Introduction
Temporomandibular joint disorders (TMD) are one of
the most prevalent types of joint disease, which cause persistent
and recurrent joint pain with eventual loss of joint function
(1). The methods of diagnosis and
treatment of TMD have improved in the past three decades; however,
the cellular and molecular mechanisms underlying the initiation and
progression of TMD remain to be elucidated (2). Interleukin-1β (IL-1β) is an important
cytokine that contributes to the severe inflammatory reactions
observed in TMD (3).
Histopathological studies have demonstrated that the synovial
membranes of patients with TMD exhibit inflammatory cell
infiltration, hyperplasia, and high-density vascularization
(4,5). In addition, it has been suggested
that inflammation-induced angiogenesis in the synovial membranes
has an important role in the progression of TMD (6).
Angiogenesis is a common process associated with the
induction of cancer and other diseases (7). Numerous molecules are activated
during the complex process of angiogenesis in the synovial membrane
(7,8). Our previous studies demonstrated that
vascular endothelial growth factor (VEGF), a vascular permeability
factor and selective endothelial mitogen, accumulates in the
synovial membrane and fluid during the inflammatory response of TMD
(9,10). Previous in vivo and in
vitro studies have reported that hypoxia inducible factor-1α
(HIF-1α) is activated in the synovial membrane of patients with TMD
(9,11), and implicated in the regulation of
important aspects of angiogenesis (12,13).
Although the expression levels of angiogenic factors such as HIF-1α
and VEGF have been shown to be increased in the synovium of joint
diseases, the mechanisms that underlie increased angiogenesis,
specifically in the case of TMD, have yet to be elucidated.
Dickkopf-related protein 1 (DKK-1) is a secreted
protein, which inhibits the Wnt/β-catenin signaling pathway, and is
implicated in various developmental and physiological processes
(14,15). A previous study demonstrated that
DKK-1 promotes angiogenesis during development, tumorigenesis, and
inflammation (16). In addition,
DKK-1 has been shown to enhance the angiogenic properties of human
endothelial colony-forming cells, and increase tumoral angiogenesis
in breast cancer (17). In
osteoarthritic knee joints, DKK-1 was associated with angiogenesis
and cartilage matrix proteinase secretion (15). However, DKK-1 expression and the
biological role of DKK-1 in the synovium of TMD are, to the best of
our knowledge, rarely studied.
In the present study, the expression of DKK-1 was
determined in synovial tissues from a cohort of patients with TMD
and from healthy patients. The association between DKK-1 and VEGF
was also examined. In addition, the effects of DKK-1 on the
angiogenesis in the was investigated in vitro.
Materials and methods
Patients
Synovial tissue samples were obtained from the
Department of Oral and Maxillofacial Surgery, School and Hospital
of Stomatology, Wuhan University (Wuhan, China). All experiments
and synovial tissue sample collections were performed in accordance
with the Helsinki Declaration of 1975, and approved by the
institute review board of the Ethics Committee of the Hospital of
Stomatology, Wuhan University. Written informed consent was
obtained from all patients. The patients with TMD were diagnosed
and classified according to the Research Diagnostic Criteria for
TMD (18,19). Patients who received any oral
medication within the previous 6 months, or any other
intra-articular treatment, were excluded from the present study.
The clinical data and descriptive characteristics of the patients
are shown in Table I. Synovial
tissue samples were collected bilaterally from six patients, and
unilaterally from 44 patients. All of the patients were sorted into
anterior disc displacement with reduction (ADDwR), anterior disc
displacement without reduction (ADDw/oR), and osteoarthritis (OA)
TMD groups, according to their clinical characteristics,
radiographic examination and arthrography. The ADDwR and ADDw/oR
subgroups formed the anterior disc displacement (ADD) group. A
total of seven samples from healthy volunteers were included as the
control group. The synovial fluid harvesting method was carried out
as previously described (20).
Briefly, the patients received 2% lidocaine (Chengdu No. 1
Pharmaceutical Group Co. Ltd., Chengdu, China) in the preauricular
region by subcutaneous infiltration, then 2 ml saline solution was
injected into the superior joint space. The synovial fluid was then
mixed once the patient opened and closed his or her mouth. The
saline solution was subsequently aspirated and collected, and an
arthrography examination was carried out at the same time. The
synovial tissue samples from the control subjects were collected in
a similar manner, except that they did not receive
temporomandibular joint (TMJ) arthrography. The synovial tissue
samples were centrifuged at 4°C (1,000 x g, 5 min) to remove the
cell and tissue debris, prior to being stored at −70°C.
 | Table IPatients and TMD characteristics. |
Table I
Patients and TMD characteristics.
| Group | No. of patients | Female | Male | Age (years) | Mean age (years) | No. of TMJs |
|---|
| Control | 7 | 4 | 3 | 25–34 | 27.3 | 7 |
| ADDwR | 18 | 10 | 8 | 17–42 | 29.5 | 22 |
| ADDw/oR | 20 | 11 | 9 | 18–53 | 26.6 | 22 |
| OA | 12 | 6 | 6 | 21–55 | 31.7 | 12 |
ELISA
The concentration levels of DKK-1 and VEGF in the
synovial fluid from the patients with TMD were measured using a
Human DKK-1 ELISA kit (Wuhan ColorfulGene Biological Technology
Co., Ltd, Wuhan, China) and a Human VEGF ELISA kit (Pierce
Biotechnology, Inc., Rockford, IL, USA) according to the
manufacturer's instructions. The detailed methods have previously
been described (20).
Cell culture and reagents
Synovial fibroblasts were cultured from the synovial
tissue samples of four patients (24, 24, 28 and 30 years-old) from
the OA groups, as previously described (21). The cells were maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 20%
fetal bovine serum (FBS) (both from Thermo Fisher Scientific Inc.,
Beijing, China), in a humidified atmosphere containing 95% air and
5% CO2 at 37°C. Cell passages 3–8 were used in the
present study. Pooled HUVECs were purchased from Lonza
(Walkersville, MD, USA), and grown in endothelial growth medium 2
(Lonza), with passages 3–8 being used in the present study. IL-1β,
DKK-1, human DKK-1 affinity purified rabbit polyclonal antibody
(DKK-1 inhibitor), and recombinant human DKK-1 (rhDKK-1) were
purchased from R&D systems, Inc. (Minneapolis, MN, USA). The
rabbit monoclonal anti-HIF-1α (Epitomics Inc., Burlingame, CA, USA;
ab190197; 1:1,000) and rabbit polyclonal anti-VEGF antibodies
(GeneTex Inc., Irvine, CA, USA; GTX102643; 1:1,000) were obtained
from Cell Signaling Technologies, Inc. (Danvers, MA, USA). Rabbit
monoclonal DKK-1 was purchased from Abcam (Abcam, Cambridge, CA,
USA; ab109416; 1:1000). Anti-GAPDH was purchased from Santa Cruz
Biotechnology Biotechnology, Inc. (Dallas, TX, USA).
