Introduction
Platanus belongs to the family Platanaceae
and grows in a wide distribution. Kosisky et al (1) investigated airborne pollen allergens
between 1998 and 2007 by quantitative collection in downtown
Washington, WA, USA and the results demonstrated that the major
pollen types include oak, cypress, Pinaceae, Morus,
Betulaceae, Acer, Platanus, Fraxinus and
Gramineae. Tree and grass pollens accounted for 91.2 and 7% of the
total pollen count, respectively. Aira et al (2) demonstrated that Platanus and
Olea are important local sources of pollen in Iberian
Peninsula, located in the northwest of Spain. Ture et al
(3) reported that Bilecik
Pinus, Cupressaceae, Platanus, Quercus and
Salix are the predominant types of regional airborne pollen
in Turkey and the highest concentration of airborne pollen was
observed in May. In northwestern Turkey, Celenk et al
(4) revealed that Pinus,
Olea, Platanus, Cupressaceae, Quercus,
Poaceae, Urticaceae and Castanea are important in the airborne
spread of pollens, of which concentrations also peaked in May. The
Platanus pollen has a higher airborne concentration and also
causes widespread allergy (4). Liu
et al (5) investigated
airborne pollen in Hubei, China, in which 2,300 patients with hay
fever received skin allergen testing and seasonal incidence was
analyzed. In Hubei, the pollen with the highest positive allergenic
rate was Platanus in spring and Artemisia and ragweed
in fall. Lu et al (6)
screened 168 patients with allergic asthma for pollen blood
allergens and the result suggested that mugwort, Platanus,
blite, Humulus and Gramineae were the predominant allergenic
pollens in Xi’an, China. Lauer et al (7) found that the allergen immunoglobulin
E response rate of Platanus pollen is between 27.3 and 63.8%
in the Mediterranean region and is the major regional airborne
pollen allergen. A number of surveys and clinical epidemiological
data have suggested that Platanus pollen is an important
cause of hay fever in China and western countries (8–10).
It has been revealed that in Platanus pollen
allergen extracts, Pla A1, Pla A2 and Pla A3 are the major
allergenic proteins. The Pla a1 protein is detected in the serum of
92% of patients with Platanus-induced hay fever, while the
Pla a2 and Pla a3 response rates are 84 and 63.8%, respectively
(7,11,12).
Few studies investigating the expression and purification of the
major allergens in Platanus pollen have been performed. The
present study successfully expressed, purified and identified the
Pla a1 protein and provided the basis for preparations of allergens
with high purity, recombinant hypoallergenic allergens and allergen
nucleic acid vaccines.
Materials and methods
Materials
T4 DNA ligase and a pET-44a vector were purchased
from Promega Corporation (Madison, WI, USA). The host E.
coli JM109 and Rosetta cells were purchased from Novagen
(Darmstadt, Germany). DNA synthesis and DNA sequencing were
performed by Shanghai Boshang Biological Company (Shanghai, China)
and rTaq polymerase, 10X polymerase chain reaction (PCR) buffer,
dNTP mixture, isopropyl β-D-1-thiogalactopyranoside (IPTG) and the
DL 2,000 DNA marker were purchased from Takara Biotechnology Co.
Ltd. (Dalian, China). Agarose, yeast and SYBR Green fluorescent dye
were purchased from Gene Company (Chai Wan, Hong Kong). A
QIAquick® PCR Purification kit and Gel Extraction kit
were purchased from Qiagen (Hilden, Germany) and polyvinylidene
fluoride (PVDF) membranes were purchased from Merck Millipore
(Darmstadt, Germany). The prestained protein molecular weight
marker was purchased from Bio-Rad Laboratories, Inc. (Hercules, CA,
USA) and skim milk powder was purchased from Sigma-Aldrich (St.
Louis, MO, USA). The StrepTrap™ HP 1-ml columns were purchased from
GE Healthcare Life Sciences (Shanghai, China).
Serum collection
Venous blood (5 ml) was obtained from 18 patients (6
males and 12 females, 12–44 years old) with positive skin tests of
Platanus pollen allergen at The Second Affiliated Hospital
of Xi’an Jiaotong University (Xi’an, China). Following
centrifugation at 2,260 × g for 30 min at room temperature, the
serum was obtained. The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Xi’an Jiaotong
University. Written informed consent was obtained from all
participants.
Codon optimization and primer design
The nucleotide and amino acid sequences of PLa a1
were obtained, according to the AJ427413.2 ID number in the GenBank
database (http://www.ncbi.nlm.nih.gov/genbank/). The open
reading frames were 156 amino acids and 468 base pairs long.
NdeI, PstI and Xho sites and Strep-TagII were
introduced during vector construction. The preferred prokaryotic
expression vector of E. coli and the RNA secondary structure
was considered, the initial efficiency of translation was improved
and codon optimization was completed for facilitating protein
translation using DNASTAR Lasergene software (version 7.1;
http://wwwbioo.com/soft/biosoft/2009/6790.html).
Following codon optimization, the GC content increased between 44.3
and 47.6%. The sequences of the optimized full genome were
synthesized by Shanghai Boshang Biological Company.
