Introduction
An estimated 18,000 new cases of brain and central
nervous system tumors are diagnosed annually, and ~13,000 people
will succumb to their disease in the United States (1). Despite the availability of the latest
neuroimaging techniques and advances in treating primary tumors,
cases of brain cancer continue to rise. The primary treatment
option for these patients is complete or partial brain irradiation.
Radiotherapy is a well-established modality for the treatment of a
number of types of cancer. It is estimated that approximately half
of the patients with brain cancer receive radiotherapy as part of a
treatment strategy. Each year, ~200,000 patients with brain cancer
are treated with partial or whole brain irradiation. However, the
therapeutic effects are limited by the harmful consequences of the
post-irradiation injuries sustained by healthy normal cells
(2–5). In the case of brain cancer
irradiation, these injuries may give rise to irreversible cognitive
impairment, which is accompanied by an increase in mortality and
morbidity. This cognitive impairment is hypothesized to be a result
of the oxidative stress that occurs as a result of irradiation. The
free radicals present are predominantly reactive oxygen species
(ROS) that are generated as a result of ionizing radiation leading
to DNA destruction, such as single or double-strand breaks, base
damage and DNA-DNA or DNA-protein cross-links. The DNA double
strand breaks are hypothesized to be the most damaging events that
occur following the administration of ionizing radiation, and have
been found to be the principal mechanism of irradiation-induced
cell death. It has also been reported that DNA damage due to
irradiation leads to apoptosis. Apoptosis is an important mechanism
of neuronal cell death in rapidly and slowly progressive brain
diseases. Experimental evidence suggests that radiation triggers
the formation of microglial cocultures as well as astrocytes in
vitro, elevating the expression of cyclooxygenase-2 (COX-2),
interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, which
are key proinflammatory mediators (6–13).
There are currently no effective, evidence-based
treatments available for radiation-induced brain injury leading to
cognitive impairment and other brain diseases. A number of
botanical extracts have been investigated in this context. Examples
include the different extracts of Ginkgo biloba, Centella
asiatica, Hippophae rhamnoides, Osimum sanctum,
Panax ginseng, Podophyllum hexandrum, Tinospora
cordifolia, Piper longum, Mentha arvensis and
Mentha piperita. Numerous natural and herbal products, which
are of medicinal use have become important ingredients in the human
diet. The capacity of dietary ingredients to provide protection
against radiation-induced injury has until now remained unexplored.
Radioprotective dietary supplements may be an ideal form of
treatment, as they are used frequently as part of a normal diet, as
they are non-toxic and have minimal side effects (14).
Rheum officinale Baill. (a member of the
Polygonaceae family) is a perennial herb, the dried roots and
rhizomes of which are commonly termed rhubarb. Rheum
officinale is also called Chinese rhubarb and is used in
traditional Chinese medicine, where it is termed ‘Yao yong
dahuang’. Anthraquinones, dianthraquinones, stilbenes,
flavonoids and polyphenols are the principal phytoconstituents of
the majority of rhubarbs. Emodin
(1,3,8-trihydroxy-6-methylanthraquinone) is a naturally occurring
anthraquinone present in the roots and rhizomes of Chinese herbs,
including Rheum officinale and Rheum emodi. Rhubarb
has been reported to have several pharmacological effects,
including anti-inflammatory, antibacterial, purgative and
anticancer properties. Certain species of Rheum have been
used against radiation-induced immune damage in rats (15–17).
The aim of the present study was to evaluate the
radioprotective action of Rheum officinale extract against
neuronal apoptotic cell death and ROS generation induced by
ionizing radiation. Phytochemical analysis by liquid
chromatography-mass spectrometry (LC-MS) and high performance
liquid chromatography (HPLC) were also conducted, which led to the
identification of five chemical constituents that were present in
the extract.
Materials and methods
Materials
Bisbenzimide was obtained from Sigma-Aldrich (St.
Louis, MO, USA). Phosphate-buffered saline (PBS) and fetal bovine
serum (FBS) were purchased from Thermo Fischer Scientific (Waltham,
MA, USA).