Transient transfection with small
interfering RNA (siRNA)
siRNA specific for human HIF-1α and a non-specific
control siRNA (scramble) were purchased from Shanghai GenePharma
Co., Ltd. (Shanghai, China). HIF-1α siRNA scramble siRNA:
5′-UAGCGACUAAACACAUCAA-3′; HIF-1a si1: 5′-GCCGCUCAAUUUAUGAAUATT-3′;
and HIF-1a si2: 5′-CCAGUUAUGAUUGUGAAGUUA-3′. The cells were grown
to 60–80% confluence in 12-well plates and were transfected using
Lipofectamine® 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA). The HIF-1α and scramble siRNA (60 µmol)
were transfected into the cells with Lipofectamine®
2000, according to the manufacturer's instructions. All experiments
were performed 24 h post-transfection. Total RNA was extracted 48 h
post-transfection, and protein was extracted 72 h
post-transfection.
Western blotting
Western blot analysis was performed as described
previously (9). Briefly, the
synovial fibroblasts were treated with IL-1β or rhDKK-1 in DMEM
supplemented with 2% FBS for 24 h. The cells were then lysed using
M-PER Mammalian Protein Extraction buffer (Pierce Biotechnology,
Inc., Rockford, IL, USA) containing protease inhibitor tablets
(complete, EDTA-free; Roche Applied Science, Shanghai, China), and
the cell extracts were separated by 10 or 12% SDS-PAGE, prior to
being transferred onto polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
skimmed milk for 1 h, and subsequently incubated overnight at 4°C
with the corresponding primary antibodies, in 5% w/v bovine serum
albumin (Beyotime Institute of Biotechnology, Nantong, China),
followed by incubation with a horseradish peroxidase-conjugated
secondary antibody (Pierce Biotechnology, Inc.). The blots were
then developed using the West Pico Super Enhanced Chemiluminescence
Detection kit (Thermo Fisher Scientific Inc., Waltham, MA, USA).
GAPDH was used as a loading control and was detected on the same
membrane. Western blots were analyzed by densitometry using Image J
software, version 1.47c (National Institutes of Health, Bethesda,
MD, USA).
Immunocytochemistry and confocal
microscopy
Immunocytochemistry was performed as previously
described (22). Briefly, the
synovial fibroblasts 1×106/well were plated in six-well
culture plates that contained carry sheet glass. Once the cells on
the glass slide had reached 70% confluence, they were incubated
with a mixture of the IL-1β or rhDKK-1 and serum-deprived DMEM.
Following 24 h incubation, the cells were fixed for 10 min with 4%
formaldehyde in phosphate-buffered saline (PBS; pH 8.0). Following
three washes with PBS, the cells were permeabilized for 5 min with
0.5% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) in PBS, prior
to being thoroughly washed again with PBS. PBS supplemented with
10% FBS was used to block non-specific binding for 60 min. The
cells were subsequently incubated with HIF-1α (1:100) and DKK-1
antibodies (1:50) overnight at 4°C. The cells were washed
thoroughly three times with PBS, and then incubated with
Cy3-conjugated donkey anti-mouse antibody (1:200; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) at room
temperature for 60 min, followed by further washing three times
with PBS. The nuclei were then stained with DAPI, and images of the
cells were captured and analyzed by CLSM-310 confocal laser
scanning microscopy (Carl Zeiss Inc., Thornwood, NY, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Invitrogen Life
Technologies) was used to extract total RNA, according to the
manufacturer's instructions. The reaction conditions were as
follows: 95°C for 5 min, then 95°C for 30 sec, 60°C for 30 sec and
72°C for 30 sec for 35 cycles, 72°C for 10 min and 15°C for ∞. The
purity and yield of the isolated RNA were determined using an ND
1000 NanoDrop spectrophotometer (Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA). Aliquots of total RNA were
reverse-transcribed using a SYBR PrimeScriptTM RT
Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China).
RT-qPCR was performed using a SYBR PrimeScriptTM RT-PCR
kit (Takara Biotechnology Co., Ltd.) and an ABI PRISM 7500
Real-Time PCR system (Applied Biosystems Life Technologies, Foster
City, CA, USA). β-actin was used as an internal control. The
following gene-specific primers were used for the reaction: Human
VEGF forward, 5′-CCCACTGAGGAGTCCAACAT-3′, and reverse,
5′-TTATACCGGGATTTCTTGCG-3′; human HIF-1α forward,
5′-CAGGACACAGATTTAGACTGGG-3′, and reverse,
5′-TGGTAGTGGTGGCATTAGC-3′; human DKK-1 forward,
5′-GAGTCCTTCTGAGATGATGG-3′, and reverse,
5′-TTGATAGCGTTGGAATTGAG-3′; and β-actin forward,
5′-CCATCGTCCACCGCAAAT-3′, and reverse, 5′-CCTGTAACAACGCATCTCATA-3′
Primers were purchased from (Sangon Biotech Co. Ltd., Shanghai,
China). β-actin was used as an internal control. The results were
obtained as threshold cycle (ΔCt) values. No amplification occurred
in the non-template controls. The 2ΔΔCt method was used
to analyze the mRNA expression levels (23).