Construction of the Pla a1 gene
expression vector
Based on the entire coding region sequence of the
optimized Pla a1 gene, a pair of primers was designed. The primers,
synthesized by Shanghai Boshang Biological Company, were as
follows: Forward 5′-CTCATATGGCCGATATTGTCCAGGG-3′ and reverse
5′-GCCTGCAGAGCACCAAGCAGTTT-3′. The annealing temperature of the
forward primer was 68.2°C and the reverse primer was 69.1°C. The
underlined sections highlight NdeI and PstI
restriction sites, respectively. The PCR amplification program was
as follows: 95°C pre-denaturation for 5 min; 7 cycles of 95°C
denaturation for 30 sec, 63.3°C annealing for 30 sec
(1°C/touchdown/cycle), 72°C extension for 30 sec, 25 cycles of 95°C
denaturation for 30 sec, 57.3°C annealing for 30 sec, 72°C
extension for 30 sec and 72°C extension for 5 min. Glycerol
bacteria TOP10-Puc57-pla a1 was synthesized by Gene Company and
used for monoclonal colony PCR. The PCR results were detected by 1%
agarose gel electrophoresis. The positive monoclonal colonies were
cultured and the plasmids were extracted and double digested using
NdeI and PstI at 37°C for 4 h followed by agarose gel
electrophoresis. The transformed pET44a plasmid vectors (vector
containing Strep-TagII) were also double digested to obtain
identical sticky ends. Following 2% agarose gel electrophoresis,
the PLa a1 target fragment and the pET-44a vector were recovered
and ligation was performed at a ratio of three target fragments to
one vector. The T4 DNA ligases were attached to the pET44a-pla a1
recombinant plasmid at 4°C for 16 h and the ligation product was
transformed into competent E. coli JM109 cells using KCM
containing 0.5 mol/l KCl, 0.15 mol/l CaCl2 and 0.25
mol/l MgCl2. Following transformation, positive colonies
were screened for using lysogeny broth (LB) plates (Shanghai Sangon
Biological Engineering Technology and Services Co., Ltd., Shanghai,
China) containing 50 µg/ml ampicillin (Amp) for Amp
resistance. The positive clones were identified using colony PCR
and were subsequently cultured at 37°C overnight prior to
sequencing (LB liquid medium; Shanghai Boshang Biological Co.,
Shanghai, China). The pET44a-pla a1 plasmid was transformed into
competent Rosetta cells with efficient expression and screened, as
previously, for Amp resistance and PCR detection and culture for
sequencing. The monoclonal colonies with the correct sequence were
selected to induce protein expression.
Expression and identification of the pla
a1 protein
The E. coli Rosetta cells containing the
positive recombinant pET44a-pla a1 plasmid were inoculated in LB
medium (10 g/l tryptone, 5 g/l yeast extract and 10 g/l NaCl)
supplemented with 50 mg/l Amp, at 37°C with agitation for 12 h.
Following incubation, the cells with 1% concentration were
inoculated into fresh LB medium containing 50 mg/l Amp and 4%
glucose until the optical density (OD) at 600 nm reached between
0.6 and 0.7. The OD value was determined using a 752
spectrophotometer (Nanjing Analytical Instrument Factory Co., Ltd.,
Nanjing, China). Subsequently, 0.5 mmol/l IPTG was added to induce
plasmid expression (4 h at 37°C). The bacteria were collected and
SDS-PAGE (12% separating gel, 4% stacking gel; Sigma-Aldrich) was
used to estimate the expression of Pla a1, as described previously
(13). Briefly, 1.5 ml bacterial
culture was centrifuged at 1,200 × g for 5 min for collection of
bacteria and 100 µl 2X loading buffer containing 20 g/l SDS,
500 ml/l glycerol, 62.5 mmol/l Tris-HCl (pH 6.8), 20 ml/l
β-mercaptoethanol, 0.1 g/l bromophenol blue, 30 mmol/l NaCI and 1
mmol/l EDTA, was added and mixed prior to boiling for 5 min. The
samples were then centrifuged at 1,200 × g for 5 min and SDS-PAGE
electrophoresis was performed.
Protein expression form
Protein expression was induced using the method
described above. The bacterial supernatants were collected by
centrifugation at 10,000 rpm for 6 min at 4°C. The bacterial wet
weight was set as 5 ml/1 g and binding buffer, containing 100 mM
Tris-HCl, 150 mM NaCl and 1 mM EDTA (pH 8), was added to resuspend
the bacteria. Sonication was performed in an ice-water bath (lysis
for 5 sec, termination for 5 sec, 50% strength at 100 W, lysis for
10 min). The supernatant and precipitate were collected by
centrifugation at 1,200 × g for 30 min at 4°C. The precipitate was
centrifuged again, as previously, and the subsequent precipitates
were resuspended in binding buffer of an equal volume with 6 M urea
and agitated overnight at 4°C. The supernatant was collected by
centrifugation at 1,800 × g for 30 min at 4°C and the inclusion
bodies of the expressed proteins were identified through
SDS-PAGE.
Purification of the expressed Pla a1
protein
The denatured proteins were dialyzed for 12 h in
binding buffer containing 4, 2 and 1 M urea, respectively.
Following dialysis, the supernatants were collected by
centrifugation at 18,000 rpm for 10 min at 4°C to prepare for
protein purification. StrepTrap HP prepacked columns (1 ml) were
connected to a GE-048 AKTA FPLC system (GE Healthcare, Waukesha,
WI, USA). The columns were washed using binding buffer with a 1
ml/min flow rate. The three supernatants were passed through the
column and then the columns were washed using binding buffer. The
penetration peaks were collected and calculated by determining the
OD value using the spectrophotometer (Nanjing Analytical Instrument
Factory Co., Ltd.). The columns were washed using elution buffer
containing 2.5 mM desulfurization biotin, 100 mM Tris-HCI, 150 mM
NaCl and 1 mM EDTA (pH 8.0). The elution peaks were collected and
calculated by the same method.
