Introduction
Dendritic cells (DCs) are potent antigen-presenting
cells (APCs), which can prime naïve T cells to differentiate into
antigen-specific cytotoxic T lymphocytes (CTLs), which are involved
in the immune reaction (1). By
utilizing this function of DCs, DC-based cellular immunotherapy has
been developed for use in anti-tumor therapy and has been approved
for use in prostate cancer by the Food and Drug Administration
(Silver Spring, MD, USA) (2). In
addition to therapy involving direct administration of DCs to
patients, adoptive specific cellular immunotherapy has been
developed. This involves the infusion of antigen-specific CTLs
generated by priming lymphocytes with antigen-presenting DCs in
vitro into patients, which has been demonstrated to be a
promising novel therapeutic treatment strategy in cancer (3). Although previous studies have
supported this treatment option, it is technically difficult and
labor-intensive to expand what is currently known about
antigen-specific CTLs in vitro. Adoptive immunotherapy using
antigen-specific CTLs may be more practical for anti-cancer
treatment if the potent APCs were available at any time.
In a previous study by our group, a leukemic
plasmacytoid dendritic cell (pDC) line, PMDC05, was established
from pDC leukemia cells (4). The
PMDC05 cells were antigen-presenting, which was shown to be
enhanced by stimulation with lipopolysaccharides (LPS) (4). Previously, PMDC05 cells were
demonstrated to undergo a morphological, surface-phenotypical and
functional transformation from pDC to a myeloid DC (mDC) lineage
following the stimulation with interleukin (IL)-3 or LPS (5). Furthermore, PMDC05 cells have been
indicated to possess a potent ability to generate tumor
antigen-specific CTLs from normal peripheral blood (PB)
CD8+ T cells in vitro (6). Yamahira et al (7) identified that an antigen-presenting
ability equivalent to that of maximally activated PMDC05 could be
invoked in PMDC05 cells via lentiviral vector-mediated transduction
of CD80 genes. CD80 gene-transduced PMDC05 cells were termed PMDC11
in the present study, and their antigen-presenting ability was
potentiated by stimulation with LPS. The present study aimed to
establish efficient and potent APCs by transducing the
constitutively active (ca) toll-like receptor 4 (TLR4) gene into
PMDC11 using the Tet-On system with a lentiviral vector.
Materials and methods
Cell culture
PMDC05 cells (4)
and PMDC11 cells (7) were
established in our laboratory (Laboratory of Hematology and
Oncology, Graduate School of Health Sciences, Niigata University,
Niigata, Japan). The cells were cultured in Iscove’s modified
Dulbecco’s medium (IMDM; Invitrogen Life Technologies, Carlsbad,
CA, USA) with 10% fetal bovine serum (FBS; Nichirei Biosciences,
Inc., Tokyo, Japan). For establishing the PMDC05 cells, and using
both of the cell lines, written informed consent was obtained from
the patient’s spouse. The present study was approved by the ethics
committee of the Faculty of Medicine, Niigata University (March 25,
2011).
Lentivirus production and titer
determination
Virus production was conducted using transient
co-transfection into 293T cells (American Type Culture Collection,
Manassas, VA, USA) according to the method described previously
(8). The lentiviruses were
concentrated by ultracentrifugation at 82,700 × g for 120 min at
4°C, typically resulting in titers of >108
transduction units/ml. The lentiviral titer was determined via the
assessment of the viral p24 antigen concentration using an ELISA
(Cell Biolabs, Inc., San Diego, CA, USA), and is hereafter
expressed as µg of p24 equivalent units/ml. A measure of 1
µg p24 equivalent per ml corresponds to ~1–5×107
transduction units/ml using 293T cells as a reference line. p24
concentrations of pLVX-Tet-ON Advanced and
pLVX-Tight-TLR4-IRES-GFP-PGK-Puro lentiviruses (Clontech
Laboratories, Inc., Mountain View, CA, USA) used in the present
study were 19.1 and 11.3 µg/ml, respectively. The
lentiviruses were prepared by Dr Noriyuki Kasahara (CURE Vector
Core & JCCC Vector Shared Resource Facility, University of
California, Los Angeles, CA, USA).
Lentiviral vector transduction
Transduction of pLVX-Tet-ON Advanced vector into the
PMDC11 cells was performed as previously described (9). A total of 5×105 PMDC11
cells were suspended in a 15-ml tube containing 200 µl
lentiviral vector (19.1 µg/ml p24 core protein by ELISA) and
protamine sulfate (Sigma-Aldrich, St. Louis, MO, USA) at a final
concentration of 5 µg/ml. Cells were centrifuged at 800 × g
for 30 min and suspended in 800 µl 10% FBS-containing IMDM,
and were subsequently transferred to a 12-well plate. The 12-well
plate containing the cells was centrifgued at 800 × g for 30 min
(PlateSpinII; Kubota, Tokyo, Japan), in order to enhance the
transduction efficiency, and the cells were then incubated at 37°C
overnight in the presence of 5% CO2. Cells were then
carefully collected in 15-ml tubes and diluted with fresh media and
centrifuged at 200 × g for 5 min. The supernatant was removed and 2
ml fresh medium was added to the tube. Cells were then transferred
into a 35-mm culture dish and cultured for 24 h. PMDC11 cells
transduced with pLVX-Tet-ON Advanced vector were selected by adding
400 mg/ml G418 (Sigma-Aldrich) to the culture dish on the next day,
followed by further culturing for 7–12 days. G418-selected PMDC11
cells were transduced with pLVX-Tight-TLR4-IRES-GFP-PGK-Puro
lentiviral vector using an almost identical method, with the
exception of using two selection reagents [1 µg/ml puromycin
(Sigma-Aldrich) and G418]. The concentrations of G418 and puromycin
used in the present study were confirmed to be appropriate for
PMDC11 cells by a titration assay (10). The selected PMDC11 cells were
termed caTLR4-PMDC11.
