Introduction
Human teeth are highly specialized organs of the
human body. Two series of teeth grow in humans comprised of 20
temporary teeth and 32 permanent teeth; however, the second series
of teeth hardly show any further growth following their maturation.
If the teeth are broken through physical impact, bacterial
degradation or aging factors in adults, they are not able to
regenerate and are currently replaced with artificial prosthetics
or implants (1). However,
regenerative medicinal studies are currently investigating the
feasibility of tooth regeneration using stem cells, and therefore,
it is important to study development mechanisms of human teeth for
further tooth regeneration (2).
Pluripotent stem cells have been isolated from
embryos or various tissues, and they have displayed tremendous
potential in regenerative medicine due to their characteristics of
self-renewal and multi-differentiation under certain conditions. In
2000, Gronthos et al (3)
isolated cells from the dental pulp of odontogenic teeth and proved
the characteristics of self-renewal and potency for multilineage
differentiation of these cells, and finally named these cells as
dental pulp stem cells (DPSCs) (3). The discovery of DPSCs provided the
tool to study mechanisms of dental development.
In the past ten yeas, studies on DPSCs have led to
the development of isolation and culturing techniques. Miura et
al (4) successfully isolated
stem cells from human exfoliated deciduous teeth (SHED) and
identified that they had the general characteristics of DPSCs. Wang
et al (5) also isolated
SHED and found that their capability of mineralization and mRNA
expression levels of inflammatory cytokines were higher compared
with those of DPSCs (5).
Furthermore, Kerkis and Caplan (6)
described that the derivation of induced pluripotent stem (iPS)
cells from DPSCs was significantly more efficient compared with
that of human fibroblasts. In addition, stem cells from the third
molar were shown to have a lower population doubling time, higher
colonogenic activity and an improved growth curve compared with
those from the deciduous incisor, indicating that stem cells from
the human third molar are appropriate candidates for use in
experimental, pre-clinical and even clinical setups (7). However, a study by Govindasamy et
al (8) indicated that the
proliferation rate of the stem cells derived from human deciduous
teeth was higher than that from human permanent teeth. Furthermore,
stem cells from deciduous and permanent teeth display differences
in their differentiation ability, particular into neuronal
lineages, as well as differential expression of extracellular
matrix proteins that are involved in the odontoblast lineage
differentiation and in dentin mineralization (9,10).
Although these differences in proliferation and gene
expression between stem cells from deciduous and permanent teeth
have been identified, the reasons accounted to these differences
have remained elusive. Studies on adult stem cells have provided
conflicting information, with certain studies reporting no change
(11–14) while others identified an
age-associated decline of the above-mentioned parameters (15). It is not known whether aging of
DPSCs affects their differentiation ability, but the donor age for
deciduous stem cells is always less than that for permanent stem
cells; however, there is still no detailed study on the effects of
donor age on the DPSCs.
In addition, expression of cell surface maker genes
varied in adult stem cells from different tissues, except for a
number of pluripotent stem cell marker genes. Abramova et al
(16) proved that gene expression
was dependent on the embryonal development stage in acutely
isolated central nervous system progenitor cells from mice. In
long-term cultured mesenchymal stem cells (MSCs), Halfon et
al (17) found that cell
surface marker genes were downregulated with increasing passage and
indicated its close association with the quality of MSCs. However,
although certain cell surface marker genes were shown to be
differentially expressed under different conditions, the effect of
the donor age on marker gene expression on DPSCs has remained
elusive.
The present study aimed to investigate the
derivation of DPSCs from human teeth of donors of different age and
compared cell surface marker gene expression among them. The teeth
were assessed regarding the presence of stem cells, and the latter
were examined for their potential to proliferate, differentiate and
to be used in regenerative dental medicine.
Materials and methods
Isolation and culture of DPSCs
The present study was approved by the ethics
committees of Tianjin University and Tianjin General Hospital
(Tianjin, China). Human dental pulp tissues were obtained from
clinically healthy extracted deciduous and permanent teeth (due to
trauma), including six deciduous teeth from children aged 4–8
years, four permanent teeth from adolescents aged 12–20 years, five
permanent teeth from adults aged 30–50 years and six permanent
teeth from aged individuals aged 55–67 years. The DPSCs were
isolated and cultured as previously described (18). The teeth were fractured with a
dental surgical elevator (model:1141-1; Rongwei Ltd. Corp.,
Shanghai, China) and pulp tissue was gently separated with a
sterile dentinal excavator (Gladent; Henki Medical Instrument
Factory, Foshan, China) from the crown and root. The dental pulp
was gently rinsed in phosphate-buffered saline (PBS; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), dissected into
1-mm2 tissue sections and implanted into culture dishes
(BD Biosciences, New York, NY, USA) in 3 ml culture medium. After
three to four days, soma fibroblast-like cells grew around the mass
and were digested with 0.5 g/l trypsin and 0.53 mmol/l EDTA (both
Thermo Fisher Scientific, Inc.) for 3–5 min at 37°C. The
fibroblast-like cells were further incubated for propagation and
later passaged at ~5×104 cells/cm2. Cells
were incubated at 37°C under 5% CO2 and 80% humidity.
Penicillin and streptomycin (Thermo Fisher Scientific, Inc.) were
used in all solutions to minimize bacterial contamination.
Cell proliferation analysis
The cell number was quantified using a Scepter
Handheld automated cell counter with 60-µm Scepter Sensors
(both Merck-Millipore, Billerica, MA, USA), which employs the
Colter principle of impedance-based particle detection. The
concentration of viable cells was identified based on cell
volume/size measurements using Scepter software Pro 2.0
(Merck-Millipore).
Fluorescence-activated cell sorting
(FACS) analysis
Cell surface marker expression was analyzed by FACS.
Briefly the cells of the different cell lines were digested using
0.25% trypsin and then rinsed with PBS. The harvested cells were
stained with the monoclonal antibodies for overnight and analyzed
by FACSAria II (BD Biosciences, San Jose, CA, USA). The following
antibodies were used: CD14-fluorescein isothiocyanate (FITC),
CD29-phycoerythrin (PE), CD31-PE, CD34-PE, CD44-PE, CD73-PE,
CD90-allophycocyanine (APC), CD166-PE, CD45-PerCP-Cy5,
HLA-DR-PerCP-Cy5, CD105-PE and CD133-APC.