Western blot analysis of the purified
protein
The purified fusion proteins (15 µl)
separated by SDS-PAGE were transferred to PVDF membranes (constant
current 45 mA, 26 min), prior to blocking with 50 g/l skim milk
dissolved in phosphate-buffered saline (PBS; Sigma-Aldrich)
containing 8 g/l NaCl, 0.28 g/l KCl, 0.28 g/l
KH2PO4, 2.98 g/l
Na2HPO4·12H2O (pH 7.4) and 1 ml/l
Tween 20 (PBST; Sigma-Aldrich) for 2 h at room temperature. The
membrane was then incubated with Platanus pollen allergy
serum primary antibody (diluted 1:1; collected from the Respiratory
Disease Laboratory of the Second Affiliated Hospital of Xi’an
Jiaotong University) overnight at 4°C. The membrane was then washed
four times with PBST (5 min each) prior to incubation with
horseradish peroxidase-labeled goat anti-human immunoglobulin E
antibody (1:500; Shanghai Sangon Biological Engineering Technology
and Services Co., Ltd.) at room temperature for 1 h. The membrane
was then washed twice with PBST (5 min each) followed by washing
twice with PBS. Diaminobenzidine (Sigma-Aldrich) was used as a
chromogenic substrate (10 min) to detect the antigenicity of Pla
a1.
Sequencing of the purified protein
Following SDS-PAGE, the PVDF membrane was cut to an
appropriate size and electroblotting was performed under 45 mA at
room temperature for 28 min. The membrane was stained using 0.1%
Coomassie Brilliant Blue R-250 (Shanghai Sangon Biological
Engineering Technology and Services Co., Ltd.) dissolved in 40%