Evaluation of Tet-On system
The operation of the lentiviral Tet-On system with
pLVX-Tet-ON Advanced vector and pLVX-Tight-TLR4-IRES-GFP-PGK-Puro
vector was evaluated by culturing caTLR4-PMDC11 cells in
G418/puromycin-containing medium with various concentrations of
doxycycline for 6 h to 3 days and analyzing the expression of green
fluorescent protein (GFP) on doxycycline-exposed caTLR4-PMDC11
cells using a FACSCalibur flow cytometer (BD Biosciences, San Jose,
CA, USA).
Stimulation of PMDC11 and
caTLR4-PMDC11
PMDC11 cells (1.0×106 cells/ml) were
stimulated with 0.1 µg/ml LPS for 24 h (PMDC11 + LPS),
whereas caTLR4-PMDC11 cells (1.0×106 cells/ml) were
activated with 1 µg/ml doxycycline for 24 h
(caTLR4-PMDC11*Dox) prior to further analysis.
Phenotype analysis
PMDC11 cells and caTLR-PMDC11 cells were stained
with the following phycoerythrin (PE)-conjugated monoclonal
antibodies: mouse CD1a (cat. no. 1590; Immunotech, Marseille,
France), rat CD40 (cat. no. 732138; Immunotech), mouse CD80 (cat.
no. 560925; BD Biosciences), mouse CD83 (cat. no. 305307;
BioLegend, San Diego, CA, USA), mouse CD86 (cat. no. 305405;
BioLegend) and mouse HLA-DR (cat. no. 307605; BioLegend). Stained
cells were assessed using the FACSCalibur flow cytometer and data
were analyzed using CellQuestPro software (BD Biosciences).
Allogeneic mixed leukocyte culture
(MLC)
MLC was performed as described previously (4). Briefly, PMDC11 cells or caTLR4-PMDC11
cells were irradiated with 30 Gy of 137Cs generated
gamma irradiation (PS-3000SB Cs-137; Pony Industry Co., Ltd.,
Osaka, Japan) immediately prior to MLC. A total of 1×105
allogeneic peripheral blood mononuclear cells (PB-MNCs) were
co-cultured in a 96-well flat-bottom microtiter plate (BD
Biosciences) with graded numbers (0, 1,250, 2,500 and 5,000) of
irradiated PMDC11 cells or caTLR4-PMDC11 cells. The co-cultured
cells were pulsed with 0.5 µCi (18.5 KBq)/well of
[methyl-3H]-thymidine (Perkin-Elmer, Inc., Waltham, MA,
USA) on day five of culture and harvested with a cell harvester
(Labo Mash; Futaba Medical, Inc., Tokyo, Japan) following overnight
culture. Cellular proliferation was measured by
3H-thymidine incorporation with a liquid scintillation
counter (LSC-5100; Hitachi Aloka Medical Ltd., Tokyo, Japan). The
experiments were conducted in triplicate.
Interferon (IFN)-γ assay using cytometric
bead array (CBA)
IFN-γ in the MLC supernatant was quantified using
the CBA Human IFN-γ Flex Set Assay (BD Biosciences) according to
the manufacturer’s instructions. Briefly, the provided standard
IFN-γ (BD Biosciences) or culture supernatant was added to IFN-γ
Capture Beads and the mixture was incubated for 1 h. PE-conjugated
anti-human IFN-γ antibody (IFN-γ PE Detection Reagent) was added to
the mixture, which was then incubated for 2 h. The mixture was
washed and centrifuged at 300 × g for 10 min at room temperature.
The centrifuged pellet was then resuspended in 300 ml wash buffer.
The FACSCalibur flow cytometer was set up with BD FACSComp version
5.1 software and BD Calibrite beads (BD Biosciences). IFN-γ
concentrations were indicated by their fluorescent intensities
(Fl-2) and were quantified using the standard reference curve
provided for IFN-γ.
CTL induction using PMDC11 or
caTLR4-PMDC11
CD8+ T cells were separated from PB-MNCs
of a normal person with HLA-A*24:02 using the fluorescein
isothiocyanate (FITC)-conjugated CD8 monoclonal antibody and
anti-FITC microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany) according to the manufacturer’s instructions. PMDC11 cells
and caTLR4-PMDC11 cells were incubated with 10 mg/ml mutant (m)
9mer WT1 peptides with antigenicity in the HLA-A*24:02+
individual (CYTWNQMNL; NeoMPS, Inc., San Diego, CA, USA) for 24 h.
caTLR4-PMDC11 cells were exposed to 1 mg/ml doxycycline during this
incubation period for operating Tet-On system. CD8+ T
cells (5×105/well) were co-cultured with 30
Gy-irradiated PMDC11 cells or caTLR4-PMDC11 cells at a cell ratio
of 2:1 in a six-well plate containing 2.5 ml 5% autologous
serum-containing RPMI 1640 medium (Invitrogen Life Technologies).
IL-2 (Shionogi & Co., Ltd., Osaka, Japan) and IL-7 (R&D
Systems, Inc., Minneapolis, MN, USA) were added to the co-culture
on day three at a final concentration of 50 U/ml and 10 ng/ml,
respectively. A proportion of two thirds of the medium containing
IL-2 and IL-7 was replenished every 2–3 days throughout the culture
period. The co-culture was stimulated repeatedly every week with
the same mWT1 peptide-pulsed PMDC11 cells or caTLR4-PMDC11 cells.
mWT1 tetramer analysis of the co-cultured lymphocytes was performed
every week immediately prior to the addition of PMDC cells
(11).
Cytotoxicity assay of co-cultured
CD8+ T cells
A cytotoxicity assay was conducted using flow
cytometry with co-cultured CD8+ T cells as effector
cells and T2A24 cells [transporter associated with antigen
processing-deficient, HLA-A24-expressing human T, B-hybridoma
cells] as the target cells. The T2A24 cells were donated by Dr
Yoshiki Akatsuka (Department of Hematology, Fujita Health
University, Toyoake, Aichi, Japan). Lentivirally GFP
gene-transduced T2A24 cells, which were pulsed with mWT1 peptides
for 24 h, were cultured with effector cells in RPMI 1640 without
phenol red for 4 h at 37°C in a fully humidified 5% CO2
atmosphere. Co-cultured cells (consisting of effector cells and
target cells) were stained with 7-amino-actinomycin D (7AAD;
Sigma-Aldrich) immediately prior to flow cytometric analysis. Cells
were acquired for 120 sec in all the samples and viable target
cells (GFP+/7AAD−) were gated in an FL-1
(GFP)/FL-3 (7AAD) dot plot. The percentage cytotoxicity of the
assay was calculated with the following formula: %
Cytotoxicity=[(absolute number of viable target cells in the tube
containing target cells only - absolute number of viable target
cells in the sample tube)/absolute number of viable target cells in
the tube containing target cells only] ×100.
