Introduction
Premature ovarian failure (POF), an ovarian disorder
of multifactorial origin, is defined as the occurrence of
amenorrhea, hypergonadotropism and hypoestrogenism in females
<40 years old (1). POF results
in fertility problems and leads to an increased incidence of
cardiovascular disease, stroke and osteoporosis, a severe endocrine
disorder (2). It has been reported
that POF may result from either a reduced number of follicles
formed during ovarian development or an increased rate of follicle
loss (3). Previous studies have
demonstrated that various factors, including androgens (4), can induce apoptosis in ovarian
granulosa cells and result in follicle loss (5). Thus, the apoptosis of ovarian
granulosa cells is important in POF and understanding the
regulatory mechanism underlying ovarian granulosa cell apoptosis
may be advantageous for the management of POF.
MicroRNAs (miRNAs), a class of gene negative
regulators, are short (~22 nt) noncoding RNAs. miRNAs repress the
expression of target genes by degrading or destabilizing their mRNA
in a sequence-specific manner (6).
In this way, miRNAs affect various cellular processes, including
cell proliferation, differentiation and apoptosis under normal and
diseased conditions (7).
Increasing evidence suggests that miRNAs have a
regulatory function in oocyte maturation and ovarian follicular
development (8). Numerous
differentially expressed miRNAs have been identified in
ovary-associated diseases. Fiedler et al identified 13
differentially expressed miRNAs in mouse granulosa cells prior to
and 4 h after human chorionic gonadotropin treatment and further
investigation indicated that miR-21 could inhibit apoptosis of
mouse periovulatory granulosa cells (9). A previous study demonstrated
differential miRNA expression profiles in the plasma of patients
with POF and healthy females by miRNA microarray analysis. A total
of 10 upregulated miRNAs were identified, including miR-146a
(8). Nevertheless, the signaling
pathways that regulate the expression of miRNAs during POF and the
function of individual miRNAs in granulosa cell apoptosis remain to
be elucidated.
Interleukin (IL)-1 receptor-associated kinase
(IRAK1) and tumor necrosis factor receptor-associated factor 6
(TRAF6), two key adaptor/scaffold proteins in the IL-1 and
Toll-like receptor (TLR) signaling pathway, are known to positively
regulate nuclear factor (NF)-κB activity, which is presented by
phosphorylation of IκBα. IRAK1 and TRAF6 are proposed to be
targeted by miR-146a as a part of the NF-κB-induced negative
feedback loop (10,11). NF-κB has been demonstrated to be
involved in the immune response, inflammation, apoptosis and other
biological processes (12). The
caspase cascade is an important pathway for apoptosis. Activation
of caspase-8 and caspase-9 initiates cell apoptosis, which causes
the executor of cell apoptosis caspase-3 to be cleaved and degrades
the substrate poly (ADP-ribose) polymerase (PARP) (13). Thus, it was hypothesized that
miR-146a contributes to the apoptosis of ovarian granulosa cells
via the caspase cascade by directly targeting IRAK1 and TRAF6.
The present study generated gain-of and loss-of
function cell models of miR-146a, and various inhibitors of
apoptosis were used. The aim of the study was to explore the
functional role of miR-146a in POF and identify the possible
molecular mechanism for the modulation of miR-146a in POF.
Materials and methods
Tissue samples
Six plasma samples from different patients with POF
and two age-matched normal plasma samples were obtained from the
Xiangya Hospital, Central South University (Changsha, China) and
diagnosis was confirmed by routine pathological examination. All
experiments were conducted following obtaining approval from the
Ethics Committee of Central South University. Each patient provided
written informed consent.
Isolation of human granulosa cells from
patients with POF
Isolation of human ovarian granulosa cells from
follicular fluid was performed as previously described (8). Briefly, all granulosa cells were
disaggregated and incubated with 10% hyaluronidase for 15 min at
37°C. Subsequently, percoll (Sigma-Aldrich, St. Louis, MO, USA) was
used to separate granulosa cells from red blood cells and
lymphocytes by density gradient centrifugation at 1,000 × g for 15
min. The granulosa cells were harvested and cultured in RPMI-1640
medium (Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum and 1% (w/v) penicillin/streptomycin in a 5%
CO2 humidified atmosphere at 37°C. In the present study,
the cells were termed POFC-112 and POFC-38.
