRNA
in PCa and BPH
DNMT1 mRNA was highly expressed in the majority of
PCa tissues (the positive rate in poorly-differentiated tissue was
90.0%), whereas a low level of expression was observed in BPH
tissues (the positive rate was 40.0%). This difference was
statistically significant (P<0.05). GSTP1 and APC mRNA was
highly expressed in the majority of BPH tissues (the positive rates
were 90.0% and 80.0%, respectively), while a low level of
expression was observed in the majority of PCa tissues (the
positive rate in poorly-differentiated tissues was 23.3% and 33.3%,
respectively; P<0.05; Fig. 2
and Table II).
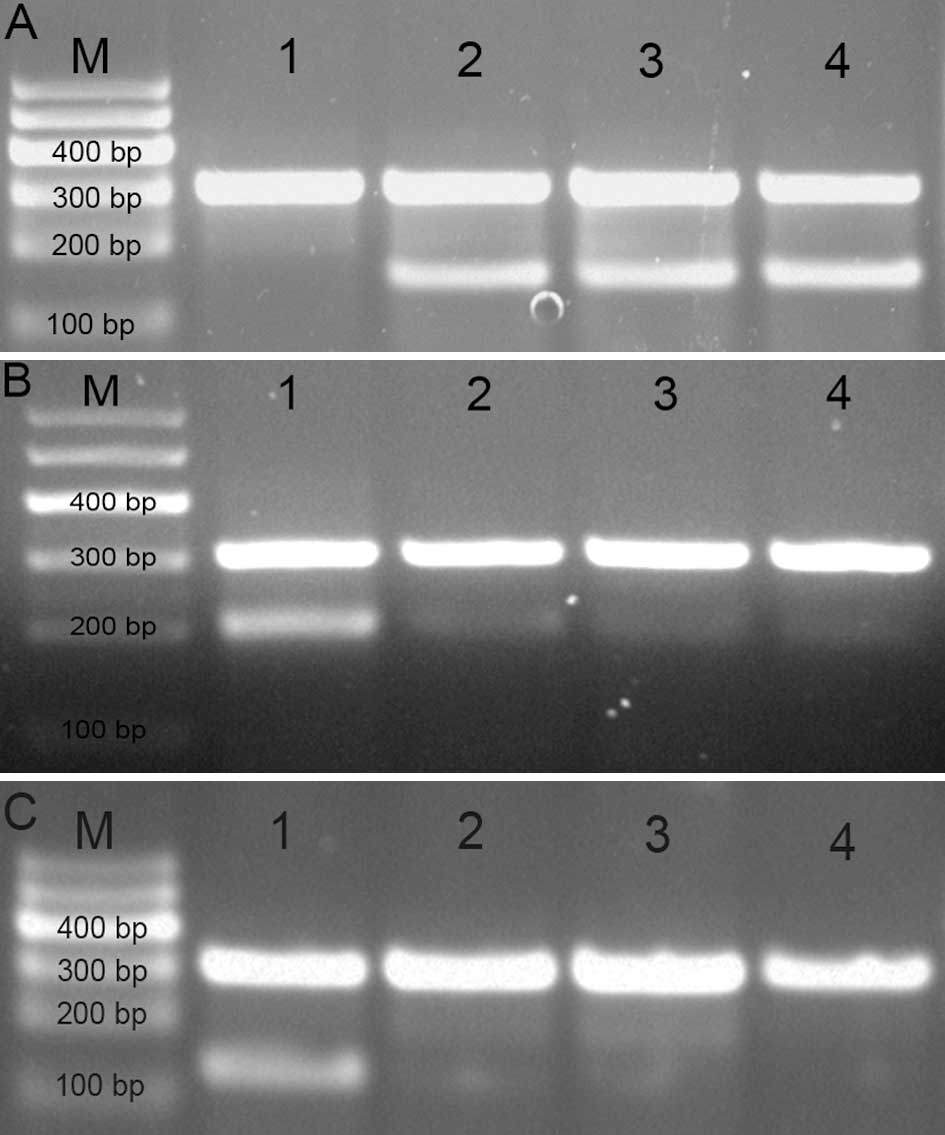 | Figure 2Expression of DNMT1, GSTP1 and APC
mRNA in PCa and BPH. (A) mRNA expression of DNMT1 mRNA (152 bp);
(B) GSTP1 mRNA (209 bp); and (C) APC mRNA (127 bp). The lanes are
as follows: M, marker; 1, BPH; 2, well-differentiated PCa; 3,
moderately- differentiated PCa; and 4, poorly-differentiated PCa.