Wound-healing assay, Boyden chamber
migration assay, and tube formation assay
Conditioned medium (CM) from synovial fibroblasts
treated with either DKK-1 and HIF-1α siRNA, or with DKK-1 alone was
harvested. HUVECs (2×106/well) were plated in six-well
culture plates and grown to 90% confluence. A scratch was made
across the cell layer using a sterile pipette tip to simulate wound
healing. The cells then received the indicated culture
supernatants. Following 12 h incubation, the cells were fixed and
stained using acridine orange (Sigma-Aldrich), and cells that had
migrated into the gap were counted under a microscope (CLSM-310;
Carl Zeiss AG, Jena, Germany). Transwell Boyden chambers (Corning
Life Sciences, Corning, NY, USA) containing a 6.5 mm diameter
polycarbonate filter (8 µm pore size) were used to determine
the motility of endothelial cells. Serum-starved HUVECs
(5×104 cells/well) were plated in the upper wells,
whereas the indicated culture supernatants, used as a
chemo-attractants, were added to the lower wells. The cells were
then incubated for 12 h at 37°C. Using a cotton swab, the HUVECs on
the upper surface were carefully removed, and the cells that had
adhered to the underside of the membrane were fixed with 4%
formaldehyde, stained with crystal violet (Beyotime Institute of
Biotechnology), and observed under a microscope (BHS-313; Olympus,
Tokyo, Japan). For the capillary-like tube formation assay, the
HUVECs (1×104/well) were seeded into 96-well plates
coated with Matrigel (BD Biosciences, San Jose, CA, USA) in culture
supernatants. The cells were then cultured for 6 h at 37°C, and the
capillary-like structures were counted under a microscope (BHS-313
Olympus).
Statistical analysis
GraphPad Prism 5.00 for Windows (GraphPad Software,
Inc., La Jolla, CA, USA) was used for statistical analysis. A
Pearson correlation analysis was used to determine the correlation
between DKK-1 and VEGF expression. Student's t-tests were performed
to analyze the results of HIF-1α nuclear translocation. A one-way
analysis of variance with post-Tukey analysis was used to analyze
the results of the wound-healing assay, transwell assay, tube
formation, and RT-qPCR. All data were presented as the mean ±
standard error of the mean. P<0.05 was considered to indicate a
statistically significant difference.
Results
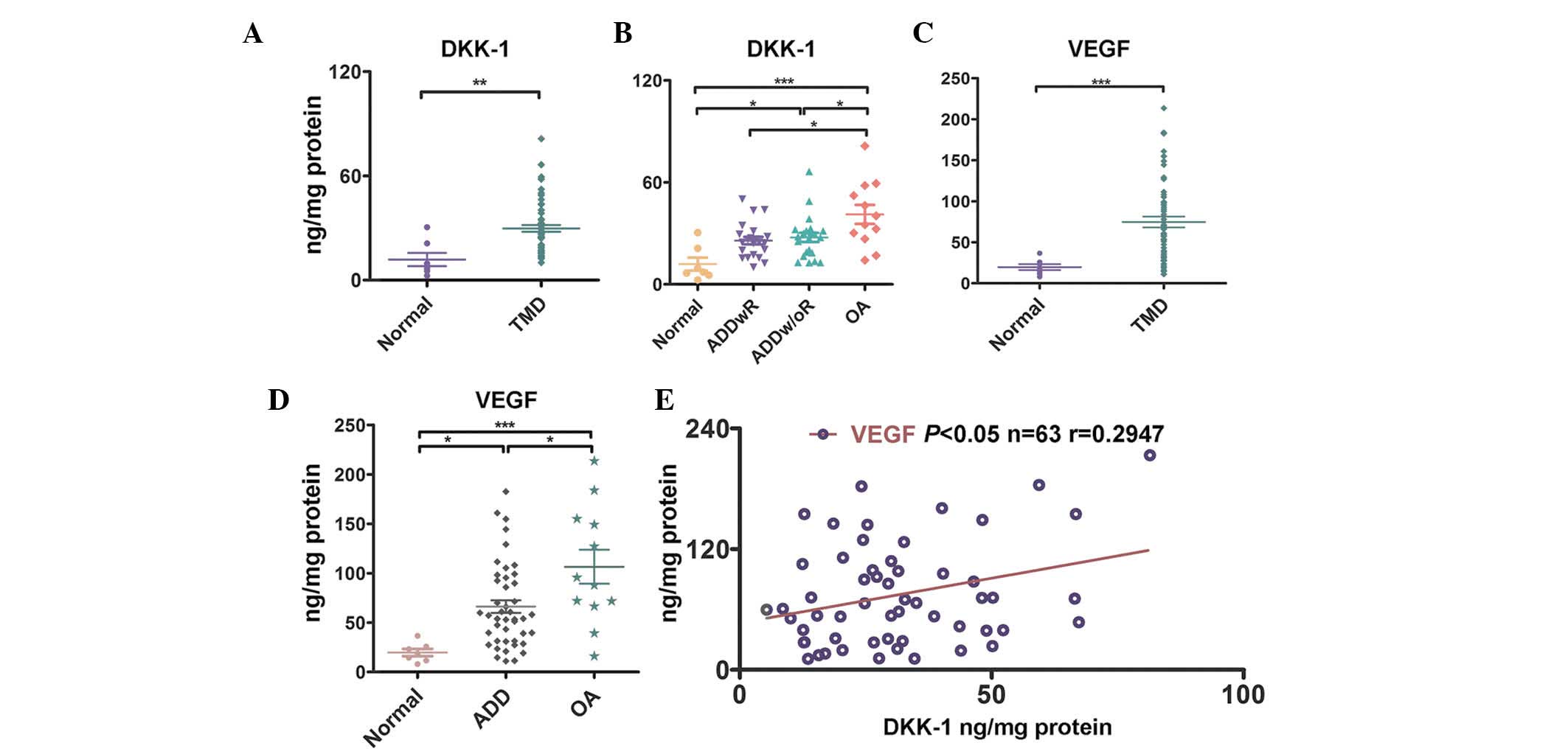
Elevated expression levels of DKK-1 are
associated with increased expression levels of VEGF in the synovial
fluid of patients with TMD
To determine the expression levels of DKK-1 in the
synovial fluid of patients with TMD, the synovial fluid from 50
patients and seven healthy controls were collected for analysis.
The expression levels of DKK-1 in the patients with TMD were
significantly higher, as compared with the control subjects
(P<0.01 Fig. 1A). The
expression levels of DKK-1 in the TMD-OA group were significantly
higher, as compared with those of the TMD-ADDwR and TMD-ADDw/oR
groups (P<0.05), and with those of the control group
(P<0.001, Fig. 1B). The
expression levels of DKK-1 in the synovial fluid of the TMD-ADDw/oR
group was significantly increased, as compared with those of the
control group (P<0.05, Fig.
1B). However, the difference between the mean expression levels
of DKK-1 in patients with TMD-ADDwR and the control subjects was
not significant (Fig. 1B). As
shown in Fig. 1C, the expression
levels of VEGF in the synovial fluid of patients with TMD was
significantly higher, as compared with the control group
(P<0.001). The expression levels of VEGF in the OA group were
significantly higher, as compared with the ADD groups (P<0.05)
and control group (P<0.001, Fig.