methanol/1% acetic acid for 30 sec. The membrane was de-stained
with 1% acetic acid and 50% methanol and washed with deionized
water. The corresponding bands were cut from the membrane and
sealed in a 1.5 ml centrifuge tube for sequencing (Proteome
Research Analysis Center, Institute of Biochemistry and Cell
Biology, Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences, Shanghai, China).
Results
Identification of the recombinant
expression vector
The recombinant vector was constructed successfully
from the T7 promoter and the target fragments were identified
between the NdeI and PstI restriction sites.
Subsequent to the PstI enzyme cutting site, a Strep-TagII
comprising eight amino acids, a double terminal codon TAATAA and
XhoI sites were observed. The PET44a-pla a1 recombinants
were transferred into JM109 cells and the resistent bacterial
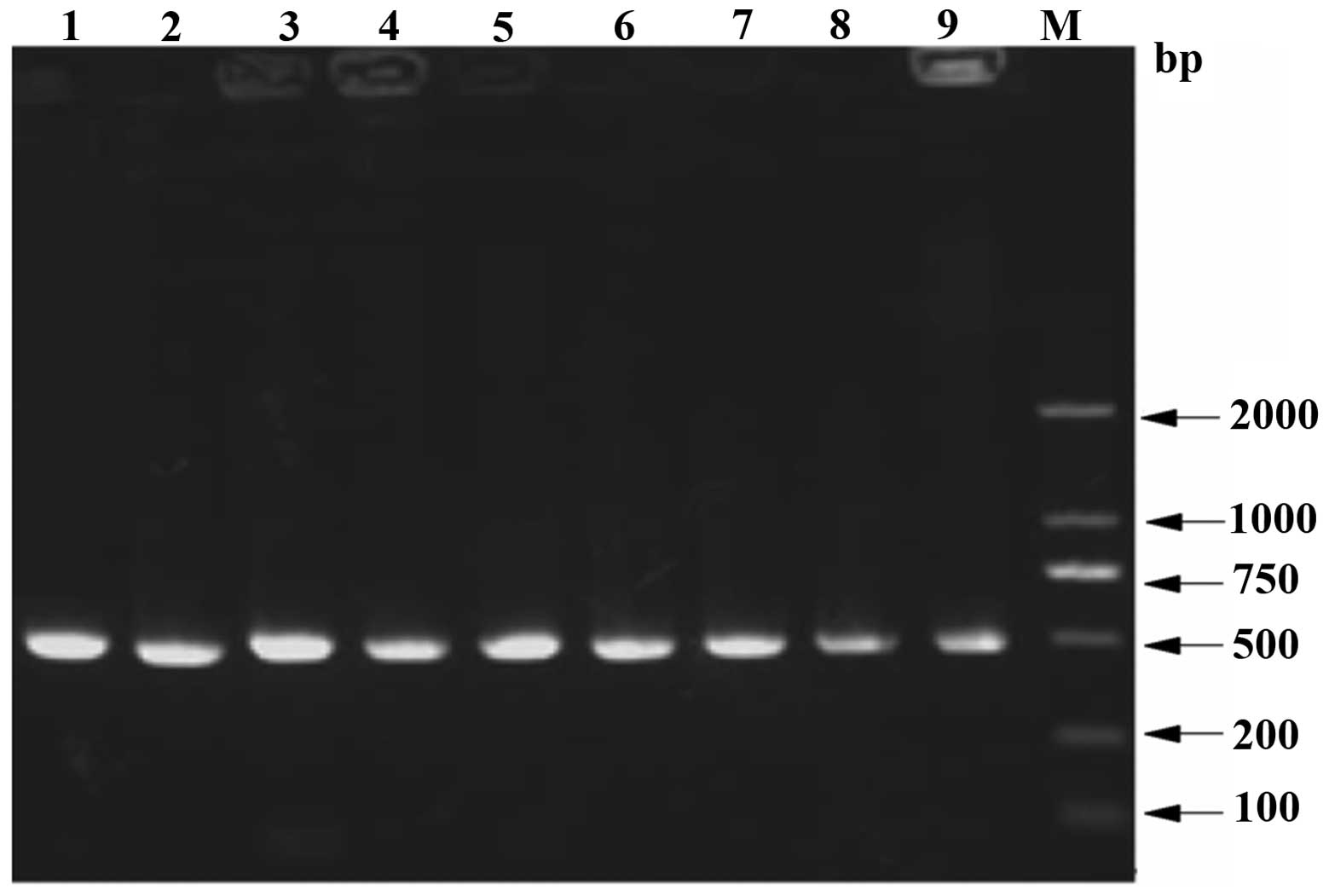
colonies were used for PCR. The results are shown in Fig. 1, in which lanes 1–9 contained
target fragments of ~500 bp, which matched the expected fragment
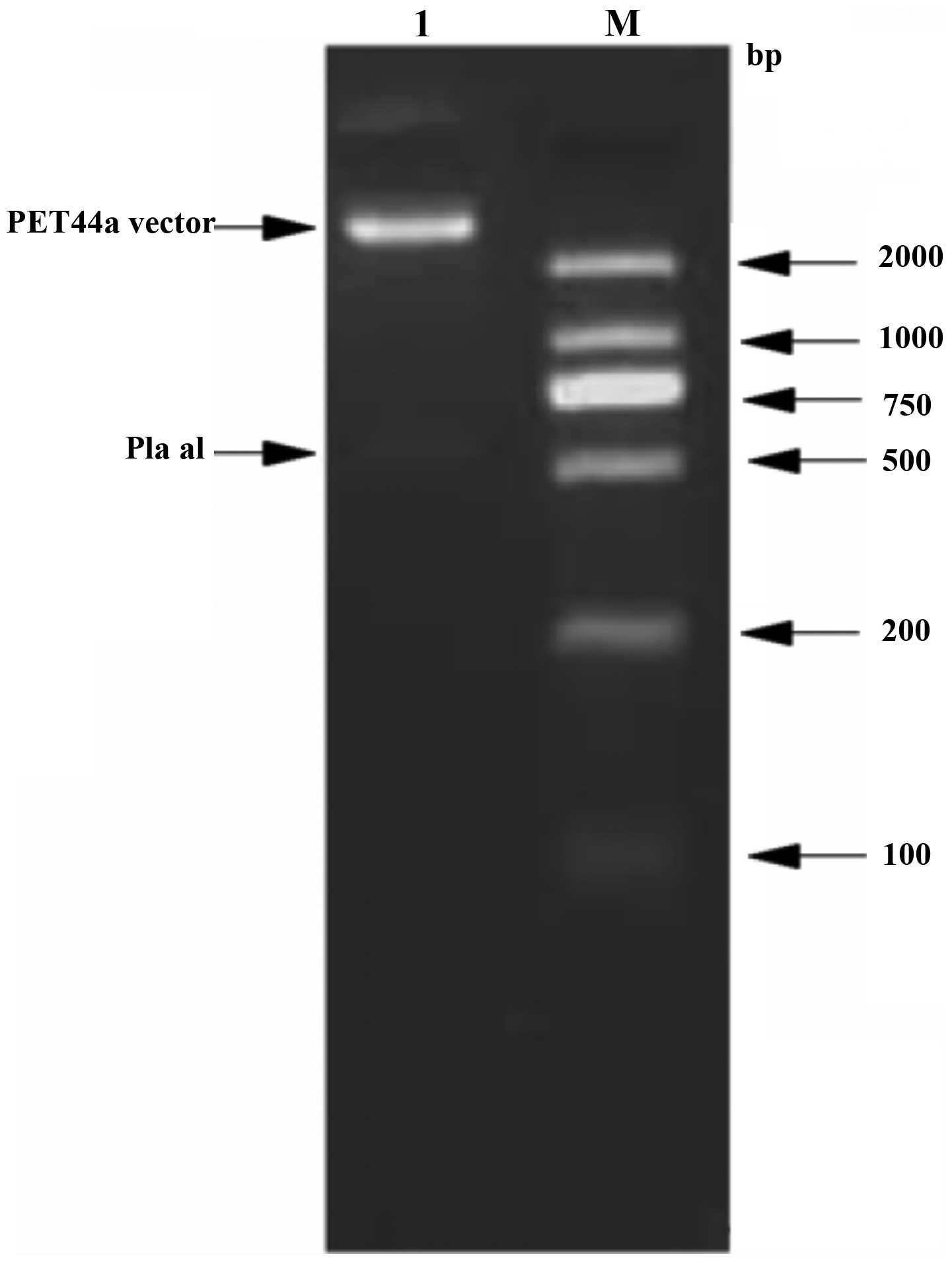
size. The PET44a-pla recombinants were double digested using
NdeI and XhoI (Fig.
2). The sizes of the target fragments were consistent,
demonstrating successful construction of the PET44a carrier
containing the target gene. The homology between the sequencing
results and the original sequences was 100%, therefore,
construction of an expressive prokaryote strain may be considered
in the future.