DPSC differentiation in vitro
The DPSCs were stimulated to differentiate in
vitro into neuronal cells and osteoblasts according to previous
methods (19,20). For neuronal differentiation, the
DPSCs were cultured in a specific induction medium [Dulbecco's
modified Eagle's medium-high glucose, 10% fetal bovine serum (FBS),
5 ml/l B27 supplemented with 10 ng/ml epidermal growth factor and
20 ng/ml basic fibroblast growth factor; Thermo Fisher Scientific,
Inc.] for the first 15 days, and then the cells were transferred
into the second differentiation medium supplemented with neuronal
growth factors [10 ng/ml neurotrophin-3, 10 ng/ml nerve growth
factor, 25 ng/ml brain-derived neurotrophic factor (BDNF), 10 mM
butylated hydroxyanisole, 25 mM 3-isobutyl-1-methylxanthine (IBMX),
0,5 mM all-trans retinoic acid and 10 nM progesterone; Thermo
Fisher Scientific, Inc.] for another 15 days, and finally, the
differentiated cells were incubated in the third differentiation
medium supplemented with 50 ng/ml BDNF; 5 mg/ml insulin; 200 mM
indomethacin and 0.5 mM IBMX (all Thermo Fisher Scientific, Inc.)
for one day. The differentiated neuronal cells were identified by
immunofluorescence.
For osteogenic differentiation, the DPSCs were
cultured in differentiation medium consisting of α-minimum
essential medium supplement with 10% FBS, 10 nM dexamethasone, 10
mM b-glycerophosphate (Thermo Fisher Scientific, Inc.) and 100 mM
Sigma A-7506 L-ascorbate-2-phosphate (Sigma-Aldrich, St. Louis, MO,
USA). After 20 days, mineralized deposits were observed in the
culture medium and identified by reverse transcription polymerase
chain reaction (RT-PCR).
Immunofluorescence analysis of cells
The DPSCs and the differentiated cells were
collected, fixed with 4% paraformaldehyde and then permeabilized
with 0.25% Triton X-100 (all Thermo Fisher Scientific, Inc.). At
passage 10, the cells were blocked and stained with the primary
antibody [anti-micro-tubule-associated protein 2 (MAP2) or
anti-neuron-specific class III beta-tubulin (TUJ1)] overnight at
4°C followed by the appropriate secondary antibodies detecting
mouse and rabbit immunoglobulin G, respectively. The sample was
then rinsed three times using PBS and dried at room temperature. A
cover slip was placed on the sample and the slide was observed
under a confocal microscope (LSM 510; Zeiss, Oberkochen, Germany)
and images were captured.
Karyotype analysis
Karyotype analysis (G-banding) was performed on at
least 20 cells from each sample. The cells were harvested and
centrifuged (speed, 300 × g for 5 mins at room temperature)
following colchicine treatment, and the cells were then re-suspened
in 70 mM KCl (Sigma-Aldrich) for 20 min at 37°C, and then
centrifuged again (as above). The cells were fixed with
methanol/acetic acid (3:1) for 5 min at room temperature. The cells
were centrifuged again (as above) and the cell pellet was incubated
overnight at 4°C. The cells were finally placed on a glass slide
and stained with Giemsa solution (Sigma-Aldrich).
RT-PCR
Cells were harvested using trypsin. After washing
with PBS, mRNA was isolated by using an RNeasy Miniprep kit
(Qiagen, Hilden, Germany). Reverse transcription was performed
using an Invitrogen SuperScript First-strand Synthesis System kit
(Invitrogen Life Technologies, Carlsbad, CA, USA). The following
primer pairs were used to detect the gene-specific mRNAs: Forward,
5′-GAGCAAAACCCGGAGGAGT-3′ and reverse, 5′-TTCTCTTTCGGGCCTGCAC-3′
for human Oct4. A Mastercycler nexus (Eppendorf Ltd., Hamburg,
Germany), was used and PCR was performed for 35 cycles.
Quantification of PCR products was performed using a JY-SCZ2
electrophoresis chamber (Junyidongfang Ltd., Beijing, China) with
A-6013 agarose (Sigma-Aldrich).
Measurement of apoptotic cells
Call apoptosis was measured at passage 10. The cells
were harvested and suspended in Annexin V-binding buffer (BioVision
Inc., Milpitas, CA, USA). Annexin V-FITC (BioVision) was added to
the cell suspensions, which were incubated at room temperature for
5 min in the dark. The suspensions were analyzed using a FACSAria
cell sorter.
Statistical analysis
Cell growth/proliferation and apoptosis assay was
repeated three times. Values are expressed as the mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance with Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference
between values. Data were analyzed using SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA).
Results
A total of six DPSC lines were derived from
deciduous teeth of six children, four DPSC lines were derived from
permanent teeth of four adolescents, three DPSC lines were derived
from five permanent teeth from adults and two DPSC lines were
derived from six permanent teeth from aged donors. The derivation
efficiency was decreased with increasing donor age (Table I).
 | Table IInformation on established human
dental pulp stem cell lines. |
Table I
Information on established human
dental pulp stem cell lines.