1D). The expression levels of VEGF in the TMD-ADDwR group and
TMD-ADDw/oR group were not significantly different, as compared
with the control group (data not shown). Furthermore, the
expression levels of DKK-1 were positively associated with higher
expression levels of VEGF (P<0.05, r=0.2947, n=63, Fig. 1E). These results suggest that the
expression levels of DKK-1 are significantly increased in the
synovial fluid of patients with TMD, and that DKK-1 expression may
have an important role in TMD angiogenesis.
DKK-1 increases the expression levels of
inflammation-induced VEGF in synovial fibroblasts
To assess the expression levels of DKK-1 in
inflammatory primary synovial fibroblasts, the fibroblasts were
initially stimulated with inflammatory IL-1β. Previous studies have
suggested that the expression levels of IL-1β are increased in the
synovial fluid of TMD patients, and that IL-1β is regarded as a key
inflammatory response regulator (3,19,24).
Following treatment with IL-1β, both the mRNA and protein
expression levels of DKK-1 increased, as compared with the control
(Fig. 2A–C). The fluorescence
intensity of DKK-1 in the synovial fibroblasts following treatment
with IL-1β are shown in Fig. 2B.
Images of each group (0. 5, and 10 µg/ml IL-1β) were
captured using the same parameters. In the untreated group,
fluorescence intensity was lower, as compared with that of the
IL-1β-treated group. The expression levels of DKK-1 were
dose-dependently elevated in both the cytoplasm and nucleus of the
synovial fibroblasts following treatment with IL-1β. These results
were concordant with the results obtained from the ELISA. The
present study also detected the expression levels of VEGF following
treatment with IL-1β. IL-1β dose-dependently increased both the
protein and mRNA expression levels of VEGF (Fig. 2A and C). To determine whether DKK-1
increased the expression levels of VEGF, the synovial fibroblasts
were co-treated with IL-1β and anti-DKK-1 antibody. Treatment with
anti-DKK-1 antibody inhibited the stimulatory effects of IL-1β on
VEGF protein expression (Fig. 2D).
Furthermore, the expression levels of VEGF were induced by rhDKK-1.
These results suggest that DKK-1 is upregulated during
inflammation, and that DKK-1 induces VEGF expression in synovial
fibroblast cells.