Plant material and extraction
procedure
Rheum officinale was collected between August
and September of 2012 from a local site in Guangzhou, China. The
identity of the plant material was confirmed by an experienced
taxonomist. The rhizomes of Rheum officinale were thoroughly
washed with tap water, shade dried and cut into small sections.
Ethanol (95%) was used for hot extraction, which was conducted over
3 h using a soxhlet extraction apparatus (Jianhu Shendi Glass
Instruments Co., Ltd., Yancheng, China). The extract was then
concentrated under reduced pressure in a rotary evaporator
(Hangzhou Greatcool Refrigeration Equipment Co., Ltd., Hangzhou,
China) at 40°C and maintained in a refrigerator at 4°C prior to
use. Emodin and aloemodin were obtained from Sigma-Aldrich.
Animals
Female Sprague-Dawley rats were obtained from the
Experimental Animal Centre of Sichuan University (Chengdu, China).
Animals were treated in accordance with the Guide for Animal Care
and Use of Laboratory Animals (National Institute of Health, 1996).
All procedures were approved by the Ethics Committee of the General
Hospital of Chengdu Military Region (Chengdu, China).
Primary neuronal cultures
Primary neuronal cultures were obtained from the
cortex of rat embryos aged 20 days. Pregnant rats were sacrificed
by decapitation and the embryos were removed aseptically. Cortices
were removed from the embryos using a dissection microscope (Ningbo
Zhanjing Optical Instruments Co., Ltd., Ningbo, China). Tissues
were ground and trypsinized using 0.21% trypsin-EDTA in 0.2 M PBS
for 10 min at 37°C. Following centrifugation, tissues were
suspended in modified Eagle’s medium (MEM; Hangzhou Sijiqing
Biological Products Co., Ltd., Hangzhou, China) containing 10% FBS
and triturated with a Pasteur pipette (Runlab Labware Manufacturing
Co., Ltd., Taizhou, China). The resulting single-cell suspension
was collected and the cell density of the suspension was measured
using a hemocytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Cells were cultured onto poly-D-lysine coated 96-well plates
(1×106 cells/well). Cell cultures were maintained at
37°C in a CO2 incubator. The resultant culture comprised
95% neuronal cells, 3.5% astrocytes, 0.5% oligodendrocytes, 0.6%
microglia and 0.4% ependymal cells.
Exposure of primary cultures to ionizing
radiation
Following culture for seven days, cells were treated
with 20 μg/ml rhubarb extract, 10 μM emodin and 10
μM aloemodin in MEM and 10% FBS. The primary cultures were
then subjected to irradiation in a 137Cs irradiator
(J.L. Shepherd and Associates, San Fernando, CA, USA) with a 2 Gy
γ-ray dose. Following irradiation, cultures were maintained for 24
h at 37°C in a 7.5% CO2 incubator prior to analysis.
Measurement of ROS generation
ROS generation was measured using a
2′,7′-dichlorofluorescein (DCFH-DA) probe as described previously
(18). DCFH-DA enters cells where
it is cleaved by cellular esterases and becomes impermeable. The
probe becomes fluorescent on oxidization by ROS. Cell cultures were
washed with PBS, supplemented with 0.15 g/l CaCl2 and
0.2 g/l MgCl2, incubated with 10 μM DCFH-DA for
30 min, washed with PBS to remove any excess probe and incubated
with 10 μg/ml rhubarb extract. After 3 h, the cells were
irradiated in a 137Cs irradiator with a single dose of 2
Gy γ-rays. At 1 h following irradiation, ROS generation was
measured using a FACS BD Calibur (BD Biosciences, Bedford, MA,
USA), and BD Cell Quest Pro 6.0 software was used to analyze the
data.
Lactate dehydrogenase (LDH) assay
At 24 h post-irradiation, the cell culture medium
was harvested and rendered cell-free using centrifugation (15,000 x
g for 5 min at 4°C). LDH Cytotoxicity Assay kit (Roche Diagnostics
Corporation, Indianapolis, IN, USA) was used to measure the release
of LDH from the cells. After a 40 min incubation at room
temperature using the LDH kit, the LDH release was measured at a
wavelength of 495 nm using a microplate system enzyme-linked
immunospot assay reader (MHM-96B; Changchun MH Medical Co., Ltd.,
Changchun, China). Three different concentrations (2, 5 and 10
μg/ml) of the rhubarb extract were used to evaluate its
effect on lactate dehydrogenase release in neuronal cell
cultures.
