Introduction
Acute myocardial infarction (AMI) is a type of
myocardial ischemia caused by acute coronary and persistent hypoxic
ischemia. AMI is associated with electrocardiographical changes,
which may be complicated by cardiac arrhythmia, shock or heart
failure, and are often life-threatening (1). AMI causes initial structural changes
to the infarcted and non-infarcted areas of the heart, followed by
expansion of the ventricles, resulting in cardiac dysfunction
(2). The formation of myocardial
fibrosis and scar tissue following AMI is an important pathological
alteration that induces heart failure. The formation of the
myocardial scar is associated with the speed of myocardial
ischemia, size and location of infarct-associated vessels, and the
presence of reperfusion (3).
Surrounding the infarcted area and in the area away from normal
tissue, ventricular reconstruction is conducted to maintain cardiac
output and to lower wall tension. This adaptation process includes
left ventricular dilatation, left ventricular hypertrophy,
myocardial cell hypertrophy and fibrosis of the intercellular
matrix. Previous pharmacological studies have indicated that
numerous types of cardioprotective drugs treat cardiovascular
disease via anti-inflammatory and anti-oxidant signaling pathways
(4).
Recently, an upregulated inflammatory response was
identified as being essential for the pathophysiology of AMI
(5). Overexpression of
inflammatory cytokines have been identified in the adipose tissue
of rat models of AMI, including tumor necrosis factor-α (TNF-α),
interleukin (IL)-1β and IL-6 (6).
Furthermore, TNF-α has been shown to be overexpressed in the muscle
tissue of obese humans or mice (7). TNF-α is able to increase the
phagocytosis of neutrophils, promote the secretion of IL-1 and IL-6
by endothelial cells, and strengthen the adhesion of neutrophils
and endothelial cells, thereby stimulating local inflammatory
expansion and the response to AMI (8,9). Of
note, TNF-α is responsible for mediating the cytoprotective effects
through nuclear factor (NF)-κB. Since NF-κB mediates cytoprotective
responses, NF-κB activation is considered to exhibit cytoprotective
effects in AMI (2). Accordingly,
the upregulation of anti-inflammatory signaling pathways may have
cardioprotective effects in rats following AMI.
Furchgott et al (10) and Palmer et al (11) demonstrated that vascular
endothelial cells are able to synthesize and secrete
endothelium-derived relaxing factor, which is also known as nitric
oxide (NO). NO is produced in a multi-step oxidation and reduction
reaction in which L-arginine is catalyzed by nitric oxide synthase
(NOS) and reacts with oxygen molecules. NOS can be broadly divided
into two types: Constructive nitric oxide synthase and inducible
nitric oxide synthase (iNOS). iNOS is expressed not only in immune
cells, including macrophages and neutrophils, but also in
fibroblasts, keratinocytes, endothelial cells and vascular smooth
muscle cells (12). Excessive NO
is usually accompanied by inflammation and immune disorders, pain,
neurological disorders, atherosclerosis and cancer (13). Following myocardial infarction in
mice, increased expression levels of iNOS have been detected, which
results in the induction of excessive NO, decreased cardiac
function and increased mortality (14). Therefore, iNOS inhibitors may be
used clinically in order to reduce iNOS expression and decrease NO
production. iNOS and NO exist in the cytoplasm of all tissues,
which is one of the important indicators for AMI diagnosis.
Therefore, it was hypothesized that inhibiting the iNOS signaling
pathways may have a cardioprotective effect in a rat model of AMI
(15).
Paeoniflorin (PF) is a monoterpene glycoside
compound. Recently, PF has been shown to increase the levels of
superoxide dismutase and reduce the malondialdehyde content in
ischemic brain tissue (16). PF
has a significant anti-inflammatory effect, which can reduce the
abnormally increased phagocytic function of peritoneal macrophages
in models of rheumatoid arthritis and lower the levels of TNF-α,
IL-1 and IL-6 (17,18). Previous studies have also
demonstrated that PF is able to inhibit the gene expression of iNOS
(17,19). However, whether PF can ameliorate
AMI in rats remains to be elucidated. Since PF has a critical role
in the protection against ischemic insults, the present study
hypothesized that PF may provide a potential therapeutic target for
cardioprotection. Therefore, the present study aimed to determine
the cytoprotective effects of PF in a rat model of AMI, and to
further explore the potential underlying mechanisms.
Materials and methods
Ethical approval
All of the experimental procedures of the present
study were approved by the First Affiliated Hospital of Dalian
Medical University (Dalian, China). The present study was performed
in accordance with the recommendations in the Guide for the Care
and Use of Laboratory Animals of the National Institutes of Health
(Bethesda, MD, USA).
Experimental animals
A total of 30 Sprague-Dawley rats (8–10 weeks-old;
weighing 250–300 g) were purchased from the Experimental Animal
Center of The First Affiliated Hospital of Dalian Medical
University (Dalian, China), housed under a 12 h/12 h dark and light
cycle, at 23±1°C with a relative humidity of 50%. The rats were
allowed to acclimatize in plastic cages with ad libitum
access to food and clean drinking water.
Animal models
All experimental rats were subjected to surgery
following anesthesia with sodium pentobarbitone [40 mg/kg
intraperitoneally; Tiangen Biotech (Beijing) Co., Ltd., Beijing,
China]. The rats were bound to a rat table, and under anesthesia,
the trachea of each rat was separated, intubated and artificially
ventilated with a respirator (S9 VPAP ST; ResMed Inc., Bella Vista,
NSW, Australia) at a tidal volume of 4–5 ml per breath. Needle
electrodes were connected to a normal electrocardiogram device
(ECG1200G; Contec Medical Systems Co., Ltd., Qinhuangdao, China).