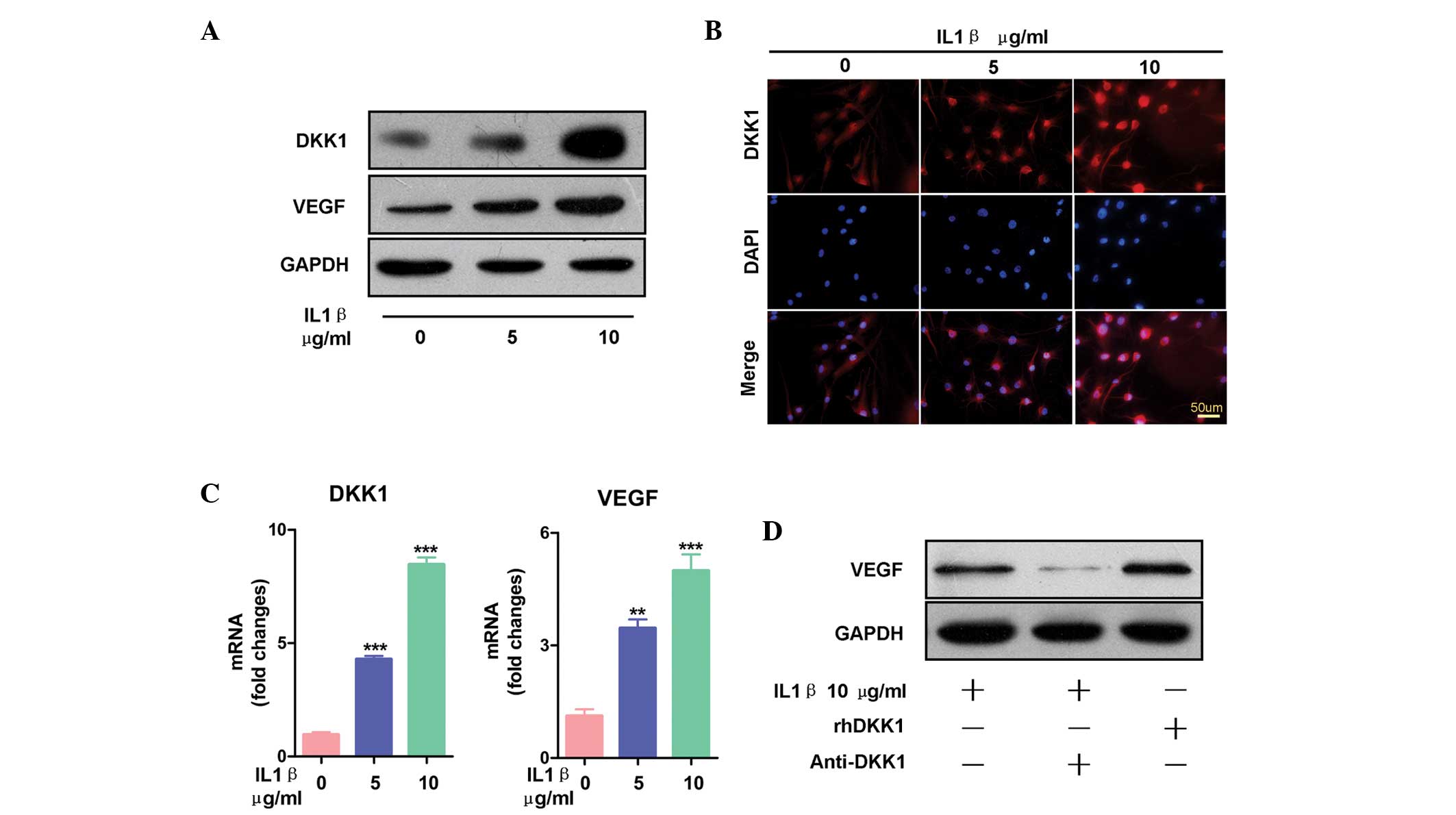 | Figure 2Dickkopf-related protein 1 (DKK-1)
increased the expression levels of inflammation-induced vascular
endothelial growth factor (VEGF) in the synovial fibroblasts. (A)
Treatment with interleukin (IL)-1β for 24 h dose-dependently
increased the protein expression levels of both VEGF and DKK-1, as
determined by western blot analysis. (B) Synovial fibroblasts
treated with IL-1β (0, 5, or 10 µg/ml) for 24 h increased
the expression levels of DKK-1, as demonstrated by
immunofluorescence. The images of each group (0, 5, and 10
µg/ml IL-1β) were captured using the same parameters. Scale
bars, 25 µm. (C) Treatment with IL-1β at the indicated
concentrations (0. 5, and, 10 µg/ml IL-1β) increased the
mRNA expression levels of DKK-1 and VEGF. The data are presented as
the mean ± standard error of the mean. **P<0.01, and
***P<0.001, vs. the vehicle group, as determined by
one-way analysis of variance. (D) Synovial fibroblasts treated with
10 µg/ml recombinant human (rh)DKK-1 for 24 h exhibited
increased expression levels of VEGF, and synovial fibroblasts
treated with 10 µg/ml anti-DKK-1 monoclonal antibody
exhibited decreased expression levels of VEGF, even when combined
with IL-1β treatment. |
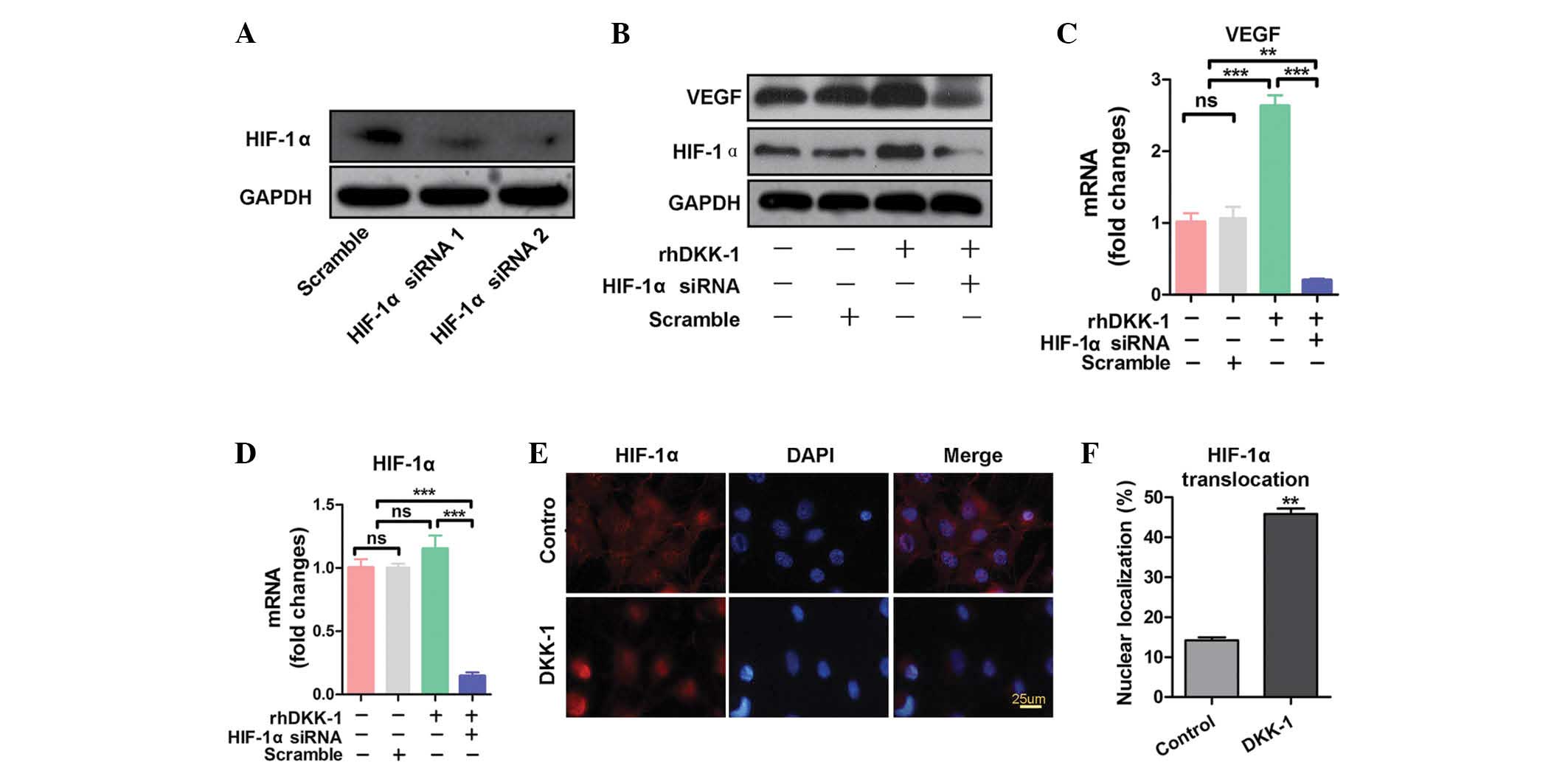
HIF-1α mediates DKK-1 regulation of VEGF
expression
To investigate the mechanisms underlying DKK-1
regulation of VEGF expression, the expression levels of HIF-1α, an
upstream inducer of VEGF, were analyzed. HIF-1α siRNA 2 markedly
inhibited the expression of HIF-1α (Fig. 3A). HIF-1α siRNA 2 was therefore
chosen as the target sequence. As shown in Fig. 3B, the protein expression levels of
HIF-1α were increased in synovial fibroblasts treated with rhDKK-1,
as compared with the negative control. The protein expression
levels of VEGF exhibited similar results. However, the upregulatory
effects of rhDKK-1 on VEGF and HIF-1α protein expression were
inhibited by HIF-1α siRNA (Fig.
3B). The mRNA expression levels of HIF-1α and VEGF were also
analyzed (Fig. 3C and D). The mRNA
expression levels of VEGF were concordant with its protein
expression levels (Fig. 3C).
Notably, no significant differences in the mRNA expression levels
of HIF-1α were observed between the negative control group and the
rhDKK-1 treatment group (Fig. 3D).