DNMT1, DNA (cytosine-5)-methyltransferase 1; GSTP1, glutathione
S-transferase-P1; APC, adenomatous polyposis coli; PCa, prostate
cancer; BPH, benign prostatic hyperplasia. |
 | Table IIExpression of DNMT1, GSTP1, APC mRNA
in prostate cancer and benign prostatic hyperplasia (%), as
determined by semi-quantitative polymerase chain reaction. |
Table II
Expression of DNMT1, GSTP1, APC mRNA
in prostate cancer and benign prostatic hyperplasia (%), as
determined by semi-quantitative polymerase chain reaction.
| Tissue | n | DNMT1 mRNA | GSTP1 mRNA | APC mRNA |
|---|
| Poorly-differentiated
PCa | 30 | 27 (90.0) | 7 (23.3) | 10 (33.3) |
|
Moderately-differentiated PCa | 17 | 14 (82.4) | 7 (41.2) | 9 (52.9) |
| Well-differentiated
PCa | 9 | 6 (66.7) | 5 (55.6) | 7 (77.8) |
| BPH | 10 | 4 (40.0)a | 9 (90.0)a | 8 (80.0)a |
Methylation status of the GSTP1 and APC
genes in BPH and PCa tissues
The MS-PCR method was used to detect the methylation
status of the GSTP1 and APC genes in 10 samples of BPH tissue and
56 samples of PCa tissue. GSTP1 and APC exhibited hypermethylation
in the majority of the PCa samples (the positive rate in
poorly-differentiated tissues was 83.3% and 73.3%, respectively),
while hypomethylation (or demethylation) was observed in BPH
samples (the positive rate was 10.0% and 20.0%, respectively,
P<0.05; Fig. 3, Table III).
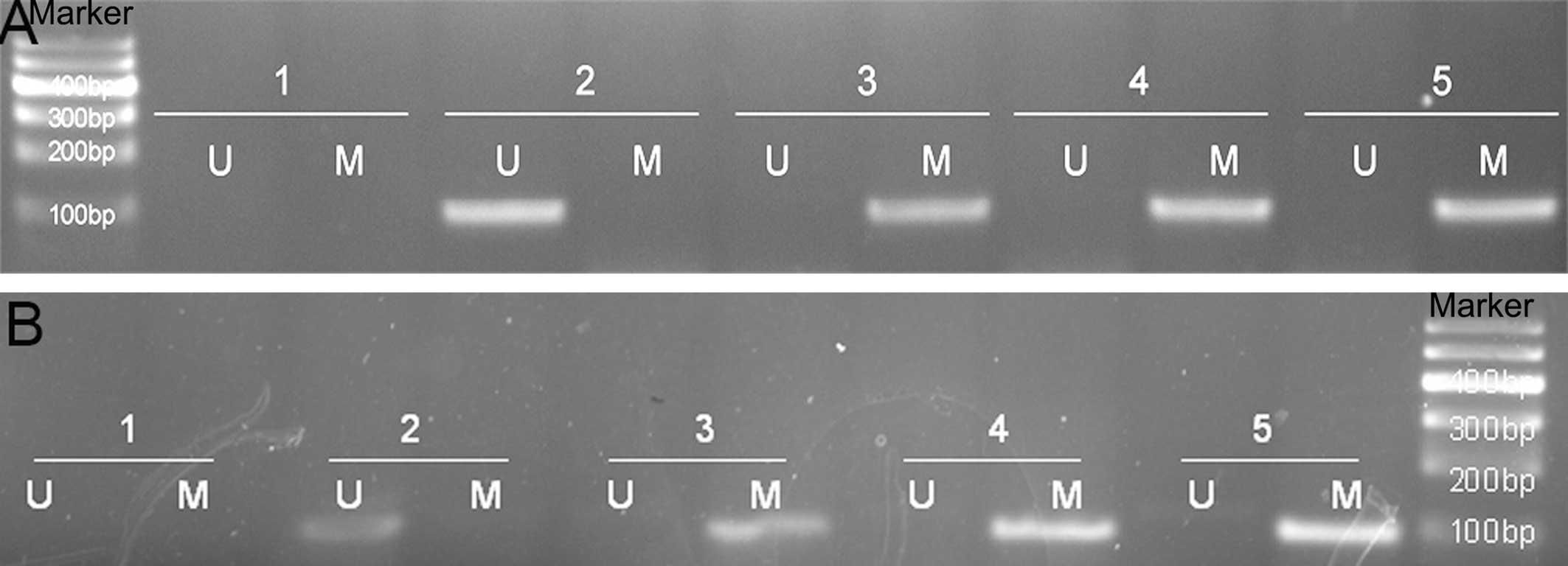 | Figure 3Methylation-specific polymerase chain
reaction analysis of the methylation status of GSTP1 and APC in BPH
and PCa. (A) methylation status of the GSTP1 (U: 97 bp; M: 93 bp);
(B) APC (U: 111 bp; M: 98 bp). The lanes are as follows: Marker; 1,
H2O; 2, BPH; 3, well-differentiated PCa; 4,
moderately-differentiated PCa; and 5, poorly-differentiated PCa. U,
unmethylated; M, methylated; BPH, benign prostatic hyperplasia;
DNMT1, DNA (cytosine-5)-methyltransferase 1; GSTP1, glutathione
S-transferase-P1; APC, adenomatous polyposis coli; PCa, prostate
cancer. |
 | Table IIIMethylation status of the GSTP1 and
APC genes in BPH and PCa (%), as determined by methylation-specific
polymerase chain reaction. |
Table III
Methylation status of the GSTP1 and
APC genes in BPH and PCa (%), as determined by methylation-specific
polymerase chain reaction.
| Tissue | n | mGSTP1 (+) | mAPC (+) |
|---|
| Poorly-differentiated
PCa | 30 | 25 (83.3) | 22 (73.3) |
|
Moderately-differentiated PCa | 17 | 11 (64.7) | 9 (52.9) |
| Well-differentiated
