Introduction
Ovarian cancer accounts for 3% of cases of cancer in
females and is the leading cause of gynecological
malignancy-related mortality (1,2).
Approximately 70% of patients with ovarian cancer are not diagnosed
until the late stages (stages III and IV), due to the lack of
characteristic symptoms and effective screening methods (3). Although histologically, ovarian
cancer encompasses multiple different types, epithelial ovarian
cancer (EOC) accounts for the majority of malignant ovarian tumors
and can further be classified into eight distinct histological
subtypes (4). The standard
treatment for EOC is cytoreduction, with first-line platinum-based
chemotherapy. Although EOC is highly responsive to the initial
chemotherapy, the majority of patients experience relapse, due to
the intrinsic and acquired resistance of the cancer cells to
cytotoxic drugs, and the 5-year survival rate is <30% for
patients in the advanced stages (5,6).
Therefore, novel strategies that enhance sensitivity or reverse
resistance to chemotherapy are urgently required for patients with
EOC, particularly those that develop recurrent disease.
There are numerous non-protein-coding RNA (ncRNA)
genes in the human genome. In contrast to other types of ncRNAs,
such as microRNAs, whose roles have been greatly investigated,
little is known regarding long ncRNAs (7,8). The
various roles served by the long ncRNAs in malignant transformation
and tumor growth are becoming increasingly recognized (9). Among these long ncRNAs exists one
that was identified by a custom tilling array of the HOXC locus
(12q13.13) (2), termed Hox
transcript antisense intergenic RNA (HOTAIR).
HOTAIR has been observed to be overexpressed in
several types of tumorous tissues, including breast, colorectal,
hepatocellular, pancreatic and non-small cell lung cancer, compared
with the levels in normal tissues. The high expression level has
been observed to be correlated with increased cell invasiveness and
enhanced cancer metastasis (10–14).
A previous study has demonstrated that HOTAIR represses the
transcription of specific genes by binding to the poly-comb
repressive complex (PRC)2, retargeting it to the locus and leading
to H3K27me3 (10). Studies have
suggested that HOTAIR-mediated suppression of genes in cancer is
PRC2-dependent and PRC2-independent (10,13,15).
Thus far, HOTAIR is recognized as a negative prognostic factor in
various types of cancer, but the role HOTAIR serves, and its
association with chemoresistance, remain to be investigated in
EOC.
The aim of the present study was to investigate the
role of HOTAIR in epithelial ovarian cancer and the correlation
between HOTAIR and cisplatin resistance.
Materials and methods
Patient samples
A total of 80 freshly frozen ovarian specimens were
obtained with informed consent from patients at the time of
surgery, including 50 samples from patients with primary EOC and 30
samples of benign ovarian tissues for controls, obtained during
hysterectomies for benign diseases. Surgery was performed at the
Department of Obstetrics and Gynecology of the Second Affiliated
Hospital of Harbin Medical University (Harbin, China) between
October 2011 and November 2012. None of the participants had
received chemotherapy prior to surgery. Patient data, including
histo-pathological diagnosis, tumor grade and FIGO stages were
obtained from medical records. The present study was approved by
the ethics committee of Harbin Medical University.
Cells and reagents
The SKOV-3CDDP/R cisplatin-resistant epithelial
ovarian cancer cell line and its parental variant SKOV-3 were
purchased from the cell collection of the China Academy of Chinese
Medical Sciences (Beijing, China). All cells were cultured in
HyClone RPMI-1640 containing 10% HyClone fetal bovine serum medium
(GE Healthcare Life Sciences, Logan, UT, USA) with 1% penicillin
and streptomycin (Genom Biotech Pvt., Ltd., Shanghai, China) in a
humidified 5% CO2 atmosphere at 37°C. Cisplatin was
purchased from Beyotime Institute of Biotechnology (Shanghai,
China) and administered to cells at doses of 0, 2.5, 5, 10 and 20
μg/ml.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from frozen tissues or cell
lines using TRIzol reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. cDNA was
synthesized using Oligo(dT)15 primers with M-MLV Reverse
Transcriptase (Promega Corporation, Madison, WI, USA). HOTAIR
expression levels were evaluated using qPCR with the AccuPower 2X
GreenStar master mix solution (Bioneer Corporation, Daejeon, Korea)
in a StepOnePlus Real-Time PCR system (Applied Biosystems Life
Technologies, Foster City, CA, USA). The primer sequences for
HOTAIR and β-actin (internal control) were as follows: Forward:
5′-TGGGGAACTCTGACTCGC-3′ and reverse: 5′-TCGCCGCCGTCTGTAACT-3′ for
HOTAIR; and forward: 5′-GTCAGGTCATCACTATCGGCAAT-3′ and reverse:
5′-AGAGGTCTTTACGGATGTCAACGT-3′ for β-actin. The reaction conditions
were as follows: 5 min at 94°C, followed by 40 cycles of 94°C for
30 sec, 56°C for 30 sec and 72°C for 30 sec. The melting curves for
the two genes were analyzed to confirm the purity of the amplified
products. Samples were analyzed in triplicate in three independent
experiments. HOTAIR expression values were normalized to β-actin.