Measurement of apoptosis
Bisbenzimide nuclear staining was used to detect DNA
fragmentation. Cells were plated onto 96-well plates coated with
poly-D-lysine for bisbenzimide staining. Cells were fixed with 5%
paraformaldehyde in 0.1 M PBS, washed with 0.1 mM PBS and stained
with bisbenzimide (10 μg/ml), which is a fluorescent
DNA-binding dye, for 10 min at 25°C. Three different concentrations
(2, 5 and 10 μg/ml) of the rhubarb extract were used to
investigate its effect on neuronal apoptosis. Cells were examined
under an optical fluorescence microscope (Ningbo Sunny Instruments
Co. Ltd., Zhejiang, China). The number of cells with apoptotic
bodies per total cell number was calculated from 8–10 random fields
of 6×103 cells/well. Three wells were assessed per
treatment.
LC-ESI-MS-MS/HPLC analysis
LC-MS equipment consisted of a chromatographic
system (LC-MS QqQ-6410B Agilent, 1260 Infinity series (Agilent
Technologies, Santa Clara, CA, USA) coupled with an Agilent Triple
Quad mass spectrometer fitted with an electrospray ionization
source, using the following MS conditions: MS range, 100–1,200 Da;
MS spectra obtained using positive and negative modes; nebulizer
gas, 45 ψ; and capillary voltage, 4000 V.
HPLC analysis was conducted using an Agilent 1260
infinity series (Agilent Technologies). A chromolith RP-18e column
(4.6 mm ID, 50 mm length) was used. The mobile phase consisted of
(A) aqueous acetic acid (0.5%) and (B) 70% methanol. The mobile
phase gradient was as follows: 0–8 min, linear gradient from 10 to
25% of B; 8–12 min, isocratic conditions at 25% of B; 12–16 min,
linear gradient from 25 to 40% of B; 16–40 min, linear gradient
from 40 to 50% of B; and 40–50 min, linear gradient from 50 to 100%
of B. The flow rate used was 1 ml/min.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. One way analysis of variance was used. All statistical
analyses were conducted using SPSS version 11.5 (SPSS Inc.,
Chicago, IL, USA). P≤0.05 was considered to indicate a
statistically significant difference.
Results
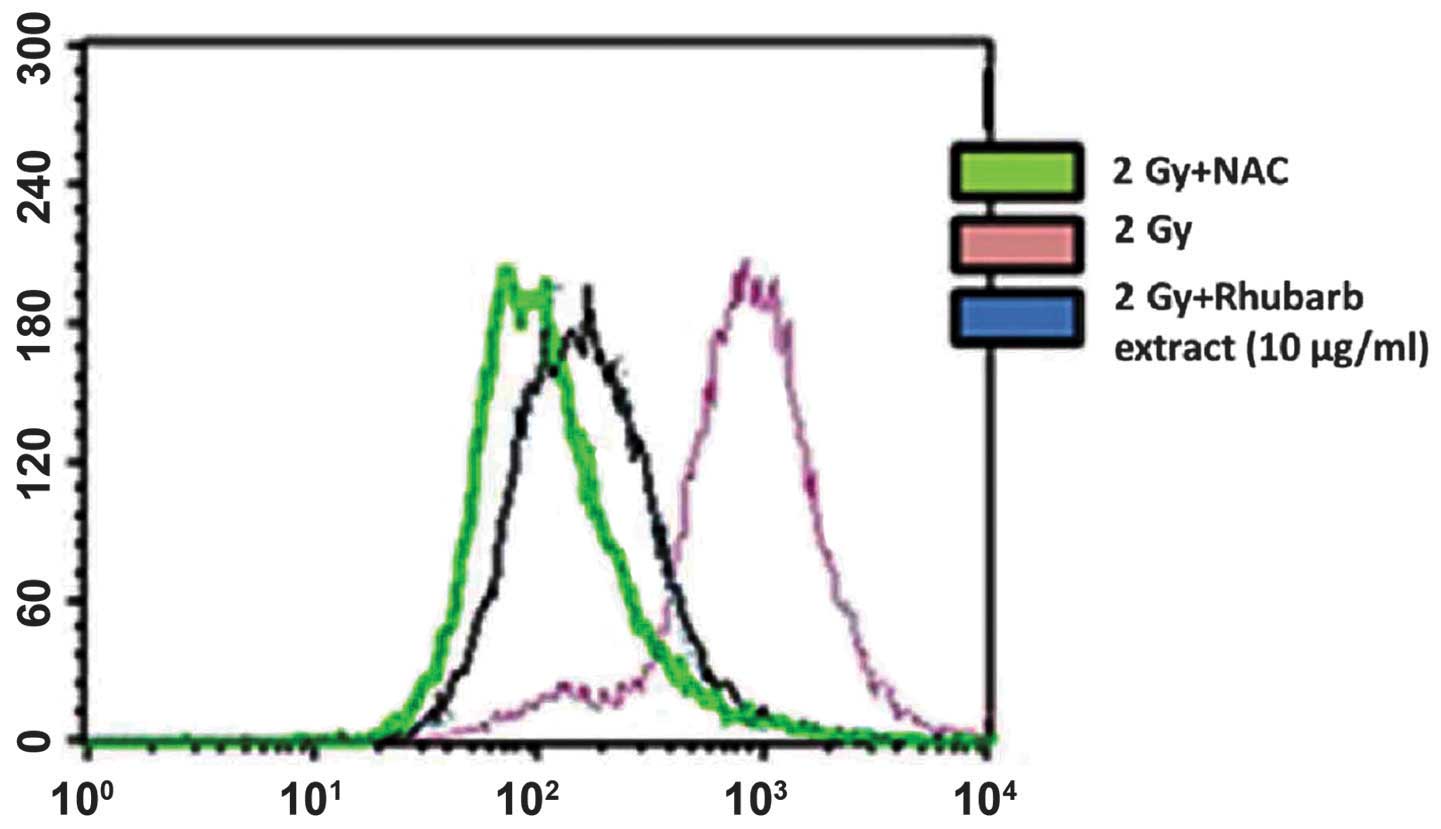
ROS generation
The present study used the oxidation-sensitive probe