| Donor age
(years) | DPSCs | Derivation efficiency
(%) |
|---|
| Children (n=6) |
| 4 | Y | 100.0 |
| 6 | Y | |
| 5 | Y | |
| 5 | Y | |
| 8 | Y | |
| 4 | Y | |
| Adolescents
(n=4) |
| 15 | Y | 100.0 |
| 12 | Y | |
| 16 | Y | |
| 20 | Y | |
| Adults (n=5) |
| 33 | Y | 60.0 |
| 38 | N | |
| 45 | N | |
| 32 | Y | |
| 49 | Y | |
| Aged (n=6) |
| 62 | N | 33.3 |
| 63 | Y | |
| 55 | Y | |
| 62 | N | |
| 67 | N | |
| 59 | N | |
| Total (n=21) | 15 | 71.4 |
The derived DPSCs were identified by specific marker
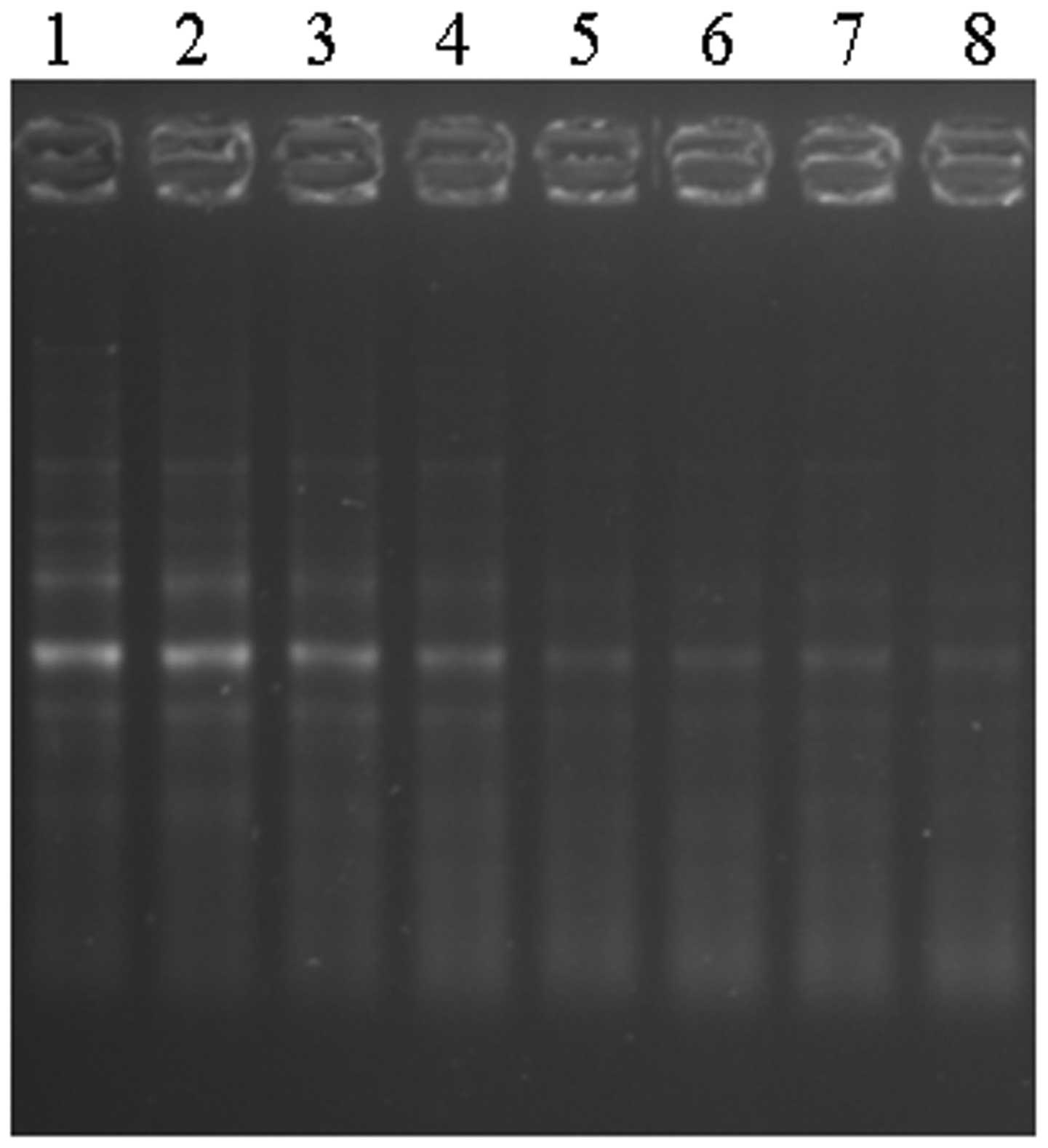
gene expression. The pluripotent transcript factor Oct4 was