Normal human ovarian granulosa cells, COV434, were
purchased from the Peking Union Medical College Cell Bank (Beijing,
China). The cells were cultured in RPMI-1640 medium (Gibco-BRL)
supplemented with 10% fetal bovine serum and 1% (w/v)
penicillin/streptomycin in a 5% CO2 humidified
atmosphere at 37°C.
Cell treatment
To investigate the role of miR-146a in POF, ectopic
expression of miR-146a was achieved by transfecting Lv-pre-miR-146a
or Lv-anti-miR-146a using Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA). Pre-miR scrambled negative
(pre-scramble) or anti-miR scrambled negative (anti-scramble) was
also transfected into the cells and used as a negative control. To
analyze the downstream molecule associated with miR-146a, COV434
cells were treated with the caspase-8 inhibitor Z-LEHD-FMK
(Sigma-Aldrich) and the caspase-9 inhibitor Z-IEHD-FMK
(Sigma-Aldrich) at a concentration of 100 mM (diluted with dimethyl
sulfoxide) for 72 h.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) for miRNA
qPCR for miRNA was performed using IQ SYBR Green
Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Briefly,
3 µg of total RNA was reverse transcribed using a specific
looped RT primer for each miRNA using a RevertAid First Strand cDNA
synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA). The
following amplification was performed using IQ SYBR Green Supermix
in a CFX connect Real-Time PCR System (Bio-Rad Laboratories, Inc.).
β-actin was used as an internal control. The primers used were as
follows: Has-miR-146a, forward TTATTAAGTATCCAGTGCAGGGTCCGAGG and
reverse TTGCGGGACATCTAATACTGCCTGGTAATG; β-actin, sense
5′-AGGGGCCGGACTCGTCATACT-3′ and antisense
5′-GGCGGCACCACCATGTACCCT-3′. The cycle threshold value was used to
calculate the normalized expression of miR-146a using Bio-Rad CFX
Manager 3.1 software (Bio-Rad Laboratories, Inc.).
qPCR for mRNA
TRIzol reagent (Invitrogen Life Technologies) was
used to extract total RNA according to the manufacturer's
instructions. Total RNA (0.3 µg) was reverse transcribed
using a RevertAid First Strand cDNA synthesis kit (Thermo Fisher
Scientific). The following PCR amplification was performed in a
Thermal Cycler Dice Real-time system using SYBR Green qPCR mix
(Bio-Rad Laboratories, Inc.). For each sample, the relative mRNA
level was normalized to β-actin. The following primer pairs were
used: IRAK1, sense 5′-CCTCCACCTTCCTCTCCCCA-3′ and anti-sense
5′-CACCGTGTTCCTCATCACCG-3′; TRAF6, sense
5′-AGTGTCTGTGTCCGTCCTCTA-3′ and anti-sense
5′-CTTGCTCTGATTCTCTCCTTC-3′; β-actin, sense
5′-AGGGGCCGGACTCGTCATACT-3′ and antisense
5′-GGCGGCACCACCATGTACCCT-3′.
Dual luciferase reporter assay
A wild type 3′-UTR of IRAK1 (wt-IRAK1) and it's
mutant type 3′-untranslated region (UTR) of IRAK1 (mut-IRAK1), and
a wild type 3′-UTR of TRAF6 (wt-TRAF6) and it's mutant type 3′-UTR
of TRAF6 (mut-TRAF6) were constructed into the dual luciferase
reporter vector, respectively. For the luciferase assay,
105 cells were seeded in 6-well plates for 12 h.
Subsequently, the cells were co-transfected with the indicated dual
luciferase reporter vector and miR-146a mimic (pre-miR-146a) or
miR-146a inhibitor (anti-miR-146a), or the negative control
(pre-scramble and anti-scramble), respectively. Following 5 h
incubation with transfection reagent, the medium was refreshed with
fresh completed medium. Following transfection for 48 h, the
luciferase activities in each group were measured using a dual
luciferase reporter gene assay kit (Promega Corporation, Madison,
WI, USA) and detected using an LD400 luminometer (Promega
Corporation). Renilla luciferase activity was normalized to
firefly luciferase activity.