DCFH-DA to determine ROS generation in neuronal cells that had been
incubated with the rhubarb extract prior to irradiation with a
single dose of 2 Gy γ-rays. As hypothesized, irradiating the cells
resulted in enhanced ROS production even at 1 h post-irradiation.
This increased ROS generation was inhibited in cells treated with
rhubarb extract at a dose of 10 μg/ml. This result was
confirmed by incubating the cells with N-acetylcysteine, a known
ROS scavenger. This also inhibited the radiation-induced increase
in DCF fluorescence, which corresponded to decreased ROS generation
(Fig. 1).
Cell apoptosis and lactate dehydrogenase
release
Significant cell death, as measured by lactate
dehydrogenase release, was observed following irradiation at a dose
of 2 Gy. Irradiation reduced the numbers of neuronal cells by
50–55% and apoptosis was observed at 24 h post-irradiation. Using
bisbenzimide staining, condensed and fragmented DNA was detected in
the apoptotic cells. The extract exhibited a dose-dependent
inhibition of radiation-induced apoptotic neuronal cell death, with
the 10 μg/ml dose of the extract resulting in the greatest
degree of inhibition (P<0.05; Figs.
2 and 3). Furthermore, in the
bisbenzimide stained cultures, the rhubarb extract significantly
reduced the number of neurons with condensed and fragmented DNA,
particularly at the higher concentration (10 μg/ml). As
shown in Fig. 2, healthy surviving
neurons have a large, round and intact nucleus without any
deformation, whereas in apoptotic neurons the chromatin is
condensed and fragmented. As shown in Fig. 2, in the control cell cultures no
apoptotic neurons were observed. In the cell cultures treated with
rhubarb extract, few apoptotic neurons were visible. Administration
of the extract also prevented the increase in lactate dehydrogenase
release induced by irradiation (P<0.05). Three different
concentrations (2, 5 and 10 μg/ml) of the extract were used
in the experiments that observed lactate dehydrogenase release and
apoptosis. The extract exhibited a dose-dependent inhibition of
lactate dehydrogenase release. The 10 μg/ml dose inhibited
lactate dehydrogenase release by >85% (Fig. 4).