positively expressed in all of the DPSC lines from donors of
different age (Fig. 1). The
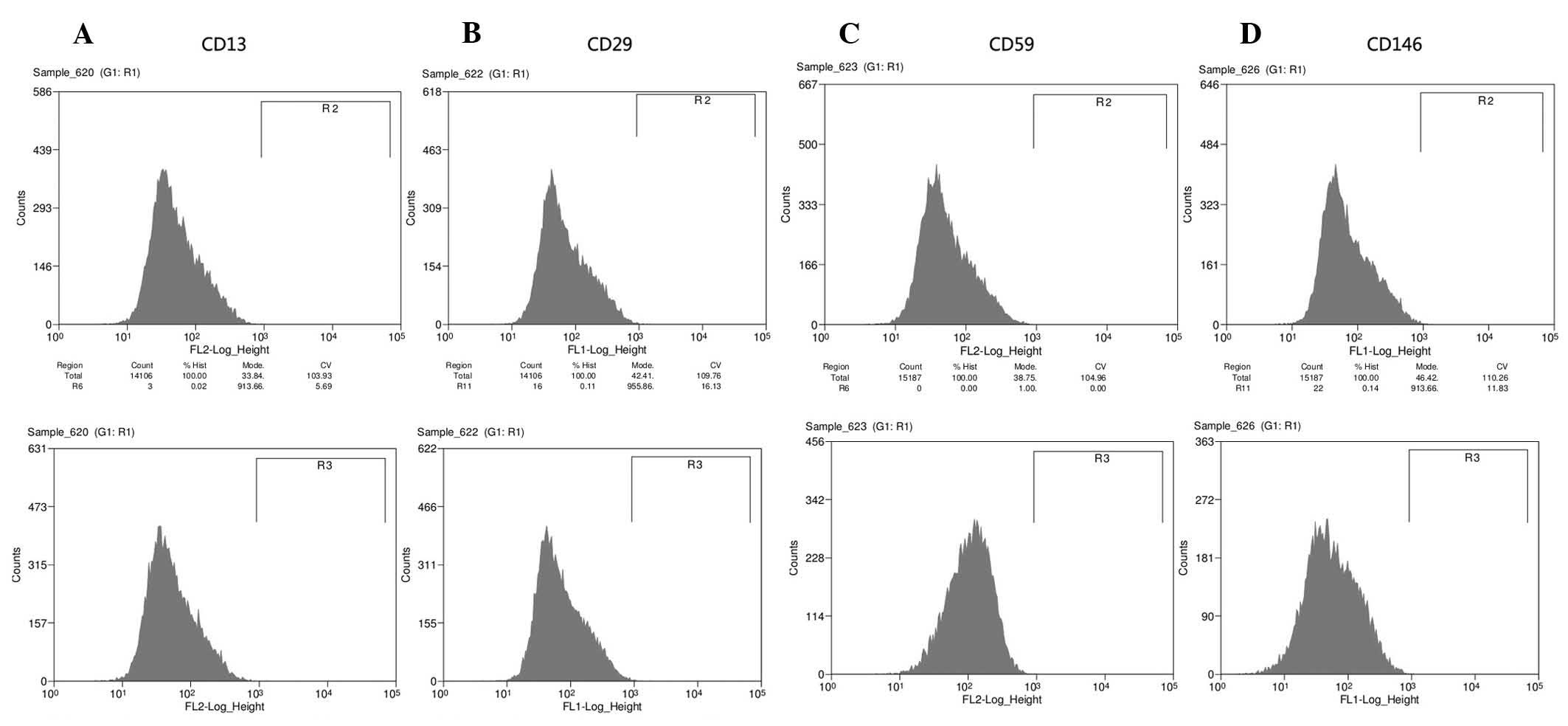
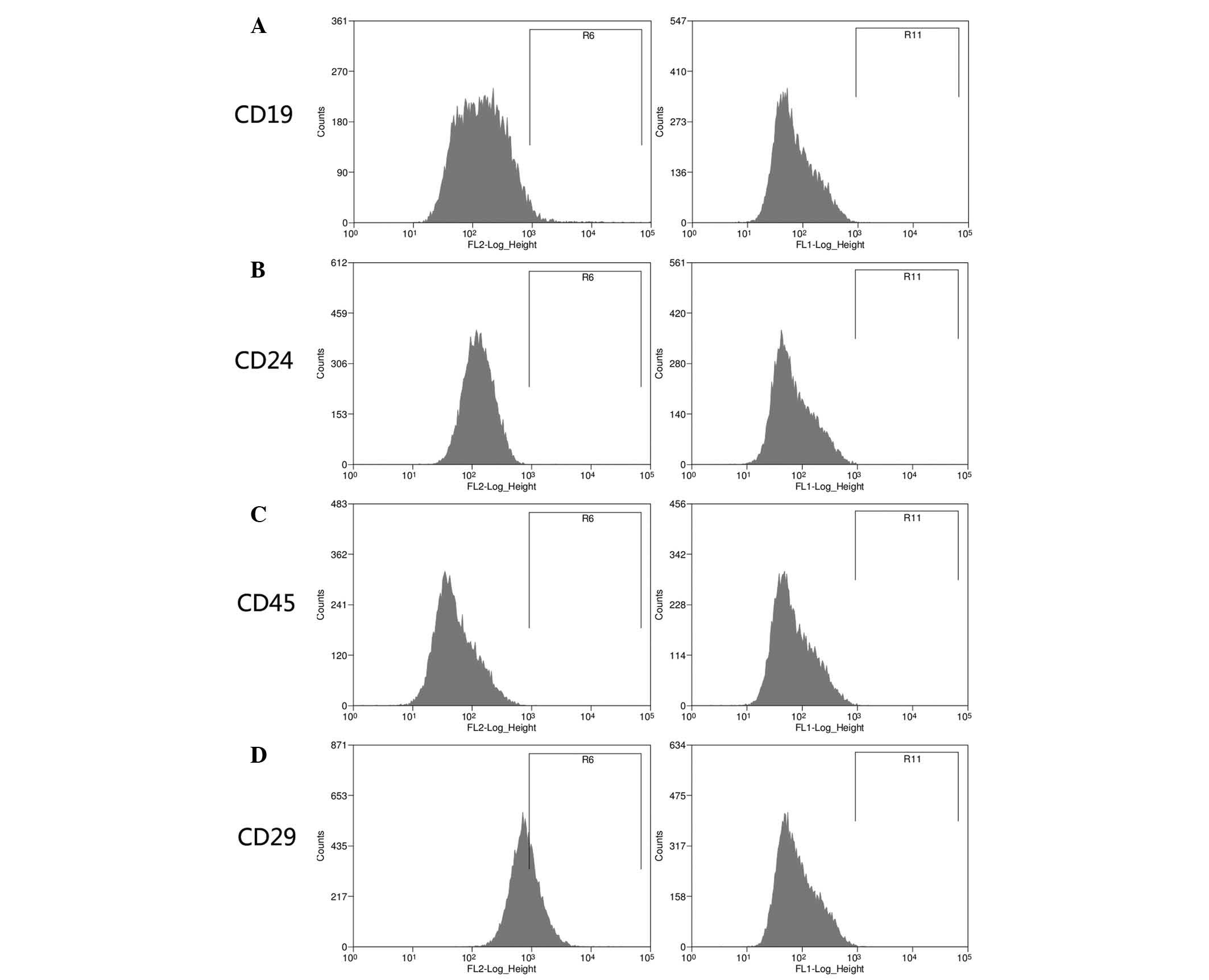
results of FACS analysis indicated that the specific cell surface
markers (CD13, CD29, CD59 and CD146; Fig. 2A–D, respectively) were positively
expressed and negative makers (CD19, CD24 and CD45; Fig. 3A–C, respectively) could not be
observed in the DPSC lines. However, the expression intensity of
CD29 was decreased with the propagation until passage ten in the
DPSCs from permanent teeth of aged donors (Fig. 3D).