These results suggest that DKK-1 may increase HIF-1α protein
translation or decrease HIF-1α degradation, rather than upregulate
the transcription levels of HIF-1α. The nuclear localization of
HIF-1α was also investigated. As shown in Fig. 3E and F, rhDKK-1 significantly
increased HIF-1α nuclear translocation, as compared with the
untreated group. These results indicate that HIF-1α translocates to
the nucleus following DKK-1 exposure, and mediates DKK-1-induced
VEGF expression.
HIF-1α siRNA inhibits DKK-1-induced
elevation of HUVEC migration
The synovial fibroblasts were treated with rhDKK-1
and/or HIF-1α siRNA, following which the CM was collected and, the
cells were cultured in fresh serum-deprived medium for 24 h. To
further assess the biological role of DKK-1 in angiogenesis, the
effects of DKK-1 on HUVECs were investigated using a wound healing
assay. As shown in Fig. 4A,
treatment of the HUVECs with CM from synovial fibroblasts
pretreated with rhDKK-1 increased migration 6-fold, as compared
with the control. Furthermore, similar results were observed in the
transwell and tube formation assays (Fig. 4A–D). In order to reveal the precise
molecular mechanism underlying DKK-1-induced angiogenesis
induction, the present study investigated whether HIF-1α, a key
regulator of angiogenesis, mediated DKK-1-induced endothelial cell
function. As expected, the CM from the synovial fibroblasts
pretreated with HIF-1α siRNA significantly suppressed HUVEC
migration, as compared with rhDKK-1 treatment alone, or scramble
alone. These results were also demonstrated by the wound-healing
assay, transwell assay, and tube formation (Fig. 4A–D).
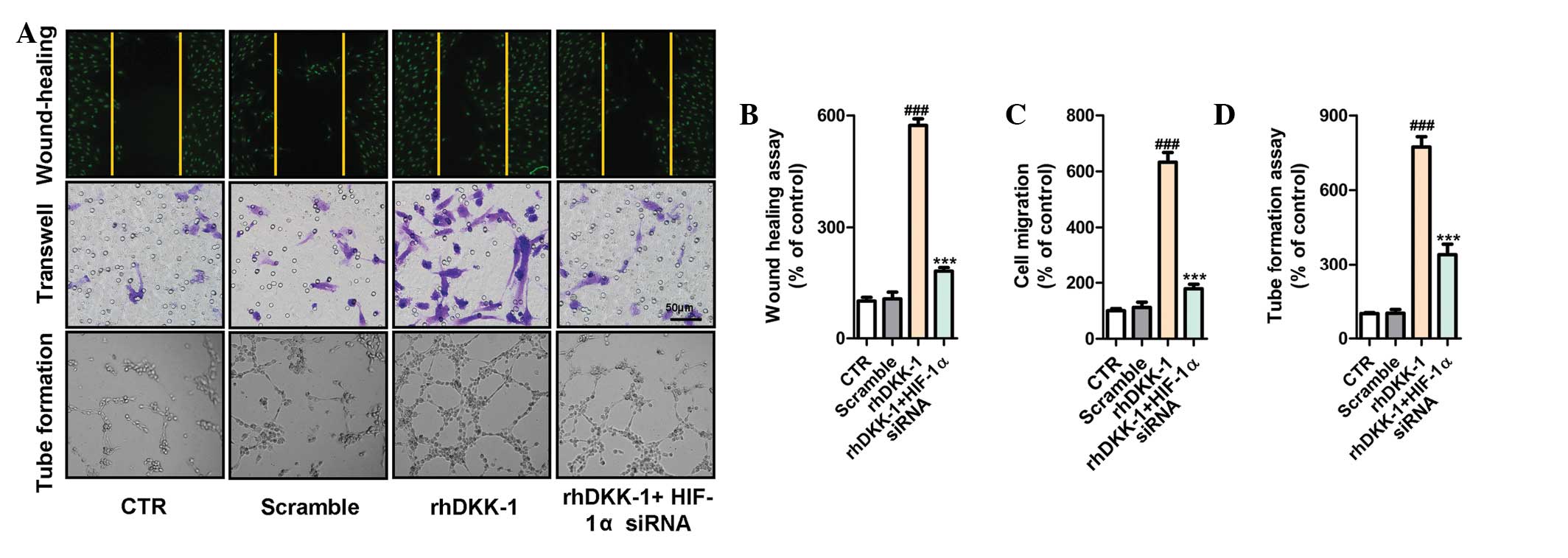 | Figure 4Hypoxia inducible factor-1α (HIF-1α)
small interfering (si)RNA inhibits dickkopf-related protein 1
(DKK-1)-induced increase of human umbilical vein endothelial cells
(HUVEC) migration. To collect the conditioned medium (CM), the
synovial fibroblasts were treated with the indicated agents, prior
to being cultured in fresh serum-deprived medium for 24 h, followed
by collection of the CM. (A) Recombinant human (rh)DKK-1
accelerated the migration of endothelial cells as visualized by the
wound-healing, transwell (8 µm pore size; scale bars, 50
µm), and tube formation assays. Pre-treatment with HIF-1α
siRNA significantly inhibited the upregulatory effects of rhDKK-1.
Quantification of HUVEC migration and tube formation in each group.
The data are presented as the mean ± standard error of the mean.
###P<0.001, vs. the control group (CTR) or scramble
group, and ***P<0.001, vs. the rhDKK-1 treatment
group; one-way analysis of variance. (B) Wound-healing assay, (C)
transwell assay, and (D) tube formation assay. |
Discussion
DKK-1 is a potent secreted Wnt antagonist, which is
a member of the DKK family that includes DKK-1, 2, 3, 4 and the
DKK3-related protein, Soggy (25,26).
DKK family members are implicated in various developmental,
pathological, and physiological processes (16,27).
In prostate cancer, DKK-1 acts as a marker of carcinogenesis, and
may provide a novel method of prostate cancer prediction for
patients with negative primary biopsy results, who may otherwise
progress to bone metastasis (28).
Recent studies have demonstrated that overexpression of DKK-1 may
be associated with increased angiogenesis (14,15).
However, the mechanism underlying the role of DKK-1 in the process
of angiogenesis remains to be elucidated. The results of the
present study demonstrated that DKK-1 promotes angiogenic factor
secretion in synovial fibroblasts from patients with TMD, and
promotes migration of endothelial cells. The present study
provided, to the best of our knowledge, the first evidence that
DKK-1 is highly expressed in the synovial fluid of patients with
TMD, and is positively correlated with VEGF expression. Treatment
of the synovial fibroblasts with rhDKK-1 and anti-DKK-1
demonstrated that VEGF expression was regulated by DKK-1. In
addition HIF-1α nuclear localization was implicated in the
DKK-1/VEGF axis.