Expression and identification of the Pla
a1 protein
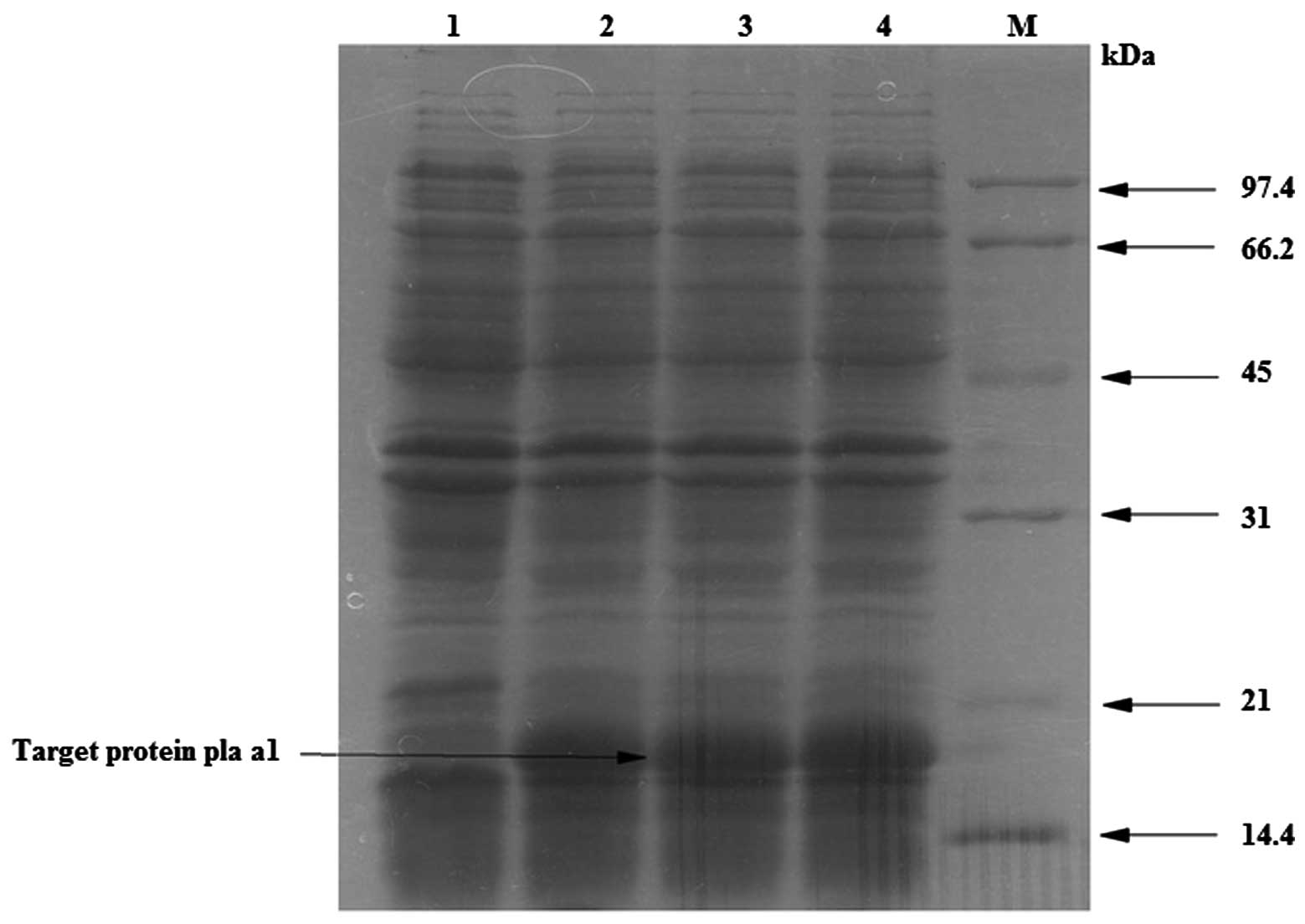
As shown in Fig. 3,
expression of Pla a1 was observed in the Rosetta strain. The
expression of Pla a1 was observed following 0.5 mmol/l IPTG (lanes
2–6) compared with the non-induced control (lane 1). The expression
of the target protein (18 kDa) was significantly higher in the
induced lanes compared with the control, indicating that the
exogenous gene expressed the Pla a1 protein. The marker used was an
03–04 protein marker.
Identification of the expressed protein
form
Following the induction of protein expression, the
bacteria were harvested and non-denaturing lysis buffer was added
prior to sonication. The supernatants and precipitates were
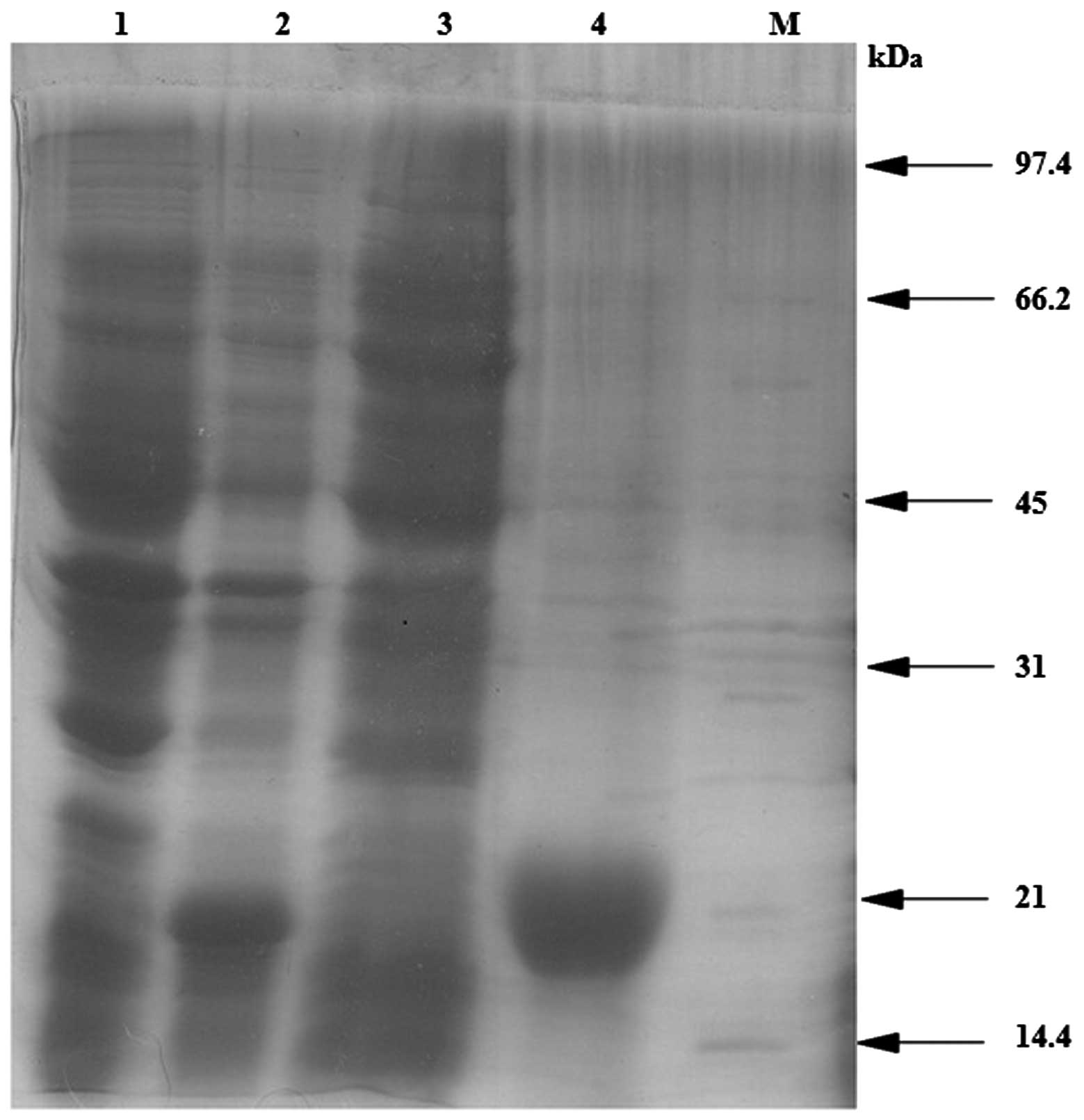
analyzed by SDS-PAGE. As shown in Fig.