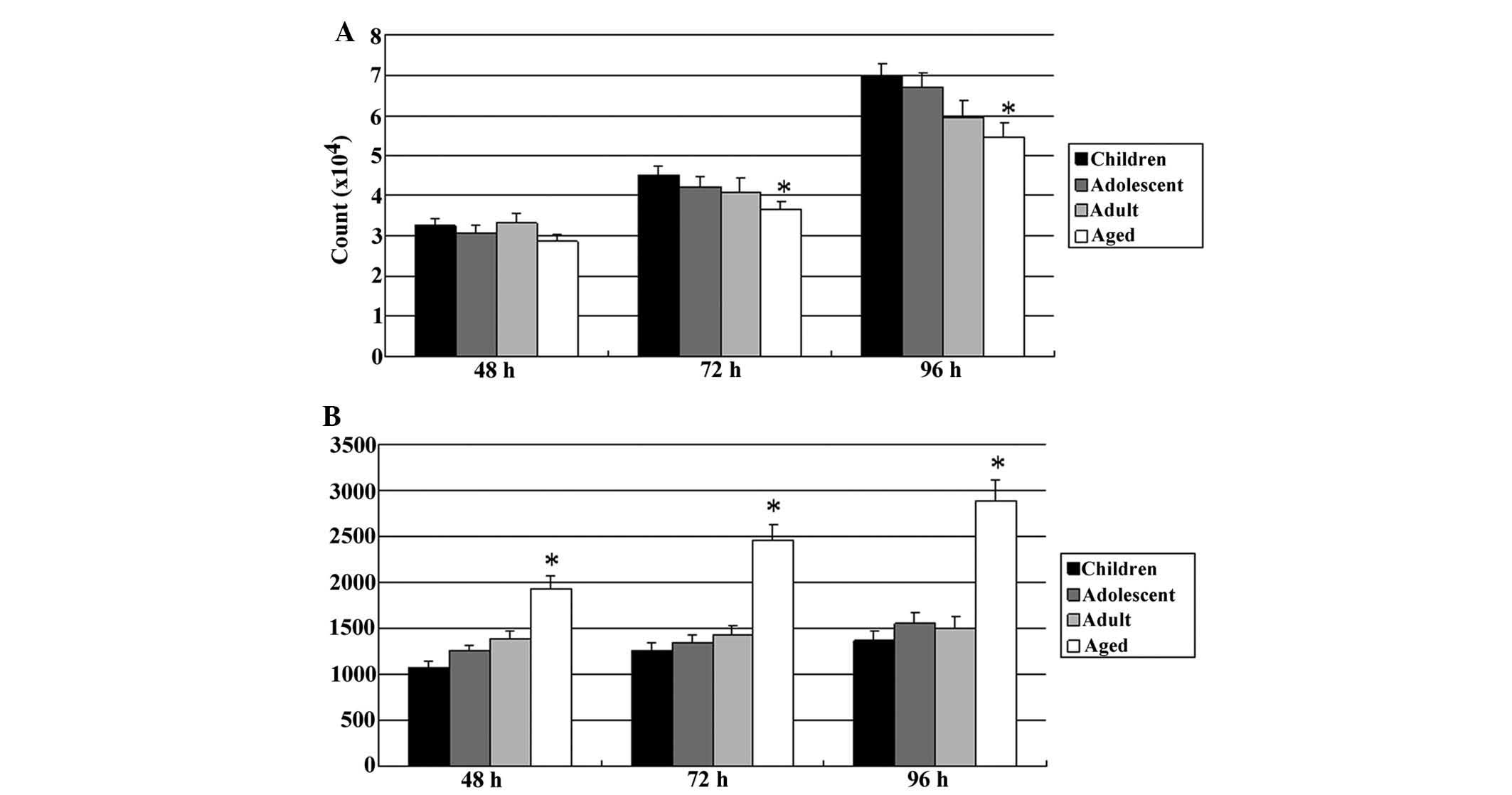
The proliferation of DPSCs in each group was
evaluated at 48, 72, and 96 h. There were no differences among the
DPSCs in the four age groups at 48 h, but at 72 h, the
proliferation rate of DPSCs from aged donors was significantly
lower than that in the other three groups. Furthermore, at 96 h,
the proliferation rate of DPSCs from aged donors was significantly
decreased, and the proliferation rate of DPSCs from adult donors
was also significant lower than that of the DPSCs from deciduous
teeth of children and permanent teeth from adolescents (Fig. 4A). Furthermore, as shown in
Fig. 4B, the population of annexin
V-positive (apoptotic) cells was increased in the DPSCs from aged
donors at 48 h, and the apoptotic cells were continuously increased
later at 72 and 96 h, whereas the population of annexin V-positive
cells did not increase in the other groups.
