Introduction
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) is commonly used to measure the expression
levels of a selected gene of interest by quantifying the mRNA
transcripts. RT-qPCR technology provides highly sensitive and
accurate expression profiles (1,2).
However, numerous parameters need to be controlled in order to
obtain reliable quantitative expression measurements. These
parameters include variations in the initial sample amount, RNA
recovery, RNA integrity, efficiency of cDNA synthesis, and
differences in the overall transcriptional activity of the tissue
or cells analyzed (3). Therefore,
reference genes are commonly used as internal controls. The
reference gene is submitted to the same experimental protocol as
the target gene in order to normalize qPCR data, and in order to
reduce possible errors generated during the quantification of gene
expression. The expression levels of the target gene are then
normalized according to the values of the internal controls.
Therefore, selecting a suitable reference gene is important for the
correct interpretation of RT-qPCR analyses. Typically, reference
genes satisfy the following criteria: They are highly expressed in
the cells of interest, variability in the expression levels of the
reference gene between samples is minimal, and the reference gene
expression is not influenced by experimental conditions (2,4).
However, it has recently become apparent that no single gene is
constitutively expressed in all cell types and under all
experimental conditions, implying that the expression stability of
the intended control gene must be verified prior to each
experiment. Therefore, the selection of a suitable reference gene
is largely dependent on the experimental tissue samples.
At present, GAPDH, ACTB, and
18S rRNA are the most commonly used reference genes.
However, previous studies have demonstrated that these reference
genes are not always suitable, as they exhibit different behaviors
in various types of cells and tissue (5,6),
particularly in sheep. Due to their unique advantages (such as
their similarity to humans in anatomy, physiology and wound repair
mechanisms), sheep are widely used in medical experimental studies.
Previous studies have successfully identified the most suitable
reference genes for ovine nervous tissue, spleen, ileum, lung,
mesenteric lymph node, pulmonary tissue, and blood (7–10).
However, little is currently known regarding the most suitable
reference genes for bone, specifically for the sheep mandibular
condyle.
The present study aimed to identify a set of
reference genes suitable for the normalization of sheep mandibular
condyle RT-qPCR data. A total of 12 commonly-used reference genes
belonging to various functional classes were selected, and their
expression stability was determined in both normal and fractured
sheep mandibular condyles. RefFinder was used to validate the
reference genes.
Materials and methods
Animals
Nine healthy sheep (six male and three female; age,
1 year; weight, 30–40 kg) were included in the present study. The
sheep were obtained from the Animal Experimental Center and housed
at the School of Stomatology, both at The Fourth Military Medical
University (Xi'an, China). Condylar fractures on the right side of
the sheep mandibular were created as previously reported (11). Briefly, the zygomatic arch and the
panniculus carnosus muscles at the surface of the capsule of the
temporomandibular joint were exposed via a curved pre-auricular
skin incision. A horizontal incision was subsequently made through
the capsule at the condylar neck to open the inferior joint space.
An oblique vertical osteotomy was made from the lateral pole of the
condyle to the medial side of the condylar neck, using an
ultrasound osteotome (Guilin Woodpecker Medical Instrument Co.,
Ltd., Guilin, China). The left side of the sheep mandibular served
as a control. Three sheep were postoperatively sacrificed at the
end of the second, fourth and twelfth week by dissecting the
carotid artery (under a general anaesthetic) resulting in acute
hemorrhagic death. The bones surrounding the fracture line on the
right side, and the bones from the corresponding area on the left
side were subsequently collected for further experimentation.
The present study was approved by the Ethics
Committee of the School of Stomatology, The Fourth Military Medical
University. All surgical procedures were conducted under
satisfactory anesthesia (0.1 ml/kg xylazine hydrochloride; Military
Veterinary Research Institute of Military Medical Science,
Changchun, China). The sheep were cared for according to the
guidelines set by the Laboratory Animal Research Centre of the
Fourth Military Medical University.