4, the predominant inclusion body expression of the target
protein was observed in the induction lane (lane 2) compared with
the control and sonicated supernatant samples.
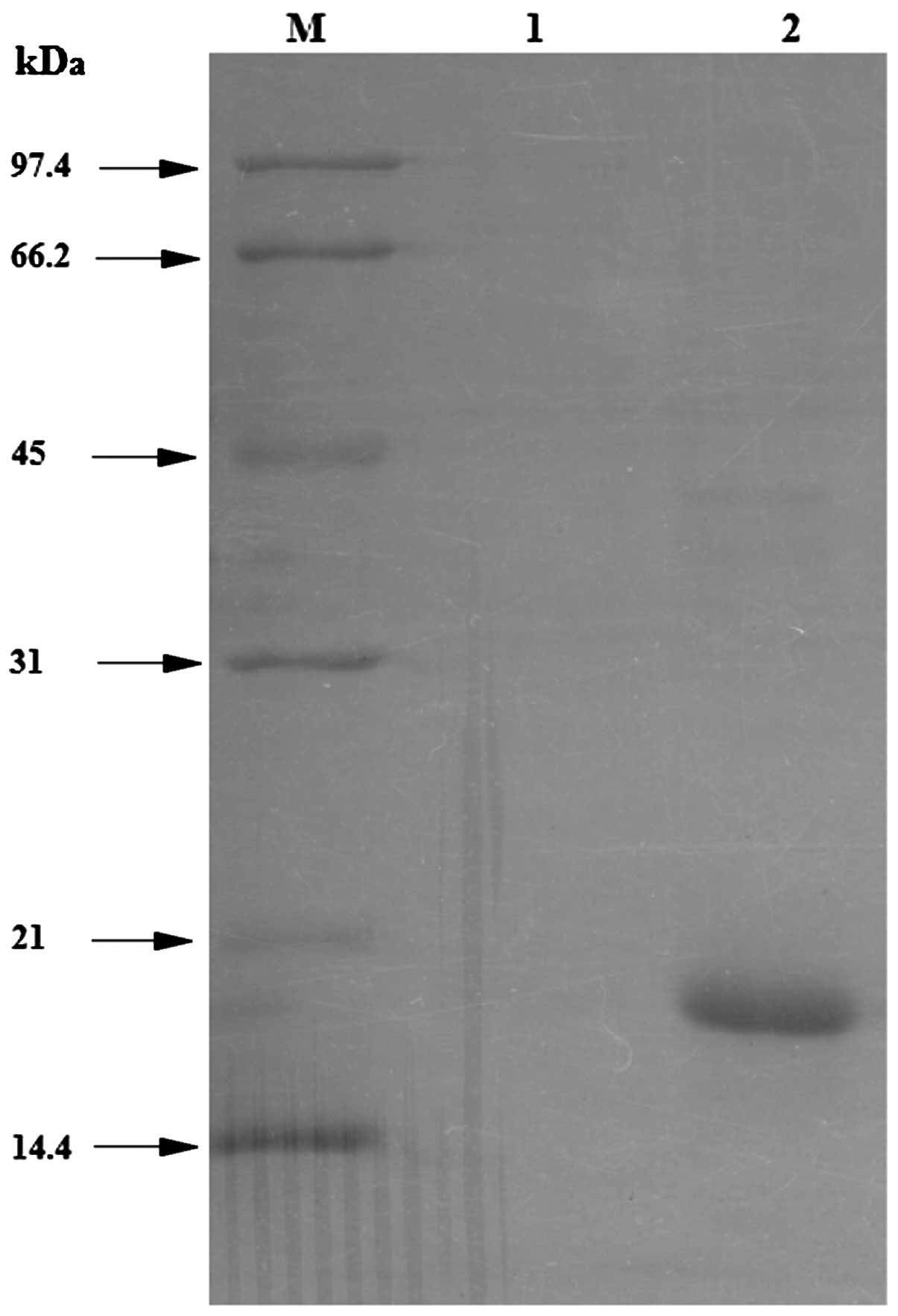
Purification of the Pla a1 fused
protein
The supernatents were collected using 1 ml StrepTrap
HP prepacked columns. The supernatants (100 µl) were added
to 20 µl 6X loading buffer containing β-mercaptoethanol and
boiled for 6 min using the AKTA FPLC system. Following
centrifugation at 10,000 rpm for 5 min, 10 µl supernatant
was added to an SDS-PAGE gel. As shown in Fig. 5, the penetration peak was observed
in lane 1, whereas the penetration peak of the target protein was
observed in lane 2, which was obtained at the UV absorption peak
(>50 mA) under the protein elution.
