Introduction
Gastric cancer remains the second leading cause of
cancer-associated mortality worldwide, with an overall 5-year
survival rate of <25% (1,2).
There are ~930,000 newly diagnosed cases and ~700,000 patients
succumb to the condition each year (2). Therefore, novel strategies for
gastric cancer treatment are urgently required.
Calcium (Ca2+) is a multifunctional
messenger, which controls several cellular processes ranging
between short-term responses, including muscle contraction and
secretion, and long-term regulation, including cell growth and
proliferation (3,4). Store-operated Ca2+ entry
(SOCE) is a major mechanism for Ca2+ entry across the
cell membrane, which is stimulated in response to the depletion of
Ca2+ from intracellular Ca2+ stores,
primarily the endoplasmic reticulum (ER), and mediated via the
activation of specific plasma membrane channels, termed
store-operated channels (SOCs) (5). Stromal interacting molecule 1 (STIM1)
is a highly conserved type-I membrane and ER-resident protein,
containing a luminal EF-hand Ca2+-binding domain and
several cytosolic protein-protein interaction domains, and serves a
dual role as an ER Ca2+ sensor and activator of SOCE
(6–8).
The role of STIM1 in regulating cancer progression
remains controversial. In previous studies, which were performed
prior to the elucidation of its role in Ca2+ signaling,
STIM1 was described as a tumor suppressor, as it caused growth
arrest in human G401 rhabdoid tumor cells and human RD
rhab-domyosarcoma cells (9,10).
However, subsequent studies have revealed a potential role of STIM1
as an oncogene, as it is upregulated in several types of human
cancers, including breast cancer (11), glioblastoma (12,13)
and cervical cancer (14). Thus,
further investigation is required to fully determine the role of
STIM1 in tumorigenesis, which might vary in different types of
tumor. In the present study, plasmid-mediated short hairpin (sh)RNA
was used to suppress the expression of STIM1 in SGC7901 cells, and
the effects of STIM1 knockdown on cell proliferation, apoptosis,
cell cycle progression, migration and invasion were examined.
Elucidation of the role of STIM1 in regulating cancer cell
progression provides aimed to establish whether STIM1 may serve as
a therapeutic target for gastric cancer.
Materials and methods
Chemicals and suppliers
Disposable culture equipment was purchased from
Corning Glass Works (Corning, NY, USA). Cell culture media and
fetal bovine serum (FBS) were obtained from Gibco Life Technologies
(Carlsbad, CA, USA). OPti-MEM medium, thapsigargicine (TG),
Fura-2AM and Lipofectamine™ 2000 were obtained from
Invitrogen Life Technologies (Carlsbad, CA, USA). All other
biochemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
The rabbit polyclonal antibody against STIM1 (1:1,000; #4916) was
purchased from Cell Signaling Technology, Inc. (Danvers, MA). Mouse
β-actin monoclonal antibody (1:500; sc-47778) and the secondary
antibodies, goat anti-mouse immunglobulin (Ig)G conjugated to
horseradish peroxidase (1:2,000; sc-2005), and goat anti-rabbit IgG
conjugated to horseradish peroxidase (1:5,000; sc-2004) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Cell culture
Human gastric cancer SGC7901 cells were obtained
from American Type Culture Collection (Manassas, VA, USA) and
cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing
10% heat-inactivated FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin. The cells were maintained in a 95% humidified air, 5%
CO2 incubator at 37°C.
Plasmid construction and
transfection
For plasmid construction, three shRNAs targeting the
STIM1 gene were designed and synthesized by Invitrogen Life
Technologies. The shRNA deemed most applicable by comparison of
interference effects, shSTIM1, was used in the subsequent
experiments. The sequences of shSTIM1 were as follows: Sense 5′-CA
C CAG AAG GAG CTA GAA TCT CAC TTC AAG AGA GTG AGA TTC TAG CTC CTT
CTT TTTTG-3 and antisense 5′-GAT CCA AAA AAG AAGGAG CTA GAA TCT CAC
TCT CTT GAA GTG AGA TTC TAG CTC CTTCT-3′. The sequence of the
scrambled shRNA (negative control) was sense 5′-CAC CGA CGC TGA AGA
CTC TTG GCT TCA AGA GAG CCA AGA GTC TTC AGC GTC TTT TTTG-3′ and
antisense: 5′-GAT CCA AAA AAG ACG CTG AAG ACT CTT GGC TCT CTT GAA
GCC AAG AGT CTT CAG CGTC-3′. The shRNA was constructed by annealing
the synthetic DNA oligonucleotide primers, which were cooled to
room temperature and inserted between the BbsI and BamHI sites of
the pGPU6/green fluorescent protein (GFP)/Neo eukaryote expression
vector, which contained the GFP gene as a reporter, with an
internal cytomegalovirus promoter. The recombinant vector was then
transfected into competent DH5α cells. Clone identity was verified
by sequencing at Invitrogen Life Technologies (Shanghai, China),
and analyzing the results with DNAssist version 2.2 (http://dnassist.en.softonic.com/).
The SGC7901 cells (0.8~1×106 cells/dish)
were transiently transfected using Lipofectamine™ 2000, according
to the manufacturer’s instructions. The medium containing the
transfection reagents was replaced 4–6 h following transfection
with DMEM supplemented with 10% FBS. The cells were collected 48 h
after transfection, processed in the following experiments and
prepared for protein extraction. The silence efficiency of STIM1
was assessed using western blot analysis.
Protein extraction and western blot
analysis
Protein extraction and western blot analysis were
performed, as previously described (15). In brief, total cell lysates were
obtained by scraping the plates and centrifuging at 450 × g for 5
min at 4°C. The supernatant was removed and the pellet was rinsed
with 4 ml cold PBS, then centrifugation was conducted at 450 × g
for 5 min at 4°C. Lysis buffer (Pierce Biotechnology, Inc.,
Rockford, IL, USA) was added in the pellet and the cells were
sonicated, incubating on ice for 40 min and subsequently
centrifuging at 10,460 × g for 12 min at 4°C. The protein
concentrations in the supernatant were calculated using a
bicinchoninic acid protein assay (Pierce Biotechnology, Inc.). The
supernatants were boiled for 5 min and were then subjected to
electrophoresis on 10% SDS-PAGE gels (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The proteins were transferred to polyvinylidene
difluoride filters, which were incubated at room temperature
(~25°C) for 1 h in 5% non-fat dry milk and Tris-buffered saline
with 0.5% Tween 20 (TBS-T). Immunological evaluation was performed
either for 1 h at room temperature or overnight in 4°C using 5%
milk TBS-T containing 1–2 μg/ml the STIM1, antibody,
according to the manufacturer’s instructions. The filters were then
washed with 1X TBS-T and incubated for 1 h at room temperature with
the goat anti-rabbit HRP-conjugated secondary antibody. Following
multiple washes with TBS-T, the filters were developed using
enhanced chemiluminescence.