Statistical analysis
The statistical significance of differences between
values was evaluated via one- and two-way analysis of variance,
with GraphPad Prism version 5 software (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Antigen presentation-associated molecules
of caTLR4-PMDC11 cells
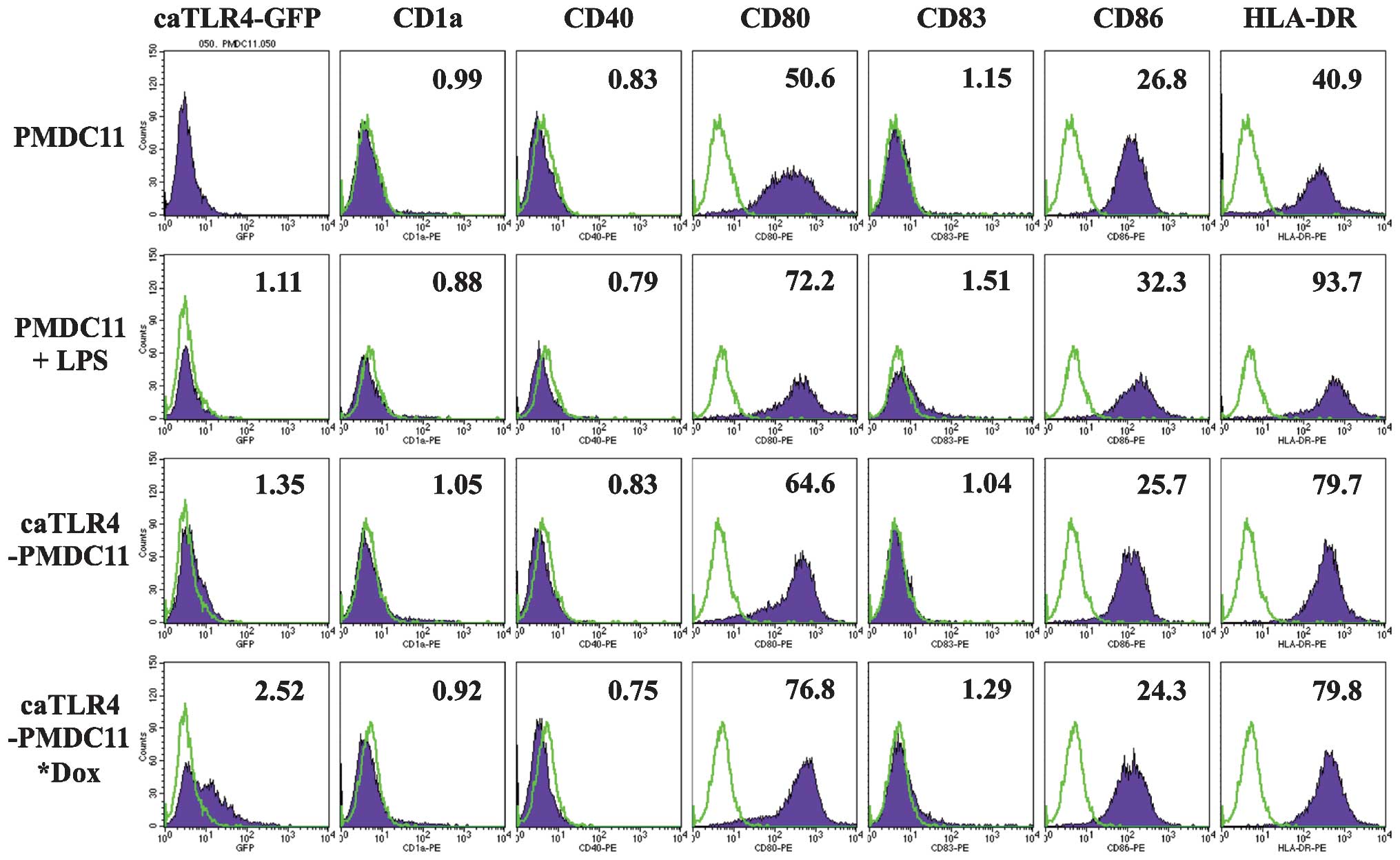
With regard to the establishment of the conditions
for driving the Tet-On system, caTLR4 was observed to be highly
expressed on caTLR4-PMDC11 following 24-h incubation with 1 µg/ml
doxycycline, in the presence of which caTLR4-PMDC11 cells were
viable. Therefore, caTLR4 expression was induced with 1
µg/ml of doxycycline for 24 h in the subsequent
experiments.
The expression of antigen presentation-associated
molecules, including CD80, CD83, CD86 and HLA-DR, on PMDC11 cells
was demonstrated to be enhanced by the stimulation with LPS for 24
h. caTLR4-PMDC11 cells without doxycycline exposure exhibited a
marginal increase in CD80 and HLA-DR expression, which was
suggested to be due to a spontaneous and partial expression of
caTLR4 in addition to GFP when the Tet-On system was inactive. By
driving the Tet-On system with doxycycline in caTLR4-PMDC11, which
was confirmed by the expression of GFP, the expression of CD80,
CD83 and HLA-DR was enhanced to the level of LPS-stimulated PMDC11
cells (Fig. 1).