Total RNA extraction and cDNA
synthesis
Total RNA was extracted using the Total RNA kit I
(Omega Bio-Tek, Inc., Norcross, GA, USA), according to the
manufacturer's instructions. Contaminating genomic DNA was removed
by on-column treatment of each sample with DNase I (Omega Bio-Tek,
Inc., Norcross, GA, USA). The purity and quality of the extracted
RNA was evaluated using a Nanodrop 2000 spectrophotometer (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at a wavelength of 260
nm. Total RNA (500 ng) was reverse transcribed in a final volume of
50 µl using PrimeScript® RT Reagent kit (Takara
Bio, Inc., Otsu, Japan), according to the manufacturer's
instructions. The cDNA was subsequently stored at −20°C.
Gene selection and primer design
A total of 12 genes belonging to various functional
classes, which are frequently used as references genes in RT-qPCR
studies, were selected for experimentation. The following genes
were included in the present study: ACTB, YWHAZ,
HPRT, TFRC, SDHA, B2M, PGK1,
GAPDH, G6PD, RPL19, RPS24 and
18S. The primer sequences are specified in Table I (Augct, Beijing, China). The
primer sequences for PGK1, SDHA, and G6PD were
based on previous publications (7,9).
 | Table IPrimer sequences of the 12 candidate
reference genes. |
Table I
Primer sequences of the 12 candidate
reference genes.
| Gene name | Primer sequences |
|---|
| RPL19 | F: 5′
AGCCTGTGACTGTCCATTCC 3′
R: 5′ ACGTTACCTTCTCGGGCATT 3′ |
| YWHAZ | F: 5′
AGACGGAAGGTGCTGAGAAA 3′
R: 5′ CGTTGGGGATCAAGAACTTT 3′ |
| PGK1 | F: 5′
ACTCCTTGCAGCCAGTTGCT 3′
R: 5′ AGCACAAGCCTTCTCCACTTCT 3′ |
| HPRT | F: 5′
TTTATTCCTCATGGACTAATTATGGA 3′
R: 5′ CCACCCATCTCCTTCATCAC 3′ |
| TFRC | F: 5′
TTCTGGGCAGACCTCAAATC 3′
R: 5′ CAGCTTCACGTGGGACATAA 3′ |
| G6PD | F: 5′
TGACCTATGGCAACCGATACAA 3′
R: 5′ CCGCAAAAGACATCCAGGAT 3′ |
| B2M | F: 5′
CTGTCGCTGTCTGGACTGG 3′
R: 5′ TTTGGCTTTCCATCTTCTGG 3′ |
| ACTB | F: 5′
CCAACCGTGAGAAGATGACC 3′
R: 5′ CCAGAGGCGTACAGGGACAG 3′ |
| SDHA | F: 5′
CATCCACTACATGACGGAGCA 3′
R: 5′ ATCTTGCCATCTTCAGTTCTGCTA 3′ |
| RPS24 | F: 5′
TTTGCCAGCACCAACGTTG 3′
R: 5′ AAGGAACGCAAGAACAGAATGAA 3′ |
| 18S | F: 5′
TGGTCGCTCGCTCCTCTCC 3′
R: 5′ CGCCTGCTGCCTTCCTTGG 3′ |
| GAPDH | F: 5′
CCAGAGGCGGACTACTACG 3′
R: 5′ CCG AGA GAGCAACACAGG 3′ |
RT-qPCR
All RT-qPCR reactions were performed in a 20
µl volume using SYBR® Premix Ex Taq™ II (Takara
Bio, Inc.). The RT-qPCR was performed according to the instructions
of the ABI 7500 real-time PCR system (Applied Biosystems Life
Technologies, Foster City, CA, USA), which were as follows: Holding
stage, 95°C for 3 min; 40 cycles of 95°C for 15 sec and 55°C for 34
sec; melt curve stage, 95°C for 15 secs, 60°C for 1 min, 95°C 30
sec and 60°C for 15 sec. A melt curve was included at the end of
each RT-qPCR reaction.