The device was subcutaneously penetrated into four limbs of the rat
via a transducer attached to a multi-channel recorder. A left
sternal border thoracotomy was performed between the third and
fourth intercostal spaces. The pericardium was removed, separating
the heart and blood vessels on the left ventricular surface. The
left anterior descending coronary artery was ligated below the left
atrial appendage, using a 1–2 mm 5–0 silk suture. Penicillin and
streptomycin were then injected into the rats, in order to prevent
infection. The rat model of AMI was prepared by ligation of the
left anterior descending coronary artery. A total of six rats
underwent a sham surgery, which involved the identical surgical
procedure without the coronary artery ligation. A total of 24 rats
successfully underwent the experimental surgery. Successful
establishment of the AMI model was confirmed by regional cyanosis
of the myocardial surface, which was represented by ST-segment
elevation (SPR-838; Millar Instruments, Houston, TX, USA) and
analyzed using analysis software (PVAN 2.9; Millar Instruments)
(20).
Group design and drug administration
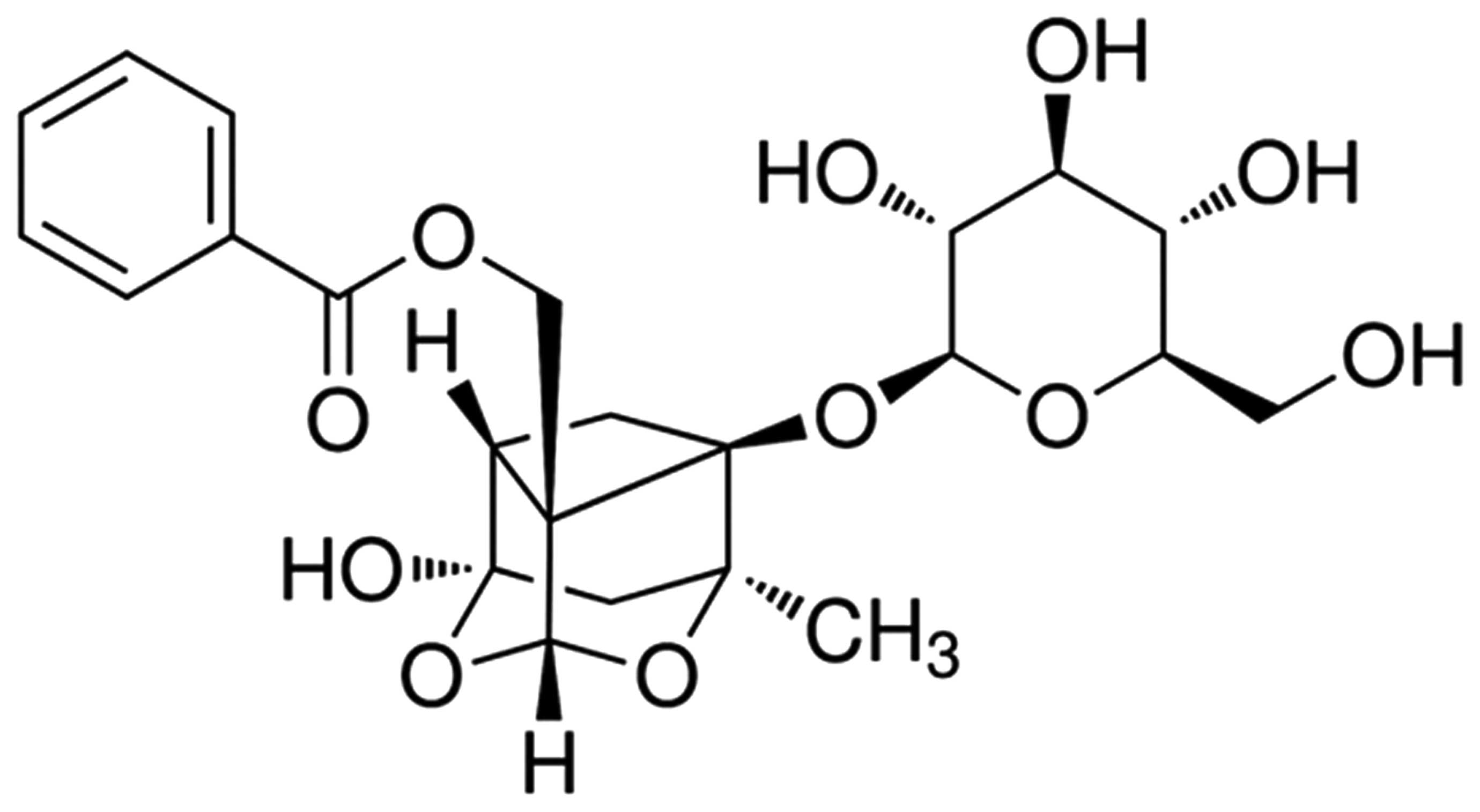
The chemical structure of PF is presented in
Fig. 1. PF (Sigma-Aldrich, St.