Antigen-presenting ability of
caTLR-PMDC11
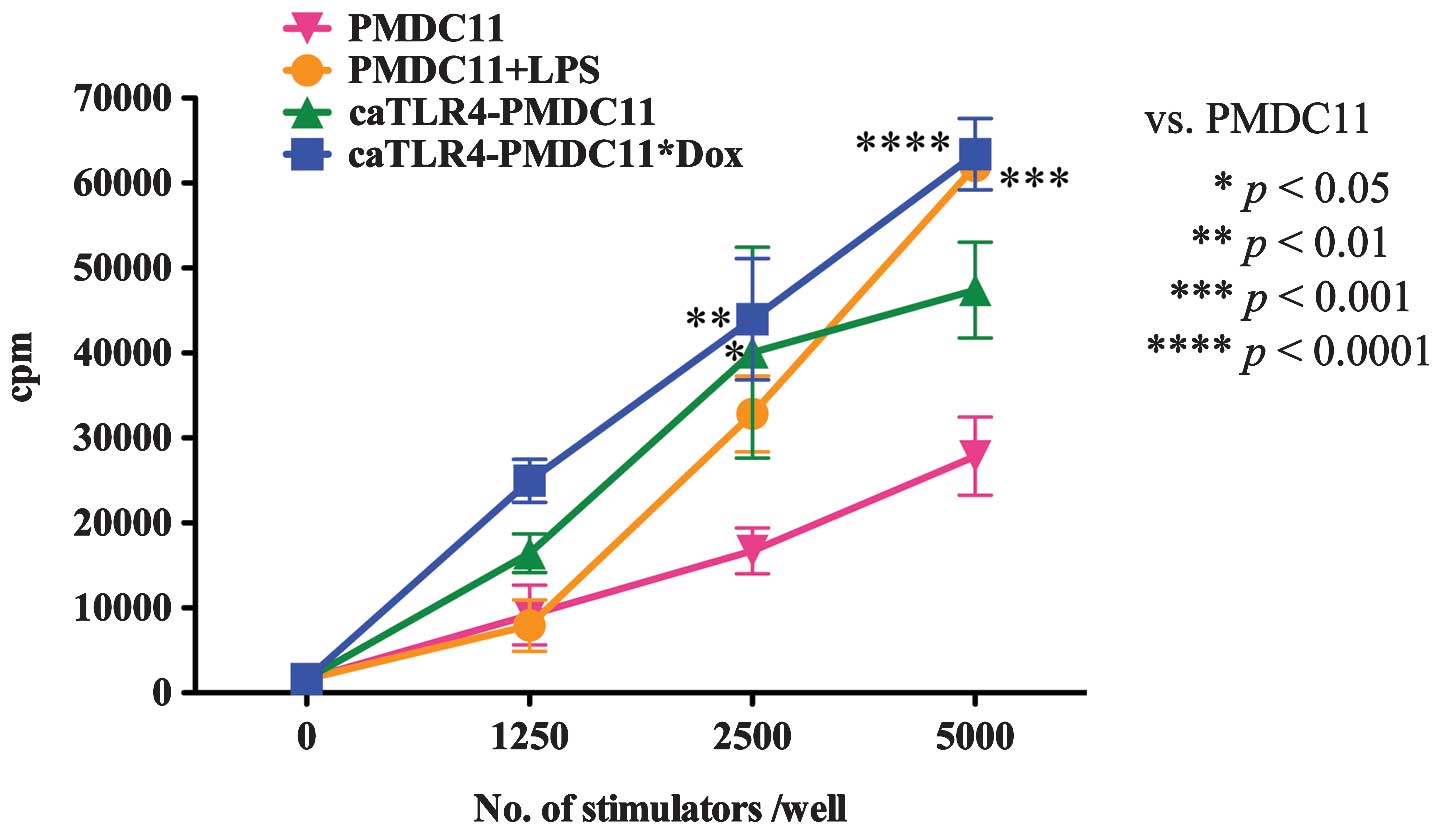
The antigen-presenting ability of caTLR4-PMDC11
cells with or without doxycycline exposure was compared with that
of PMDC11 cells with or without LPS stimulation by performing MLC
with 3H-thymidine incorporation. The antigen-presenting
ability of PMDC11 cells was identified to be enhanced by
stimulation with LPS. By transduction with the caTLR4 gene, the
antigen-presenting ability of PMDC11 cells was observed to increase
(caTLR4-PMDC11 without doxycycline exposure). In addition,
following exposure to doxycycline, the antigen-presenting ability
of caTLR4-PMDC11 was increased. caTLR4-PMDC11 cells possessed
greater than or equal antigen-presenting ability compared with that
of the LPS-stimulated PMDC11 cells (Fig. 2). This enhanced antigen-presenting
ability of caTLR4-PMDC11 cells without doxycycline exposure
compared with that of PMDC11 cells was hypothesized to be due to
spontaneous and partial expression of caTLR4 in the state of Tet-On
system inactivity.