Data analysis
RefFinder (http://www.leonxie.com/referencegene.php?type=reference)
was used in order to validate the reference genes. RefFinder is a
user-friendly web-based comprehensive tool developed for evaluating
and screening reference genes from extensive experimental datasets.
RefFinder integrates the following currently available
computational algorithms: geNorm, NormFinder, BestKeeper, and the
comparative ΔCt method, in order to compare and rank experimental
candidate reference genes. Based on the rankings from each program,
RefFinder assigns an appropriate weight to an individual gene, and
calculates the geometric mean of these weights in order to generate
a final overall ranking. In addition to analyses of the test and
control groups, the expression data of the two groups were also
combined to form a combined group.
Results
Expression levels of reference genes
All 12 reference genes expressed in the mandibular
condyle of the sheep exhibited a single marked increase in the
melt-curve. RPL19 and 18S exhibited the highest
expression levels, with a comparative threshold (Ct) mean of 17.46
and 26.09, respectively, whereas B2M showed the lowest
expression levels, with a Ct mean of 29.78. Ct refers to the cycle
number at which the fluorescence intensity exceeds the set
threshold. A high Ct value represents a low original template
concentration, while a low Ct value represents a high original
template concentration.
geNorm analysis
The stability values (M) of the reference genes were
calculated using the geNorm algorithm. High M-values indicated high
gene expression variability, whereas the most stable genes
exhibited M-values <1.5. The M-values of the reference genes in
the normal and fractured area of the sheep mandibular condyle as
determined by geNorm, are shown in Table II and Fig. 1. In the control group, the most
stable genes were RPL19 and ACTB, with an average
M-value of 0.754. The second most stable gene was PGK1, with
an average M-value of 0.948, whereas the least stable genes were
SDHA and G6PD. However, in the test group, the most