Louis, MO, USA; 98% purity; 5, 10 and 20 mg/kg) was dissolved in
physiological saline. The 30 rats were randomized into five groups,
each containing six rats: Sham group, Vehicle group and PF
treatment groups (various doses of PF were used: 5, 10 and 20
mg/kg) (21). The sham group was
the control group that did not undergo the coronary ligation. The
vehicle group underwent the AMI procedure prior to injection with
the same volume of physiological saline (Luye Pharma Group Ltd.,
Dalian, China). The PF treatment groups underwent the AMI procedure
prior to injection with PF through the tail vein. PF and
physiological saline were injected for seven consecutive days. The
dosage and dosing frequency were determined on the basis of a
previous study (22). The rats
underwent the coronary ligation 30 min after the last
administration.
Infarct size measurement
Rats were anesthetized by injecting chloral hydrate.
All experimental rats were sacrificed by decapitation. The hearts
were immediately cannulated via the aorta and washed with
physiological saline. Six hours after the coronary artery was
ligated, the left ventricles were incubated at −80°C for 5 min, and
then sliced into 2-mm sections. Infarct size was estimated as a
percentage of area at risk following an the incubation with
2,3,5-triphenyltetrazolium chloride (1.5%; Sigma-Aldrich) (23,24).
The area of the heart without color indicated ischemic heart
muscle, whereas the area stained brick red indicated normal
myocardium. The size of the infarct area was measured by the volume
and weight as a percentage of the left ventricle.
Measurement of creatine kinase (CK), MB
isoenzyme of CK (CK-MB), lactate dehydrogenase (LDH) and cardiac
troponin (cTnT) levels
Rat serum samples were taken from the vena cava 6 h
after the ligation of the coronary artery. The samples were
centrifuged at 3,500 × g for 5 min in order to determine the levels
of myocardial-specific enzymes: CK, CK-MB, LDH and cTnT. The CK
activities were determined using a creatine kinase assay kit (cat.
no. A032; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China). The activities of CK-MB and serum cTnT were quantified by
CK-MB and cTnT ELISA (cat. nos. ARB10700 and ARB13662; Rapidbio,
West Hills, CA, USA). A colorimetric assay was used to analyze the
activities of LDH, according to the manufacturer's instructions
(Nanjing Jiancheng Bioengineering Institute, China).
Measurement of NF-κB, TNF-α, IL-1β and
IL-6 levels
Following the 3-h ischemic period, whole blood
samples were allowed to clot in a serum separator tube for 30 min.
The serum samples were then centrifuged at 1,000 × g for 25 min,
and were maintained at −80°C until further use. For the measurement
of the serum levels of NF-κB, TNF-α, IL-1β and IL-6, a commercially
available ELISA kit was used (Uscnlife, Wuhan EIAab Science Co.,
Ltd, Wuhan, China), according to the manufacturer's
instructions.
Western blot analysis for the detection
of iNOS protein
Western blot analysis was performed for the
detection of iNOS protein expression (25). Proteins were extracted from the
cardiac samples, which had been stored at −80°C. Briefly, the
samples were homogenized in ice-cold lysis buffer [Tiangen Biotech
(Beijing) Co., Ltd.]. Following centrifugation at 13,200 × g for 20
min at 4°C, the supernatant was collected and total protein levels
were quantified using a bicinchoninic acid (BCA) protein assay kit
(Beyotime Institute of Biotechnology, Haimen, China). Protein
samples (50–70 µg) were then separated by SDS-PAGE (BeastBio,
Shanghai, China) and transferred onto nitrocellulose membranes
(Millipore Corporation, Bedford, MA, USA). The membranes were
blocked with 5% non-fat milk (Yili Group., Hohhot, China) and 0.1%
Tween 20 [Tiangen Biotech (Beijing) Co., Ltd.] in 10 mM Tris-HCl
(Invitrogen Life Technologies, Carlsbad, CA, USA) for 1–2 h at room
temperature, and then incubated with anti-iNOS (cat. no. 2982;
1:500; Cell Signaling Technology, Inc., Danvers, MA, USA) and
anti-β-actin (cat. no. sc-47778; 1:2,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), at 4°C overnight. After three washes with
Tris-buffered saline containing Tween 20, the membranes were
incubated with horseradish peroxidase-labeled goat anti-rabbit
immunoglobulin G (1:5,000; Santa Cruz Biotechnology, Inc.) at room
temperature for 2 h. The bound antibodies were visualized using an
enhanced chemiluminescence system (Pierce Biotechnology Inc.,
Rockford, IL, USA) and exposed to X-ray film. β-actin was used as
an internal reference for relative quantification. The expression
levels of each sample were analyzed using Image-Pro plus software
6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
iNOS activity assay
iNOS activity in the ischemic myocardium was
determined using an NOS assay kit (Nanjing Jiancheng Bioengineering
Institute) (26). Myocardium
samples (100–120 mg) were separated from the anterior wall of the
left ventricle and placed in a centrifuge tube, alongside ~10
volumes of ice-cold phosphate-buffered saline (pH 7.4; BeastBio).
The tissue was homogenized for 1–2 min on ice and centrifuged at
1,200 × g for 15 min at 4°C. The supernatant was collected and
stored at −80°C until further use. The protein concentration was
measured using a BCA assay. The supernatant was then incubated with
0.6 ml reaction buffer and 1 mmol/l ethylene glycol tetraacetic
acid (BeastBio) for 15 min at 37°C. iNOS activity was determined
using the plate reader (Infinite M200; Tecan, Männedorf,
Switzerland) by measuring the absorbance at a wavelength of 530
nm.