Relative gene expression was calculated using the 2−ΔΔCt
method.
Transfection of siRNA
siRNA oligonucleotides (50 nM) were transfected into
the two types of cells with Lipofectamine 2000 transfection reagent
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. The transfection solution was removed after 6 h of
incubation and replaced with fresh growth medium. After 48-h
incubation, the cells were assayed for the expression level of
HOTAIR post-transfection. An siRNA targeting HOTAIR and a negative
control (NC) siRNA (silencer NC siRNA) were purchased from Bioneer
Corporation. The sequences of the siRNAs were as follows: siHOTAIR:
5′-UCAGUGUCAGAAAAUGCUU-3′ and siNC: 5′-CCUACGCCAAUUUCGU-3′.
Analysis of cell viability and
apoptosis
WST-8 dye from a Cell Counting kit-8 (CCK-8;
Beyotime Institute of Biotechnology) was used to detect cell
viability according to the manufacturer’s instructions. Cell
staining was conducted with an Annexin V-FITC Apoptosis Detection
kit, including propidium iodide (BD Biosciences, San Jose, CA,
USA). Staining was quantified by flow cytometry analysis using a
FACSCalibur cell sorting system (BD Biosciences). Cells in the
early and late stages of apoptosis were evaluated. Samples were
analyzed in triplicate in three independent experiments.
Analysis of cell invasiveness
A cell-invasion assay was conducted using a Costar
24-well Transwell chamber with a pore size of 8 μm (Corning
Life Sciences, Corning, NY, USA), and the upper membrane surfaces
were coated with 30 μl Matrigel (1:2 dilution; BD
Biosciences). Cells (1×105) were seeded in the
compartment chamber in serum-free medium subsequent to RNA
interference. The lower compartment was filled with cell culture
medium (RPMI-1640; HyClone) supplemented with 10% FBS. After 24 h,
cells on the upper membrane surface were removed and the invaded
cells on the bottom surface were fixed with methanol and stained
with 1% crystal violet. The invading cells were examined, counted
and images were captured using digital microscopy (P6000; Nikon
Corporation, Tokyo, Japan). Four fields were counted per filter in
each chamber.
Statistical analysis
The differences in the continuous data between two
groups were analyzed with the independent t-test and results