A previous study demonstrated that VEGF is involved
in TMD (9). VEGF is a selective
endothelial mitogen and vascular permeability factor, which is
upregulated in patients with chronic closed lock (CCL) of the TMJ,
and the expression levels of VEGF in the synovial fluid of patients
with TMD reflects the clinical status of patients with CCL.
Furthermore, VEGF may be an important molecular target for future
chemotherapy treatment of TMD and CCL (29). Our previous study demonstrated that
expression of VEGF121 and VEGF165 in synovial fibroblasts promoted
inflammatory angiogenesis in TMD (9). These results suggest that VEGF has an
important role in TMD initiation and progression, and further
elucidates the molecular mechanism underlying VEGF upregulation.
The results of the present study indicated that VEGF expression was
induced by rhDKK-1, and downregulated by anti-DKK-1. In numerous
cell types, HIF-1α regulates VEGF expression (30–32).
Notably, the results of our studies have also suggested that VEGF
is associated with the activation of HIF-1α. DKK-1, in the present
study, caused a rapid increase in the protein expression levels of
HIF-1α. The results of the present study also demonstrated that
there was no significant difference in the mRNA expression levels
of HIF-1α between the negative control group and the rhDKK-1
treatment group; these effects are likely due to DKK-1-induced
protection of HIF-1α stability, and promotion of HIF-1α nuclear
translocation in the synovial fibroblasts, rather than an increase
in HIF-1α transcription. Therefore, DKK-1-induced HIF-1α
upregulation in the synovial fibroblasts of TMJ, may be associated
with HIF-1α protein stability.
In the present study, rhDKK-1 was able to induce
endothelial cell migration and chemotaxis, which are important
processes in angiogenesis. Furthermore, pretreatment with HIF-1α
siRNA significantly inhibited rhDKK-1-induced HUVEC migration and
chemotaxis. A previous study reported that DKK-1 was able to
promote angiogenesis of endothelial progenitor cells through C-X-C
chemokine receptor type 4 and VEGF receptor 2 signaling (17). DKK-1 is a regulator of vasculogenic
progenitors that enhance neovascularization in Matrigel plugs
(33). The present study
demonstrated that knockdown of HIF-1α was able to decrease
DKK-1-induced enhancement of angiogenic factor expression. These
results were concordant with the hypothesis that DKK-1 promotes
angiogenesis in TMD. However, the present study carried out these
investigations in synovial fibroblasts alone, and DKK-1 also
promotes the upregulation of VEGF expression via HIF-1α in a
classic angiogenesis pathway. Achieving further understanding of
the molecular mechanism underlying DKK-1 promotion of angiogenesis
in TMD may provide the basis of a treatment strategy that may delay
or halt TMD progression. This mechanism requires further
investigation.
In conclusion, the present study demonstrated that
DKK-1 is highly expressed in the synovial fluid of patients with
TMD, and DKK-1 expression is positively correlated with VEGF
expression. The results of the present study also demonstrated that
upregulation of DKK-1 expression is associated with angiogenic
activities in the synovial fluid of TMD. Furthermore, HIF-1α was
associated with DKK-1-induced HUVEC functional activation. The
present study provides further understanding regarding the
mechanism underlying TMD development.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundations of China to Professor Xing
Long (grant no. 81271171), and Professor Wei Fang (grant no.
81200804). The authors of the present study are grateful to
EssayStar for its linguistic assistance during the preparation of
the manuscript.
References
|
1
|
Aliko A, Ciancaglini R, Alushi A, Tafaj A
and Ruci D: Temporomandibular joint involvement in rheumatoid
arthritis, systemic lupus erythematosus and systemic sclerosis. Int
J Oral Maxillofac Surg. 40:704–709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Boer EW, Dijkstra PU, Stegenga B, de
Bont LG and Spijkervet FK: Value of cone-beam computed tomography
in the process of diagnosis and management of disorders of the
temporomandibular joint. Br J Oral Maxillofac Surg. 52:241–246.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Satoh K, Ogura N, Akutsu M, Kuboyama N,
Kuyama K, Yamamoto H and Kondoh T: Expression of cyclooxygenase-1
and -2 in IL-1beta-induced synovitis of the temporomandibular
joint. J Oral Pathol Med. 38:584–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Varol A, Basa S, Topsakal A and Akpinar I:
Assessment of synovial vascularization by power Doppler
ultrasonography in TMJ internal derangements treated
arthroscopically. Br J Oral Maxillofac Surg. 46:625–630. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Camejo Fde A, Almeida LE, Doetzer AD,
Caporal KS, Ambros V, Azevedo M, Alanis LR, Olandoski M, Noronha L
and Trevilatto PC: FasL expression in articular discs of human
temporomandibular joint and association with osteoarthrosis. J Oral
Pathol Med. 43:69–75. 2014. View Article : Google Scholar
|
|
6
|
Sato J, Segami N, Nishimura M, Yoshimura
H, Demura N, Yoshitake Y and Nishikawa K: Correlation between the
arthroscopic diagnosis of synovitis and microvessel density in
synovial tissues in patients with internal derangement of the
temporomandibular joint. J Craniomaxillofac Surg. 31:101–106. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dejana E and Lampugnani MG: Differential
adhesion drives angiogenesis. Nat Cell Biol. 16:305–306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ke J, Liu Y, Long X, Li J, Fang W, Meng Q
and Zhang Y: Up-regulation of vascular endothelial growth factor in
synovial fibroblasts from human temporomandibular joint by hypoxia.
J Oral Pathol Med. 36:290–296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song J, Cao L and Li Y: RNA
interference-mediated inhibition of survivin and VEGF in pancreatic
cancer cells in vitro. Mol Med Rep. 7:1651–1655. 2013.PubMed/NCBI
|
|
11
|
Gao W, Sweeney C, Connolly M, Kennedy A,
Ng CT, McCormick J, Veale DJ and Fearon U: Notch-1 mediates
hypoxia-induced angiogenesis in rheumatoid arthritis. Arthritis
Rheum. 64:2104–2113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liao HY, Wang GP, Huang SH, Li Y, Cai SW,
Zhang J, Chen HG and Wu WB: HIF-1α silencing suppresses growth of
lung adenocarcinoma A549 cells through induction of apoptosis. Mol
Med Rep. 9:911–915. 2014.PubMed/NCBI
|
|
13
|
She Q, Xia S, Deng SB, Du JL, Li YQ, He L,
Xiao J and Xiang YL: Angiogenesis in a rat model following
myocardial infarction induced by hypoxic regulation of
VEGF165 gene-transfected EPCs. Mol Med Rep. 6:1281–1287.