Intracellular calcium imaging
To visualize intracellular Ca2+, the
SGC7901 cells (0.8~1×106 cells/dish), transfected at 90%
confluence with either 1.6 μg pGPU6-shSTIM1 or
pGPU6-shNC/well were cultured with 6 μM Fura-2 AM and 0.02%
pluronic acid for 60 min at 37°C. The medium was then removed, and
the cells were plated onto glass-bottomed perfusion chambers, which
were washed three times in isotonic buffer without Ca2+
(KH buffer: 132 mM NaCl, 5 mM KCl, 10 mM dextrose, 10 mM HEPES,
1.05 mM MgCl2). Ringer’s solution, containing 2 mM
Ca2+ (150 mM NaCl, 4.5 mM KCl, 10 mM d-glucose, 2 mM
CaCl2, 1 mM EGTA, 1 mM MgCl2, and 5 mM HEPES)
was used to induce intracellular Ca2+ flux; and 1
μM thapsigargin in Hanks’ balanced salt solution was used to
deplete ER Ca2+ stores. Images were captured every 20
sec using an IX71 inverted microscope (Olympus, Center Valley, PA,
USA), at xcitation wavelengths of 340 nm and 380 nm, and emission
wavelngth of 508 nm, and monitored using Cell^R (Olympus). The
data, comprising relative intracellular Ca2+
concentrations are reported as 340/380 ratios.
Cell viability and proliferation
assay
The cell viability and proliferation activity was
examined using an MTT assay, as described previously (16). Following transfection of the cells
with either pGPU6-shSTIM1 or scrambled shRNA for 48 h, 100 ml of
the cell suspension (1×103 cells) was plated in a
96-well microtitre plate in triplicate. At 0, 24, 48 and 72 h, the
cells were washed with warm PBS, and 20 μl MTT (5 mg/ml in
PBS) was added into each well, following incubation for 4 h at
37°C. The media was then removed and 200 μl dimethyl
sulfoxide (Sigma-Aldrich) was added to dissolve the formazan
crystals. The absorbance of the blue formazan derivative was
measured at 570 nm using a microplate reader (Bio-Rad Laboratories,
Inc.). The results are expressed as the mean ± standard deviation
of three independent experiments.
Apoptosis assay
The apoptosis of the SGC7901 cells was analyzed
using an Annexin V-fluorescein isothiocyanate (FITC)/Propidium
iodide (PI) Apoptosis Detection kit (KeyGEN Biotech Co, Ltd.,
Nanjing, China). Briefly, the cells were cultured in 6 cm dishes
(0.8~1×106 cells/well) and transfected with
pGPU6-shSTIM1 or scrambled shRNA for 48 h. The cells were then
trypsinized using 0.25% Typsin. The cells were collected and washed
twice with PBS, and suspended in 200 μl binding buffer and
10 μl annexin V-FITC for 20 min in the dark. Thereafter, 300
μl binding buffer and 5 μl PI were added to each
sample. The apoptotic cells were determined using a flow cytometer
(FACSCalibur; BD Biosciences) with FCS Express V3 (De Novo
Software, Glendale CA, USA).
Cell cycle
The effect of STIM1 on cell cycle distribution was
determined using flow cytometry (17). Briefly, the plasmid-transfected
SGC7901 cells were harvested and washed with PBS and fixed
overnight with 75% ice-cold ethanol at −20°C. The fixed cells were
stained with a solution containing PI (50 μg/ml), RNase A
(100 μg/ml) and Triton X-100, and analyzed using flow
cytometry (BD Biosciences, USA). The fraction of cells in G0/G1, S,
and G2/M phases were analyzed with FCS Express V3 (De Novo
Software, CA).
Cell migration and invasion assay
Transwell invasion and migration assays were
performed, as described previously (18,19).
Invasion assays were Performed at 37°C for 12 h using a 12-well
Transwell apparatus (8-μm pore size with a polycarbonate
membrane; Corning Costar, Lowell, MA, USA), coated with 200
μg Matrigel (BD Biosciences). Following rehydration of the
chambers, the transfected cells were trypsinized and seeded into
the upper Transwell chamber. The lower chamber contained 500
μl DMEM with 10% FBS. The number of migratory cells, which
invaded the Matrigel and migrated through the membrane, was
measured as the number of cells, which invaded from a defined area
of the microfilter through the micropores in 24 h. The micropore
filters were fixed in paraformaldehyde (Sigma-Aldrich) and stained
with crystal violet. Image of four randomly-selected fields were
captured, and the number of cells were counted to calculate the
average number of cells/field that had transmigrated. The cell
migration assays were performed in a similar manner, but without
the Matrigel coating.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS 13
software. Comparisons were assessed using Student’s t-test, and
differences between three or more groups were assessed using
Bonferroni’s test. P<0.05 was considered to indicate a
statistically significant difference.