expressed as the mean ± standard deviation. The analysis of HOTAIR
expression levels in the different groups of ovarian tissues was
performed using nonparametric tests (Mann-Whitney U test or
Kruskal-Wallis H test). P<0.05 was considered to indicate a
statistically significant difference. Each experiment was conducted
at least three times in triplicate. Statistical analyses were
performed with SPSS software, version 16.0 for Windows (SPSS, Inc.,
Chicago, IL, USA).
Results
Expression of HOTAIR in clinical ovarian
tissues
In the current study, RT-qPCR was performed in order
to define the expression levels of HOTAIR in the clinical ovarian
tissues. Initially, the tissues were divided into two groups: The
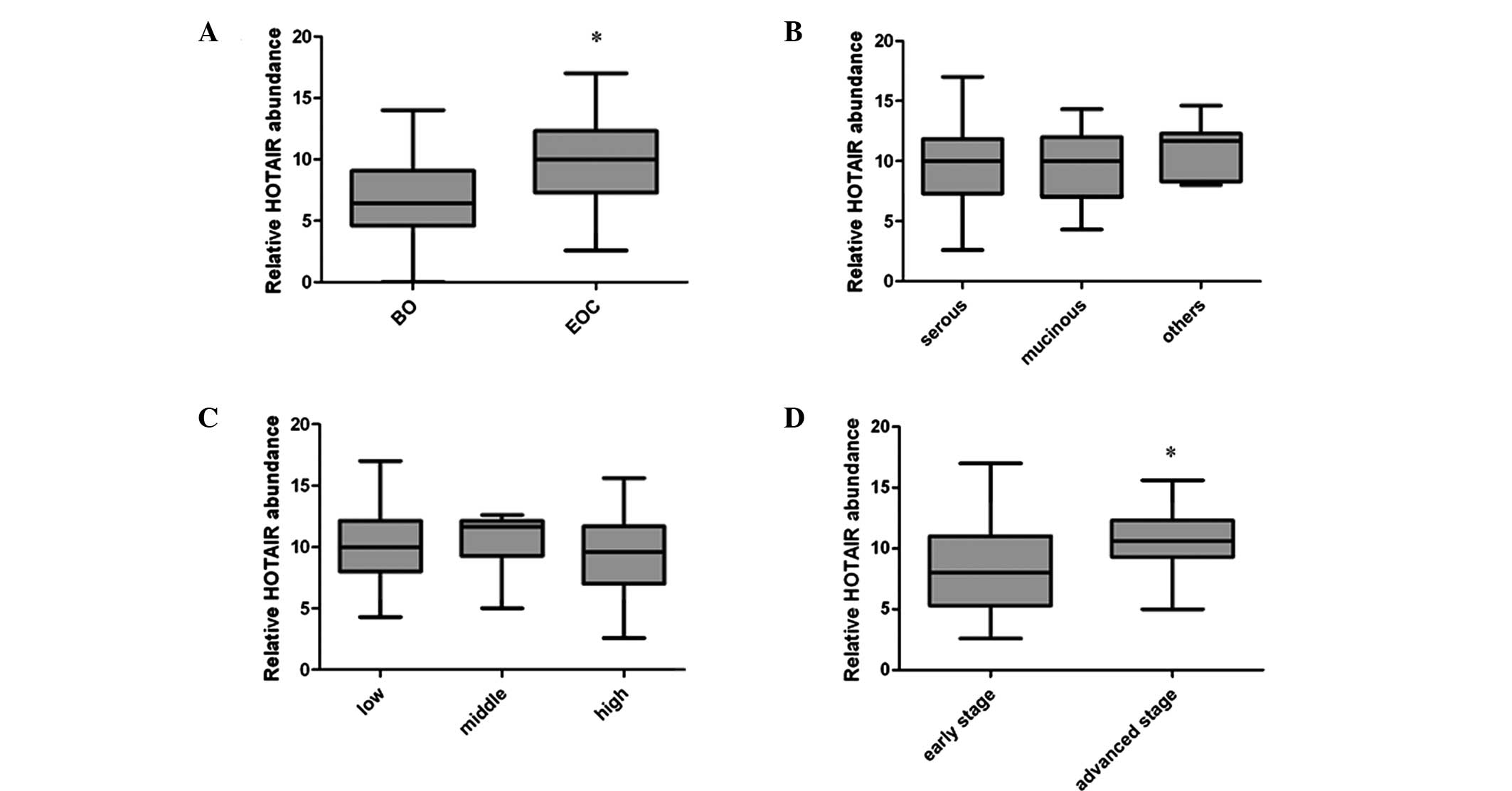
benign ovarian (BO) and EOC tissues (Fig. 1A). The results indicated that the
HOTAIR expression level was significantly higher in the EOC group
compared with the BO group (P<001). Next, the HOTAIR levels in
the 50 cases of primary malignant ovarian tissues were compared.
The tissues were divided into groups according to tumor subtype,
tumor grade and tumor stage. The observed differences in expression
levels between the different tumor subtypes or tumor grade were not
significant (P=0.466 and 0.342, respectively; Fig. 1B and C). However, a significant
difference in expression levels was identified between samples from
the early and advanced stages of EOC (P=0.027), the HOTAIR
expression level in stage III and IV EOC was significantly higher
than that of the stage I and II EOC samples (Fig. 1D).
Knockdown of HOTAIR suppresses cell
proliferation in SKOV-3 cells
To determine the effects of the HOTAIR expression
level on EOC cells, an RNA interference assay was used to modulate
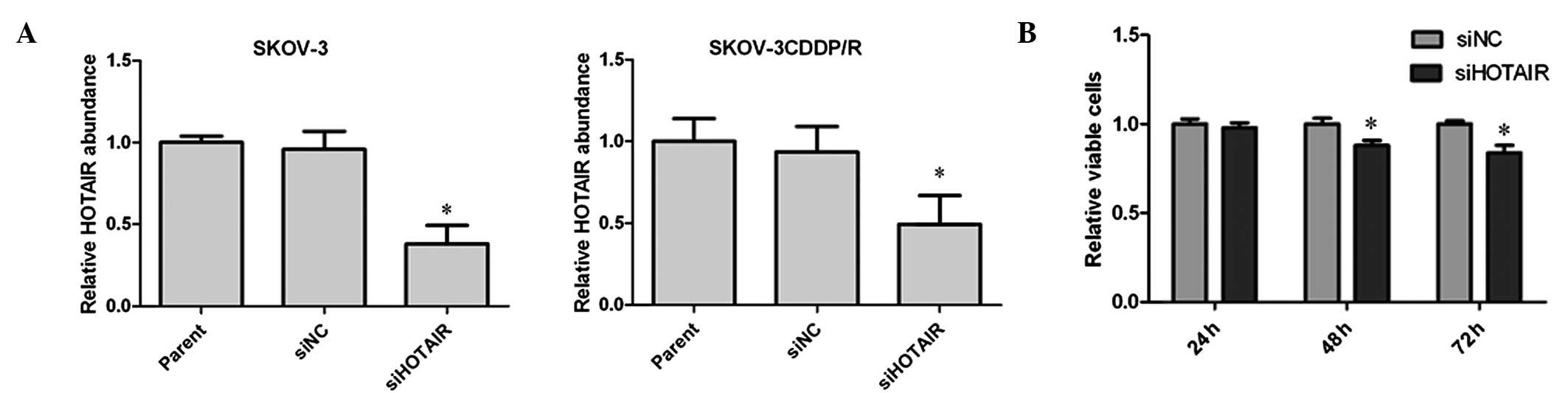
the expression of HOTAIR. As presented in Fig. 2A, the endogenous HOTAIR levels
determined by RT-qPCR in the two cell lines were effectively
reduced by siHOTAIR treatment, but not by siNC treatment. Next, the
cell viability was assessed using the CCK-8 assay kit at different
time points following transfection in SKOV-3 cells. The results
indicated that the effective knockdown of HOTAIR significantly
inhibited cell proliferation at 48 and 72 h (Fig. 2B).