Caspase-3 and caspase-9 activity
assays
Caspase-3 and caspase-9 activities were measured by
cleavage of chromogenic caspase substrates, acetyl-Asp-Glu-Val-Asp
p-nitroanilide (Ac-deVd-pnA), caspase-3 and caspase-9 substrate.
The activities of caspase-3 and caspase-9 were determined using
caspase-3 and caspase-9 colorimetric assay kits (Beyotime Institute
of Biotechnology), according to the manufacturer's instructions.
Cardiac cytosolic protein (~50 µg) was incubated in a solution
buffer at 37°C for 30 min. The caspase reaction was then initiated
by adding 2 mM Ac-DEVD-pNA, and the samples were incubated at 37°C
for 4 h. The change in fluorescence (excitation, 400 nm) was
detected at a wavelength of 405 nm.
Statistical analysis
All data were analyzed by SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA) and are presented as the mean ± standard
deviation. Differences were analyzed by one-way analysis of
variance, followed by Dunnett's test for individual comparisons
between each group. P<0.05 was considered to indicate a
statistically significant difference.
Results
Infarct size in a rat model of AMI
The infarct size in the vehicle group was
42.15±2.11%. Following treatment with PF (5, 10 and 20 mg/kg), the
infarct size was significantly reduced to 34.86±1.61% (P<0.01;
n=6), 31.94±1.79% (P<0.01; n=6) and 27.43±1.89% (P<0.01;
n=6), respectively, as compared with that in the vehicle group
(Fig. 2).
CK, CK-MB, LDH and cTnT levels in a rat
model of AMI
The levels of serum CK, CK-MB, LDH and cTnT in the
sham, vehicle and PF-treated (5, 10 and 20 mg/kg) groups are
summarized in Table I. The levels
of CK, CK-MB, LDH and cTnT were significantly increased in the
serum of the operated rats (P<0.01, n=6), as compared with those
in the sham group. Pre-treatment with PF at 5 mg/kg (P<0.01;
n=6), 10 mg/kg (P<0.01; n=6) and 20 mg/kg (P<0.01, n=6)
markedly reduced the levels of CK, CK-MB, LDH and cTnT in the serum
of the AMI rat model as compared with those in the vehicle
group.
 | Table IActivities of CK, CK-MB and LDH, and
levels of cTnT in a rat model of AMI. |
Table I
Activities of CK, CK-MB and LDH, and
levels of cTnT in a rat model of AMI.
| Group | CK (U/ml) | CK-MB (IU/l) | LDH (U/l) | cTnT (U/ml) |
|---|
| Sham | 0.25±0.04 | 82.36±6.98 | 1698.69±342.21 | 0.08±0.04 |
| Vehicle | 0.87±0.07a | 199.02±8.97a |
5731.63±421.13a | 0.51±0.08a |
| PF (5 mg/kg) | 0.43±0.04c | 114.52±7.86c |
3125.74±308.53b | 0.17±0.06c |
| PF (10 mg/kg) | 0.35±0.05c | 97.26±8.96c |
2712.42±377.42c | 0.15±0.04c |
| PF (20 mg/kg) | 0.30±0.03c | 85.72±6.69c |
2289.37±358.93c | 0.13±0.04c |
TNF-α, IL-1β, IL-6 and NF-κB activities
in a rat model of AMI
In order to corroborate the effects of PF on the
inflammatory mediators in a rat model of AMI, the activities of
NF-κB, TNF-α, IL-1β and IL-6 were evaluated (Fig. 3A–D). NF-κB activity was enhanced in
the vehicle group to 56.11±3.28 ng/mg protein (P<0.01; n=6), as
compared with 11.8±1.03 ng/mg protein in the sham group (Fig. 3A). Following treatment with PF (5,
10 and 20 mg/kg), NF-κB activity was markedly decreased to
38.21±2.29 (P<0.01; n=6), 32.25±2.89 (P<0.01; n=6) and
29.18±3.01 ng/mg protein (P<0.01; n=6) respectively, as compared
with that in the vehicle group (56.11±3.28 ng/mg protein) (Fig. 3A).