stable genes were S24 and B2M with an average M-value
of 1.657, whereas the least stable genes were HPRT and
YWHAZ. A comprehensive analysis of the combined group by
geNorm identified the most stable genes to be RPL19 and
ACTB, with an average M-value of 1.081. The second most
stable gene in the combined group was PGK1, with an average
M-value of 1.359. The remaining genes exhibited high M-values
>1.5, with the least stable genes being SDHA and
YWHAZ. Notably, SDHA and YWHAZ are considered
conventional housekeeping genes in ovine blood (12).
 | Table IIExpression stability of candidate
reference genes, as determined by geNorm. |
Table II
Expression stability of candidate
reference genes, as determined by geNorm.
| Gene name | M-value
|
|---|
| Control | Test | Combined |
|---|
| ACTB | 0.745 (1) | 1.778 (3) | 1.081 (1) |
| RPL19 | 0.745 (1) | 1.868 (4) | 1.081 (1) |
| PGK1 | 0.948 (3) | 2.110 (6) | 1.359 (3) |
| 18S | 1.074 (4) | 2.665 (9) | 2.206 (7) |
| GAPDH | 1.235 (5) | 2.001 (5) | 1.535 (4) |
| B2M | 1.518 (6) | 1.657 (1) | 1.743 (5) |
| HPRT | 1.713 (7) | 3.182 (11) | 2.774 (10) |
| S24 | 1.877 (8) | 1.657 (1) | 1.996 (6) |
| TFRC | 2.037 (9) | 2.480 (8) | 2.387 (8) |
| YWHAZ | 2.258 (10) | 3.861 (12) | 4.058 (12) |
| G6PD | 2.473 (11) | 2.350 (7) | 2.553 (9) |
| SDHA | 2.742 (12) | 2.943 (10) | 3.059 (11) |
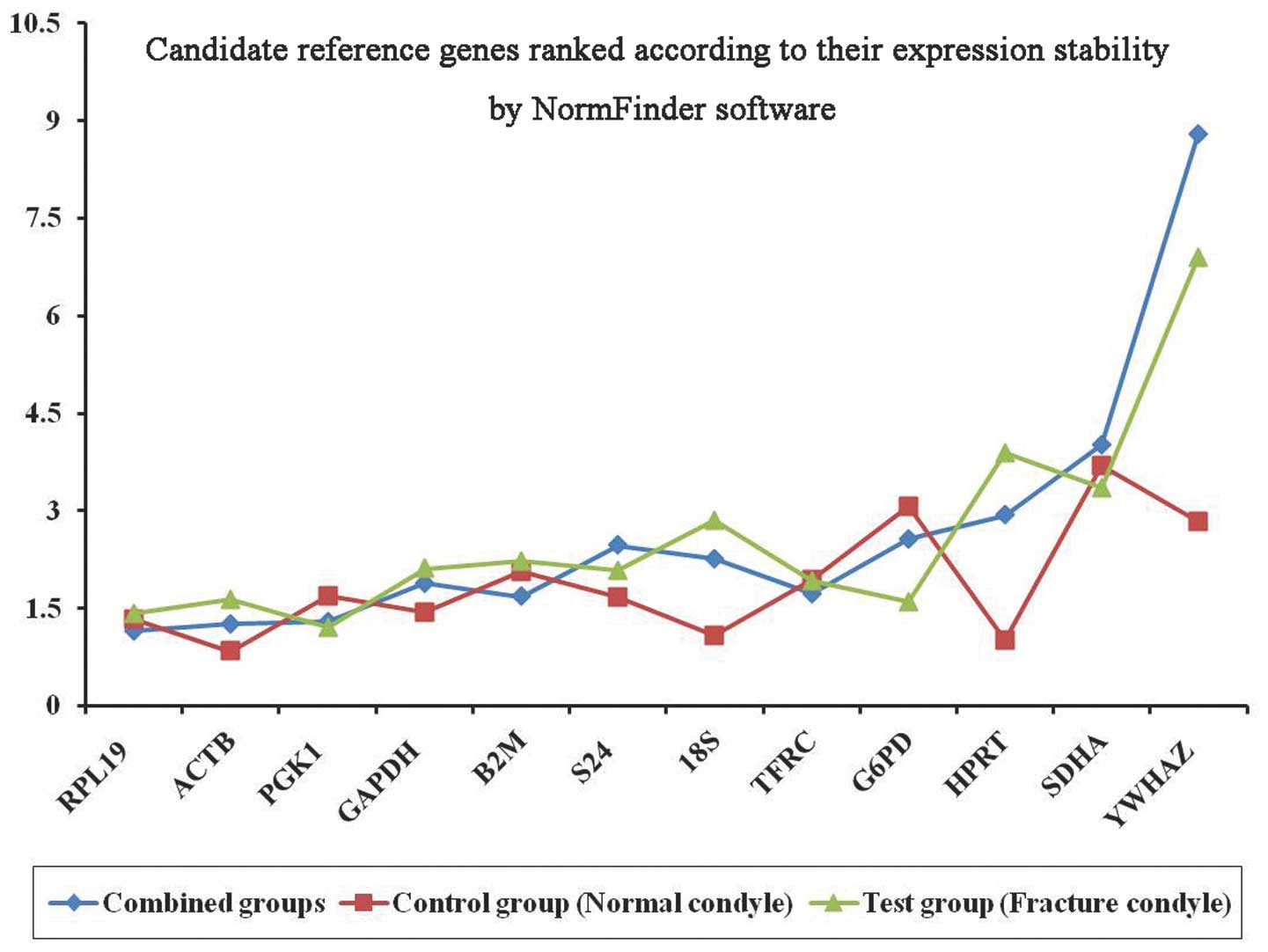
NormFinder analysis
NormFinder ranks candidate genes according to their
expression stability values (ϱ) based on the similarity of their
expression profiles. Lower values are assigned to the most stable
genes. The candidate gene expression stabilities, as determined by
NormFinder, are shown in Table
III and Fig. 2. In the control
group, the expression stability analysis demonstrated that
ACTB was the most stable gene, with a ϱ-value of 0.838. In
the test group, PGK1 was identified as the most stable
reference gene, with a ϱ-value of 1.209. When the expression data
were combined (combined group), the results confirmed that
RPL19 was the most stable gene, with a ϱ-value of 1.146.