PCa | 9 | 5 (55.6) | 3 (33.3) |
| BPH | 10 | 1 (10.0)a | 2 (20.0)a |
The association between the expression of DNMT1, and
the methylation status of GSTP1 and APC in PCa tissue was assessed
using Spearman’s rank correlation analysis. This demonstrated a
significant positive correlation between DNMT1 expression and the
methylation status of GSTP1 in PCa tissue (rs=0.817,
P<0.0001). In addition, the correlation between DNMT1 expression
and the methylation status of APC in PCa tissues was also
significantly positive (rs= 0.671, P<0.0001)
Discussion
Within the human genome, 70–80% of all CpG
dinucleotides are methylated, and occur in repetitive and
non-transcribed DNA regions (9).
CpG dinucleotides also occur in transcribed DNA regions, which are
clustered in CpG islands. CpG islands (typically 0.5–2 kb long) are
located in the proximal promoter regions of numerous human genes
(10). Cancer-dependent epigenetic
regulation of genes involved in cell cycle control, DNA damage
repair, tumor-cell metastasis/adhesion and hormonal responses, in
addition to the silencing of these genes, is associated with tumor
initiation, development and progression, thereby, increasing the
risk of developing PCa (11).
The pattern of epigenetic change or altered gene
transcription/expression is important in a number of types of
cancer, including breast cancer and PCa (12). DNMTs transfer the methyl group from
s-adenosylmethionine, thereby generating patterns of genomic
methylation (13–15). To date, the following DNMTs have
been identified: DNMT1, DNMT2, DNMT3a, DNMT3b and DNMT3L (16). DNMT1 is primarily responsible for
the maintenance of DNA methylation. It has been observed in PCa,
that there are numerous CpG islands exhibiting hypermethylation. In
the present study, the expression of DNMT1 was examined in PCa and
BPH using immunohistochemical methods. The expression of DNMT1 mRNA
was lower in BPH than that in PCa tissues. In addition, the
positive rate was significantly higher in poorly-differentiated PCa
tissue, compared with that in BPH tissue, and this difference was
statistically significant (P<0.05). The positive rate of GSTP1
and APC mRNA expression in BPH tissues was higher than that in
poorly-differentiated PCa tissues; a difference that was also
statistically significant (P<0.05). DNMT1 expression increased
in PCa and this increased activity was associated with an increased
degree of malignancy in the PCa cells.