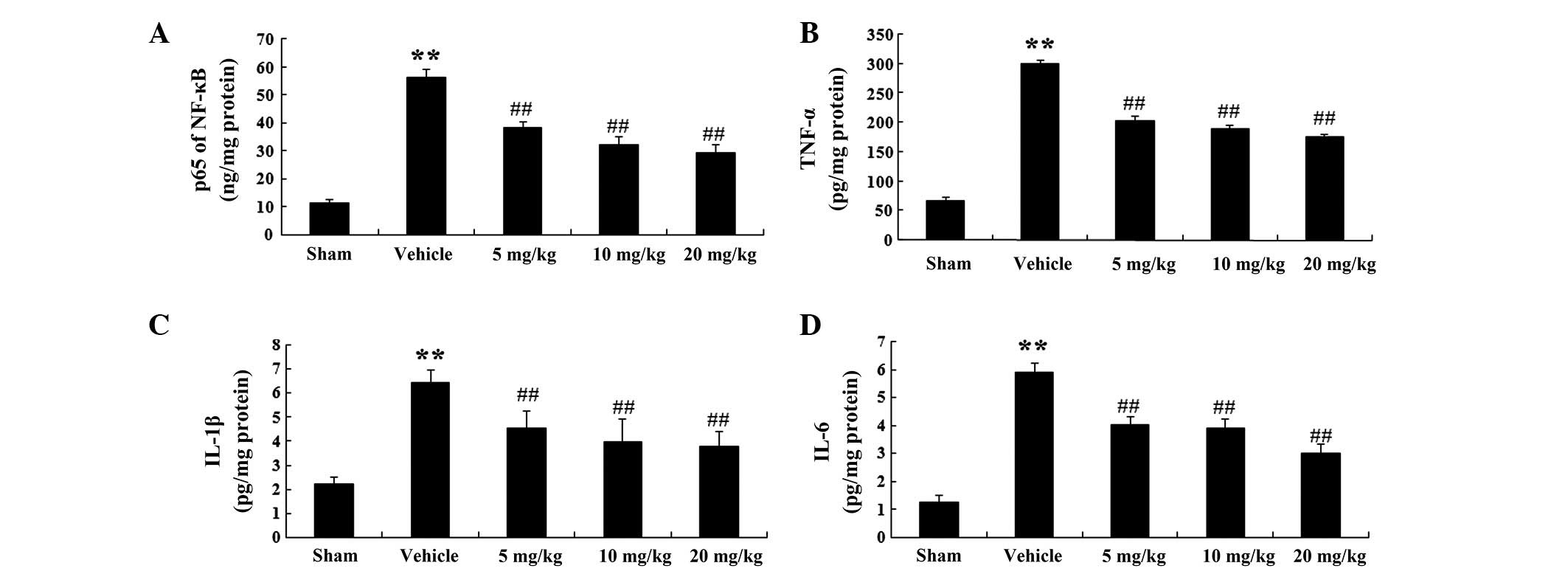 | Figure 3NF-κB, TNF-α, IL-1β and IL-6 activity
in a rat model of acute myocardial infarction. (A) NF-κB, (B)
TNF-α, (C) IL-1β and (D) IL-6 activity in the various groups.
Values are expressed as the mean ± standard deviation (n=6).
**P<0.01 vs. the sham group; ##P<0.01
vs. the vehicle group. Sham, sham-operated; Vehicle,
vehicle-treated; 5 mg/kg, PF (5 mg/kg)-treated; 10 mg/kg, PF (10
mg/kg)-treated and 10 mg/kg, PF (20 mg/kg)-treated groups. NF-κB,
nuclear factor-κB; TNF-α, tumor necrosis factor-α; IL, interleukin;
PF, paeoniflorin. |
Similarly, TNF-α activity in the vehicle group was
markedly increased to 299.42±8.03 pg/mg protein (P<0.01; n=6) as
compared with that in the sham group (66.87±6.32 pg/mg protein)
(Fig. 3B). In the PF treatment
groups (5, 10 and 20 mg/kg), TNF-α activity was significantly
reduced to 202.13±9.11 (P<0.01; n=6), 187.8±8.41 (P<0.01;
n=6) and 175.32±6.32 pg/mg protein (P<0.01; n=6), respectively,
as compared with that in the vehicle group (299.42±8.03 pg/mg
protein) (Fig. 3B).
Furthermore, IL-1β activity in the vehicle group was
markedly elevated to 6.46±0.52 pg/mg protein (P<0.01, n=6), as
compared with that in the sham group (2.22±0.28 pg/mg protein)
(Fig. 3C). In the PF treatment
groups (5, 10 and 20 mg/kg), IL-1β activity was significantly
decreased to 4.53±0.71 (P<0.01; n=6), 3.99±0.93 (P<0.01; n=6)
and 3.78±0.62 pg/mg protein (P<0.01; n=6), respectively, as
compared with that in the vehicle group (6.46±0.52 pg/mg protein)
(Fig. 3C).
In addition, IL-6 activity in the vehicle group was
significantly increased to 5.89±0.32 pg/mg protein (P<0.01, n=6)
as compared with that in the sham group (1.28±0.21 pg/mg protein)
(Fig. 3D). In the PF treatment
groups (5, 10 and 20 mg/kg), IL-6 activity was significantly
decreased to 4.01±0.31 (P<0.01; n=6), 3.89±0.33 (P<0.01; n=6)
and 0.33±0.33 pg/mg protein (P<0.01; n=6) as compared with that
in the vehicle group (45.26±0.65 pg/mg protein) (Fig. 3D).
Protein expression levels of iNOS and
iNOS activity in a rat model of AMI
The protein expression levels of iNOS were
determined in the rat model of AMI using western blot analysis.
iNOS expression was detected in bands located at 130 kDa (Fig. 4A). The protein expression levels of
iNOS were significantly increased in the vehicle group (P<0.01;
n=6), as compared with those in the sham group. In addition, the
protein expression levels of iNOS were markedly decreased following
treatment with PF at 5 mg/kg (P<0.01; n=6), 10 mg/kg (P<0.01;
n=6) and 20 mg/kg (P<0.01; n=6), as compared with those in the
vehicle group (Fig. 4B).
Furthermore, iNOS activity in the vehicle group was
markedly elevated to 61.35±2.23 ng L-citrulline/mg protein/30 min
(P<0.01; n=6), as compared with that in the sham group
(24.18±2.14 ng L-citrulline/mg protein/30 min) (Fig. 4C). In the PF treatment groups (5,
10 and 20 mg/kg), iNOS activity was significantly decreased to
40.25±2.19 (P<0.01; n=6) 35.89±2.25 (P<0.01; n=6) and
30.11±3.24 ng L-citrul-line/mg protein/30 min (P<0.01; n=6) as
compared with that in the vehicle-treated group (61.35±2.23 ng
L-citrul-line/mg protein/30 min) (Fig.