IFN-γ production of caTLR-PMDC11
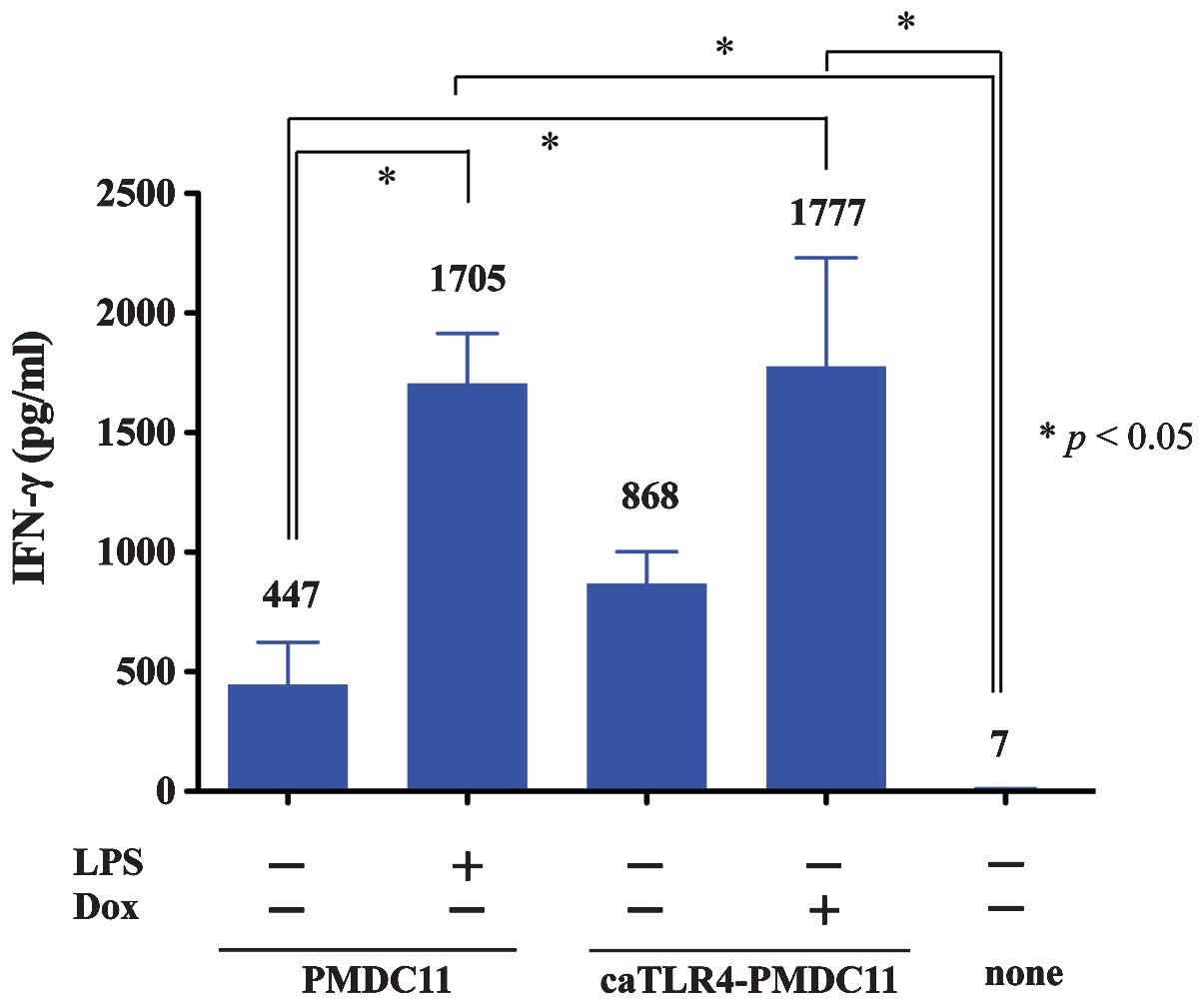
IFN-γ production of lymphocytes stimulated by
caTLR4-PMDC11 cells with or without doxycycline exposure was
compared with that produced by PMDC11 cells with or without LPS
stimulation by using the CBA Human IFN-γ Flex Set assay (Fig. 3). IFN-γ production of
PMDC11-stimulated lymphocytes was observed to be enhanced by the
culture of PMDC11 cells with LPS. By transduction with the caTLR4
gene, the lymphocyte-stimulating ability of PMDC11 cells leading to
the production of IFN-γ was increased (caTLR4-PMDC11 without
doxycycline exposure). In addition, in the presence of doxycycline,
the lymphocyte-stimulating ability of caTLR4-PMDC11 cells leading
to the production of IFN-γ was demonstrated to increase.
Doxycycline-treated caTLR4-PMDC11 cells possessed equivalent levels
of potency in mediating the production of IFN-γ by lymphocytes
compared with those of LPS-stimulated PMDC11 cells (Fig. 3). The enhanced
lymphocyte-stimulating ability of caTLR4-PMDC11 without doxycycline
exposure leading to the production of IFN-γ compared with that of
PMDC11 cells was suggested to be due to spontaneous and partial
expression of caTLR4 in the state of Tet-On system inactivity.
mWT1-specific CTL induction by
caTLR4-PMDC11 cells
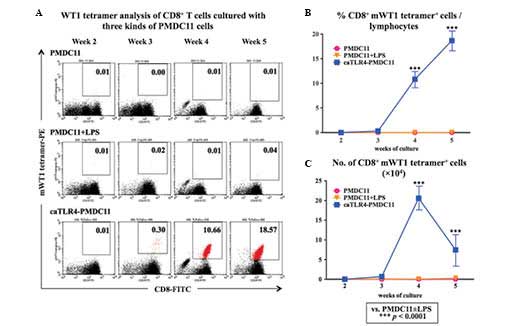
The mWT1-specific CTL-inducing ability of normal
PB-CD8+ T cells was compared between the PMDC11,
LPS-stimulated PMDC11 and doxycycline-treated caTLR4-PMDC11 cells
as the stimulator cells. Following three to five weeks of
co-culture of CD8+ T cells and PMDC11 cells,
doxycycline-treated caTLR4-PMDC11 cells generated an increased
percentage and number of mWT1-specific CTLs compared with that of
PMDC11 or LPS-stimulated PMDC11 cells (Fig. 4).