Western blot analysis
Total protein was extracted from the indicated cells
using RIPA lysis buffer (Auragene Bioscience Inc., Changsha,
China). A BCA Protein Assay kit (Thermo Fisher Scientific) was used
to determine the protein concentration. Total proteins (60
µg) mixed in loading buffer were denaturalized, separated by
SDS-PAGE and transferred onto a polyvinylidene fluoride membrane
(Millipore, Billerica, MA, USA). Following incubation with the
appropriate primary antibodies at 37°C for 3 h, the membrane was
washed and incubated with horseradish peroxidase-conjugated
secondary antibody. The signal was visualized by the Chemi-Lumi One
system (Nacalai Tesque, Kyoto, Japan). Data were analyzed by
densitometry using Image-Pro plus software 6.0 (Media Cybernetics,
Rockville, MD, USA) and normalized to β-actin expression. The
following primary antibodies (1:1,000 dilution) were purchased from
Abcam (Cambridge, MA, USA): Rabbit polyclonal anti-IRAK1 (cat. no.
ab190234), rabbit polyclonal anti-TRAF6 (cat. no. ab181622), rabbit
polyclonal anti-NFκB-p65 (cat. no. ab16502), rabbit polyclonal
anti-pNFκB-p65 (cat. no. ab86299), rabbit polyclonal anti-IKBα
(cat. no. ab7217), mouse monoclonal anti-pIKBα (cat. no. ab12135),
rabbit polyclonal anti-cleaved caspase3 (cat. no. ab2302), rabbit
monoclonal anti-cleaved PARP (cat. no. ab32064), rabbbit polyclonal
anti-caspase-9 (cat. no. ab69514) and rabbit polyclonal
anti-caspase-8 (cat. no. ab25901). Mouse monoclonal anti-β-actin
(1:500 dilution; cat. no. YM3028) was purchased from Immunoway
(Newark, DE, USA) and horseradish peroxidase-conjugated secondary
anti-rabbit IgG (cat. no. 7074S) and anti-mouse IgG (cat. no.
3420S) were obtained from Cell Signaling Technology, Inc. (Danvers,
MA, USA).
Apoptosis assay
Cells were transfected with 80 nM miR-146a precursor
(pre-miR-146a) or 80 nM miR-146a inhibitor (anti-miR-146a),
respectively, and 48 h after transfection the cells were then
subjected to an apoptosis assay. Apoptosis was detected by annexin
V/propidium iodide staining with the apoptosis detection kit (BD
Biosciences, San Jose, CA, USA). Briefly, 106 treated
cells were incubated with annexin V/propidium iodide for 20 min at
25°C. The cells were then analyzed by flow cytometry.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
A TUNEL assay was also used to detect the apoptosis
of COV434 cells transfected with pre-miR-146a or anti-miR-146a.
Briefly, the cells were smeared on slides. Following 30 min fixed
with 4% paraformaldehyde at room temperature, the slides were
incubated with 0.1% Triton X-100 for 10 min, rinsed with
phosphate-buffered saline (PBS), incubated with 3%
H2O2 to block endogenous peroxidase activity,
and rinsed with PBS. Following that, an apoptosis detection kit (BD
Biosciences) was used for TUNEL staining in accordance with the
manufacturer's instructions. The TUNEL-stained slides were observed
under a fluorescence microscope [AE31; Motic Xiamen (China),
Fujian, China].
Statistical analysis
Statistical evaluation for data analysis was
determined by unpaired Student's t-test. Data are presented as the
mean ± standard deviation of three independent experiments. Data
were analyzed using SPSS 17.0 statistical software (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-146a is frequently upregulated in
plasma and human granulosa cells from patients with POF
The present study focused on the role of miR-146a in
POF. The present study initially detected the expression of
miR-146a in peripheral plasma from patients with POF. As shown in
Fig. 1A, the results of qPCR for
miR-146a in plasma samples demonstrated that compared with the
normal control, the expression of miR-146a was significantly
upregulated in patients with POF. Furthermore, the expression of
miR-146a was also detected in granulosa cells from patients with
POF. In accordance with the results from plasma, an increase in
miR-146a expression was observed in granulosa cells obtained from
patients with POF (Fig. 1B). This
result indicated that there was a correlation between miR-146a and
POF.