4C).
Caspase-3 and caspase-9 activity in a rat
model of AMI
A colorimetric analysis was conducted, and PF was
shown to decrease the activity of caspase-3 (Fig. 5A). Caspase-3 activity in the
vehicle group was markedly increased by 6.26±0.18 (P<0.01; n=6),
as compared with that in the sham group. In the PF treatment groups
(5, 10 and 20 mg/kg), there was a marked decline in caspase-3
activity by 4.02±0.22 (P<0.01; n=6), 3.56±0.24 (P<0.01; n=6)
and 2.89±0.31 (P<0.01; n=6) respectively, as compared with that
in the vehicle-treated group (Fig.
5A).
In addition, treatment with PF was able to decrease
caspase-9 activity (Fig. 5B).
Caspase-9 activity in the vehicle group was significantly increased
by 8.12±0.34 (P<0.01, n=6), as compared with that in the sham
group. In the PF treatment groups (5, 10 and 20 mg/kg), there was a
marked decrease in caspase-9 activity by 5.12±0.27 (P<0.01;
n=6), 4.25±0.61 (P<0.01; n=6) and 3.99±0.58 (P<0.01; n=6),
respectively, as compared with that in the vehicle-treated group
(Fig. 5B).
Discussion
PF is the main active component of the commonly used
Traditional Chinese Medicine peony, Paeonia Suffruticosa,
which exhibits anti-oxidative, anti-inflammatory and anti-apoptotic
activities. Previous studies have demonstrated that PF is able to
significantly reduce the expression levels of NF-κB and B-cell
lymphoma-2 in a dose-dependent manner (18,19,27).
PF has a protective effect on the blood-brain barrier after
cerebral ischemia, local cerebral blood flow and brain edema. It
has also been indicated that PF has a significant protective effect
on focal cerebral ischemic injury. It has been suggested that PF
may inhibit intracellular calcium and free radical overload, and
improve cerebral vasomotor dysfunction caused by ischemia and
anoxia. Furthermore, PF is able to protect the blood-brain barrier
following cerebral perfusion during ischemia, and promote the
recovery of cerebral blood flow in the early period of reperfusion.
PF can also reduce iNOS, TNF-α, IL-β and IL-6 activation. The
results of the present study demonstrated that treatment with PF
had a cardioprotective effect in a rat model of AMI, which may be
associated with modulation of inflammation and iNOS signaling
pathways.
The infarct size of AMI and the presence of
myocardial specific enzymes (CK, CK-MB, LDH and cTnT) are regarded
as important indices for assessing the cardiac damage caused by
AMI. Numerous studies have indicated that CK, CK-MB and cTnT are
widely spread over the cytoplasm of myocardial cells, and the
activities of CK, CK-MB and cTnT in rats underwent AMI (27–29).
cTnT activity is a more sensitive and specific marker of AMI in
rats, as compared with CK-MB (30). The levels of LDH in the culture
supernatant are a very sensitive and specific indicator for
detecting AMI. However, LDH is a less sensitive and specific marker
of AMI in rats, as compared with CK-MB, and has also been observed
in non-cardiac conditions (31).
The present study demonstrated that treatment with PF was able to
decrease the myocardial infarct size as well as CK, CK-MB, LDH and
cTnT activities in a rat model of AMI, thus suggesting that PF
exerts cardioprotective effects on AMI.
Recent studies have suggested that the inflammatory
response has an important role throughout the development and
progression of ischemic heart disease. It is well known that NF-κB,
which is an inflammatory factor in cardiac tissues during AMI, is a
transcription factor associated with various biological processes
(32). NF-κB-p65 is the primary
trans-activating transcriptional activator of NF-κB and has
regulatory functions in the inflammatory process (33). Pro-inflammatory cytokines have been
shown to be upregulated during AMI, with TNF-α, IL-1β and IL-6
being the most important (34).
Activation of TNF-α, IL-1β and IL-6 is modulated by NF-κB. TNF-α
can increase the phagocytosis of neutrophils and also promote the
secretion of IL-1β and IL-6 from endothelial cells. TNF-α, IL-1β
and IL-6 are synthesized rapidly and released by immune cells in
rat models of AMI. The results of the present study demonstrated
that treatment with PF was able to attenuate the excessive
activation of NF-κB and the release of TNF-α, IL-1β and IL-6 in a
rat model of AMI. Previous studies have also suggested that PF may
significantly reduce the expression of NF-κB, TNF-α, IL-1 and IL-6
(17,27). These findings indicated that
treatment with PF may have a cardio-protective anti-inflammatory
effect following AMI.
NOS is a catalytic enzyme which synthesizes NO, and
NO is known to have a wide range of important physiological and
pathological functions. NO has an important regulatory role in the
cardiovascular, immune, nervous and digestive systems (35). Due to the reactivity and short
half-life of NO, numerous studies have focused on NOS. Although NO
is able to maintain mucosal vasodilation and vascular permeability,
excessive NO can also directly cause cell toxicity and lead to
peroxidation, causing tissue damage. TNF-α, IL-1β, IL-6, and NF-κB
are able to stimulate the cells in blood vessel walls to express
iNOS, which is accompanied by the release of NO and a decline in
vascular tension (36,37). These cytokines increase the
catalytic activity of iNOS through transcriptional,
post-transcriptional and translational regulation, and affect
signal transduction pathways, in order to promote NO synthesis.