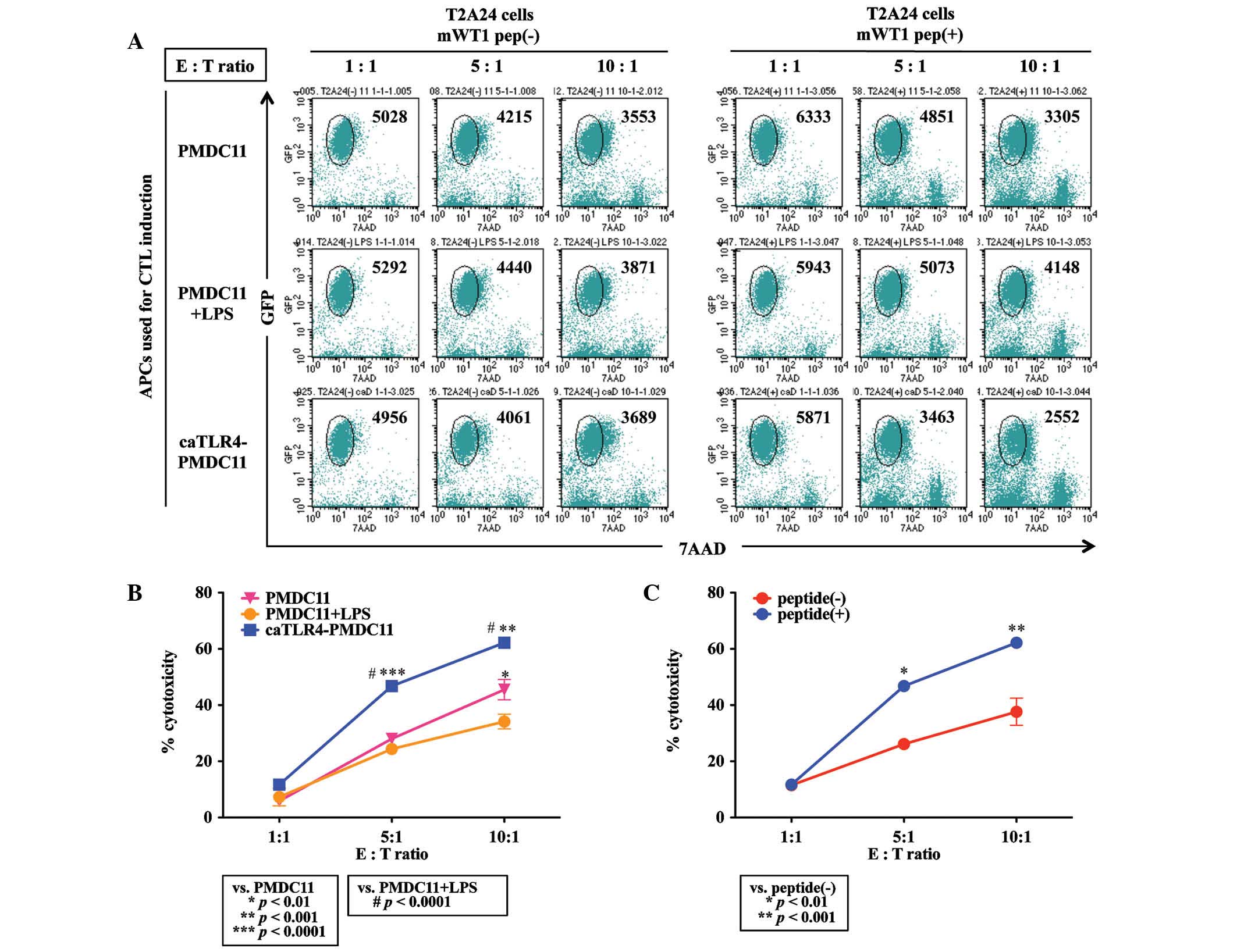
Cytotoxicity of mWT1-specific CTLs
induced by caTLR4-PMDC11
Antigen-specific cytotoxicity of CTLs generated from
normal CD8+ T cells by priming with mWT1 peptide-pulsed
PMDC11 cells, LPS-stimulated PMDC11 cells or doxycycline-exposed
caTLR4-PMDC11 cells was evaluated by flow cytometric analysis using
mWT1 peptide-pulsed and GFP-transduced T2A24 cells as target cells.
CD8+ T cells co-cultured with mWT1 peptide-pulsed
caTLR4-PMDC11 cells for four weeks demonstrated an increased
cytotoxicity against mWT1 peptide-pulsed target cells compared with
that of CD8+ T cells co-cultured with mWT1
peptide-pulsed PMDC11 or LPS-stimulated PMDC11 cells (Fig. 5A and B). The differences among the
PMDC11, LPS-stimulated PMDC11 and doxycycline-exposed caTLR4-PMDC11
cells were suggested to be due to the increased percentage of
mWT1-specific CTLs present in the co-cultured cells. Although
non-specific cytotoxicity against T2A24 cells without mWT1 peptide
loading was observed in CD8+ T cells co-cultured with
mWT1 peptide-pulsed caTLR4-PMDC11 cells, the percentage
cytotoxicity against T2A24 cells loaded with the mWT1 peptide was
significantly greater than that of non-loaded target cells at E:T
ratios of 5:1 and 10:1 (P<0.01; Fig. 5C).
Discussion
Freely available APCs with standardized quality are
required for the establishment of an efficient adoptive cellular
immunotherapy method for use in tumors and severe viral infections.
Although autologous monocyte-derived DCs (moDCs) are commonly used
as APCs for use in immunotherapy, the preparation of a sufficiently
large quantity is complex and the quality of moDCs varies between
individuals (12). Artificial APCs
using either acellular (13–16)
or cell-based systems [e.g., Drosophila (17), mouse fibroblast (18,19),
human erythro-myeloid (20,21)
or breast carcinoma (22) cell
lines] have been introduced in place of moDCs. In these cell-based
artificial APCs, antigen presentation-associated molecules,
including major histocompatibility class I (17–21),
CD80 (17–19,21,22),
intercellular adhesion molecule 1 (CD54) (17–19,21),
lymphocyte function-associated antigen 3 (CD58) (18,19,21)
or CD83 (21) require expression
via cDNA transfection in order to reach sufficient levels of
antigen-presenting ability on these artificial APCs.
Additionally, a DC line with antigen-presenting
ability may be used for the induction of anti-tumor or anti-viral
CTLs. In order to do this, three cell lines are available, one of
which is the PMDC05 cell line, which is derived from pDC leukemia
cells. Another is a pDC line, GEN2.2, which has been reported to
require a monolayer of irradiated (60 Gy) MS-5 feeder cells for
growth (23); however, an
additional study has suggested that it may be able to grow without
feeder cells (24). The third cell
line is CAL-1, which was originally established from a patient with
blastic natural killer cell lymphoma. CAL-1 cells are very similar
to PMDC05 cells in morphology and surface phenotypes, in addition
to producing a small quantity of IFN-α (25). Recently, CAL-1 was used for
evaluating the efficacy of anti-nuclear factor (NF)-κB-treatment on
blastic plasmacytoid dendritic cell neoplasms, which was associated
with aberrant activation of the NF-κB pathway (26). With regard to GEN2.2, Aspord et
al (27) demonstrated that the
GEN2.2 cell line was able to stimulate increased levels of
tumor/viral antigen-specific and functional cytotoxic T cell
responses in vitro. They additionally noted that this cell
line resulted in an inhibition of tumor growth in a humanized mouse
in vivo model. Furthermore, Aspord et al (27) indicated that the GEN2.2 vaccine had
a greater efficacy than conventional myeloid DC-based vaccines and
suggested that an allogeneic pDC line-based vaccine may be a novel
therapeutic tool for clinical application in cancer treatment
(27). GEN2.2 cells have
additionally been observed to secrete Type I and III IFN in
response to the hepatitis C virus pathogen-associated molecular
patterns (28).