GSTP1 acts to protect cells from DNA damage and the
development of cancer, as it is associated with the detoxification,
metabolism and elimination of potentially genotoxic exogenous
compounds. Inhibition of the activity of GSTP1 may lead to
increased DNA damage and susceptibility to cancer (17,18).
The methylation of the GSTP1 gene promoter in PCa was first
reported in 1994 (19) and is the
most commonly detected epigenetic alteration, occurring in >90%
of cancerous samples and ~70% of prostatic intraepithelial
neoplasia (PIN) samples. However, it is rarely observed in BPH or
normal prostate tissues (20). It
has also been detected in proliferative inflammatory atrophy (PIA)
lesions, although the mechanism by which GSTP1 subsequently alters
methylation and silences transcription during progression from PIA
to PIN remains to be elucidated (21). The expression of GSTP1 was examined
in the BPH and PCa tissue samples using the same
immunohistochemical method as a previous study by this group
(22), in which it was
demonstrated that with an increased level of methylation, the
expression of GSTP1 is decreased, and a higher degree of malignancy
is observed. It is hypothesized that GSTP1 promoter CpG island
hypermethylation is one of the factors leading to its
inactivation.
APC is an important tumor suppressor gene located on
chromosome 5q21 (23). The APC
protein performs a number of functions, including the regulation of
β-catenin, which is an important component of the Wnt signaling
pathway. APC regulates β-catenin stability by mediating β-catenin
degradation. Loss of APC or β-catenin function leads to the
activation of certain downstream target genes, including cyclin D1,
c-myc and matrilysin (24). One
possible mechanism for this is functional inactivation of the APC
gene caused by promoter hypermethylation. In the present study, the
expression of APC was examined in BPH and PCa tissue samples using
an immunohistochemical method. It was shown that in PCa, compared
with BPH, the APC methylation levels are increased and its
expression is decreased. Thus, methylation may be associated with
the loss of expression of APC and may have an important role in the
development of PCa.
In conclusion, high expression of DNMT1 and low
expression of GSTP1 and APC in PCa, indicates that promoter region
hypermethylation of these genes is associated with tumor suppressor
gene inactivation. GSTP1 and APC promoter CpG island
hypermethylation, in particular that of GSTP1, may be used in the
molecular diagnosis of PCa as an early marker of this disease
(25). In addition, DNA
methylation of does not alter the nucleotide sequence. Instead, it
changes the level of chromosome structure and composition of
protein acetylation, gene transcription indirectly causes
inhibition (3). Thus, it is
hypothesized that if a reduction in DNMT activity has the potential
to restore the expression of certain tumor suppressor genes, an
alteration in the methylation of this gene may be of use in the
development of novel cancer therapies.
Acknowledgments
This study was supported by the Henan Science and
Technology Committee Grant (grant no. 052SGYS33209) and the Young
Foundation of the First Affiliated Hospital of Zhengzhou
University.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodriquez C, Calle EE, Miracle-McMahill
HL, Tatham LM, Wingo PA, Thun MJ and Heath CW Jr: Family history
and risk of fatal prostate cancer. Epidemiology. 8:653–657. 1997.
View Article : Google Scholar
|
|
3
|
Neugut AI, Chen AC and Petrylak DP: The
skinny on obesity and prostate cancer prognosis. J Clin Oncol.
22:395–398. 2004. View Article : Google Scholar
|
|
4
|
Esteller M, Corn PG, Baylin SB and Herman
JG: A gene hypermethylation profile of human cancer. Cancer Res.
61:3225–3229. 2001.PubMed/NCBI
|
|
5
|
Li LC, Okino ST and Dahiya R: DNA
methylation in prostate cancer. Biochim Biophys Acta. 1704:87–102.
2004.PubMed/NCBI
|
|
6
|
Richiardi L, Fiano V, Vizzini L, De Marco
L, Delsedime L, Akre O, Tos AG and Merletti F: Promoter methylation
in APC, RUNX3, and GSTP1 and mortality in prostate cancer patients.