Cisplatin-induced cytotoxicity in SKOV-3
and SKOV-3CDDP/R cell lines
In order to examine the association of HOTAIR
expression patterns with drug sensitivity, the cisplatin-resistant
cell line SKOV-3CDDP/R and its parental cell line SKOV-3 were
selected. The CCK-8 assay was employed to compare the effects of
cisplatin on the proliferation of the SKOV-3 and SKOV-3CDDP/R
cells. A range of doses (0–20 μg/ml) of cisplatin were
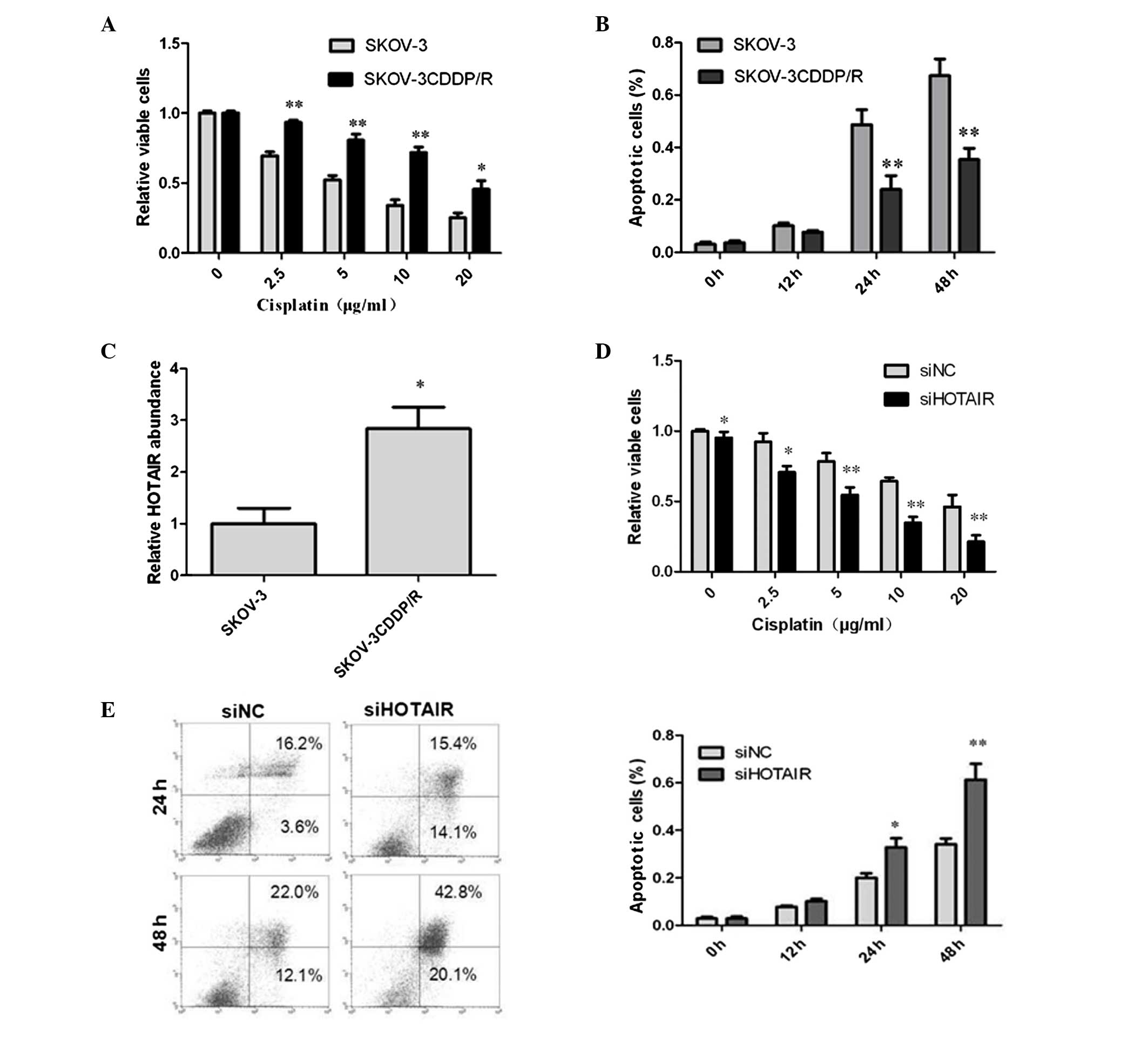
administered to the cells for 24 h. The results indicated that the
viabilities of the SKOV-3 and SKOV-3CDDP/R cells were reduced by
cisplatin in a dose-dependent manner (Fig. 3A). Additionally, SKOV-3CDDP/R cells
exhibited greater resistance to cisplatin than the SKOV-3 cells. As
evaluated using the IC50 values, SKOV-3 cells exhibited a reduction
of ~4-fold in viability compared with SKOV-3CDDP/R cells
(P<0.05). The greatest difference in cisplatin-induced
cytotoxicity between the two types of cells was observed when cells
were exposed to 5 μg/ml cisplatin (P<0.001). This result
verifies the chemosensitivity of the two cell lines; cisplatin
resistance in SKOV-3CDDP/R cells and cisplatin sensitivity in the
parental SKOV-3 cells. In order to further assess the cisplatin
sensitivity, flow cytometry was used to define cisplatin-induced
apoptosis in the cells with 5 μg/ml cisplatin at different
time points (Fig 3B). The results
demonstrated that there was a greater level of cisplatin-induced
apoptosis in the SKOV-3 cells compared with the SKOV-3CDDP/R
cells.
HOTAIR is more abundant in resistant
cells than in cisplatin-sensitive cells
The HOTAIR expression level was examined by qPCR in
the paired cell lines. There was a significant difference in the
levels of HOTAIR expression between the two cell lines (Fig. 3C). The expression level of HOTAIR