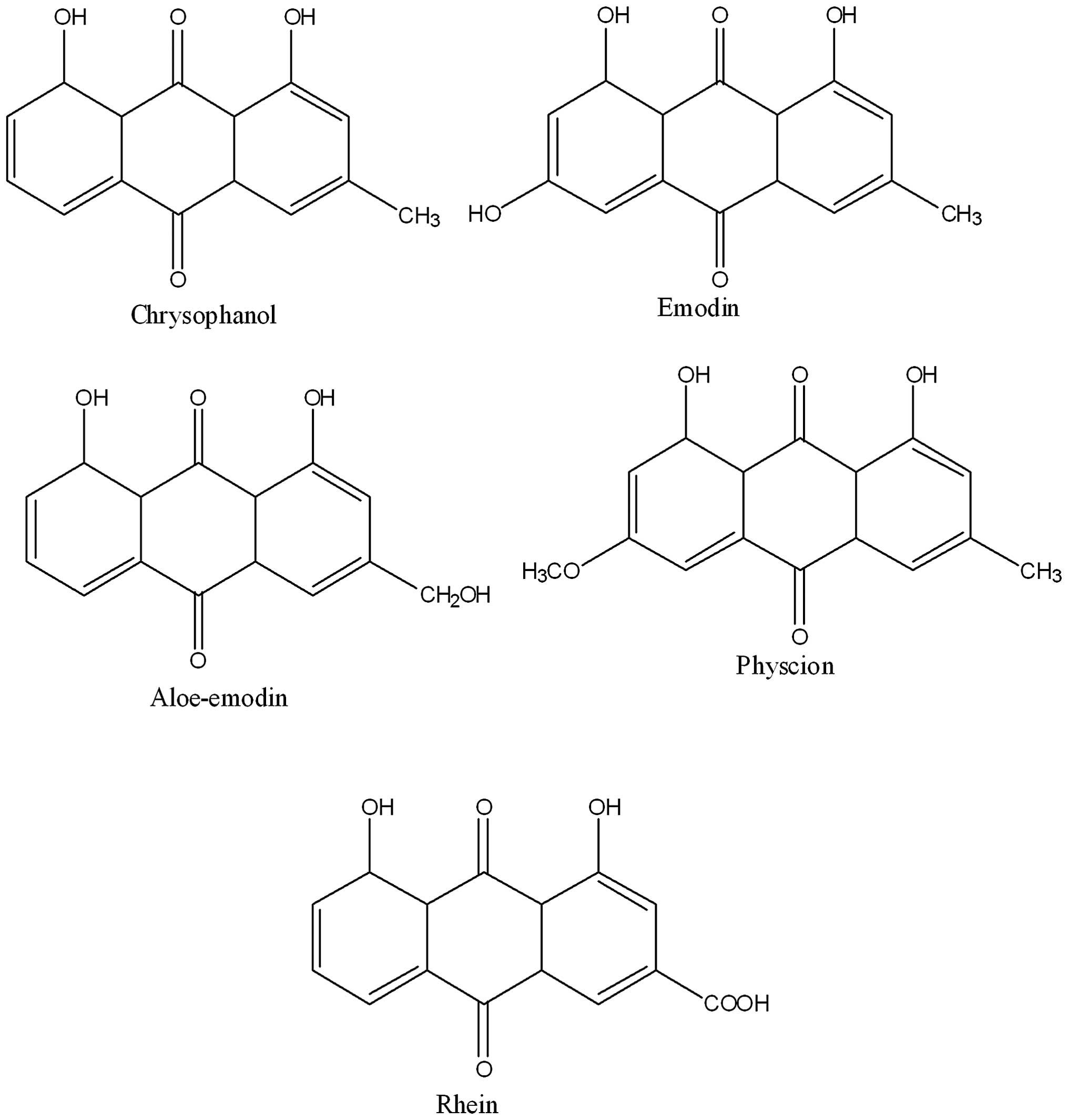
LC-MS-MS/HPLC analysis
The phytochemical analysis of the Rheum
officinale extract was conducted by LC-ESI-MS and HPLC with
diode-array detection techniques. The extract was run under
positive and negative ESI-MS conditions and it showed several major
and minor ionic species. Fragmentation of the major peaks was used
for the identification of compounds present in the extract. The
identification of the chemical compounds was also conducted by
comparing the molecular ion peaks, along with the MS fragmentation
patterns, with those in the literature. The five chemical
constituents identified were emodin, aloe-emodin, chrysophanol,
physcion and rhein (Fig. 4).