Results
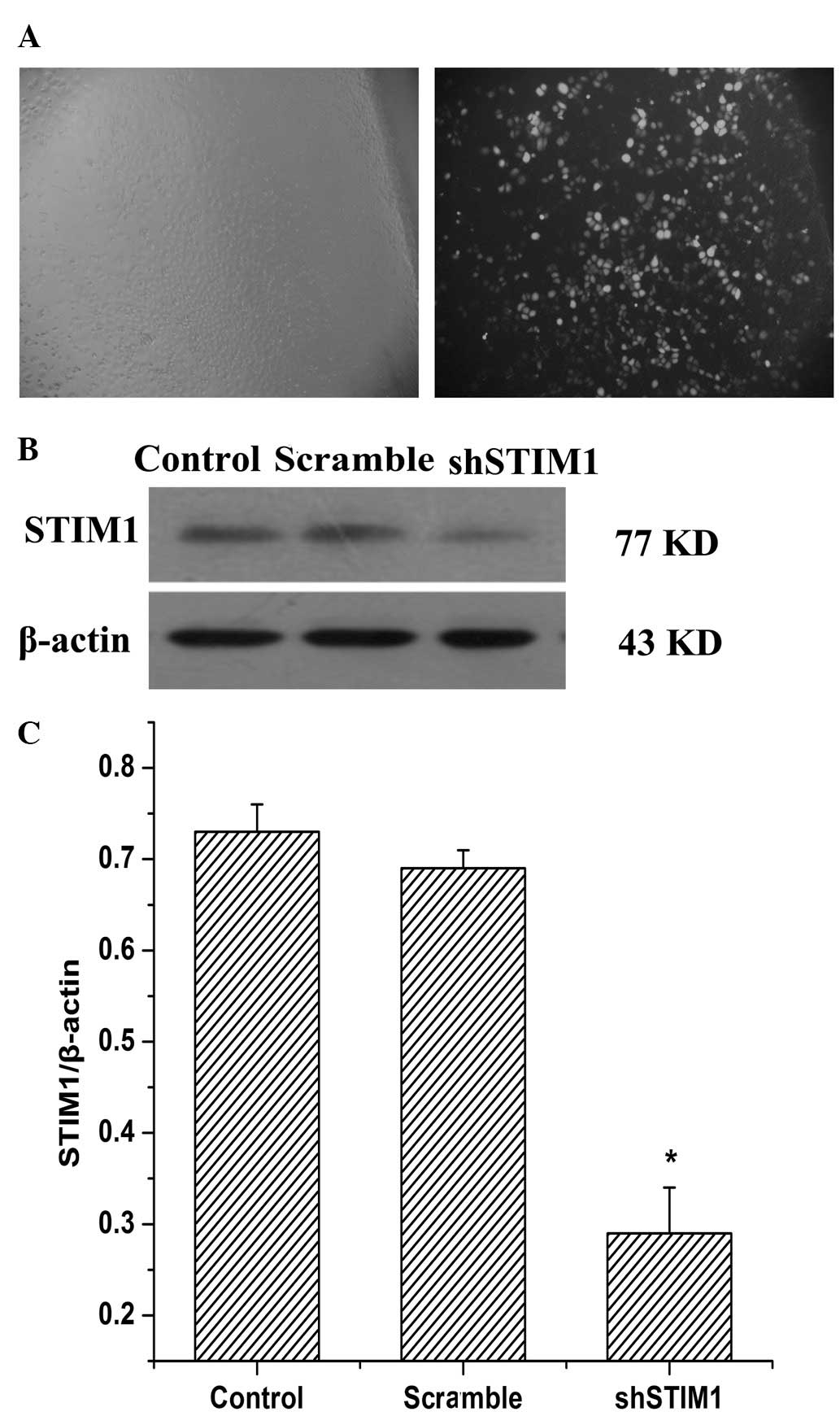
Plasmid-mediated shRNA targeting STIM1
inhibits the expression of STIM1 in SGC7901 cells
To determine whether STIM1 offers potential use a
therapeutic target for gastric cancer, the present study used RNA
interference (RNAi) to inhibit the expression of STIM1 in the
SGC7901 cells. The efficiency of plasmid transfection in the
SGC7901 cells was examined using fluorescent microscopy, and
>80% of the cells were infected with shSTIM1 after 48 h
(Fig. 1A). To determine the
knockdown efficiency of STIM1, western blot analysis was performed.
As shown in Fig. 1B, western blot
analysis was also performed 48 h after plasmid transfection. The
protein expression level of STIM1 was significantly reduced in the
shSTIM1 group compared with the scramble group. These results
indicated that plasmid-mediated shRNA suppressed the expression of
STIM1 in the SGC7901 cells, efficiently and specifically.
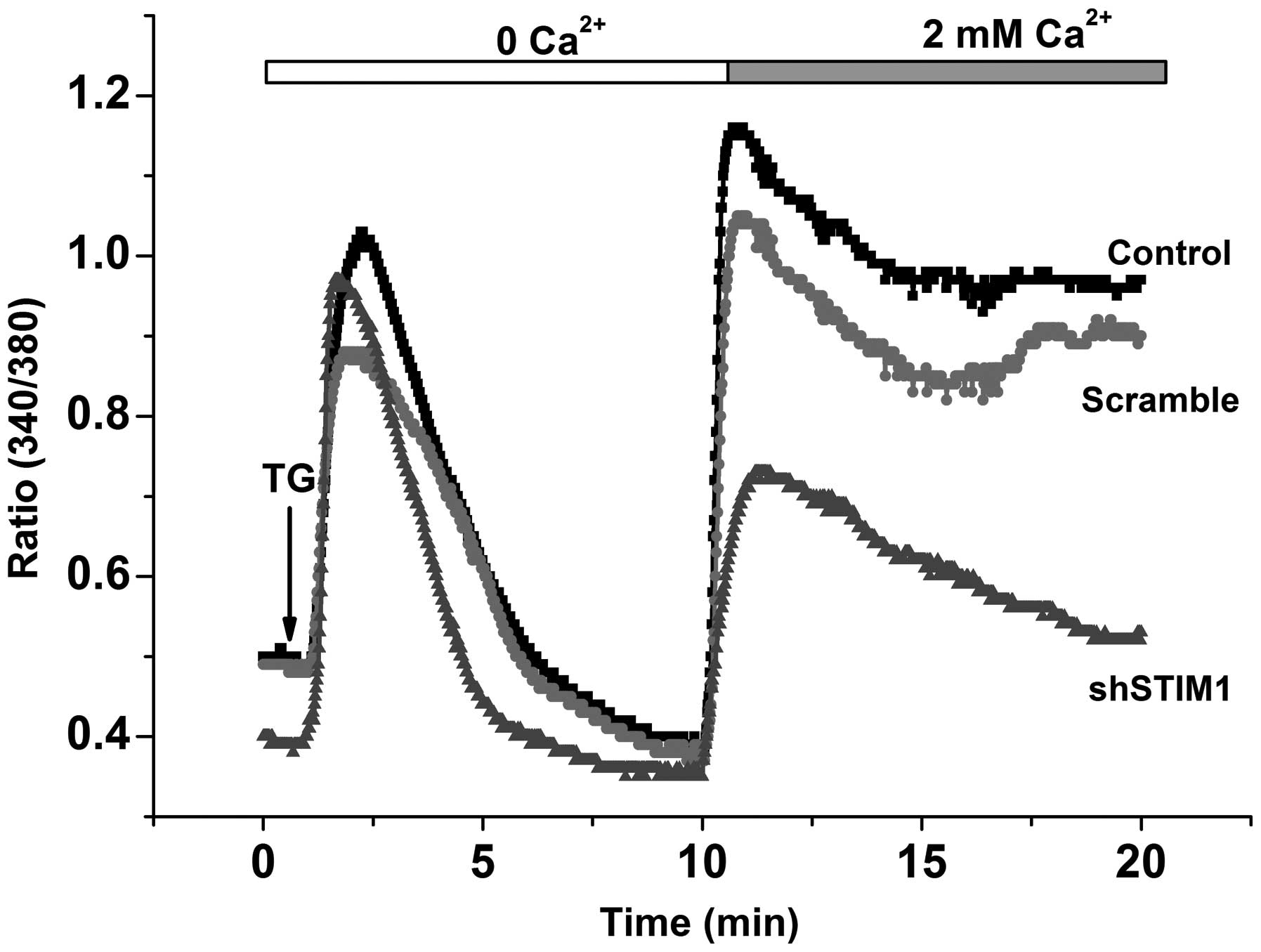
Suppression of STIM1 inhibits
Ca2+ entry in SGC7901 cells
To examine the role of STIM1 protein on SOCE, the
SGC7901 cells were transfected with shRNA, designed against STIM1.