miR-146a regulates cell apoptosis in
COV434 cells
To further determine the role of miR-146a in POF,
gain- and loss-functional experiments were performed by
transfecting pre-miR-146a or anti-miR-146a into COV434 cells. The
present study examined the apoptosis of COV434 cells following
upregulating or downregulating miR-146a by flow cytometry. As shown
in Fig. 2A, upregulation of
miR-146a promoted cell apoptosis, whereas downregulation of
miR-146a reduced cell apoptosis. In addition, a TUNEL assay was
used to confirm the results of flow cytometry. Consistent with the
results of flow cytometry, it was found that overexpression of
miR-146a increased the number of TUNEL-positive cells compared with
the negative control, and reducing the expression of miR-146a
decreased the number of TUNEL-positive cells compared with the
negative control (Fig. 2B). Thus,
the data confirmed that miR-146a regulates cell apoptosis in COV434
cells in vitro.
miR-146a directly targets IRAK1 and
TRAF6
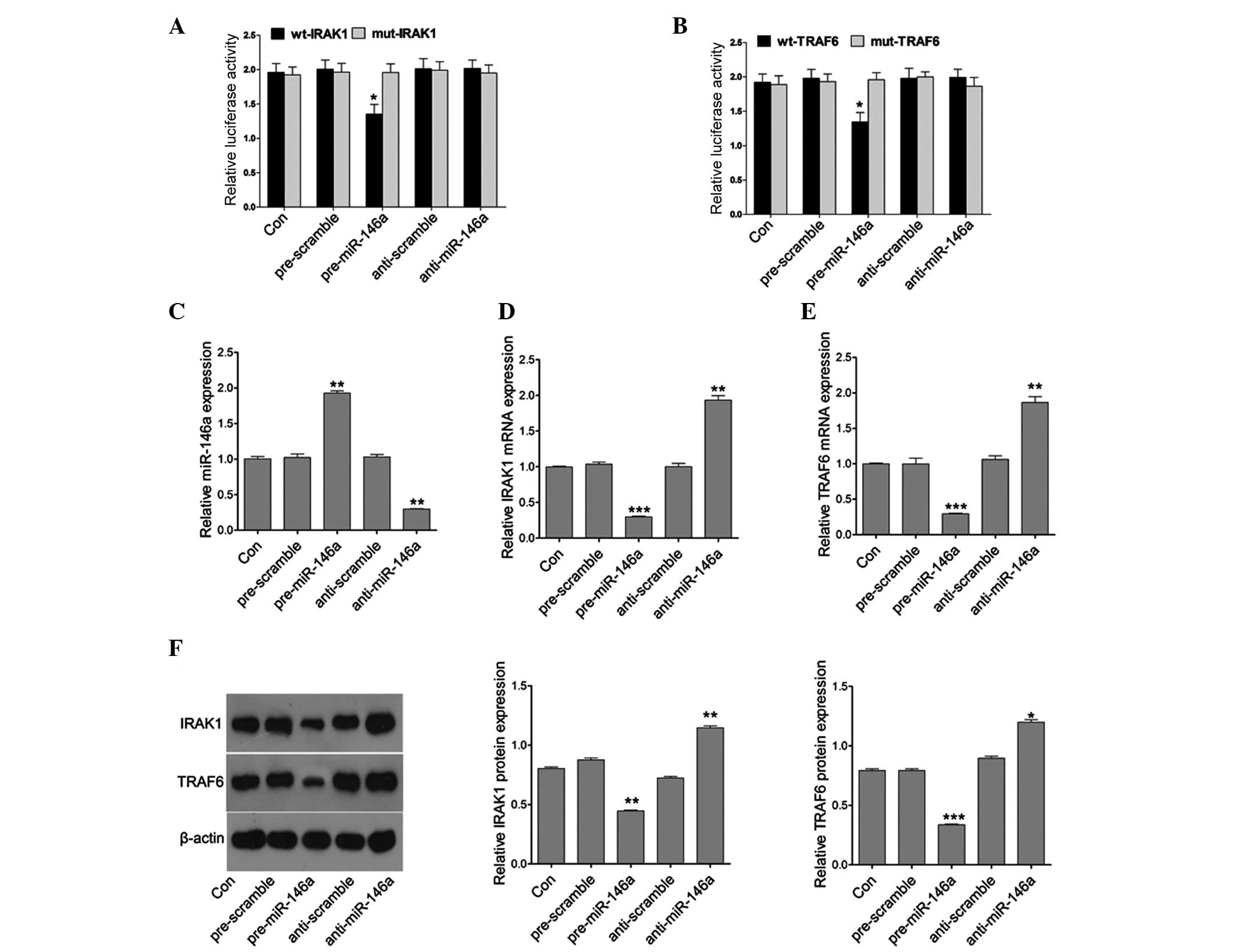
Since miR-146a is upregulated in patients with POF,
the target genes of miR-146a were next examined, as miRNAs must
produce their effects through their target genes. Bioinformatics is
a useful tool for screening the targets of miRNAs. Using the
TargetScan and microRNA website (www.targetscan.org), two putative targets, IRAK1 and
TRAF6, of miR-146a were selected. To confirm whether miR-146a
directly targeted the 3′UTR of IRAK1 and TRAF6, the 3′UTR of IRAK1
and TRAF6 was cloned downstream to a luciferase reporter gene
(wt-IRAK1 and wt-TRAF6). The wt-IRAK1 or wt-TRAF6 vector was
co-transfected with the miR-146a mimics or inhibitor into COV434
cells, respectively. The luciferase activity of miR-146a
mimic-transfected cells was significantly decreased compared with
cells co-transfected with their mutant type and scramble control
cells (Fig. 3A and B). The present
study then assessed whether miR-146a directly regulates the
expression of IRAK1 and TRAF6. qPCR was used to detect the
expression of miR-146a following miR-146a mimic or inhibitor
treatment. The results demonstrated that miR-146a mimics
efficiently induced the expression of miR146a, and the miR-146a
inhibitor markedly downregulated the expression of miR-146a
(Fig. 3C). Furthermore, miR-146a
mimics efficiently reduced the mRNA expression of IRAK1 and TRAF6
whereas the miR-146a inhibitor markedly upregulated the mRNA