 | Table IIIExpression stability of candidate
reference genes, as determined by NormFinder. |
Table III
Expression stability of candidate
reference genes, as determined by NormFinder.
| Gene name | ϱ-value
|
|---|
| Control | Test | Combined |
|---|
| ACTB | 0.838 (1) | 1.642 (4) | 1.252 (2) |
| HPRT | 1.015 (2) | 3.887 (11) | 2.933 (10) |
| 18S | 1.073 (3) | 2.851 (9) | 2.266 (7) |
| RPL19 | 1.325 (4) | 1.413 (2) | 1.146 (1) |
| GAPDH | 1.439 (5) | 2.110 (7) | 1.884 (6) |
| S24 | 1.675 (6) | 2.086 (6) | 2.468 (8) |
| PGK1 | 1.696 (7) | 1.209 (1) | 1.296 (3) |
| TFRC | 1.937 (8) | 1.928 (5) | 1.723 (5) |
| B2M | 2.064 (9) | 2.233 (8) | 1.679 (4) |
| YWHAZ | 2.832 (10) | 6.895 (12) | 8.789 (12) |
| G6PD | 3.060 (11) | 1.602 (3) | 2.563 (9) |
| SDHA | 3.691 (12) | 3.351 (10) | 4.009 (11) |
BestKeeper analysis
Analysis of the reference gene expression levels was
carried out using the BestKeeper software (Table IV and Fig. 3). In the control group, the results
showed that ACTB was the most stable gene, with a stability
value of 0.78. In the test group, PGK1 was identified as the
most stable reference gene, with a stability value of 0.93. In the
combined group, PGK1 was determined to be the most stably
expressed reference gene with a stability value of 0.96, whereas
YWAZH was the least stable reference gene.
 | Table IVExpression stability of candidate
reference genes, as determined by BestKeeper. |
Table IV
Expression stability of candidate
reference genes, as determined by BestKeeper.
| Gene name | Stability value
|
|---|
| Control | Test | Combined |
|---|
| ACTB | 0.78 (1) | 1.39 (2) | 1.18 (2) |
| 18S | 0.87 (2) | 2.51 (9) | 1.88 (6) |
| PGK1 | 1.00 (3) | 0.93 (1) | 0.96 (1) |
| RPL19 | 1.08 (4) | 1.66 (4) | 1.46 (3) |
| GAPDH | 1.42 (5) | 1.81 (5) | 1.80 (5) |
| S24 | 1.46 (6) | 1.51 (3) | 1.74 (4) |
| HPRT | 1.50 (7) | 4.16 (12) | 2.69 (10) |
| B2M | 1.98 (8) | 2.28 (8) | 2.16 (9) |
| TFRC | 2.07 (9) | 2.16 (7) | 1.94 (7) |
| YWHAZ | 2.23 (10) | 3.23 (11) | 4.93 (12) |
| G6PD | 3.56 (11) | 1.92 (6) | 2.14 (8) |
| SDHA | 4.08 (12) | 2.51 (9) | 2.98 (11) |
ΔCt analysis
The ΔCt method ranks a set of genes according to the
repeatability of the gene expression differences among various
samples (STDEV average). The results of the ΔCt analysis are shown
in Table V and Fig. 4. In the control group, ACTB
was the most stable reference gene followed by 18S, with
stability values of 2.07 and 2.21, respectively. In the test group,
G6PD and RPL19 were the highest stably expressed
genes with stability values of 2.28 and 3.09, respectively. The
combined results (combined group) identified RPL19 and
ACTB as the most stable genes, with stability values of 3.02
and 3.06, respectively. YWHAZ and SDHA were the least
stably expressed genes, with stability values of 9.05 and 4.88,
respectively.