in SKOV-3CDDP/R cells was significantly greater than that in SKOV-3
cells (>2.5 fold) indicating that HOTAIR may be a factor in the
reduction of chemosensitivity in SKOV-3CDDP/R cells.
Knockdown of HOTAIR restores the
cisplatin sensitivity of cisplatin-resistant cells
The siRNAs were utilized to investigate the effects
of RNA interference on the cisplatin sensitivity of the
SKOV-3CDDP/R cisplatin-resistant cell line. Following the transient
transfection of siRNAs, the cells were treated with various doses
of cisplatin (0–20 μg/ml). After 24 h, the CCK-8 assay was
applied to assess the cell viability as in the prior assay. For the
groups of cells exposed to 0 μg/ml cisplatin for 24 h,
siHOTAIR-treated cells displayed a significant difference in cell
viability compared with siNC treated cells (P=0.48). However, with
greater concentrations of cisplatin, the difference in the cell
viability distinctly increased between the two groups. These
results demonstrate that the cells treated with siHOTAIR were more
sensitive to cisplatin than the cells treated with siNC (Fig 3D). It is clear that the knockdown of
HOTAIR suppressed levels of cell proliferation and more notably
resensitized the SKOV-3CDDP/R cells to cisplatin. To further
investigate the role of HOTAIR in drug sensitivity, flow cytometry
was used to define cisplatin-induced apoptosis following
transfection of SKOV-3CDDP/R cells with cisplatin (5 μg/ml)
at different time points (Fig 3E).
The results indicated that the level of apoptosis was higher in the
HOTAIR knockdown group compared with the negative control group,
and the differences increased in a time-dependent manner. Hence,
the alteration of HOTAIR expression levels in ovarian carcinoma
cells markedly alters cisplatin-induced cytotoxicity and
susceptibility to apoptosis.