expression of IRAK1 and TRAF6 (Fig. 3D
and E). In addition, the protein expression of IRAK1 and TRAF6
was further analyzed in cells transfected with the miR-146a mimics
or inhibitor. Similarly, the protein expression of IRAK1 and TRAF6
was decreased or induced by the miR-146a mimics or inhibitor,
respectively (Fig. 3F). These
results suggested that miR-146a regulated IRAK1 and TRAF6
expression at the transcriptional and translational levels.
miR-146a regulates the expression of
pNFκB-p65, pIκBα, caspase-8, caspase-9, cleaved caspase-3 and
cleaved PARP
To examine the mechanism underlying the regulation
of cell apoptosis by miR-146a in COV434 cells, the downstream
target genes associated with apoptosis of COV434 cells were
detected. Considering the role of miR-146a in cell apoptosis, the
molecules involved in apoptosis were detected in granulosa cells.
The expression levels of pNFκB-p65, pIκBα, caspase-8, caspase-9,
cleaved caspase-3 and cleaved PARP were measured by western
blotting in pre-miR-146a or anti-miR-146a-transfected COV434 and
negative control cells. As shown in Fig. 4A, the expression of pNFκB-p65 and
pIκBα was reduced following pre-miR-146a transfection at the
protein level, whereas it was markedly induced by anti-miR-146a
transfection compared with the negative control. However,
upregulation and downregulation of miR-146a did not alter the
expression of NFκB-p65 and IκBα. Caspase signaling is important for
apoptosis. Thus, the protein levels of caspase-8, caspase-9,
cleaved caspase-3 and cleaved PARP were next detected by western
blotting in pre-miR-146a or anti-miR-146a-transfected COV434 and
control cells. Compared with the negative control, the protein
level of caspase-8, caspase-9, cleaved caspase-3 and cleaved PARP
was significantly increased in pre-miR-146a-transfected cells,
whereas it was markedly reduced in anti-miR-146a-transfected cells
(Fig. 4B). These results indicated
that miR-146a contributed to granulosa cell apoptosis.
 | Figure 4miR-146a regulates the expression of
pNFκB-p65, pIκBα, caspase-8, caspase-9, cleaved caspase-3 and
cleaved PARP. (A) Western blot analysis was used to analyze the
protein expression of NFκB-p65, pNFκB-p65, IκBα and pIκBα. (B)
Western blot analysis was used to analyze the protein expression of
caspase-8, caspase-9, cleaved caspase-3 and cleaved PARP. Data are
presented as the mean ± standard error of the mean.