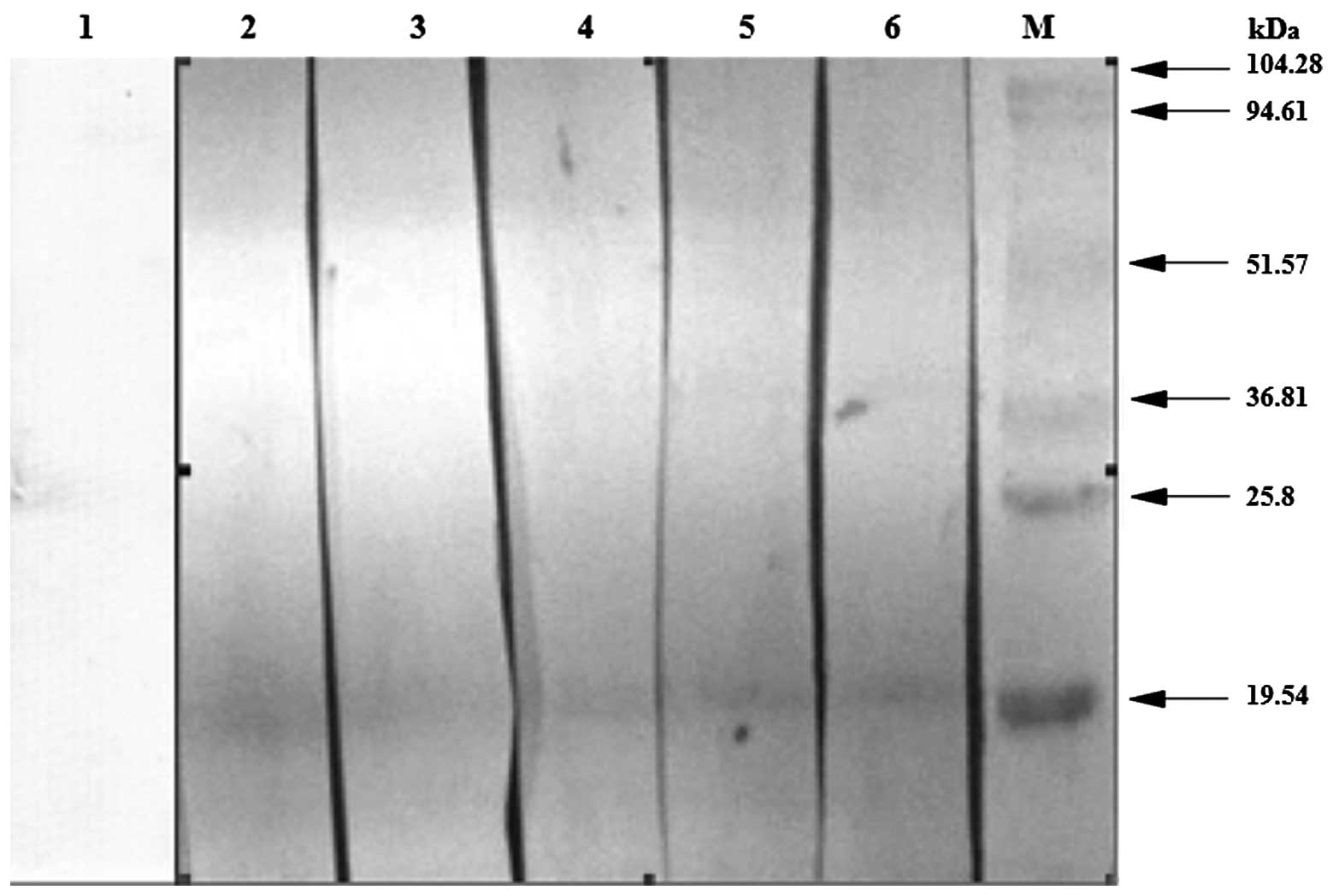
Western blot analysis of the purified
proteins
The induced, purified proteins were used for western
blot analysis and the serum from the patients with Platanus
pollen allergy was used as the primary antibody. As shown in
Fig. 6, in which lane 1 contained
normal serum as a control, the serum in lanes 2–6 were detected by
ImmunoCAP and the Platanus pollen allergen-specific
immunoglobulin E antibodies were grade two or above. Lanes 2–6
exhibited a distinct band at the target site, demonstrating that
the target protein contained the Platanus pollen
allergen-specific immunoglobulin E antibodies.
Sequencing of the purified fusion
protein
The purified fusion proteins were transferred onto
PVDF membranes for sequencing (Proteome Research Analysis Center of
the Institute of Biochemistry and Cell Biology, Shanghai Institutes
for Biological Sciences, Chinese Academy of Sciences). The
sequencing results were consistent with the predicted gene
sequences in GenBank (ID AJ427413.2), which indicated that the
synthesized protein was the target protein.
Discussion
Epidemiological data of pollens in China, Europe and
the USA, demonstrate that Platanus pollens are widespread in
distribution (1,2,4,14).
Previous studies have suggested that Platanus pollen causes
a substantial number of cases of hay fever, including allergic
asthma, allergic rhinitis and urticaria (7,12,15–18).
However, few investigations have focused on the expression,
purification and identification of Platanus pollen.
A previous study revealed that the codon bias, mRNA
stability, initial efficiency of translation and carrier selection
are significant factors affecting the efficiency of exogenous gene
expression (19) and
investigations into codon bias has become an area of interest. If
the target gene does not match the expressive host, this reduces
the efficiency and stability of mRNA translation and can result in
early termination (20). In the
present study. the GC content was increased between 44.3 and 47.6%
through codon optimization. In an initial region of translation,
the optimal codons were preferred and the RNA secondary structure
was considered to maintain a linear open structure in this region,
facilitating protein translation.