PMDC05 cells, which were established in our
laboratory (Laboratory of Hematology and Oncology, Graduate School
of Health Sciences, Niigata University), are APCs and are able to
produce a small quantities of IFN-α when stimulated with CpG and
influenza virus (29). By
stimulation with LPS, PMDC05 cells produce IL-12p70 and the
antigen-presenting ability is markedly increased (5). PMDC05 cells are able to induce
antigen-specific CTLs efficiently from normal PB-CD8+ T
cells in the co-culture experiments using tumor antigen (mWT1
peptide) or viral antigen (CMVpp65 peptide) (6). Although PMDC05 cells have an
antigen-presenting ability without stimulation, minimal expression
of CD80 was detected on PMDC05 cells (4). Therefore PMDC05 cells were transduced
with the CD80 gene using a lentiviral vector and the
CD80-transduced PMDC05 cells were termed PMDC11 cells, which were
demonstrated to have a more potent antigen-presenting ability than
that of PMDC05 cells (7).
Preliminary investigations demonstrated that the
expression of antigen presentation-associated molecules and
antigen-presenting ability were enhanced in PMDC11 cells via the
activation of TLR4 by LPS. To further potentiate the
antigen-presenting ability and antigen-specific CTL-inducing
ability, the caTLR4 gene was transduced into PMDC11 cells in order
to constitutively activate TLR4 signaling while avoiding the use of
LPS. LPS is not preferable in a clinical setting due to its
gram-negative bacterial endotoxin-origin.
With regard to caTLR4, Cisco et al (30) reported that transfection of caTLR4
RNA into DCs induced DC maturation comparable to that driven by the
potent cytokine mixture of TNF-α, IL-6, IL-1β and prostaglandin E2.
caTLR4 RNA-transfected DCs secreted IL-12 and TNF-α and maturation
marker expression was enhanced in them, resulting in an increase in
allostimulatory and antigen-specific T-cell responses (30). Abdel-Wahab et al (31) demonstrated that co-transfection
into DC with RNA of caTLR4 and tumor antigens induced tumor
antigen-specific T-cell responses more efficiently than
transfection of tumor antigen RNA in combination with maturation
cytokines. Bonehill et al (32) reported an increase in T-cell
stimulatory capacity of moDCs by co-electroporation with differing
combinations of CD40L, CD70 and caTLR4-encoding mRNA.
In order to further potentiate the
antigen-presenting and CTL-inducing ability of CD80-expressing
PMDC11, a lentiviral vector expressing caTLR4 was transduced into
PMDC11 cells. PMDC11 cells, which were transduced with the
caTLR4-expressing vector, proliferated transiently for three weeks
and then proceeded to apoptosis. Thus, PMDC11 cells were
subsequently transduced with a caTLR4 gene regulated by the Tet-On
system (caTLR4-PMDC11). Normal CD8+ T cells were
co-cultured with mWT1 peptide-pulsed PMDC11 or caTLR4-PMDC11 and
primed with mWT1 peptide-pulsed PMDC11 or caTLR4-PMDC11 every week.
mWT1 tetramer+/CD8+ T cells were generated
subsequent to 2–3 weeks of culture and were observed to be
significantly increased in caTLR4-PMDC11-primed CD8+
T-cell culture, compared with PMDC11-primed CD8+ T-cell
culture. Identical results were obtained in experiments using
CMVpp65 peptide instead of mWT1 peptide (data not shown).
In conclusion, the results of the present study
indicated that the antigen-specific CTL-inducing ability of PMDC11
was enhanced by caTLR4 gene transduction and that caTLR4-PMDC11
cells may be applied as potent APCs in order to generate
antigen-specific CTLs for adoptive cellular immunotherapy against
tumors and severe viral infections.
Acknowledgments
The results of the present study were presented at
the 15th International Congress of Immunology in 2013
(Milan, Italy). The authors would like to thank Dr Renata Stripecke
(Department of Hematology, Hemostasis, Oncology and Stem Cell
Transplantation, Hannover Medical School, Hannover, Germany) for
providing the gene transduction protocol. The UCLA Vector Core is
supported by JCCC/P30 CA016042 and CURE/P30 DK041301.
References
|
1
|
Steinman RM and Banchereau J: Taking
dendritic cells into medicine. Nature. 449:419–426. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sims RB: Development of sipuleucel-T:
Autologous cellular immunotherapy for the treatment of metastatic
castrate resistant prostate cancer. Vaccine. 30:4394–4397. 2012.
View Article : Google Scholar
|
|
3
|
Montagna D, Turin I, Schiavo R, et al:
Feasibility and safety of adoptive immunotherapy with ex
vivo-generated autologous, cytotoxic T lymphocytes in patients with
solid tumor. Cytotherapy. 14:80–90. 2012. View Article : Google Scholar
|
|
4
|
Narita M, Watanabe N, Yamahira A, et al: A
leukemic plasmacytoid dendritic cell line, PMDC05, with the ability
to secrete IFN-alpha by stimulation via Toll-like receptors and
present antigens to naive T cells. Leuk Res. 33:1224–1232. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watanabe N, Narita M, Yamahira A, et al:
Transformation of dendritic cells from plasmacytoid to myeloid in a
leukemic plasmacytoid dendritic cell line (PMDC05). Leuk Res.
34:1517–1524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamahira A, Narita M, Nakamura T, et al:
Generation of antigen-specific cytotoxic T lymphocytes using a
leukemic plasmacytoid dendritic cell line as antigen presenting
cells. Leuk Res. 35:793–799. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamahira A, Narita M, Ishii K, et al:
Enhancement of antigen presenting ability in the leukemic
plasmacytoid dendritic cell line (PMDC05) by lentiviral
vector-mediated transduction of CD80 gene. Leuk Res. 36:1541–1546.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stripecke R: Lentiviral vector-mediated
genetic programming of mouse and human dendritic cells. Methods Mol
Biol (Clifton, NJ). 506:139–158. 2009.
|
|
9
|
Pincha M, Salguero G, Wedekind D, et al:
Lentiviral vectors for induction of self-differentiation and
conditional ablation of dendritic cells. Gene Ther. 18:750–764.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi M, Shigeno N, Takahashi H, et
al: Effects of transfection of p210bcr-abl and bcr-v-abl into the
factor-dependent human leukemia cell line HSM-911. Leuk Res.