*P<0.05, **P<0.01 and
***P<0.001 vs. control. PARP, poly (ADP-ribose)
polymerase; NFκB. nuclear factor-κB; miR-146a, microRNA146a. |
miR-146a regulates cell apoptosis via
caspase signaling
To further determine the signaling pathway of
miR-146a contributing to cell apoptosis in granulosa cells, the
caspase pathway was inhibited using a caspase-8 inhibitor
(Z-LEHD-FMK) and caspase-9 inhibitor (Z-IEHD-FMK) following
pre-miR-146a or anti-miR-146a treatment. As shown in Fig. 5A, inhibition of caspase-8 and
caspase-9 by their specific inhibitor significantly attenuated the
effect of miR-146a on apoptosis. In addition, gene expression
associated with apoptosis was detected, including cleaved caspase-3
and cleaved PARP by western blot analysis. As shown in Fig. 5B, the protein expression of cleaved
caspase-3 and cleaved PARP was induced by upregu-lating miR-146a
and decreased by the caspase-8 inhibitor and caspase-9 inhibitor.
When co-treating the caspase inhibitor with pre-miR-146a, it was
found that the increase in cleaved caspase-3 and cleaved PARP
expression was decreased by the caspase-8 inhibitor and caspase-9
inhibitor. Thus, these results indicated that miR-146a contributed
to granulosa cell apoptosis via caspase signaling.
Discussion
Although the causes of POF remain to be elucidated,
POF is characterized by increased apoptosis of ovarian granulosa
cells (14). Increasing evidence
has demonstrated that miRNAs are involved in multiple cell
processes, including survival, proliferation and apoptosis
(15). Certain differentially
expressed miRNAs in the plasma of POF patients have been
identified, including downregulated miRNAs (let-7c and miR-144) and
upregulated miRNAs (miR-202 and miR-146a) (8). Numerous previous studies have
demonstrated that miR-146a is implicated in cell apoptosis. It was
reported that upregulation of miR-146a induced apoptosis of human
chondrocytes (16) and miRNA-146a
regulated the maturation and differentiation of vascular smooth
muscle cells (17). In addition,
miR-146a induces apoptosis in several types of cancer, including
non-small cell lung cancer (18),
gastric cancer (19) and breast
cancer (20). However, the role of
miR-146a in POF remains to be elucidated. In the present study, in
line with a previous study (8), it
was confirmed that miR-146a was significantly upregulated in the
plasma of POF patients compared with the normal control.
Furthermore, in isolated ovarian granulosa cells from patients with
POF, the expression of miR-146a was markedly increased. Thus, these
results indicated that miR-146a is important in the apoptosis of
ovarian granulosa cells in patients with POF.
By gain- and loss- of function analyses, it was
found that upregulation of miR-146a induced apoptosis of ovarian
granulosa cells, whereas downregulation of miR-146a reduced
apoptosis of ovarian granulosa cells. miR-146a functions as an
apoptosis regulator by targeting the 3′UTR of target genes. Using
bioinformatic tools, two putative targets of miR-146a were found,
including IRAK1 and TRAF6. In addition, by using the dual
luciferase reporter assay, it was confirmed that miR-146a directly
targeted the 3′UTR of IRAK1 and TRAF6 in ovarian granulosa cells.
In addition, miR-146a regulated the expression of IRAK1 and TRAF6
at the transcriptional and translational levels in ovarian
granulosa cells. TLR signaling within ovarian granulosa cells has
broad implications for ovarian physiology. Woods et al
demonstrated that treatment with lipopolysaccharide in vitro
led to the differential regulation of TLRs based on the stage of
follicle maturation (21),
suggesting that follicle maturation was required for the TLR
signaling gene. IRAK-1 and TRAF-6 are two key elements involved in
the TLR signaling pathway. It was reported that miR-146a modulated
inflammatory responses by targeting IRAK1 and TRAF6 in macrophages
(22). miR-146a has been
demonstrated as a regulator of the inflammatory response (23,24).
Thus, the present study provided evidence to demonstrate that
miR-146a contributes to the apoptosis of ovarian granulosa cells
via TLR signaling.