No significant difference was observed between the basal
Ca2+ entry in the absence of TG stimulation and the
control cells. The addition of TG in Ca2+-free medium
elicited similar responses in the control and STIM1-knockdown
cells, whereas the response elicited by Ca2+ re-addition
was inhibited by 55% in the STIM1-knockdown cells (Fig. 2). These results demonstrated that
the STIM1 protein contributed to SOCE in the SGC7901 gastric cancer
cell line.
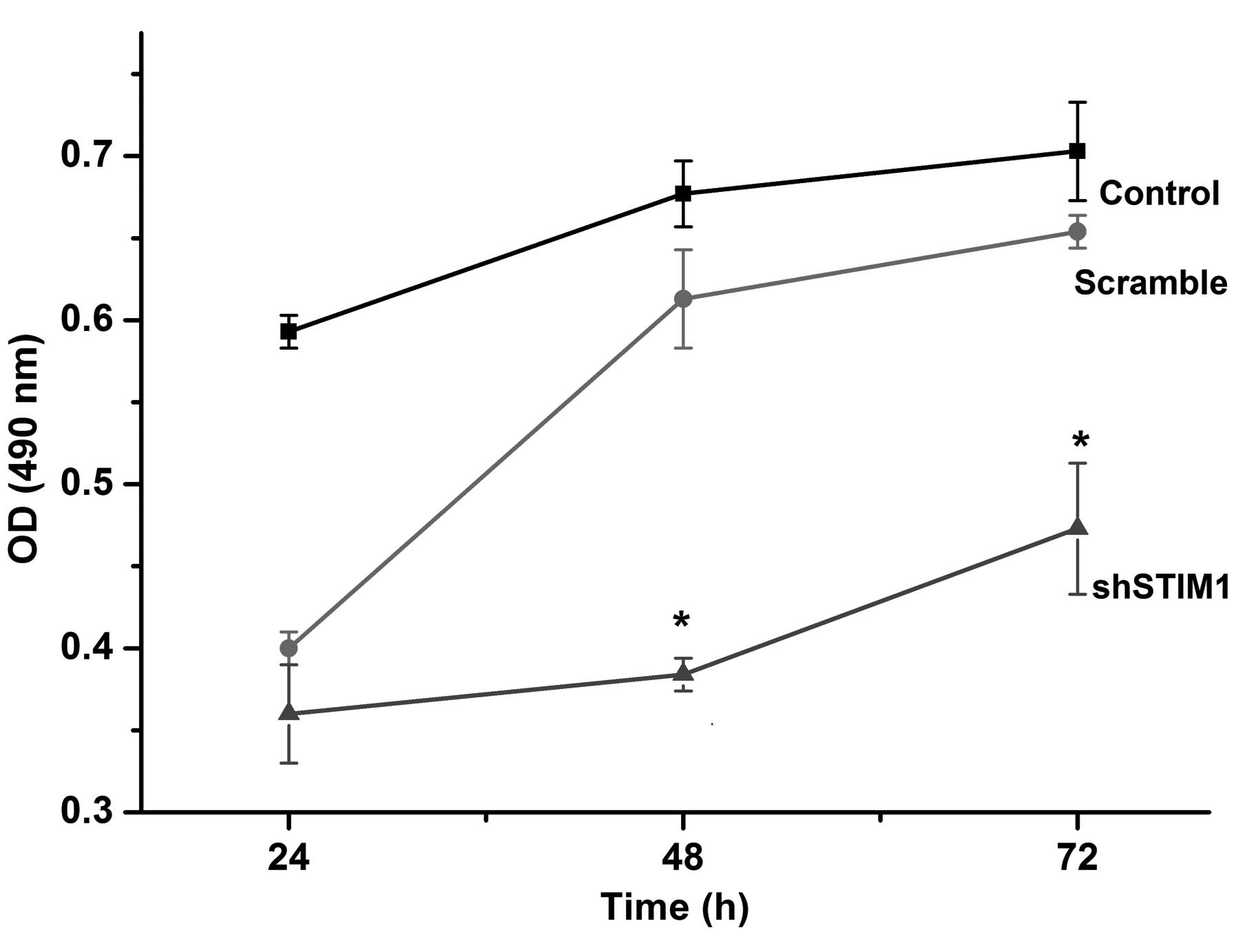
Suppression of STIM1 inhibits SGC7901
cell proliferation
The effect of downregulation of STIM1 on the
proliferation of gastric cancer cells in vitro was assessed
using an MTT assay. As shown in Fig.
3, silencing of the STIM1 gene inhibited SGGC7901 cell
proliferation in a time-dependent manner. When compared with the
scramble group, the number of cells in the shSTIM1 group was
reduced significantly by 41% 48 h after transfection. These results
demonstrated that knockdown of STIM1 by plasmid-mediated shRNA
inhibited SGC7901 cell proliferation in vitro.