All of the DPSC lines from deciduous teeth of
children and permanent teeth of adolescents were able to
differentiate into neuronal and osteogenic lineages under the
specific differentiation conditions. However, one DPSC line from
adult permanent teeth was not able to differentiate into an
osteogenic lineage, but into neurons, while two other DPSC lines
from adult permanent teeth were able to undergo neuronal and
osteogenic differentiation. Furthermore, one DPSC line from the
tooth of an aged donor differentiated into an osteogenic lineage
but did not undergo neuronal differentiation, while the other one
could not be induced to differentiate at all (Table II). Differentiation was confirmed
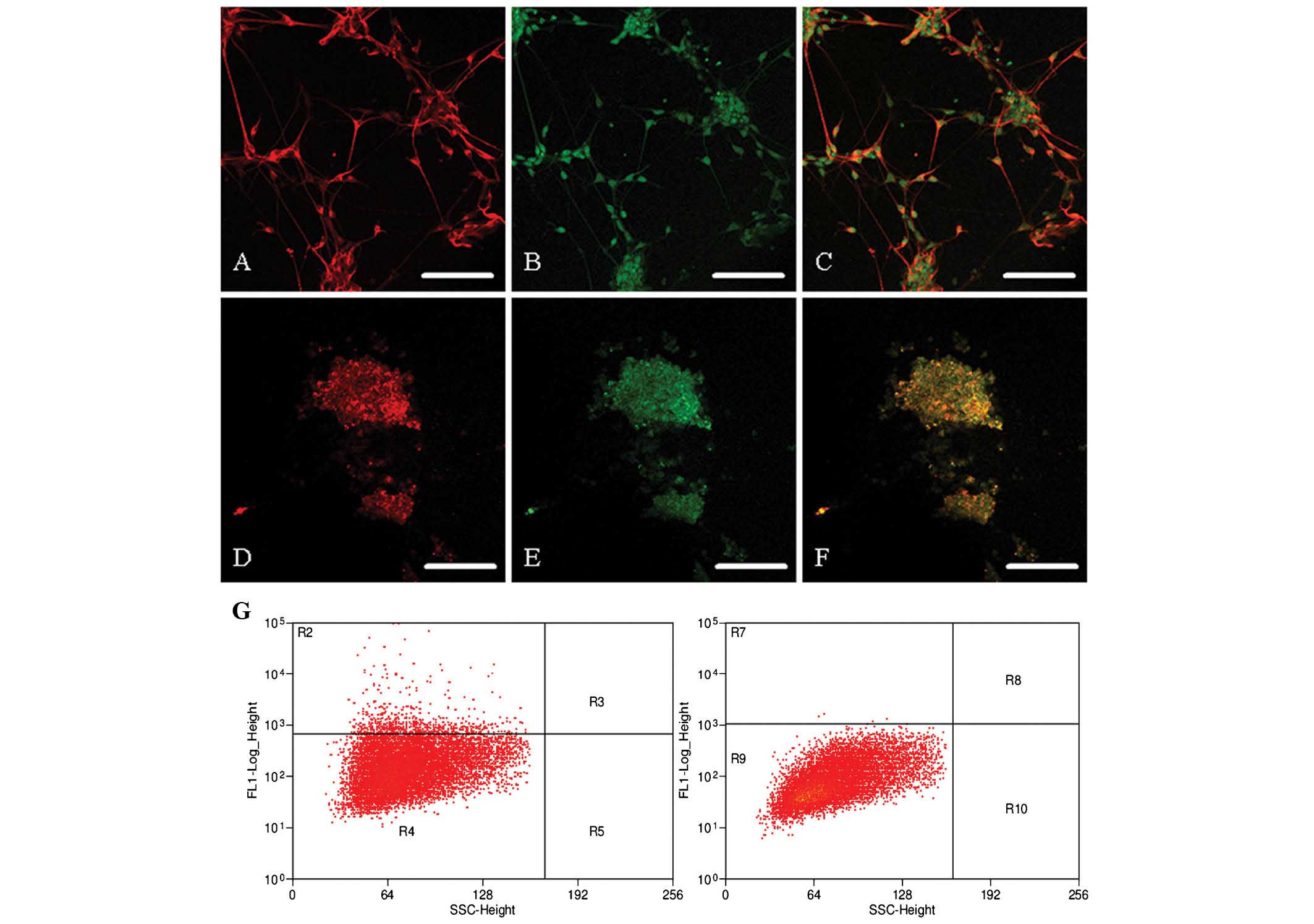
using the neuronal markers MAP2 and TUJ-1 (Fig. 5A–F) and the bone marker CD45
(Fig. 5G).
 | Table IIDifferentiation ability of human
DPSCs. |
Table II
Differentiation ability of human
DPSCs.
| DPSC lines | Neuronal
lineage | Osteogenic
lineage |
|---|
| Child-1 | Y | Y |
| Child-2 | Y | Y |
| Child-3 | Y | Y |
| Child-4 | Y | Y |
| Child-5 | Y | Y |
| Child-6 | Y | Y |
| Adolescent-1 | Y | Y |
| Adolescent-2 | Y | Y |
| Adolescent-3 | Y | Y |
| Adolescent-4 | Y | Y |
| Adult-1 | Y | Y |
| Adult-2 | Y | N |
| Adult-3 | Y | Y |
| Elderly-1 | N | Y |
| Elderly-2 | N | N |
Discussion
In the present study, DPSCs were successfully
isolated from deciduous or permanent teeth from donors of different
ages, and these cells were positive for DPSCs-specific markers
alongside typical morphologies (adherent cells resembling
fibroblasts) (21). However, the
expression of certain marker genes was decreased and the
differentiation to neuronal and osteogenic lineages was also
impaired in the DPSCs from permanent teeth of aged donors compared
with those in the other three groups.
DPSCs have been derived from deciduous teeth of
children and permanent teeth of adults; however, there are few
studies on DPSCs derived from permanent teeth of aged individuals
(22). In the permanent teeth of
aged individuals, the cell number in the dental pulp is reduced,
and the fiber as well as the collagen composition is gradually
increased (23). Furthermore,
calcification often occurs in parts of the pulp chamber and root
canal, which results in a reduction in size of the pulp chamber
caused by the continual secretion of dentin matrix by odontoblasts
(24). Mitsiadis et al
(25) showed that the dental pulp
volume decreases gradually upon aging due to the continuous
production of dentin matrix by odontoblasts and that this
age-associated pulp chamber reduction is due to the elimination of
a certain number of odontoblasts by apoptosis. These age-associated
physiological changes explain, at least in part, the lower
efficiency of DPSC line derivation from permanent teeth of aged
donors.
The DPSC proliferation rate was analyzed and the
results indicated that high donor age impaired the proliferation
rate. The possible explanation for this was the increasing levels
of apoptosis in DPSCs from teeth of aged donors. In the present
study, a significantly higher apoptotic rate was observed in aged
DPSCs using annexin V staining. Apoptosis is expected to decrease
the mitotic cell number, resulting in a decreased proliferation
rate; furthermore, age-associated DNA replication errors and
reduced genomic stability are observed in aged cells, leading to
induction of apoptosis and a reduced proliferation rate. The
activation of apoptotic signaling pathways in aged dental pulp has
been proved by Tranasi et al (26) using a micro-array method.
Furthermore, DPSC-specific cell surface marker gene
expression was identified in the present study. The transcription
factor Oct4, which is specifically expressed in pluripotent stem
cells, was positively expressed in all DPSC lines, and a number of
DPSC-specific cell surface markers were also positively or
negatively expressed as expected in the DPSCs, which proved the
DPSCs originated from dental pulp and not from MSCs (27–29).