Extraction of these phytoconstituents has previously been reported
from this and other species of Rheum. These constituents
have also been reported to possess a spectrum of biological
properties, including antioxidant, antitumor and anti-inflammatory
effects.
Discussion
The current study demonstrated that rhubarb extract
provided significant protection against radiation-induced apoptosis
and reduced ROS generation in primary neuronal cultures. These
results suggest that rhubarb extract may be useful as a medicinal
agent against radiation-induced neuronal apoptosis, which leads to
cognitive impairment. The extract may also be of use in alleviating
the oxidative stress that is induced following partial or whole
brain irradiation. Oxidative stress has been reported to induce
neuroinflammation, which is hypothesized to be involved in the
development of radiation-induced brain injury.
Plant extracts contain a range of phytochemicals and
therefore the radioprotective effects of these compounds are likely
to be mediated through a number of mechanisms. Polyphenols in
plants are involved in scavenging radiation-induced free radicals,
in particular ROS which lead to DNA damage, such as single or
double-strand breaks, base damage and DNA-DNA or DNA-protein
cross-links. The DNA double strand breaks are hypothesized to be
the most damaging events caused by ionizing radiation and are the
primary mechanism leading to cell death due to irradiation.
Polyphenols may also upregulate mRNA expression of antioxidant
enzymes, including glutathione peroxidase, glutathione transferase,
peroxidase, catalase and superoxide dismutase, and thus may
alleviate the oxidative stress induced by ionizing radiation. The
predominant medicinal constituents of rhubarb are emodin and
aloemodin, which are polyphenols. Emodin has been reported to
exhibit anti-inflammatory effects in a number of experimental
models and any molecules with a similar structure are hypothesized
to augment cancer therapy. The anti-inflammatory action of emodin
is linked to its inhibition of nitric oxide and cytokine
production. It may also inhibit superoxide production (19–21).
Emodin or emodin-containing extracts, such as rhubarb, are also
reported to promote an antioxidant status due to inhibition of free
radical formation, free radical scavenging, inhibition of lipid
peroxidation and increases in antioxidant defenses (22–25).
Emodin or emodin-containing extracts thus provide protection to
cell constituents in the presence of oxidative stress, such as that
induced by irradiation.
In conclusion, rhubarb extract significantly reduced
apoptotic neuronal cell death and inhibited ROS generation
following irradiation. As such, rhubarb extract may be amenable to
development for use as a therapeutic agent in radiation-induced
brain injury, which is a risk factor for a number of chronic brain
disorders.
Acknowledgments
This study was supported by the China Postdoctoral
Science Foundation grant (grant no. 2013M542232).
References
|
1
|
American Cancer Society: Cancer Facts and
Figures 2006. American Cancer Society; Atlanta, GA: 2006
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greene-Schloesser D and Robbins ME:
Radiation-induced cognitive impairment - from bench to bedside.
Neuro Oncol. 14(Suppl 4): iv37–iv44. 2012. View Article : Google Scholar :
|
|
5
|
Patel RR and Mehta MP: Targeted therapy
for brain metastases: improving the therapeutic ratio. Clin Cancer
Res. 13:1675–1683. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lowe XR, Bhattacharya S, Marchetti F and
Wyrobek AJ: Early brain response to low-dose radiation exposure
involves molecular networks and pathways associated with cognitive
functions, advanced aging and Alzheimer’s disease. Radiat Res.