 | Table VExpression stability of candidate
reference genes, as determined by the ΔCt method. |
Table V
Expression stability of candidate
reference genes, as determined by the ΔCt method.
| Gene name | Stability value
|
|---|
| Control | Test | Combined |
|---|
| ACTB | 2.07 (1) | 3.11 (2) | 3.06 (2) |
| 18S | 2.21 (2) | 3.91 (9) | 3.67 (8) |
| RPL19 | 2.24 (3) | 3.09 (1) | 3.02 (1) |
| HPRT | 2.30 (4) | 4.65 (11) | 4.18 (10) |
| GAPDH | 2.38 (5) | 3.38 (6) | 3.37 (4) |
| PGK1 | 2.47 (6) | 3.12 (3) | 3.16 (3) |
| S24 | 2.63 (7) | 3.35 (5) | 3.64 (7) |
| B2M | 2.77 (8) | 3.42 (8) | 3.42 (5) |
| TFRC | 2.79 (9) | 3.41 (7) | 3.49 (6) |
| YWHAZ | 3.40 (10) | 7.25 (12) | 9.05 (12) |
| G6PD | 11.00 (11) | 2.28 (4) | 3.77 (9) |
| SDHA | 12.00 (12) | 4.36 (10) | 4.88 (11) |
Comprehensive analysis
The combined results obtained from geNorm,
BestKeeper, the ΔCt method, and NormFinder were analyzed
comprehensively using RefFinder (Table VI and Fig. 5). In the control group, RefFinder
identified ACTB as the most stably expressed gene, with a
stability value of 1.00. In the test group, PGK1 was the
most stable reference gene with a stability value of 2.06. The
combined results of the two groups (combined group) identified
RPL19 as the most stably expressed reference gene with a
stability value of 1.32, whereas YWAZH was the least stable
reference gene. Candidate reference genes of the control group,
test group and combined group, ranked using five different methods,
were compared in Figs. 6Figure 7–8, respectively. Though each algorithm
employs a different method to determine the most suitable reference
gene, the results obtained from all of the methods exhibited a
similar trend.
 | Table VIExpression stability of candidate
reference genes, as determined by RefFinder. |
Table VI
Expression stability of candidate
reference genes, as determined by RefFinder.
| Gene name | Stability value
|
|---|
| Control | Test | Combined |
|---|
| ACTB | 1.00 (1) | 2.63 (3) | 1.68 (2) |
| RPL19 | 2.63 (2) | 2.38 (2) | 1.32 (1) |
| 18S | 2.63 (3) | 9.00 (9) | 6.96 (8) |
| PGK1 | 4.41 (4) | 2.06 (1) | 2.28 (3) |
| HPRT | 4.45 (5) | 11.24 (11) | 10.00 (10) |
| GAPDH | 5.00 (6) | 5.69 (7) | 4.68 (4) |
| S24 | 6.70 (7) | 3.08 (4) | 6.05 (6) |
| B2M | 7.67 (8) | 4.76 (6) | 5.48 (5) |
| TFRC | 8.74 (9) | 6.65 (8) | 6.40 (7) |
| YWHAZ | 10.00 (10) | 11.74 (12) | 2.00 (12) |
| G6PD | 11.00 (11) | 4.74 (5) | 8.74 (9) |
| SDHA | 12.00 (12) | 10.00 (10) | 11.00 (11) |
Discussion
Due to its high specificity, sensitivity, efficiency
and repetitiveness, RT-qPCR has been widely used to measure the
expression levels of target genes. The advantages of RT-qPCR also
includes rapid detection, simple design, and low cost. However, the
primary limitation of relative quantification is that a reference
gene must be included as an internal control. Numerous reference
genes, such as GAPDH, ACTB, 18S, and
G6PD, have previously been identified. However, to date, no
reference gene has been shown to be constitutively expressed in all
cell types and under all experimental conditions. Therefore,
evaluating the expression stability of candidate reference genes
and identifying the most stable gene is important in order to
ensure the authenticity and reliability of a given study. Numerous
reference genes have been identified as suitable for various ovine
tissue samples. SDHA and YWHAZ are considered to be
the most suitable internal controls for RT-qPCR analysis of ovine
blood (12), ACTB for the
cerebrum and spleen, and GAPDH for the mesenteric lymph node
(7). However, little is currently
known regarding the most suitable reference gene for the bone,
particularly for the sheep mandibular condyle. Sheep have an
important role in the study of human bone diseases, particularly
studies associated with temporomandibular diseases (13,14).