A study observed changes in MSC-specific marker expression and
epigenetic modification following long-term culture in vitro
(30). In the present study, CD29
expression was decreased at the tenth passage in the DPSCs from
permanent teeth of aged donors, but not differently expressed in
DPSCs derived from teeth of the other age groups, suggesting that
the donor age contributed to the differential gene expression
profiling for specific markers. Similar results were obtained for
human bone marrow mesenchymal stem cells by Zaim et al
(31). A study by Pruszak et
al (32) indicated that CD29
together with CD15 and CD24 was able to regulate neuronal
differentiation and proved that low expression of CD29 reduced the
population of proliferating cell types in human embryonic stem cell
differentiation. The distinct function of CD29 was associated with
β1-integrin signaling and regulatory cell adhesion by interacting
with the extracellular matrix (ECM), whereas integrin signaling has
been shown to be of functional relevance for neural crest (33) as well as mesenchymal development
(34,35). Tranasi et al (26) identified several differentially
expressed genes that were categorized as growth factors,
transcription regulators, apoptosis regulators and genes of the ECM
using a high-throughput microarray method, and indicated that in
dental pulp from young individuals, biological functions of cells,
including tissue differentiation and development as well as
proliferation and of immune, lymphatic and hematologic cells were
exerted at high rates, whereas apoptotic pathways were active in
dental pulp from aged donors (26).
CD29 is regarded to be one of the most important
cell surface markers in human neuron stem cells (36). In the present study, neither of the
two DPSC lines derived from permanent teeth of aged donors were
able to differentiate into neuron lineages, which indicated that
the neuronal differentiation ability was impaired by the lower
expression of CD29. Aanismaa et al (37) demonstrated that DPSC-derived neural
progenitor stage cells could be produced, which, however, did not
mature further into functional neuronal cells. In the present
study, the differentiated cells from DPSCs were positive for
specific markers for neurons and gliocytes. In rats, DPSCs were
able to differentiate into nestin-positive progenitors,
TUJ-1-positive neuronal cells and S100-positive glial cells
(38). Furthermore, human
postnatal dental pulp cells were demonstrated to be able to
differentiate into class III beta tubulin and TUJ-1 positive
neuronal cells (39). In addition
to neuronal cells, DPSCs were indicated to differentiate into
osteoblasts, adipose cells and smooth muscle cells (40–42).
In the present study, osteoblast markers were identified in all
DPSCs, and only one cell line derived from a permanent tooth of an
aged donor failed to form osteoblasts.
In conclusion, DPSC lines were derived from teeth of
donors of different age, and the results demonstrated that the
derivation efficiency, proliferation rate, differentiation into
neuronal lineages and the cell surface marker expression were all
impaired in the DPSCs derived from permanent teeth of aged donors.
Therefore, DPSCs should be established and stored as early as
possible for treatment of dental diseases in aged patients.
References
|
1
|
Aldrigui JM, Jabbar NS, Bonecker M and
Braga MM: a systematic review and meta-analysis. Community Dent
Oral Epidemiol. 42:30–42. 2014. View Article : Google Scholar
|
|
2
|
Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang
J, Xu GT and Liang A: Characteristics and potential applications of
human dental tissue-derived mesenchymal stem cells. Stem Cells.
33:627–638. 2015. View Article : Google Scholar
|
|
3
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miura M, Gronthos S, Zhao M, Lu B, Fisher
LW, Robey PG and Shi S: SHED: stem cells from human exfoliated
deciduous teeth. Proc Natl Acad Sci USA. 100:5807–5812. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Sha XJ, Li GH, Yang FS, Ji K, Wen
LY, Liu SY, Chen L, Ding Y and Xuan K: Comparative characterization
of stem cells from human exfoliated deciduous teeth and dental pulp
stem cells. Arch Oral Biol. 57:1231–1240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kerkis I and Caplan AI: Stem cells in
dental pulp of deciduous teeth. Tissue Eng Part B Rev. 18:129–138.
2012. View Article : Google Scholar :
|
|
7
|
Eslaminejad MB, Vahabi S, Shariati M and
Nazarian H: In vitro growth and characterization of stem cells from
human dental pulp of deciduous versus permanent teeth. J Dent
(Tehran). 7:185–195. 2010.
|
|
8
|
Govindasamy V, Abdullah AN, Ronald VS,
Musa S, Ab Aziz ZA, Zain RB, Totey S, Bhonde RR and Abu Kasim NH:
Inherent differential propensity of dental pulp stem cells derived
from human deciduous and permanent teeth. J Endod. 36:1504–1515.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harumi Miyagi SP, Kerkis I, da Costa
Maranduba CM, Gomes CM, Martins MD and Marques MM: Expression of
extracellular matrix proteins in human dental pulp stem cells
depends on the donor tooth conditions. J Endod. 36:826–831. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Butler WT: Dentin matrix proteins. Eur J
Oral Sci. 106:204–210. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alt EU, Senst C, Murthy SN, Slakey DP,
Dupin CL, Chaffin AE, Kadowitz PJ and Izadpanah R: Aging alters
tissue resident mesenchymal stem cell properties. Stem Cell Res.
8:215–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asumda FZ and Chase PB: Age-related
changes in rat bone-marrow mesenchymal stem cell plasticity. BMC
Cell Biol. 12:442011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scharstuhl A, Schewe B, Benz K, Gaissmaier
C, Bühring HJ and Stoop R: Chondrogenic potential of human adult
mesenchymal stem cells is independent of age or osteoarthritis
etiology. Stem Cells. 25:3244–3251. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tokalov SV, Gruener S, Schindler S,
Iagunov AS, Baumann M and Abolmaali ND: A number of bone marrow
mesenchymal stem cells but neither phenotype nor differentiation
capacities changes with age of rats. Mol Cells. 24:255–260.
2007.PubMed/NCBI
|
|
15
|
Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong
TH, Zhou G, Baggett LS, Mikos AG and Cao Y: Donor age and cell
passage affects differentiation potential of murine bone
marrow-derived stem cells. BMC Cell Biol. 9:602008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abramova N, Charniga C, Goderie SK and
Temple S: Stage-specific changes in gene expression in acutely
isolated mouse CNS progenitor cells. Dev Biol. 283:269–281. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Halfon S, Abramov N, Grinblat B and Ginis
I: Markers distinguishing mesenchymal stem cells from fibroblasts
are downregulated with passaging. Stem Cells Dev. 20:53–66. 2011.