IRAK-1 acts as the essential upstream adaptor that
mediates signaling to NF-κB through the TRAF6 cascade (25). The results from the present study
demonstrated that upregulation of miR-146a reduced activation of
NF-κB and IκBα through targeting IRAK1 and TRAF6. TLR signaling is
closely associated with the caspase cascade. It was reported that
activated TLR signaling in microglia was inhibited by the pan
caspase inhibitor zVAD-fmk and the caspase-8 inhibitor IETD-fmk
(26). TLR3 stimulation induced
activation of apoptotic caspases -8, -9 and -3 (27). In addition, TLR4 induces the
assembly of caspase-8-based signaling complexes (28). The present study demonstrated that
ectopic expression of miR-146a affects the caspase cascade, which
alters the expression of caspase-8, caspase-9, cleaved caspase-3
and cleaved PARP. By inhibiting caspase-8 and caspase-9 using their
specific inhibitors, it was found that apoptosis induced by
miR-146a was attenuated by caspase inhibitors. In addition, when
co-treated with the caspase inhibitor, the role of miR-146a in
promoting apoptosis was inhibited. Thus, it was demonstrated that
miR-146a mediated ovarian granulosa cell apoptosis via caspase
signaling.
In conclusion, upregulation of miR-146a in the
plasma and ovarian granulosa cells of patients with POF may act as
a biomarker for POF. Furthermore, miR-146a regulated TLR signaling
by simultaneously targeting IRAK1 and TRAF6, which affected the
activity of NF-κB and IκBα, and the caspase cascade. Thus,
miR-146a/IRAK1/TRAF6/caspase-8 signaling mediated apoptosis of
ovarian granulosa cells in POF, suggesting that inhibition of
miR-146a may be beneficial for the management of POF.
References
|
1
|
Beck-Peccoz P and Persani L: Premature
ovarian failure. Orphanet J Rare Dis. 1:92006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mittal M, Savvas M, Arya R, McEniery C,
Narvekar N, Cardozo L, Panay N and Hamoda H: A randomised
controlled trial comparing the effects of micronized progesterone
to medroxyprogesterone acetate on cardiovascular health, lipid
metabolism and the coagulation cascade in women with premature
ovarian insufficiency: Study protocol and review of the literature.
Menopause Int. 19:127–132. 2013.PubMed/NCBI
|
|
3
|
Morgan S, Anderson RA, Gourley C, Wallace
WH and Spears N: How do chemotherapeutic agents damage the ovary?
Hum Reprod Update. 18:525–535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Knet M, Tabarowski Z, Slomczynska M and
Duda M: The effects of the environmental antiandrogen vinclozolin
on the induction of granulosa cell apoptosis during follicular
atresia in pigs. Theriogenology. 81:1239–1247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ford JH: Reduced quality and accelerated
follicle loss with female reproductive aging - does decline in
theca dehydroepiandrosterone (DHEA) underlie the problem? J Biomed
Sci. 20:932013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sirotkin AV, Lauková M, Ovcharenko D,
Brenaut P and Mlyncek M: Identification of microRNAs controlling
human ovarian cell proliferation and apoptosis. J Cell Physiol.
223:49–56. 2010.
|
|
7
|
Toloubeydokhti T, Bukulmez O and Chegini
N: Potential regulatory functions of microRNAs in the ovary. Semin
Reprod Med. 26:469–478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang X, Zhou Y, Peng S, Wu L, Lin HY, Wang
S and Wang H: Differentially expressed plasma microRNAs in
premature ovarian failure patients and the potential regulatory
function of mir-23a in granulosa cell apoptosis. Reproduction.
144:235–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fiedler SD, Carletti MZ, Hong X and
Christenson LK: Hormonal regulation of MicroRNA expression in
periovulatory mouse mural granulosa cells. Biol Reprod.
79:1030–1037. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zilahi E, Tarr T, Papp G, Griger Z, Sipka
S and Zeher M: Increased microRNA-146a/b, TRAF6 gene and decreased
IRAK1 gene expressions in the peripheral mononuclear cells of
patients with Sjögren's syndrome. Immunol Lett. 141:165–168. 2012.
View Article : Google Scholar
|
|
11
|
Hung PS, Liu CJ, Chou CS, Kao SY, Yang CC,
Chang KW, Chiu TH and Lin SC: miR-146a enhances the oncogenicity of
oral carcinoma by concomitant targeting of the IRAK1, TRAF6 and
NUMB genes. PLoS One. 8:e799262013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woods DC, White YA, Dau C and Johnson AL:
TLR4 activates NF-κB in human ovarian granulosa tumor cells.