Knockdown of HOTAIR reduces the invasion
ability of ovarian carcinoma cells
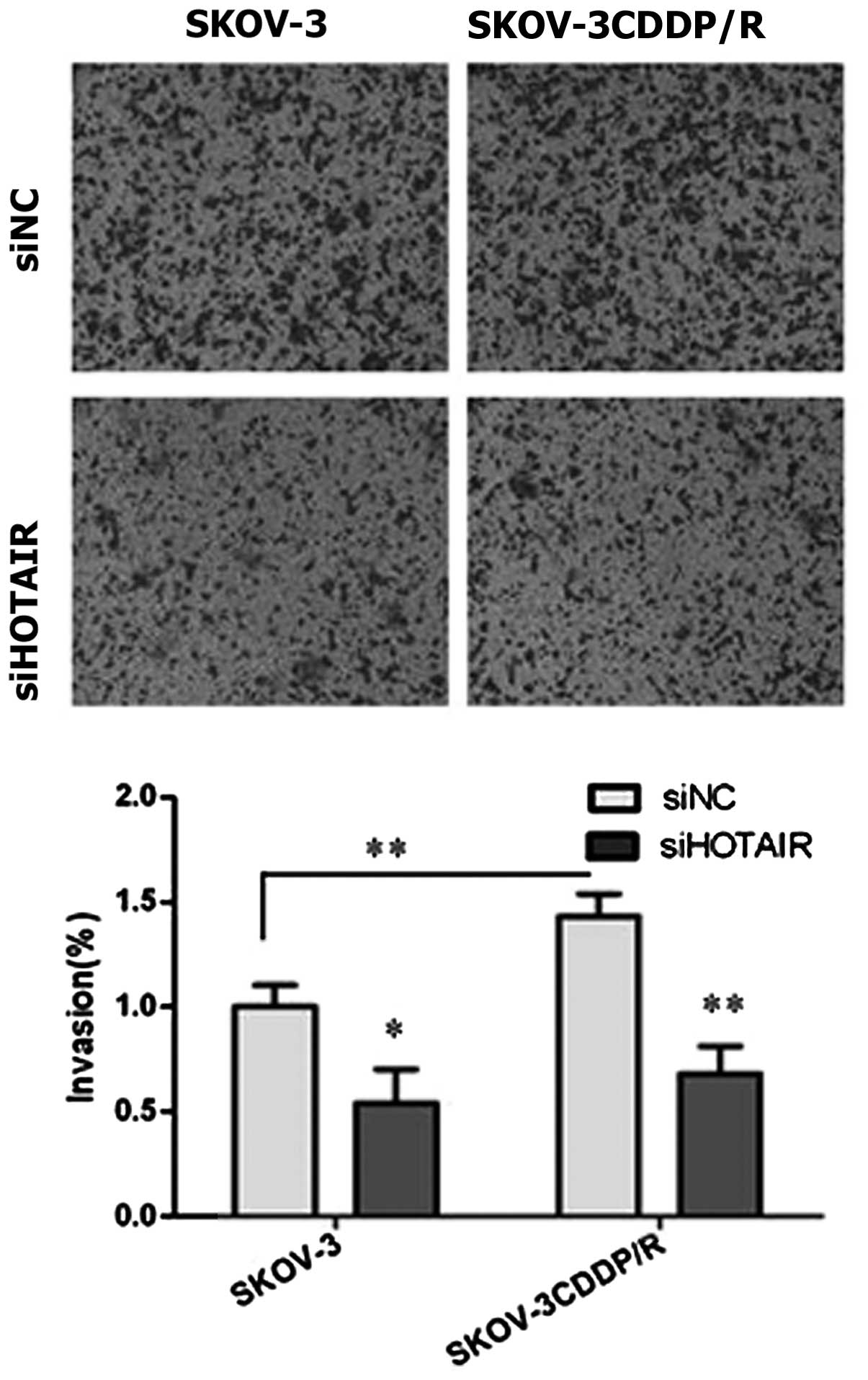
Next, the invasiveness of the cells was assessed by
a Transwell invasion assay following the knockdown of HOTAIR
(Fig. 4). The invasion capacities
were markedly reduced when HOTAIR expression was knocked down in
vitro using siRNAs in the SKOV-3 (P=0.035) and SKOV-3CDDP/R
(P<0.01) cells, as indicated by the reduced number of cells
invading through the Matrigel-coated membrane. Additionally, the
cisplatin-resistant SKOV-3CDDP/R cells that exhibited higher levels
of HOTAIR expression displayed a stronger ability for invasion
compared with the parental counterpart (P<0.01).
Discussion
Previous studies have demonstrated that HOTAIR is
overexpressed in several types of tumor tissues at a greater level
than in corresponding non-tumor tissues. One study also indicated
that HOTAIR is highly expressed in aggressive and invasive
pancreatic tumors (10–13). To the best of our knowledge, the
current study demonstrated for the first time that the expression
levels of HOTAIR in ovarian tissues are also associated with the
malignant phenotype (P<0.001). The differences in expression
levels at different tumor stages were also analyzed. The results
demonstrated that there was a significant difference in the levels
of HOTAIR in early and late stage malignant ovarian tumors. The
stage of a tumor is associated with the recurrence of ovarian
cancer, as advanced diseases present with a greater rate of
recurrence within two years (16).