View Article : Google Scholar
|
|
18
|
Jo YY, Lee HJ, Kook SY, Choung HW, Park
JY, Chung JH, Choung YH, Kim ES, Yang HC and Choung PH: Isolation
and characterization of postnatal stem cells from human dental
tissues. Tissue Eng. 13:767–773. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao S, Pan F, Prpic V and Wise GE:
Differentiation of stem cells in the dental follicle. J Dent Res.
87:767–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Walboomers XF, Shi S, Fan M and
Jansen JA: Multilineage differentiation potential of stem cells
derived from human dental pulp after cryopreservation. Tissue Eng.
12:2813–2823. 2006. View Article : Google Scholar
|
|
21
|
Ellis KM, O'Carroll DC, Lewis MD, Rychkov
GY and Koblar SA: Neurogenic potential of dental pulp stem cells
isolated from murine incisors. Stem Cell Res Ther. 5:302014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Sha XJ, Li GH, Yang FS, Ji K, Wen
LY, Liu SY, Chen L, Ding Y and Xuan K: Comparative characterization
of stem cells from human exfoliated deciduous teeth and dental pulp
stem cells. Arch Oral Biol. 57:1231–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morse DR: Age-related changes of the
dental pulp complex and their relationship to systemic aging. Oral
Surg Oral Med Oral Pathol. 72:721–745. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morse DR, Esposito JV and Schoor RS: A
radiographic study of aging changes of the dental pulp and dentin
in normal teeth. Quintessence Int. 24:329–333. 1993.PubMed/NCBI
|
|
25
|
Mitsiadis TA, De Bari C and About I:
Apoptosis in developmental and repair-related human tooth
remodeling: a view from the inside. Exp Cell Res. 314:869–877.
2008. View Article : Google Scholar
|
|
26
|
Tranasi M, Sberna MT, Zizzari V, D'Apolito
G, Mastrangelo F, Salini L, Stuppia L and Tetè S: Microarray
evaluation of age-related changes in human dental pulp. J Endod.
35:1211–1217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang GT, Sonoyama W, Chen J and Park SH:
In vitro characterization of human dental pulp cells: various
isolation methods and culturing environments. Cell Tissue Res.
324:225–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karaöz E, Doğan BN, Aksoy A, Gacar G,
Akyüz S, Ayhan S, Genç ZS, Yürüker S, Duruksu G, Demircan PC, et
al: Isolation and in vitro characterisation of dental pulp stem
cells from natal teeth. Histochem Cell Biol. 133:95–112. 2010.
View Article : Google Scholar
|
|
29
|
Lindroos B, Mäenpää K, Ylikomi T, Oja H,
Suuronen R and Miettinen S: Characterisation of human dental stem
cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun.
368:329–335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi MR, In YH, Park J, Park T, Jung KH,
Chai JC, Chung MK, Lee YS and Chai YG: Genome-scale DNA methylation
pattern profiling of human bone marrow mesenchymal stem cells in
long-term culture. Exp Mol Med. 44:503–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zaim M, Karaman S, Cetin G and Isik S:
Donor age and long-term culture affect differentiation and
proliferation of human bone marrow mesenchymal stem cells. Ann
Hematol. 91:1175–1186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pruszak J, Ludwig W, Blak A, Alavian K and
Isacson O: CD15, CD24 and CD29 define a surface biomarker code for
neural lineage differentiation of stem cells. Stem Cells.
27:2928–2940. 2009.PubMed/NCBI
|
|
33
|
Breau MA, Pietri T, Eder O, Blanche M,
Brakebusch C, Fässler R, Thiery JP and Dufour S: Lack of beta1
integrins in enteric neural crest cells leads to a
Hirschsprung-like phenotype. Development. 133:1725–1734. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fuchs BC, Fujii T, Dorfman JD, Goodwin JM,
Zhu AX, Lanuti M and Tanabe KK: Epithelial-to-mesenchymal
transition and integrin-linked kinase mediate sensitivity to
epidermal growth factor receptor inhibition in human hepatoma
cells. Cancer Res. 68:2391–2399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miller FD: Riding the waves: neural and
nonneural origins for mesenchymal stem cells. Cell Stem Cell.
1:129–130. 2007. View Article : Google Scholar
|
|
36
|
Hall PE, Lathia JD, Miller NG, Caldwell MA
and Ffrench-Constant C: Integrins are markers of human neural stem
cells. Stem Cells. 24:2078–2084. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aanismaa R, Hautala J, Vuorinen A,
Miettinen S and Narkilahti S: Human dental pulp stem cells
differentiate into neural precursors but not into mature functional
neurons. Stem Cell Discovery. 2:85–91. 2012. View Article : Google Scholar
|
|
38
|
Sasaki R, Aoki S, Yamato M, Uchiyama H,
Wada K, Okano T and Ogiuchi H: Neurosphere generation from dental
pulp of adult rat incisor. Eur J Neurosci. 27:538–548. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arthur A, Rychkov G, Shi S, Koblar SA and
Gronthos S: Adult human dental pulp stem cells differentiate toward
functionally active neurons under appropriate environmental cues.
Stem Cells. 26:1787–1795. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
d'Aquino R, Graziano A, Sampaolesi M,
Laino G, Pirozzi G, De Rosa A and Papaccio G: Human postnatal
dental pulp cells co-differentiate into osteoblasts and
endotheliocytes: a pivotal synergy leading to adult bone tissue
formation. Cell Death Differ. 14:1162–1171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Laino G, d'Aquino R, Graziano A, Lanza V,
Carinci F, Naro F, Pirozzi G and Papaccio G: A new population of
human adult dental pulp stem cells: a useful source of living
autologous fibrous bone tissue (LAB). J Bone Miner Res.
20:1394–1402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Laino G, Graziano A, d'Aquino R, Pirozzi
G, Lanza V, Valiante S, De Rosa A, Naro F, Vivarelli E and Papaccio
G: An approachable human adult stem cell source for hard-tissue
engineering. J Cell Physiol. 206:693–701. 2006. View Article : Google Scholar
|