The sera was obtained from blood samples from 18
patients with positive Platanus pollen allergen skin tests
were obtained. For ImmunoCAP assessment, nine of the cases were in
stage two or above. Purification of the exogenous target proteins
was performed for western blot analysis and the results revealed
five cases with positive reactions, exhibiting positive response
rates of >50%. Immunoblotting identified Platanus pollen
as the predominant type of allergen protein and demonstrated that
the exogenous recombinant protein had the corresponding
antigen.
With the development of molecular biology
techniques, recombinant pollen, which is prepared by genetic
engineering and is used in the clinical treatment and research of
allergic diseases, has became a strategy used in treatment of
allergies. Recombinant allergens with high purity and yield are
easily standardized and have no exogenous toxic substances or
pathogenic microbial contamination (21). In vitro investigations and
the results of skin tests have demonstrated that the majority of
recombinant gene allergen activity is consistent with that of
natural allergens (21). A
recombinant plasmid with an allergen-encoding gene requires
construction to express the functional activity of the recombinant
allergen, which may be applied in the diagnosis and treatment of
allergic diseases and be used for detailed investigations of
allergen molecular structure and pathogenic mechanisms. The present
study provided a basis for subsequent transformations of
hypoallergenic allergens and the development of allergen nucleic
acid vaccines.
Acknowledgments
This study was supported by the Key Topics of Health
Ministry: The Specific Diagnosis and Immunotherapy of Recombinant
Allergen for Bronchial Asthma (no. 2007353), the Major Issue of
National Science and Technology (no. 2008ZX08011-005) and Key
Projects (no. 2009ZX08011-004B) and Guangzhou Educational System
Research and Innovation Academic Team (no. B94118).
References
|
1
|
Kosisky SE, Marks MS and Nelson MR: Pollen
aeroallergens in the Washington, DC, metropolitan area: a 10-year
volumetric survey (1998–2007). Ann Allergy Asthma Immunol.
104:223–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aira MJ, Rodriguez-Rajo FJ,
Fernandez-Gonzalez M and Jato V: Airborne pollen of ornamental tree
species in the NW of Spain. Environ Monit Assess. 173:765–775.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ture C and Bocuk H: Analysis of airborne
pollen grains in Bilecik, Turkey. Environ Monit Assess. 151:27–35.
2009. View Article : Google Scholar
|
|
4
|
Celenk S, Canitez Y, Bicakci A, et al: An
aerobiological study on pollen grains in the atmosphere of
North-West Turkey. Environ Monit Assess. 158:365–380. 2009.
View Article : Google Scholar
|
|
5
|
Liu GH, Zhu RF, Zhang W, et al: Survey of
airborne pollen in Hubei Province. Chin Med Sci J. 23:212–217.
2008. View Article : Google Scholar
|
|
6
|
Lu JM, Sun XZ, Liu Y, et al: Survey of
airborne pollen in Xi’an City. Journal of Xi’an Jiaotong University
(Medical Science Edition). 31:472–474. 2010.
|
|
7
|
Lauer I, Miguel-Moncin MS, Abel T, et al:
Identification of a plane pollen lipid transfer protein (Pla a 3)
and its immunological relation to the peach lipid-transfer protein,
Pru p 3. Clin Exp Allergy. 37:261–269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alcázar P, García-Mozo H, Trigo Mdel M, et
al: Platanus pollen season in Andalusia (southern Spain): trends
and modeling. J Environ Monit. 13:2502–2510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mardones P, Grau M, Araya J, et al: First
annual register of allergenic pollen in Talca, Chile. Allergol
Immunopathol (Madr). 41:233–238. 2012. View Article : Google Scholar
|
|
10
|
Pérez-Badia R, Rapp A, Vaquero C and
Fernández-González F: Aerobiological study in east-central Iberian
Peninsula: pollen diversity and dynamics for major taxa. Ann Agric
Environ Med. 18:99–111. 2011.PubMed/NCBI
|
|
11
|
Asturias JA, Ibarrols I, Eraso E, et al:
The major Platanus acerifolia pollen allergen Pla a1 has sequence
homology to invertase inhibitors. Clin Exp Allergy. 33:978–985.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ibarrola I, Arilla MC, Martinez A, et al:
Identification of a polygalacturonase as a major allergen (Pla a2)
from Platanus acerifolia pollen. J Allergy Clin Immunol.
113:1185–1191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Celenk S, Bicakci A, Tamay Z, et al:
Airborne pollen in European and Asian parts of Istanbul. Environ
Monit Assess. 164:391–402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Subiza J, Cabrera M, Valdivieso R, et al:
Seasonal asthma caused by airborne Platanus pollen. Clin Exp
Allergy. 24:1123–1129. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pazouki N, Sankian M, Leung PT, et al:
Identification of cyclophilin as a novel allergen from Platanus
orientalis pollens by mass spectrometry. J Biosci Bioeng.
107:215–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernández-González D, González-Parrado Z,
Vega-Maray AM, et al: Platanus pollen allergen, Pla a 1:
quantification in the atmosphere and influence on a sensitizing
population. Clin Exp Allergy. 40:1701–1708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Varela S, Subiza J, Subiza JL, et al:
Platanus pollen as an important cause of pollinosis. J Allergy Clin
Immunol. 100:748–754. 1997. View Article : Google Scholar
|
|
19
|
Baneyx F: Recombinant protein expression
in Escherichia coli. Curr Opin Biotechnol. 10:411–421. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng BQ: Escherichia coli codon
preference overview. Silicon Valley. 3:242009.
|
|
21
|
Nikolaizik WH, Weichel M, Blaser K and
Crameri R: Intracutaneous tests with recombinant allergens in
cystic fibrosis patients with allergic bronchopulmonary
aspergillosis and Aspergillus allergy. Am J Respir Crit Care Med.
165:916–921. 2002. View Article : Google Scholar : PubMed/NCBI
|