J Clin Oncol. 27:3161–3168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang GH, Lee S, Lee H, Hwang J and
Aberrant KS: CpG island hypermethylation of multiple genes in
prostate cancer and prostatic intraepithelial. Neoplasia J Pathol.
202:233–240. 2004. View Article : Google Scholar
|
|
8
|
Phé V, Cussenot O and Rouprêt M:
Methylated genes as potential biomarkers in prostate cancer. BJU
Int. 105:1364–1370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoder JA, Walsh CP and Bestor TH: Cytosine
methylation and the ecology of intragenomic parasites. Trends
Genet. 13:335–340. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bird AP: CpG-rich islands and the function
of DNA methylation. Nature. 321:209–213. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gardiner-Garden M and Frommer M: CpG
islands in vertebrate genomes. J Mol Biol. 196:261–282. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baylin SB and Ohm JE: Epigenetic gene
silencing in cancer-a mechanism for early oncogenic pathway
addiction? Nat Rev Cancer. 6:107–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ross SA: Diet and DNA methylation
interactions in cancer prevention. Ann NY Acad Sci. 983:197–207.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brenner C and Fuks F: DNA
methyltransferases: facts, clue, mysteries. Curr Top Microbiol
Immunol. 301:45–66. 2006.
|
|
15
|
Gopisetty G, Ramachandran K and Singal R:
DNA methylation and apoptosis. Mol Immunol. 43:1729–1740. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singal R and Ginder GD: DNA methylation.
Blood. 93:4059–4070. 1999.PubMed/NCBI
|
|
17
|
Berhane K, Widersten M, Engstrom A,
Kozarich JW and Mannervik B: Detoxication of base propenals and
other alpha, betaunsaturated aldehyde products of radical reactions
and lipid peroxidation by human glutathione transferases. Proc Natl
Acad Sci USA. 91:1480–1484. 1994. View Article : Google Scholar
|
|
18
|
Nelson CP, Kidd LC, Sauvageot J, Isaacs
WB, De Marzo AM, Groopman JD, Nelson WG and Kensler TW: Protection
against 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine
cytotoxicity and DNA adduct formation in human prostate by
glutathione S-transferase P1. Cancer Res. 61:103–109.
2001.PubMed/NCBI
|
|
19
|
Lee WH, Morton RA, Epstein JI, Brooks JD,
Campbell PA, Bova GS, Hsieh WS, Isaacs WB and Nelson WG: Cytidine
methylation of regulatory sequences near the pi-class glutathione
S-transferase gene accompanies human prostatic carcinogenesis. Proc
Natl Acad Sci USA. 91:11733–11737. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brooks JD, Weinstein M, Lin X, Sun Y, Pin
SS, Bova GS, Epstein JI, Isaacs WB and Nelson WG: CG island
methylation changes near the GSTP1 gene in prostatic
intraepithelial neoplasia. Cancer Epidemiol Biomark Prev.
7:531–536. 1998.
|
|
21
|
Nakayama M, Bennett CJ, Hicks JL, Epstein
JI, Platz EA, Nelson WG and De Marzo AM: Hypermethylation of the
human glutathione S-transferase-pi gene (GSTP1) CpG island is
present in a subset of proliferative inflammatory atrophy lesions
but not in normal or hyperplastic epithelium of the prostate: a
detailed study using laser-capture microdissection. Am J Pathol.
163:923–933. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin X, Tascilar M, Lee WH, et al: GSTP1
CpG island hypermethylation is responsible for the absence of GSTP1
expression in human prostate cancer cells. Am J Pathol.
159:1815–1826. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thliveris A, Albertsen H, Tuohy T, Carlson
M, Groden J, Joslyn G, Gelbert L, Samowitz W, Spirio L and White R:
Long-range physical map and deletion characterization of the
1100-kb NotI restriction fragment harboring the APC gene. Genomics.
34:268–270. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fearnhead NS, Britton MP and Bodmer WF:
The ABC of APC. Hum Mol Genet. 10:721–733. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Woodson K, O’Reilly KJ, Hanson JC, et al:
The usefulness of the detection of GSTP1 methylation in urine as a
biomarker in the diagnosis of prostate cancer. J Urol. 179:508–511.
2008. View Article : Google Scholar
|