Biochem Biophys Res Commun. 409:675–680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park EJ, Shin JW, Seo YS, Kim DW, Hong SY,
Park WI and Kang BM: Gonadotropin-releasing hormone-agonist induces
apoptosis of human granulosa-luteal cells via caspase-8, -9 and -3
and poly-(ADP-ribose)-polymerase cleavage. Biosci Trends.
5:120–128. 2011. View Article : Google Scholar
|
|
14
|
Ebrahimi M and Akbari Asbagh F:
Pathogenesis and causes of premature ovarian failure: An update.
Int J Fertil Steril. 5:54–65. 2011.PubMed/NCBI
|
|
15
|
Kim YJ, Ku SY, Kim YY, Liu HC, Chi SW, Kim
SH, Choi YM, Kim JG and Moon SY: MicroRNAs transfected into
granulosa cells may regulate oocyte meiotic competence during in
vitro maturation of mouse follicles. Hum Reprod. 28:3050–3061.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin L, Zhao J, Jing W, Yan S, Wang X, Xiao
C and Ma B: Role of miR-146a in human chondrocyte apoptosis in
response to mechanical pressure injury in vitro. Int J Mol Med.
34:451–463. 2014.PubMed/NCBI
|
|
17
|
Dong S, Xiong W, Yuan J, Li J, Liu J and
Xu X: MiRNA-146a regulates the maturation and differentiation of
vascular smooth muscle cells by targeting NF-κB expression. Mol Med
Rep. 8:407–412. 2013.PubMed/NCBI
|
|
18
|
Chen G, Umelo IA, Lv S, Teugels E, Fostier
K, Kronenberger P, Dewaele A, Sadones J, Geers C and De Grève J:
miR-146a inhibits cell growth, cell migration and induces apoptosis
in non-small cell lung cancer cells. PLoS One. 8:e603172013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sha M, Ye J, Zhang LX, Luan ZY and Chen
YB: Celastrol induces apoptosis of gastric cancer cells by miR-146a
inhibition of NF-κB activity. Cancer Cell Int. 13:502013.
View Article : Google Scholar
|
|
20
|
Elsarraj HS, Stecklein SR, Valdez K and
Behbod F: Emerging functions of microRNA-146a/b in development and
breast cancer: MicroRNA-146a/b in development and breast cancer. J
Mammary Gland Biol Neoplasia. 17:79–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woods DC, Schorey JS and Johnson AL:
Toll-like receptor signaling in hen ovarian granulosa cells is
dependent on stage of follicle maturation. Reproduction.
137:987–996. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li S, Yue Y, Xu W and Xiong S:
MicroRNA-146a represses mycobacteria-induced inflammatory response
and facilitates bacterial replication via targeting IRAK-1 and
TRAF-6. PLoS One. 8:e814382013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu M, John CM and Jarvis GA: Induction of
endotoxin tolerance by pathogenic Neisseria is correlated with the
inflammatory potential of lipooligosaccharides and regulated by
microRNA-146a. J Immunol. 192:1768–1777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quinn EM, Wang JH, O'Callaghan G and
Redmond HP: MicroRNA-146a is upregulated by and negatively
regulates TLR2 signaling. PLoS One. 8:e622322013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong W, Liu Y, Peng J, Chen L, Zou T, Xiao
H, Liu Z, Li W, Bu Y and Qi Y: The IRAK-1-BCL10-MALT1-TRAF6-TAK1
cascade mediates signaling to NF-kappaB from Toll-like receptor 4.
J Biol Chem. 281:26029–26040. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SJ and Li J: Caspase blockade induces
RIP3-mediated programmed necrosis in Toll-like receptor-activated
microglia. Cell Death Dis. 4:e7162013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grimstad Ø, Husebye H and Espevik T: TLR3
mediates release of IL-1β and cell death in keratinocytes in a
caspase-4 dependent manner. J Dermatol Sci. 72:45–53. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Antonopoulos C, El Sanadi C, Kaiser WJ,
Mocarski ES and Dubyak GR: Proapoptotic chemotherapeutic drugs
induce noncanonical processing and release of IL-1β via caspase-8
in dendritic cells. J Immunol. 191:4789–4803. 2013. View Article : Google Scholar : PubMed/NCBI
|