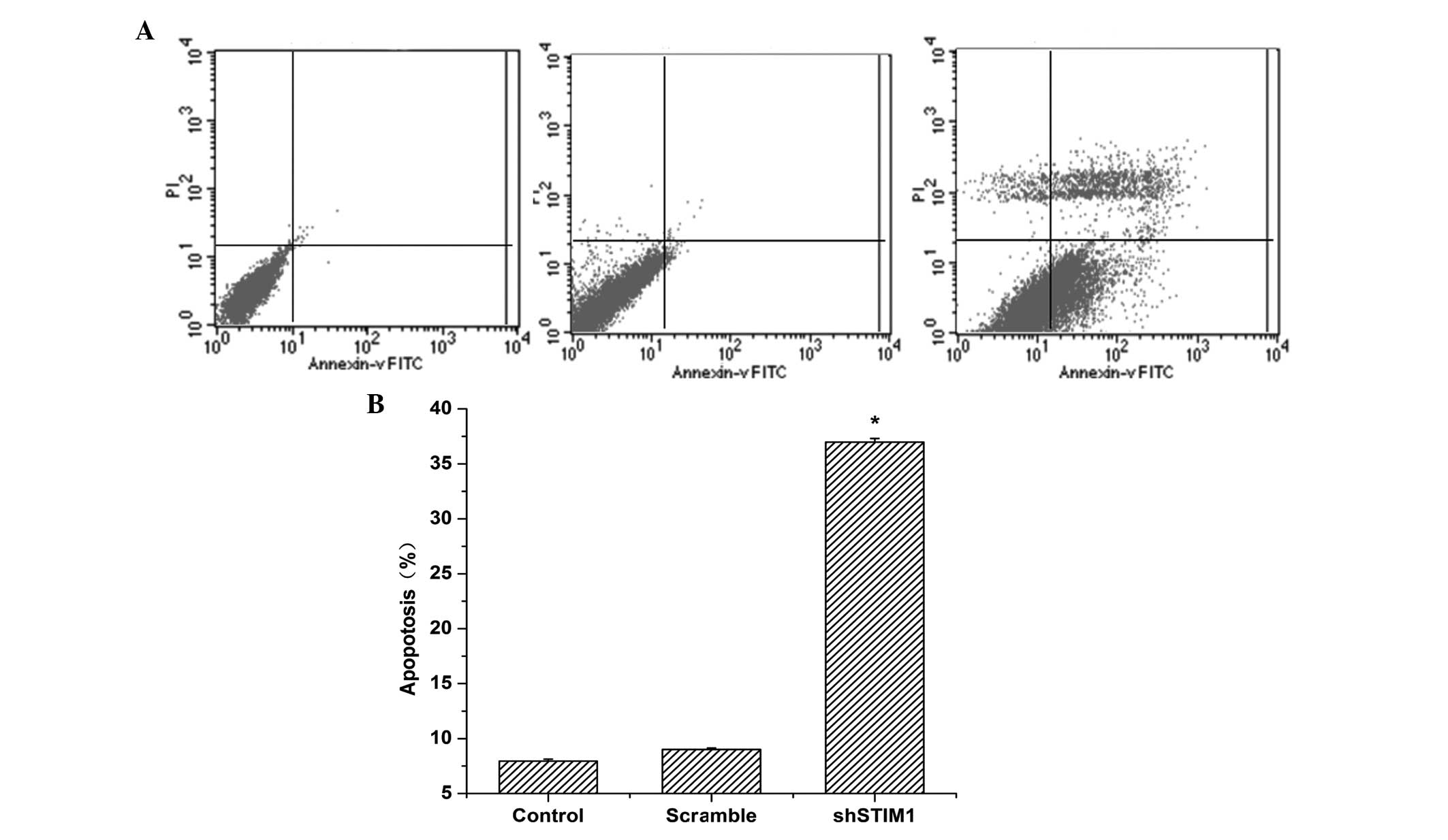
Knockdown of the expression of STIM1
induces gastric cancer cell apoptosis
As shown in Fig. 4,
the percentage of apoptotic cells infected with STIM1 shRNA was
significantly higher compared with that observed in the scramble
group (P<0.05). No significant differences were observed between
the control shRNA plasmid-infected cells and the untransfected
cells. These data indicated that knockdown of the expression of
STIM1 induced apoptosis in the gastric cancer cells.
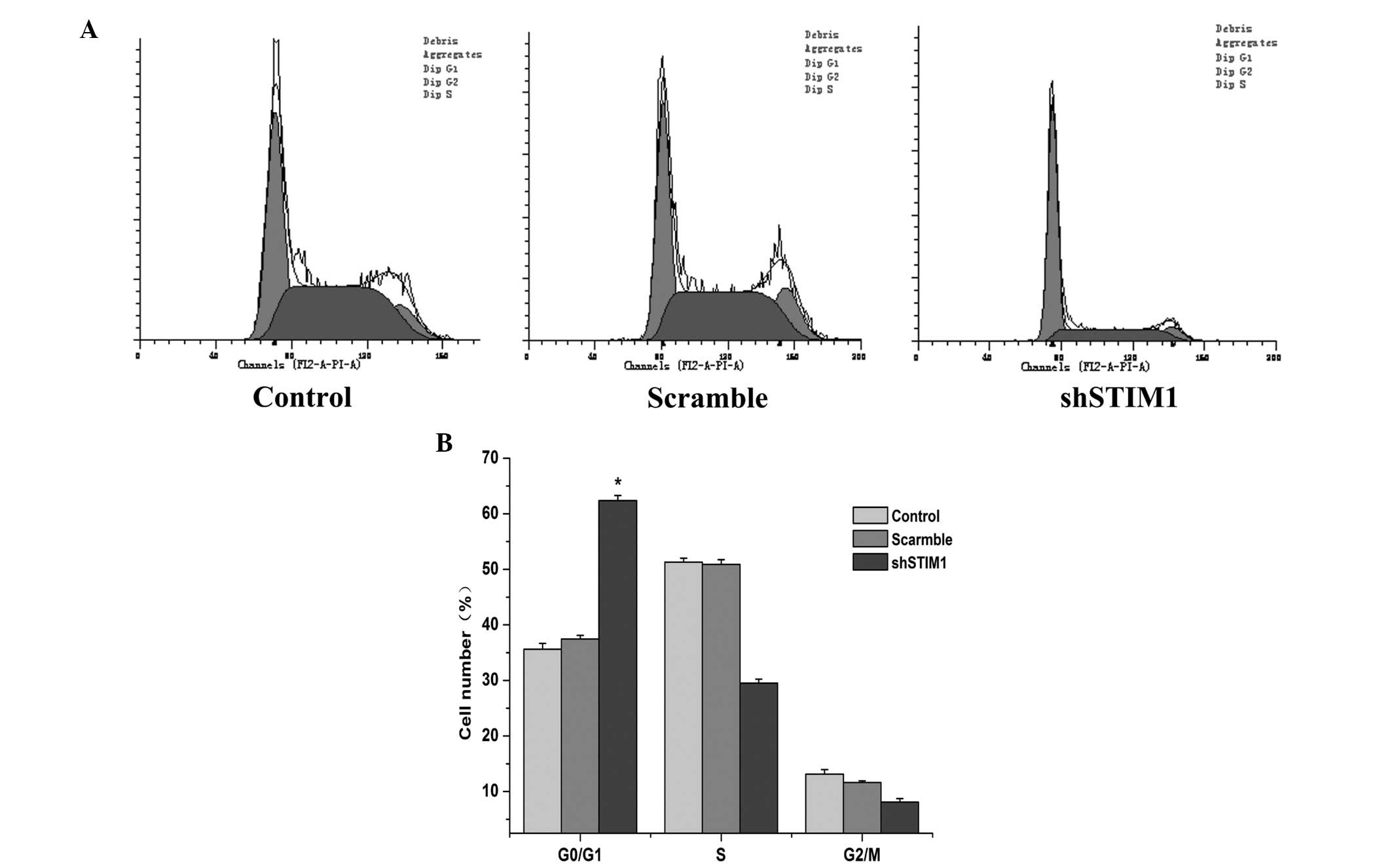
Suppression of STIM1 induces cell cycle
arrest at the G0/G1 phase in SGC7901 cells
To further elucidate the growth suppression effect
of si-STIM1 on SGC7901 cells, cell cycle distribution analysis was
performed, using flow cytometry, 48 h after transfection. As shown
in Fig. 5, STIM1 knockdown induced
cell cycle arrest at the G0/G1 phase in the SGC7901 cells. Compared
with the scramble group, the percentage of cells at the G0/G1 phase
in the shSTIM1 group was 24.92% higher at 48 h. This result
demonstrated that STIM1 silencing induced cell cycle arrest at the
G0/G1 phase.