2012.PubMed/NCBI
|
|
14
|
Park H, Jung HY, Choi HJ, Kim DY, Yoo JY,
Yun CO, Min JK, Kim YM and Kwon YG: Distinct roles of DKK1 and DKK2
in tumor angiogenesis. Angiogenesis. 17:221–234. 2014. View Article : Google Scholar :
|
|
15
|
Weng LH, Ko JY, Wang CJ, Sun YC and Wang
FS: Dkk-1 promotes angiogenic responses and cartilage matrix
proteinase secretion in synovial fibroblasts from osteoarthritic
joints. Arthritis Rheum. 64:3267–3277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Yu C, Dai J, Keller JM, Hua A,
Sottnik JL, Shelley G, Hall CL, Park SI, Yao Z, et al: Parathyroid
hormone-related protein inhibits DKK-1 expression through
c-Jun-mediated inhibition of β-catenin activation of the DKK-1
promoter in prostate cancer. Oncogene. 33:2464–2477. 2014.
View Article : Google Scholar :
|
|
17
|
Smadja DM, d'Audigier C, Weiswald LB,
Badoual C, Dangles-Marie V, Mauge L, Evrard S, Laurendeau I,
Lallemand F, Germain S, et al: The Wnt antagonist Dickkopf-1
increases endothelial progenitor cell angiogenic potential.
Arterioscler Thromb Vasc Biol. 30:2544–2552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dworkin SF and LeResche L: Research
diagnostic criteria for temporomandibular disorders: Review,
criteria, examinations and specifications, critique. J Craniomandib
Disord. 6:301–355. 1992.PubMed/NCBI
|
|
19
|
Kostrzewa-Janicka J, Jurkowski P,
Nedzi-Gora M and Mierzwinska-Nastalska E: Inflammatory markers in
temporoman-dibular joint disorders. Cent Eur J Immunol. 37:290–293.
2012. View Article : Google Scholar
|
|
20
|
Cai HX, Luo JM, Long X, Li XD and Cheng Y:
Free-radical oxidation and superoxide dismutase activity in
synovial fluid of patients with temporomandibular disorders. J
Orofac Pain. 20:53–58. 2006.PubMed/NCBI
|
|
21
|
Li J, Long X, Ke J, Meng QG, Lee WC,
Doocey JM and Zhu F: Regulation of HAS expression in human synovial
lining cells of TMJ by IL-1beta. Arch Oral Biol. 53:60–65. 2008.
View Article : Google Scholar
|
|
22
|
Zhang L, Sun ZJ, Bian Y and Kulkarni AB:
MicroRNA-135b acts as a tumor promoter by targeting the
hypoxia-inducible factor pathway in genetically defined mouse model
of head and neck squamous cell carcinoma. Cancer Lett. 331:230–238.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Y, Zhu L, Liu L, Zhang J and Peng B:
Interleukin-17A stimulates migration of periodontal ligament
fibroblasts via p38 MAPK/NF-κB-dependent MMP-1 expression. J Cell
Physiol. 229:292–299. 2014. View Article : Google Scholar
|
|
24
|
Akutsu M, Ogura N, Ito K, Kawashima M,
Kishida T and Kondoh T: Effects of interleukin-1β and tumor
necrosis factor-α on macrophage inflammatory protein-3α production
in synovial fibroblast-like cells from human temporomandibular
joints. J Oral Pathol Med. 42:491–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi HJ, Park H, Lee HW and Kwon YG: The
Wnt pathway and the roles for its antagonists, DKKS, in
angiogenesis. IUBMB Life. 64:724–731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Min JK, Park H, Choi HJ, Kim Y, Pyun BJ,
Agrawal V, Song BW, Jeon J, Maeng YS, Rho SS, et al: The WNT
antagonist Dickkopf2 promotes angiogenesis in rodent and human
endothelial cells. J Clin Invest. 121:1882–1893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jung IL, Kang HJ, Kim KC and Kim IG:
Knockdown of the Dickkopf 3 gene induces apoptosis in a lung
adenocarcinoma. Int J Mol Med. 26:33–38. 2010.PubMed/NCBI
|
|
28
|
D'Amelio P, Roato I, Oderda M, Soria F,
Zitella A, Ferracini R, Mengozzi G, Gontero P and Isaia GC: DKK-1
in prostate cancer diagnosis and follow up. BMC Clin Pathol.
14:112014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumagai K, Hamada Y, Holmlund AB, Gotoh A,
Nakaoka K, Arai G, Yamane S and Suzuki R: The levels of vascular
endothelial growth factor in the synovial fluid correlated with the
severity of arthroscopically observed synovitis and clinical
outcome after temporomandibular joint irrigation in patients with
chronic closed lock. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 109:185–190. 2010. View Article : Google Scholar
|
|
30
|
Trisciuoglio D, Gabellini C, Desideri M,
Ragazzoni Y, De Luca T, Ziparo E and Del Bufalo D: Involvement of
BH4 domain of bcl-2 in the regulation of HIF-1-mediated VEGF
expression in hypoxic tumor cells. Cell Death Differ. 18:1024–1035.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vadlapatla RK, Vadlapudi AD, Pal D,
Mukherji M and Mitra AK: Ritonavir inhibits HIF-1α-mediated VEGF
expression in retinal pigment epithelial cells in vitro. Eye
(Lond). 28:93–101. 2014. View Article : Google Scholar
|
|
32
|
Huang L, Zhang Z, Zhang S, Ren J, Zhang R,
Zeng H, Li Q and Wu G: Inhibitory action of Celastrol on
hypoxia-mediated angiogenesis and metastasis via the HIF-1α
pathway. Int J Mol Med. 27:407–415. 2011.PubMed/NCBI
|
|
33
|
Aicher A, Kollet O, Heeschen C, Liebner S,
Urbich C, Ihling C, Orlandi A, Lapidot T, Zeiher AM and Dimmeler S:
The Wnt antagonist Dickkopf-1 mobilizes vasculogenic progenitor
cells via activation of the bone marrow endosteal stem cell niche.
Circ Res. 103:796–803. 2008. View Article : Google Scholar : PubMed/NCBI
|