Following myocardial infarction in mice, increased expression of
iNOS has been detected, resulting in the induction of excessive NO,
which decreases cardiac function and increases the risk of
mortality. The results of the present study demonstrated that
treatment with PF was able to reduce AMI-induced inflammation and
iNOS signaling.
Recent studies have confirmed that the activation of
caspase-3 and caspase-9 is critical in the course of intrinsic
apoptosis (36). The initiation of
caspase-3 occurred at day 3, and was significantly increased until
day 7. The expression levels of caspase-9 have previously been
shown to be elevated 7 days after cardiac damage (38). The results of the present study
indicated that treatment with PF markedly reduced caspase-3 and
caspase-9 activity. Furthermore, the activities of caspase-3 and
caspase-9 were markedly reduced by PF treatment. In agreement with
the findings of the present study, PF treatment was previously
shown to significantly decrease caspase-3 and caspase-9 activity in
Alzheimer's disease (39).
In conclusion, the present study demonstrated that
PF treatment was able to attenuate the damage caused by AMI. The
cardioprotective effects of PF may be associated with inhibition of
inflammation and iNOS signaling. The present study was the first,
to the best of our knowledge, to identify the protective action and
the potential protective mechanism of PF in a rat model of AMI.
These results provided evidence that PF may be a potential
cardioprotective agent for the treatment of AMI.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81200173) and Liaoning
Province Science and Technology Plan Projects (grant no.
2013023026).
References
|
1
|
Ouyang J, Guzman M, Desoto-Lapaix F,
Pincus MR and Wieczorek R: Utility of desmin and a Masson's
trichrome method to detect early acute myocardial infarction in
autopsy tissues. Int J Clin Exp Pathol. 3:98–105. 2009.PubMed/NCBI
|
|
2
|
Zhang S, Liu X, Goldstein S, et al: Role
of the JAK/STAT signaling pathway in the pathogenesis of acute
myocardial infarction in rats and its effect on NF-κB expression.
Mol Med Rep. 7:93–98. 2013.
|
|
3
|
Smith RS Jr, Agata J, Xia CF, et al: Human
endothelial nitric oxide synthase gene delivery protects against
cardiac remodeling and reduces oxidative stress after myocardial
infarction. Life Sci. 76:2457–2471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan KP, Guo Y, Xing Z, et al: Dan-Shen-Yin
protects the heart against inflammation and oxidative stress
induced by acute ischemic myocardial injury in rats. Exp Ther Med.
3:314–318. 2012.PubMed/NCBI
|
|
5
|
Kain V, Ingle KA, Colas RA, et al:
Resolvin D1 activates the inflammation resolving response at
splenic and ventricular site following myocardial infarction
leading to improved ventricular function. J Mol Cell Cardiol.
84:24–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hotamisligil GS, Shargill NS and
Spiegelman BM: Adipose expression of tumor necrosis factor-alpha:
Direct role in obesity-linked insulin resistance. Science.
259:87–91. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hotamisligil GS, Arner P, Caro JF, et al:
Increased adipose tissue expression of tumor necrosis factor-alpha
in human obesity and insulin resistance. J Clin Invest.
95:2409–2415. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng P, Wang F, Chen K, et al: Hydrogen
sulfide ameliorates ischemia/reperfusion-induced hepatitis by
inhibiting apoptosis and autophagy pathways. Mediators Inflamm.
2014:9352512014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramachandran S, Liaw JM, Jia J, et al:
Ischemia-reperfusion injury in rat steatotic liver is dependent on
NFκB P65 activation. Transpl Immunol. 26:201–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furchgott RF and Zawadzki JV: The
obligatory role of endothelial cells in the relaxation of arterial
smooth muscle by acetylcholine. Nature. 288:373–376. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palmer RM, Ferrige AG and Moncada S:
Nitric oxide release accounts for the biological activity of
endothelium-derived relaxing factor. Nature. 327:524–526. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jugdutt BI: Nitric oxide and
cardioprotection during ischemia-reper-fusion. Heart Fail Rev.
7:391–405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Crowell JA, Steele VE, Sigman CC and Fay
JR: Is inducible nitric oxide synthase a target for
chemoprevention? Mol Cancer Ther. 2:815–823. 2003.PubMed/NCBI
|
|
14
|
Chen C, Zhang F, Xia ZY, et al: Protective
effects of pretreatment with Radix Paeoniae Rubra on acute lung
injury induced by intestinal ischemia/reperfusion in rats. Chin J
Traumatol. 11:37–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khan M, Mohan IK, Kutala VK, et al:
Sulfaphenazole protects heart against ischemia-reperfusion injury
and cardiac dysfunction by overexpression of iNOS, leading to
enhancement of nitric oxide bioavailability and tissue oxygenation.
Antioxid Redox Signal. 11:725–738. 2009. View Article : Google Scholar
|
|
16
|
Sun R, Yi YP, Lv LL, Zhang ZP, Sun H and
Liu GQ: Effects of paeoniflorin on pathological changes in global
brain ischemia model rats. Zhongguo Zhong Yao Za Zhi. 32:2518–2522.
2007.In Chinese.
|
|
17
|
Ding MP, Feng F and Hu HT: Effects of
puerarin on expression of nuclear factor kappaB after cerebral
ischemia/reperfusion in rats. Zhongguo Zhong Yao Za Zhi.