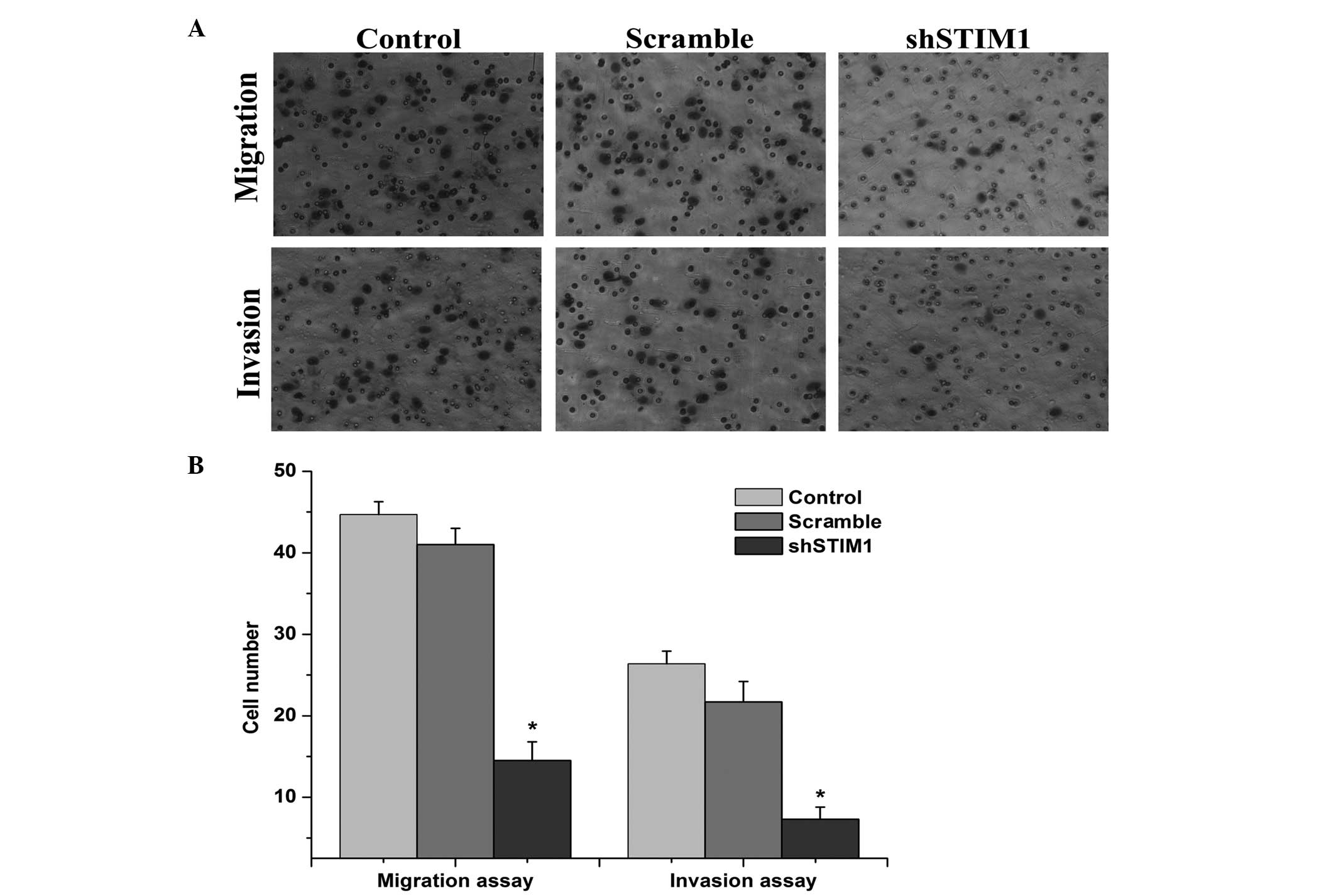
Silencing of STIM1 inhibits the migration
and invasion of SGC7901 cells
To evaluate the effects of STIM1 on cell migration
and invasion, a Matrigel invasion assay was performed (Fig. 6A). The results of the assay
revealed that the decreased expression of STIM1 inhibited the
migration of the SGC790 cells by 67.25%, compared with the scramble
group. The invasion of the cancer cells was also significantly
reduced following transfection with shSTIM1, as determined by the
invasion assay. Decreased expression of STIM1 inhibited cell
invasion by 72.24% in the SGC7901 cells compared with the scramble
group (Fig. 6B). Taken together,
these results indicated that the suppression of STIM1 inhibited the
migration and invasion ability of the SGC7901 cells.
Discussion
Gastric cancer is one of the most common types of
solid tumor worldwide and is the second highest cause of
cancer-associated mortality, despite decreases in its incidence and
mortality rates (20). Despite
curative resection treatment, patients with advanced stage gastric
cancer have poor prognoses (21).
The effects of comprehensive treatment, which combines radical
resection with chemotherapy, immunotherapy and traditional Chinese
medicine treatment, are far from ideal. The local recurrence rate
is as high as 50%and the incidence of lymph node metastasis is up
to 60% (22). Therefore,
identifying relevant therapeutic target for gastric cancer has
become an urgent requirement. Gastric cancer is a heterogeneous,
multifactorial disease. Recurrence and metastasis are the leading
causes of failure in the clinical treatment of gastric cancer
(23). If certain important genes
can be regulated to inhibit the biological behavior of tumor
invasion and metastasis, the survival rates and life quality of
patients with gastric cancer can be improved (24).