The current in vitro study has also indicated that HOTAIR
knockdown inhibits cell proliferation (P<0.05), suggesting that
HOTAIR promotes tumor progression and is associated with tumor
recurrence.
To further investigate the function of HOTAIR in
ovarian cancer recurrence, it is necessary to begin with
chemoresistance. The recurrence of ovarian cancer is mainly
attributed to the chemoresistance of tumor cells to conventional
chemotherapy, which may be due to reduced drug accumulation,
increased levels of glutathione and metallothionein and enhanced
DNA repair (17–19). In the present study, the HOTAIR
level was demonstrated to be increased in the SKOV-3CDDP/R
cisplatin-resistant cell line compared with the SKOV-3
cisplatin-sensitive cell line. These two cell lines have similar
genetic backgrounds with minor variations as the SKOV-3CDDP/R
cisplatin-resistant cell line was established by repeated in
vitro treatment of SKOV-3 cells with a clinically relevant dose
of cisplatin (20). For this
reason they were superior to other cell lines and were selected as
paired models for an in vitro investigation of drug
resistance. The silencing of HOTAIR by siRNA alone did not markedly
reduce the cell viability (reduced by <10%). However, once the
two groups were treated with cisplatin, the siHOTAIR group
demonstrated enhanced sensitivity to cisplatin indicated by
significantly reduced cell viability and an elevated rate of
apoptosis. The SKOV-3CDDP/R cells were resensitized to cisplatin
subsequent to the knockdown of HOTAIR. This indicates that HOTAIR
may not only promote tumor proliferation, but also can strengthen
the effects of antitumor drugs. This result suggests that elevated
HOTAIR expression may be responsible for drug resistance in
patients with recurrent ovarian cancer and the interference of
HOTAIR may restore this sensitivity. In addition, the preoperative
quantification of HOTAIR in tumor biopsies or ascitic fluid may aid
in predicting the tumor chemosensitivity. Although the present
study observed that the knockdown of HOTAIR resensitized
SKOV-3CDDP/R cells by inhibiting cisplatin-induced apoptosis
(Fig. 3E), further studies are
required to explore the influence of HOTAIR expression levels on
the chemosensitivity of ovarian cancer and associated tumor
recurrence. The microarray analysis between primary and recurrent
ovarian cancer may aid the investigation of the underlying
molecular mechanisms.
The present study also demonstrated that the
selective knockdown of HOTAIR markedly weakens the invasion
capacity of the two cell lines. The cisplatin-resistant cells with
higher HOTAIR expression presented higher invasion ability than
their counterparts, indicating that the HOTAIR expression level is
positively correlated to the invasion capability of ovarian
carcinoma cells with similar genetic backgrounds. This result
further verifies the fact that HOTAIR is a notable factor
associated with tumor invasion, and it is important in the
development of metastases.
Though ovarian cancer has been well-investigated,
the pathogenesis of ovarian cancer remains unclear. There are
multiple theories involving the pathogenesis of ovarian cancer; the
upsurging cancer stem cell (CSC) hypothesis is attracting an
increasing level of attention. According to this theory, cancer is
a mixed aberrant hierarchical organization of the non-tumorigenic
progeny of CSCs and the tumorigenic CSCs, which have the stem-like
ability of self-renewal and are able to generate heterogeneous
lineages of cells (21,22). This CSC hypothesis can also be
applied to recurrent ovarian cancer growth, since the stem-like
properties enable the cancer to select for more aggressive and
chemoresistant cells subsequent to chemo- and radiotherapy
(23,24). HOTAIR regulates chromatin silencing
on the HOXD locus by retargeting the PRC2, which contains three
subunits termed EZH2, EED and SUZ12. EZH2 is reported to be an
essential regulator of embryonic and adult stem cell
differentiation, and SUZ12 is also required for embryonic stem cell
differentiation (25–27). There are also studies indicating
that HOTAIR is required for the maintenance of stemness in cancer
cells lines, involving EMT triggering (28). Future studies may focus on the role
of HOTAIR in CSCs in order to assess whether the HOTAIR-mediated
retargeting of PRC2 is responsible for the differentiation of
CSCs.
In conclusion, HOTAIR serves important roles in
ovarian cancer progression, including increasing cell proliferation
and promoting cell invasion. The examination of HOTAIR levels in
tumors has the potential to be used to complement to diagnosis, as
a negative factor in prognosis and as a predictor for the risk of
recurrence.