21:1115–1123. 1997. View Article : Google Scholar
|
|
11
|
Narita M, Masuko M, Kurasaki T, Kitajima
T, Takenouchi S, Saitoh A, Watanabe N, Furukawa T, Toba K, Fuse I,
Aizawa Y, Kawakami M, Oka Y, Sugiyama H and Takahashi M: WT1
peptide vaccination in combination with imatinib therapy for a
patient with CML in chronic phase. Int J Med Sci. 7:72–81. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamanaka R, Homma J, Yajima N, et al:
Clinical evaluation of dendritic cell vaccination for patients with
recurrent glioma: Results of a clinical phase I/II trial. Clin
Cancer Res. 11:4160–4167. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Irvine DJ and Doh J: Synthetic surfaces as
artificial antigen presenting cells in the study of T cell receptor
triggering and immunological synapse formation. Semin Immunol.
19:245–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koffeman E, Keogh E, Klein M, Prakken B
and Albani S: Identification and manipulation of antigen specific
T-cells with artificial antigen presenting cells. Methods Mol Med.
136:69–86. 2007.PubMed/NCBI
|
|
15
|
Rudolf D, Silberzahn T, Walter S, et al:
Potent costimulation of human CD8 T cells by anti-4-1BB and
anti-CD28 on synthetic artificial antigen presenting cells. Cancer
Immunol Immunother. 57:175–183. 2008. View Article : Google Scholar
|
|
16
|
Steenblock ER, Wrzesinski SH, Flavell RA
and Fahmy TM: Antigen presentation on artificial acellular
substrates: modular systems for flexible, adaptable immunotherapy.
Expert Opin Biol Ther. 9:451–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai Z, Brunmark A, Jackson MR, Loh D,
Peterson PA and Sprent J: Transfected Drosophila cells as a probe
for defining the minimal requirements for stimulating unprimed
CD8+ T cells. Proc Natl Acad Sci USA. 93:14736–14741.
1996. View Article : Google Scholar
|
|
18
|
Hasan AN, Kollen WJ, Trivedi D, et al: A
panel of artificial APCs expressing prevalent HLA alleles permits
generation of cytotoxic T cells specific for both dominant and
subdominant viral epitopes for adoptive therapy. J Immunol.
183:2837–2850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Papanicolaou GA, Latouche JB, Tan C, et
al: Rapid expansion of cytomegalovirus-specific cytotoxic T
lymphocytes by artificial antigen-presenting cells expressing a
single HLA allele. Blood. 102:2498–2505. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan J, Gallardo HF, Rasalan T, et al: In
vitro expansion of Ag-specific T cells by HLA-A*0201-transfected
K562 cells for immune monitoring. Cytotherapy. 8:498–508. 2006.
View Article : Google Scholar
|
|
21
|
Butler MO, Lee JS, Ansén S, et al:
Long-lived antitumor CD8+ lymphocytes for adoptive
therapy generated using an artificial antigen-presenting cell. Clin
Cancer Res. 13:1857–1867. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sasawatari S, Tadaki T, Isogai M, Takahara
M, Nieda M and Kakimi K: Efficient priming and expansion of
antigen-specific CD8+ T cells by a novel cell-based
artificial APC. Immunol Cell Biol. 84:512–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chaperot L, Blum A, Manches O, et al:
Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of
plasmacytoid dendritic cells. J Immunol (Baltimore, Md. 150). 176.
pp. 248–255. 2006
|
|
24
|
Di Domizio J, Blum A, Gallagher-Gambarelli
M, Molens JP, Chaperot L and Plumas J: TLR7 stimulation in human
plasmacytoid dendritic cells leads to the induction of early
IFN-inducible genes in the absence of type I IFN. Blood.
114:1794–1802. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maeda T, Murata K, Fukushima T, et al: A
novel plasmacytoid dendritic cell line, CAL-1, established from a
patient with blastic natural killer cell lymphoma. Int J Hematol.
81:148–154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sapienza MR, Fuligni F, Agostinelli C, et
al: Molecular profiling of blastic plasmacytoid dendritic cell
neoplasm reveals a unique pattern and suggests selective
sensitivity to NF-κB pathway inhibition. Leukemia: official journal
of the Leukemia Society of America; Leukemia Research Fund, U.K.:
2014
|
|
27
|
Aspord C, Charles J, Leccia MT, et al: A
novel cancer vaccine strategy based on HLA-A*0201 matched
allogeneic plasmacytoid dendritic cells. PLoS ONE. 5:e104582010.
View Article : Google Scholar
|
|
28
|
Stone AE, Giugliano S, Schnell G, et al:
Hepatitis C virus pathogen associated molecular pattern (PAMP)
triggers production of lambda-interferons by human plasmacytoid
dendritic cells. PLoS Pathogens. 9:e10033162013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Narita M, Kuroha T, Watanabe N, et al:
Plasmacytoid dendritic cell leukemia with potent antigen-presenting
ability. Acta Haematol. 120:91–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cisco RM, Abdel-Wahab Z, Dannull J, et al:
Induction of human dendritic cell maturation using transfection
with RNA encoding a dominant positive toll-like receptor 4. J
Immunol (Baltimore, Md. 1950). 172:7162–7168. 2004. View Article : Google Scholar
|
|
31
|
Abdel-Wahab Z, Cisco R, Dannull J, et al:
Cotransfection of DC with TLR4 and MART-1 RNA induces
MART-1-specific responses. J Surg Res. 124:264–273. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bonehill A, Tuyaerts S, Van Nuffel AM, et
al: Enhancing the T-cell stimulatory capacity of human dendritic
cells by co-electroporation with CD40L, CD70 and constitutively
active TLR4 encoding mRNA. Mol Ther. 16:1170–1180. 2008. View Article : Google Scholar : PubMed/NCBI
|