SOCE is the principal Ca2+ entry
mechanism in non-excitable cells., and STIM1 is an ER
Ca2+ sensor, which triggers SOCE (5).
STIM1 has gained attention due to its tumor
inhibition properties and role in immunity. Previous studies have
found that STIM1 can inhibit cell growth and cause cell death in
G401 rod-shaped tumor and rhabdomyosarcoma cell clines (25); and inhibiting the expression of
STIM1 effectively relieves symptoms of spontaneous autoimmune
diseases, including rheumatoid arthritis and autoimmune
encephalomyelitis (26) in a mouse
model. STIM1 has been observed to be associated with the
proliferation and migration of vascular smooth muscle cells,
endothelial progenitor cells and normal cells (27), however, it is also closely
associated with the development of tumor cells, including
metastatic melanoma cells (28),
cervical cancer cells (14),
glioblastoma (29) and breast
cancer cells (11). This suggested
that the biological functions of STIM1 may differ from different
cell types. Abdullaev et al (30) found that the expression of STIM1
mediates SOCE, which is involved in the proliferation of human
umbilical vein endothelial cells. Knockout of STIM1 arrests
endothelial cells at the S or G2/M phases, thereby inhibiting the
proliferation of endothelial cells. In clinical specimens from
patients with a diagnosis of cervical cancer, Chen et al
(14) observed that the
Ca2+ inflow, mediated by STIM1 protein, is important in
regulating the metastasis of cervical cancer cells, otherwise, the
downregulation of STIM1 inhibits the proliferation of cervical
cancer cells, arresting the cells at the G1/S or G2/M phases. In
addition, through random ribozyme detection, Suyama E et al
(31) demonstrated that STIM1 is
linked to the migration of metastatic melanoma. In a previous study
on breast cancer cells in vitro and in vivo, Yang
et al (11) found that
STIM1 is involved in SOCE, promoting the metastasis of cancer
cells, and inhibiting the expression of STIM1 effectively inhibits
biological behaviors, including the proliferation and metastasis of
tumor cells.
The preliminary stages of the present study found
that the expression of STIM1 was almost absent in normal gastric
tissues, however, marked expression was observed in gastric cancer
tissues on primary sites, and expression levels in metastatic
gastric cancer tissues were significantly higher than those in the
primary sites (P<0.05). SGC7901 gastric cancer cells are cells
lines with high metastatic potential, isolated and cultured from
metastatic lymph nodes of gastric cancer. For this reason, the
present study selected this cell line as target cells for
transfection with shRNAs of the STIM1 gene, and the STIM1 genes
were silenced in the SGC7901 cells using an shRNA technique.
Subsequent to depleting Ca2+ in the calcium stores of
the ER and increasing the concentration of extracellular
Ca2+ between 0 and 2Mm, the extracellular
Ca2+ influx in the single-cell calcium ions was observed
using an imaging system. The results revealed rapid extracellular
Ca2+ influxes in the control and scarmble groups, while
the extracellular Ca2+ influxe was significantly
inhibited in the STIM1 knockdown group. The MTT assay demonstrated
that, following silencing of the STIM1 gene, proliferation of the
SGC7901 cells was significantly inhibited, while proliferation of
the SGC7901 cells were unaffected in the scramble and control
groups. Takahashi et al also found that inhibiting the
expression of STIM1 inhibits Ca2+ influx, thereby
inhibiting the proliferation of vascular smooth muscle cells
cultured in vitro (32).
The results of are were consistent with those of the present study.
This preliminarily indicated that STIM 1 may regulate the
proliferation and growth of SGC7901 gastric cancer cells by
regulating of extracellular Ca2+ influx.
To further examine the causes for the slow growth
and proliferation of cells following RNAi, the present study
investigated the cell cycle of the three groups of gastric cancer
cells using flow cytometry. The results revealed that all the
gastric cancer cells in STIM1 silencing group were arrested at the
G0/G1 phases, and the numbers of cells at the S and G2/M phases
were significantly decreased. These results suggested that, in the
STIM1 silencing group, gastric cancer cells increased at stationary
stages, but significantly decreased at active proliferative phases.
These results differed from those observed in the scramble and
control groups. In clinical specimens diagnosed with cervical
cancer, Chen et al (14)
found that the Ca2+ inflow, mediated by STIM1 protein,
is important in the regulation of metastasis in cervical cancer
cells, and the downregulation of STIM1 inhibits the proliferation
of cervical cancer cells, and arrest cells at the G1/S or G2/M
phases. This suggested that STIM1 silencing may inhibit the
proliferation of gastric cancer cells through arresting the tumor
cells at the G0/G1 phase.
Cell apoptosis is the process in which certain
factors trigger a stored procedure, which leads to active cell
death. Similar to the process of mitosis, apoptosis has a
regulatory role in the body’s vital functions and a stable internal
environment, while tumor development is closely associated with the
decline or failure of the mechanism of cell apoptosis (33). In the present study, flow cytometry
revealed that the apoptotic rates of gastric cancer cells were
increased significantly in the STIM1 silencing group, compared with
those in the scramble and control groups. This result was
consistent with the findings of Sun et al (34), which reported that downregulation
of STIM1 promotes the apoptosis of colon cancer cells.
The biological behaviors of malignant tumors include
invasion and metastasis, which depends predominantly on the
invasion and migration of the cells. The invasion of cells through
the basement membrane is considered an early event and predominant
feature of metastasis (35).
Through random ribozymal detection, Suyama E et al found
that STIM1 is associated with the migration of metastatic melanoma
(31); whereas a study by Yang
et al, investigating breast cancer cells in vitro and
in animal experiments, found that STIM1 is involved in SOCE and
promotes the metastasis of cancer cells (11). The results of the present study
demonstrated that, compared with the scramble and control groups,
the metastasis and invasion of SGC7901 gastric cancer cells were
significantly decreased following silencing of the STIM1 gene
(P<0.05).
However, further investigations to elucidate the
signaling pathway, which regulates the function of STIM1 in gastric
cancer.
The present study demonstrated that STIM1 is
expressed in human gastric cancer cell lines. The RNAi-mediated
gene silencing of STIM1 suppressed SGC7901 cell growth in
vitro and inhibited cell cycle progression at the G0/G1 phase.
Inhibiting the activity of STIM1 also inhibited SGC7901 cell
migration and invasion. In conclusion, the findings of the present
study indicate the significance of STIM1 in the biological behavior
of gastric cancer, and provide evidencethat STIM1 may be a
potential therapeutic target for human gastric cancer.
Acknowledgments
This study was supported by the Natural Scientific
Foundation of Jiangxi Province (no. 2010GZY0123).