Therefore, the identification of a reference gene suitable for the
normalization of sheep mandibular condyle RT-qPCR analyses is
necessary.
In the present study, the expression stability of 12
housekeeping genes were examined in both normal and fractured sheep
mandibular condyles using the following algorithms: geNorm,
NormFinder, BestKeeper, and the ΔCt method. geNorm (15) calculates the expression ratio of
two ideal internal control genes in two identical samples,
regardless of the experimental conditions or cell type. For each
control gene, the pairwise variation is determined as the standard
deviation of the logarithmically transformed expression ratios, and
the internal control gene-stability value (M) is determined as the
average pairwise variation of a particular gene with all other
control genes. NormFinder (3)
ranks the candidate genes according to their expression stability
values (ϱ) based on the similarity of their expression profiles.
BestKeeper (16) identifies the
most suitable reference genes using a pairwise correlation analysis
of all pairs of candidate genes, and calculates the geometric mean
of the most suitable genes. The ΔCt (17) method ranks a set of genes according
to the repeatability of the gene expression differences among
various samples (average of STDEV). Although each algorithm employs
a different method to determine the most suitable reference gene,
in the present study the results obtained from all five methods
exhibited a similar trend (Fig.
6Figure 7–8). All methods determined that
RPL19, ACTB, and PGK1 were the most stably
expressed reference genes in the sheep mandibular condyle,
regardless of whether the candidate genes were analyzed for the
normal group, the fracture group, or the combined group. The
results obtained from the various algorithms were comprehensively
analyzed using RefFinder. Based on the rankings obtained from each
algorithm, RefFinder assigns an appropriate weight to an individual
gene, and calculates the geometric mean of these weights, in order
to obtain an overall final ranking. The results demonstrated that
ACTB and RPL19 were the most stable reference genes
expressed in the normal sheep mandibular condyle, PGK1 and
RPL19 were the most stable reference genes expressed in the
fractured sheep mandibular condyle, whereas RPL19 and
ACTB were the most stably expressed reference genes in both
the normal and fractured sheep mandibular condyle (combined group).
The results of the present study identified RPL19 as the
most suitable reference gene for studies associated with the sheep
mandibular condyle. In addition, ACTB and PGK1 were
shown to be suitable alternatives. These results differ from
previous reports of sheep reference gene suitability, which
included the identification of SDHA and YWHAZ as the
most stable reference genes for ovine blood (12), ACTB for the cerebrum and
spleen, and GAPDH for the mesenteric lymph node (7). In conclusion, the results of the
present study suggested that if these previously reported genes
were to be used as reference genes in studies associated with the
sheep mandibular condyle, the results of such a study may be
negatively affected.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81271168).
References
|
1
|
Heid CA, Stevens J, Livak KJ and Williams
PM: Real time quantitative PCR. Genome Res. 6:986–994. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bustin SA: Quantification of mRNA using
real-time reverse transcription PCR (RT-PCR): Trends and problems.