32:2515–2518. 2007.In Chinese.
|
|
18
|
Hu W, Zhang Q, Yang X, et al: Puerarin
inhibits adhesion molecule expression in tnf-alpha-stimulated human
endothelial cells via modulation of the nuclear factor kappaB
pathway. Pharmacology. 85:27–35. 2010. View Article : Google Scholar
|
|
19
|
Hino H, Takahashi H, Suzuki Y, et al:
Anticonvulsive effect of paeoniflorin on experimental febrile
seizures in immature rats: Possible application for febrile
seizures in children. PLoS One. 7:e429202012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Korkmaz S, Loganathan S, et al:
Acute ethanol exposure increases the susceptibility of the donor
hearts to ischemia/reper-fusion injury after transplantation in
rats. PLoS One. 7:e492372012. View Article : Google Scholar
|
|
21
|
Uchida Y, Freitas MC, Zhao D, et al: The
protective function of neutrophil elastase inhibitor in liver
ischemia/reperfusion injury. Transplantation. 89:1050–1056. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Camilleri JP, Joseph D, Fabiani JN, et al:
Microcirculatory changes following early reperfusion in
experimental myocardial infarction. Virchows Arch A Pathol Anat
Histol. 369:315–333. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoda MN, Li W, Ahmad A, et al:
Sex-independent neuroprotection with minocycline after experimental
thromboembolic stroke. Exp Transl Stroke Med. 3:162011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoda MN, Siddiqui S, Herberg S, et al:
Remote ischemic perconditioning is effective alone and in
combination with intravenous tissue-type plasminogen activator in
murine model of embolic stroke. Stroke. 43:2794–2799. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ikeda U, Ikeda M, Minota S and Shimada K:
Homocysteine increases nitric oxide synthesis in
cytokine-stimulated vascular smooth muscle cells. Circulation.
99:1230–1235. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ashokkumar P and Sudhandiran G: Luteolin
inhibits cell proliferation during Azoxymethane-induced
experimental colon carcinogenesis via Wnt/β-catenin pathway. Invest
New Drugs. 29:273–284. 2011. View Article : Google Scholar
|
|
27
|
Guo RB, Wang GF, Zhao AP, et al:
Paeoniflorin protects against ischemia-induced brain damages in
rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses.
PLoS One. 7:e497012012. View Article : Google Scholar
|
|
28
|
Guo J, Li HZ, Wang LC, et al: Increased
expression of calcium-sensing receptors in atherosclerosis confers
hypersensitivity to acute myocardial infarction in rats. Mol Cell
Biochem. 366:345–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ming X, Tongshen W, Delin W and Ronghua Z:
Cardioprotective effect of the compound yangshen granule in rat
models with acute myocardial infarction. Evid Based Complement
Alternat Med. 2012:7171232012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Priscilla DH and Prince PS:
Cardioprotective effect of gallic acid on cardiac troponin-T,
cardiac marker enzymes, lipid peroxidation products and
antioxidants in experimentally induced myocardial infarction in
Wistar rats. Chem Biol Interact. 179:118–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shah H and Haridas N: Evaluation of
clinical utility of serum enzymes and troponin-T in the early
stages of acute myocardial infarction. Indian J Clin Biochem.
18:93–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sheu JJ, Sung PH, Leu S, et al: Innate
immune response after acute myocardial infarction and
pharmacomodulatory action of tacrolimus in reducing infarct size
and preserving myocardial integrity. J Biomed Sci. 20:822013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong D, Xu L, Han X, et al: Effects of the
total saponins from Rosa laevigata Michx fruit against
acetaminophen-induced liver damage in mice via induction of
autophagy and suppression of inflammation and apoptosis. Molecules.
19:7189–7206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Talasaz AH, Khalili H, Jenab Y, et al:
N-Acetylcysteine effects on transforming growth factor-β and tumor
necrosis factor-α serum levels as pro-fibrotic and inflammatory
biomarkers in patients following ST-segment elevation myocardial
infarction. Drugs R D. 13:199–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu YF, Tu SH, Chen Z, et al: Effects of
modified simiao decoction on IL-1 β and TNF α secretion in
monocytic THP-1 cells with monosodium urate crystals-induced
inflammation. Evid Based Complement Alternat Med. 2014:4068162014.
View Article : Google Scholar
|
|
36
|
Liu Y, He P, Zhang M and Wu D: Lentiviral
vector-mediated RNA interference targeted against prohibitin
inhibits apoptosis of the retinoic acid-resistant acute
promyelocytic leukemia cell line NB4-R1. Mol Med Rep. 6:1288–1292.
2012.PubMed/NCBI
|
|
37
|
Jiang P, Li C, Xiang Z and Jiao B:
Tanshinone IIA reduces the risk of Alzheimer's disease by
inhibiting iNOS, MMP-2 and NF-κBp65 transcription and translation
in the temporal lobes of rat models of Alzheimer's disease. Mol Med
Rep. 10:689–694. 2014.PubMed/NCBI
|
|
38
|
Feng J, Tao T, Yan W, et al: Curcumin
inhibits mitochondrial injury and apoptosis from the early stage in
EAE mice. Oxid Med Cell Longev. 2014:7287512014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang K, Zhu L, Zhu X, et al: Protective
effect of paeoniflorin on Aβ25–35-induced SH-SY5Y cell injury by
preventing mitochondrial dysfunction. Cell Mol Neurobiol.
34:227–234. 2014. View Article : Google Scholar
|