References
|
1
|
Jemal A, Thomas A, Murray T and Thun M:
Cancer statistics. CA Cancer J Clin. 52:23–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertone-Johnson ER: Epidemiology of
ovarian cancer: a status report. Lancet. 365:101–102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bell DA: Origins and molecular pathology
of ovarian cancer. Mod Pathol. 18(Suppl 2): S19–S32. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sood AK and Buller RE: Drug resistance in
ovarian cancer: from the laboratory to the clinic. Obstet Gynecol.
92:312–319. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGuire WP, Hoskins WJ, Brady MF, et al:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mattick JS: The genetic signatures of
noncoding RNAs. PLoS Genet. 5:e10004592009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yasuda J and Hayashizaki Y: The RNA
continent. Adv Cancer Res. 99:77–112. 2008.
|
|
9
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gupta RA, Shah N, Wang KC, Kim J, et al:
Long non-coding RNA HOTAIR reprograms chromatin state to promote
cancer metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Z, Zhou L, Wu LM, et al:
Overexpression of long non-coding RNA HOTAIR predicts tumor
recurrence in hepato-cellular carcinoma patients following liver
transplantation. Ann Surg Oncol. 18:1243–1250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kogo R, Shimamura T, Mimori K, Kawahara K,
et al: Long noncoding RNA HOTAIR regulates polycomb-dependent
chromatin modification and is associated with poor prognosis in
colorectal cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim K, Jutooru I, Chadalapaka G, et al:
HOTAIR is a negative prognostic factor and exhibits pro-oncogenic
activity in pancreatic cancer. Oncogene. 32:1616–1625. 2013.
View Article : Google Scholar
|
|
14
|
Liu XH, Liu ZL, Sun M, Liu J, Wang ZX and
De W: The long non-coding RNA HOTAIR indicates a poor prognosis and
promotes metastasis in non-small cell lung cancer. BMC Cancer.
13:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, et al: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gadducci A, Cosio S, Zola P, et al:
Surveillance procedures for patients treated for epithelial ovarian
cancer: a review of the literature. Int J Gynecol Cancer. 17:21–31.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Godwin AK, Meister A, O’Dwyer PJ, Huang
CS, Hamilton TC and Anderson ME: High resistance to cisplatin in
human ovarian cancer cell lines is associated with marked increase
of glutathione synthesis. Proc Natl Acad Sci USA. 89:3070–3074.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kelley SL, Basu A, Teicher BA, Hacker MP,
Hamer DH and Lazo J: Overexpression of metallothionein confers
resistance to anticancer drugs. Science. 241:1813–1815. 1998.
View Article : Google Scholar
|
|
19
|
Parker RJ, Eastman A, Bostick-Bruton F and
Reed E: Acquired cisplatin resistance in human ovarian cancer cells
is associated with enhanced repair of cisplatin-DNA lesions and
reduced drug accumulation. J Clin Invest. 87:772–777. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stordal BK, Davey MW and Davey RA:
Oxaliplatin induces drug resistance more rapidly than cisplatin in
H69 small cell lung cancer cells. Cancer Chemother Pharmacol.
58:256–265. 2006. View Article : Google Scholar
|
|
21
|
Pardal R, Clarke MF and Morrison SJ:
Applying the principles of stem-cell biology to cancer. Nat Rev
Cancer. 3:895–902. 2003. View
Article : Google Scholar
|
|
22
|
Curley MD, Garrett LA, Schorge JO, Foster
R and Rueda BR: Evidence for cancer stem cells contributing to the
pathogenesis of ovarian cancer. Front Biosci (Landmark Ed).
16:368–392. 2011. View
Article : Google Scholar
|
|
23
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, et
al: Glioma stem cells promote radioresistance by preferential
activation of the DNA damage response. Nature. 7:444756–760.
2006.
|
|
24
|
Neuzil J, Stantic M, Zobalova R, Chladova
J, Wang X, et al: Tumour-initiating cells vs. cancer ‘stem’ cells
and CD133: what’s in the name? Biochem Biophys Res Commun.
355:855–859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pasini D, Bracken AP, Hansen JB, Capillo M
and Helin K: The polycomb group protein Suz12 is required for
embryonic stem cell differentiation. Mol Cell Biol. 27:3769–3779.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen YH, Hung MC and Li LY: EZH2: a
pivotal regulator for controlling cell differentiation. Am J Transl
Res. 4:364–375. 2012.
|
|
27
|
Khalil AM, Guttman M, Huarte M, Garber M,
et al: Many human large intergenic noncoding RNAs associate with
chromatin-modifying complexes and affect gene expression. Proc Natl
Acad Sci USA. 106:11667–11672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pádua Alves C, Fonseca AS, Muys BR, et al:
Brief report: The lincRNA Hotair is required for
epithelial-to-mesenchymal transition and stemness maintenance of
cancer cells lines. Stem Cells. 31:2827–2832. 2013. View Article : Google Scholar
|