J Mol Endocrinol. 29:23–39. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andersen CL, Jensen JL and Ørntoft TF:
Normalization of real-time quantitative reverse transcription-PCR
data: A model-based variance estimation approach to identify genes
suited for normalization, applied to bladder and colon cancer data
sets. Cancer Res. 64:5245–5250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chari R, Lonergan KM, Pikor LA, Coe BP,
Zhu CQ, Chan TH, MacAulay CE, Tsai MS, Lam S, Ng RT and Lam WL: A
sequence-based approach to identify reference genes for gene
expression analysis. BMC Med Genomics. 3:322010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Selvey S, Thompson EW, Matthaei K, Lea RA,
Irving MG and Griffiths LR: Beta-actin - an unsuitable internal
control for RT-PCR. Mol Cell Probes. 15:307–311. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohl F, Jung M, Xu C, Stephan C, Rabien A,
Burkardt M, Nitsche A, Kristiansen G, Loening SA, Radonić A and
Jung K: Gene expression studies in prostate cancer tissue: Which
reference gene should be selected for normalization? J Mol Med
(Berl). 83:1014–1024. 2005. View Article : Google Scholar
|
|
7
|
Garcia-Crespo D, Juste RA and Hurtado A:
Selection of ovine housekeeping genes for normalisation by
real-time RT-PCR; analysis of PrP gene expression and genetic
susceptibility to scrapie. BMC Vet Res. 1:32005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lampo E, Van Poucke M, Vandesompele J,
Erkens T, Van Zeveren A and Peelman LJ: Positive correlation
between relative mRNA expression of PRNP and SPRN in cerebral and
cerebellar cortex of sheep. Mol Cell Probes. 23:60–64. 2009.
View Article : Google Scholar
|
|
9
|
Passmore M, Nataatmadja M and Fraser JF:
Selection of reference genes for normalisation of real-time RT-PCR
in brain-stem death injury in Ovis aries. BMC Mol Biol. 10:722009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lyahyai J, Serrano C, Ranera B, Badiola
JJ, Zaragoza P and Martin-Burriel I: Effect of scrapie on the
stability of housekeeping genes. Anim Biotechnol. 21:1–13. 2010.
View Article : Google Scholar
|
|
11
|
Liu CK, Liu P, Meng FW, Deng BL, Xue Y,
Mao TQ and Hu KJ: The role of the lateral pterygoid muscle in the
sagittal fracture of mandibular condyle (SFMC) healing process. Br
J Oral Maxillofac Surg. 50:356–360. 2012. View Article : Google Scholar
|
|
12
|
Peletto S, Bertuzzi S, Campanella C,
Modesto P, Maniaci MG, Bellino C, Ariello D, Quasso A, Caramelli M
and Acutis PL: Evaluation of internal reference genes for
quantitative expression analysis by real-time PCR in ovine whole
blood. Int J Mol Sci. 12:7732–7747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng FW, Hu KJ, Kong L, Zhao YT, Liu YP
and Zhou SX: Morphological evaluation of temporomandibular joint
after open and closed treatment of type B diacapsular condylar
fractures in sheep. Ann Anat. 191:288–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takaishi M, Kurita K, Hatano Y, Matsuura
H, Borg M and Goss NA: Effects of postoperative radiotherapy for
temporomandibular joint ankylosis after gap arthroplasty: An animal
study using sheep. J Oral Maxillofac Surg. 68:1763–1769. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genes. Genome Biol.
3:RESEARCH0034. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfaffl MW, Tichopad A, Prgomet C and
Neuvians TP: Determination of stable housekeeping genes,
differentially regulated target genes and sample integrity:
BestKeeper – Excel-based tool using pair-wise correlations.
Biotechnol Lett. 26:509–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Silver N, Best S, Jiang J and Thein SL:
Selection of housekeeping genes for gene expression studies in
human reticulocytes using real-time PCR. BMC Mol Biol. 7:332006.
View Article : Google Scholar : PubMed/NCBI
|