171:53–65. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Armstrong CL, Hunter JV, Ledakis GE, Cohen
B, Tallent EM, Goldstein BH, Tochner Z, Lustig R, Judy KD, Pruitt
A, Mollman JE, Stanczak EM, Jo MY, Than TL and Phillips P: Late
cognitive and radiographic changes related to radiotherapy: initial
prospective findings. Neurology. 59:40–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shaw EG, Rosdhal R, D’Agostino RB Jr,
Lovato J, Naughton MJ, Robbins ME and Rapp SR: Phase II study of
donepezil in irradiated brain tumor patients: effect on cognitive
function, mood, and quality of life. J Clin Oncol. 24:1415–1420.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jakubs K, Bonde S, Iosif RE, Ekdahl CT,
Kokaia Z, Kokaia M and Lindvall O: Inflammation regulates
functional integration of neurons born in adult brain. J Neurosci.
28:12477–12488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biscaro B, Lindvall O, Tesco G, Ekdahl CT
and Nitsch RM: Inhibition of microglial activation protects
hippocampal neurogenesis and improves cognitive deficits in a
transgenic mouse model for Alzheimer’s disease. Neurodegener Dis.
9:187–198. 2012. View Article : Google Scholar
|
|
11
|
Ekdahl CT, Kokaia Z and Lindvall O: Brain
inflammation and adult neurogenesis: the dual role of microglia.
Neuroscience. 158:1021–1029. 2009. View Article : Google Scholar
|
|
12
|
Linnik MD, Zobrist RH and Hatfield MD:
Evidence supporting a role for programmed cell death in focal
cerebral ischemia in rats. Stroke. 24:2002–2009. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cotman CW and Anderson AJ: A potential
role for apoptosis in neurodegeneration and Alzheimer’s disease.
Mol Neurobiol. 10:19–45. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jagetia GC: Radioprotective potential of
plants and herbs against the effects of ionizing radiation. J Clin
Biochem Nutr. 40:74–81. 2007. View Article : Google Scholar
|
|
15
|
Sato M, Maulik G, Bagchi D and Das DK:
Myocardial protection by protykin, a novel extract of
trans-resveratrol and emodin. Free Radic Res. 32:135–144. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuo YC, Tsai WJ, Meng HC, Chen WP, Yang LY
and Lin CY: Immune responses in human mesangial cells regulated by
emodin from Polygonum hypoleucum ohwi. Life Sci. 68:1271–1286.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peigen X, Liyi H and Liwei W:
Ethnopharmacologic study of Chinese rhubarb. J Ethnopharmacol.
10:275–293. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith PS, Zhao W, Spitz DR and Robbins ME:
Inhibiting catalase activity sensitizes 36B10 rat glioma cells to
oxidative stress. Free Radic Biol Med. 42:787–797. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang CC, Huang YJ, Chen LG, Lee LT and
Yang LL: Inducible nitric oxide synthase inhibitors of Chinese
herbs III Rheum palmatum. Planta Med. 68:869–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Yang L and Lee TJ: Oroxylin A
inhibition of lipopolysaccharide-induced iNOS and COX-2 gene
expression via suppression of nuclear factor-kappaB activation.
Biochem Pharmacol. 59:1445–1457. 2000. View Article : Google Scholar
|
|
21
|
Kuo YC, Meng HC and Tsai WJ: Regulation of
cell proliferation, inflammatory cytokine production and calcium
mobilization in primary human T lymphocytes by emodin from
Polygonum hypoleucum ohwi. Inflamm Res. 50:73–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang SS, Yeh SF and Hong CY: Effect of
anthraquinone derivatives on lipid peroxidation in rat heart
mitochondria: structure-activity relationship. J Nat Prod.
58:1365–1371. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi JS, Chung HY, Jung HA, Park HJ and
Yokozawa T: Comparative evaluation of antioxidant potential of
alaternin (2-hydroxyemodin) and emodin. J Agric Food Chem.
48:6347–6351. 2000. View Article : Google Scholar
|
|
24
|
Chiu PY, Mak DH, Poon MK and Ko KM: In
vivo antioxidant action of a lignan-enriched extract of Schisandra
fruit and an anthraquinone-containing extract of Polygonum root in
comparison with schisandrin B and emodin. Planta Med. 68:951–956.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du Y and Ko KM: Effects of emodin
treatment on mitochondrial ATP generation capacity and antioxidant
components as well as susceptibility to ischemia-reperfusion injury
in rat hearts: single versus multiple doses and gender difference.
Life Sci. 77:2770–2782. 2005. View Article : Google Scholar : PubMed/NCBI
|