References
|
1
|
Ferlay J, Shin HR, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berridge MJ, Bootman MD and Roderick HL:
Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev
Mol Cell Biol. 4:517–529. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berridge MJ, Lipp P and Bootman MD: The
versatility and universality of calcium signalling. Nat Rev Mol
Cell Biol. 1:11–21. 2000. View
Article : Google Scholar
|
|
5
|
Putney JW Jr: A model for
receptor-regulated calcium entry. Cell Calcium. 7:1–12. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liou J, Kim ML, Heo WD, et al: STIM is a
Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+
influx. Curr Biol. 15:1235–1241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roos J, DiGregorio PJ, Yeromin AV, et al:
STIM1, an essential and conserved component of store-operated Ca2+
channel function. J Cell Biol. 169:435–445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spassova MA, Soboloff J, He LP, et al:
STIM1 has a plasma membrane role in the activation of
store-operated Ca2+ channels. Proc Natl Acad Sci U S A.
103:4040–4045. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sabbioni S, Barbanti-Brodano G, Croce CM
and Negrini M: GOK: a gene at 11p15 involved in rhabdomyosarcoma
and rhabdoid tumor development. Cancer Res. 57:4493–4497.
1997.PubMed/NCBI
|
|
10
|
Sabbioni S, Veronese A, Trubia M, et al:
Exon structure and promoter identification of STIM1 (alias GOK), a
human gene causing growth arrest of the human tumor cell lines G401
and RD. Cytogenet Cell Genet. 86:214–218. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang S, Zhang JJ and Huang XY: Orai1 and
STIM1 are critical for breast tumor cell migration and metastasis.
Cancer Cell. 15:124–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Hughes JD, Rollins S, et al:
Calcium entry via ORAI1 regulates glioblastoma cell proliferation
and apoptosis. Exp Mol Pathol. 91:753–760. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scrideli CA, Carlotti CG Jr, Okamoto OK,
et al: Gene expression profile analysis of primary glioblastomas
and non-neoplastic brain tissue: identification of potential target
genes by oligonucleotide microarray and real-time quantitative PCR.
J Neurooncol. 88:281–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YF, Chiu WT, Chen YT, et al: Calcium
store sensor stromal-interaction molecule 1-dependent signaling
plays an important role in cervical cancer growth, migration and
angiogenesis. Proc Natl Acad Sci USA. 108:15225–15230. 2011.
View Article : Google Scholar
|
|
15
|
Timmons JA, Rao JN, Turner DJ, et al:
Induced expression of STIM1 sensitizes intestinal epithelial cells
to apoptosis by modulating store-operated Ca2+ influx. J
Gastrointest Surg. 16:1397–1405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gerlier D and Thomasset N: Use of MTT
colorimetric assay to measure cell activation. J Immunol Methods.
94:57–63. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nunez R: DNA measurement and cell cycle
analysis by flow cytometry. Curr Issues Mol Biol. 3:67–70.
2001.PubMed/NCBI
|
|
18
|
Albini A and Benelli R: The chemoinvasion
assay: a method to assess tumor and endothelial cell invasion and
its modulation. Nat Protoc. 2:504–511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie Y, Wolff DW, Wei T, et al: Breast
cancer migration and invasion depend on proteasome degradation of
regulator of G-protein signaling 4. Cancer Res. 69:5743–5751. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2007. CA Cancer J Clin. 57:43–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
An JY, Cheong JH, Hyung WJ and Noh SH:
Recent evolution of surgical treatment for gastric cancer in Korea.
J Gastric Cancer. 11:1–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Higuchi K, Phan A and Ajani JA: Gastric
cancer: advances in adjuvant and adjunct therapy. Curr Treat
Options Oncol. 4:413–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin Y, Ueda J, Kikuchi S, Totsuka Y, Wei
WQ, Qiao YL and Inoue M: Comparative epidemiology of gastric cancer
between Japan and China. World J Gastroenterol. 17:4421–4428. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Catalano V, Labianca R, Beretta GD, Gatta
G, de Braud F and Van Cutsem E: Gastric cancer. Crit Rev Oncol
Hematol. 54:209–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clark AJ, Diamond M, Elfline M and Petty
HR: Calicum micro-domains form within neutrophils at the
neutrophil-tumor cell synapse: role in antibody-dependent target
cell apoptosis. Cancer Immunol Immunother. 59:149–159. 2010.
View Article : Google Scholar
|
|
26
|
Schuhmann MK, Stegner D, Berna-Erro A, et
al: Stromal interaction molecules 1 and 2 are key regulators of
autoreactive T cell activation in murine autoimmune central nervous
system inflammation. J Immunol. 184:1536–1542. 2010. View Article : Google Scholar
|
|
27
|
Bisaillon JM, Motiani RK, Gonzalez-Cobos
JC, et al: Essential role for STIM1/Orai1-mediated calcium influx
in PDGF-induced smooth muscle migration. Am J Physiol Cell Physiol.
298:C993–1005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hayashi C, Rittling S, Hayata T, et al:
Serum osteopontin, an enhancer of tumor metastasis to bone,
promotes B16 melanoma cell migration. J Cell Biochem. 101:979–986.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Motiani RK, Hyzinski-García MC, Zhang X,
et al: STIM1 and Orai1 mediate CRAC channel activity and are
essential for human glioblastoma invasion. Pflugers Arch.
465:1249–1260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abdullaev IF, Bisaillon JM, Potier M, et
al: Stim1 and Orai1 mediate CRAC currents and store-operated
calcium entry important for endothelial cell proliferation. Circ
Res. 103:1289–1299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suyama E, Wadhwa R, Kaur K, et al:
Identification of metastasis-related genes in a mouse model using a
library of randomized ribozymes. J Biol Chem. 279:38083–38086.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi Y, Watanabe H, Murakami M, et
al: Upregulation of TRPC1 is involved in Angiotensin II-induced
vascular smooth muscle cell hypertrophy. J Mol Cell Cardiol.
41:10572006. View Article : Google Scholar
|
|
33
|
Xiao F, Liu B and Zhu QX: c-Jun N-terminal
kinase is required for thermotherapy-induced apoptosis in human
gastric cancer cells. World J Gastroenterol. 18:7348–7356. 2012
December 28; View Article : Google Scholar
|
|
34
|
Sun S, Li W, Zhang H, et al: Requirement
for store-operated calcium entry in sodium butyrate-induced
apoptosis in human colon cancer cells. Biosci Rep. 32:83–90. 2012.
View Article : Google Scholar
|
|
35
|
Klein CA: Cancer. The metastasis cascade.
Science. 321:1785–1787. 2008. View Article : Google Scholar : PubMed/NCBI
|