Introduction
Calcium is an important second messenger involved in
signal transduction pathways that regulate memory formation and
consolidation (1,2). In combination with
calcium/calmodulin-dependent protein kinase (CaMK), calcium
triggers autophosphorylation and maintains short-term memory for
several minutes. The CaMK-calcium complex travels into the nucleus,
where it activates the cyclic adenosine mono-phosphate response
element binding protein (CREB), promotes the synthesis of
memory-associated proteins and aids in the formation of permanent
memory that lasts from several hours to numerous years (3,4). If
the intracellular calcium concentration decreases to a level that
is insufficient to trigger the autophosphorylation of CaMKII and
the synthesis of memory-associated protein, memory is impaired.
Calcium dyshomeostasis has been observed in Alzheimer's disease
(5,6), and numerous studies have determined
that excess calcium promotes neurotoxicity and oxidative stress,
impairs mitochondrial activity and causes apoptosis (7–9);
therefore, the calcium concentration must be at a suitable level
for calcium signaling transduction. Calcium oscillations, which are
periodic fluctuations of the intracellular calcium concentration,
are a common form of information coding, and the expression levels
of various proteins are determined by the frequency of oscillation
waves as well as their amplitude and cumulative wave width
(10,11). Our group and others have
transcription and protein expression (12,13).
The synthesis of memory-associated proteins is regulated by
calmodulin, signal transducer protein and calcium channel proteins,
which are referred to as calcium memory-associated proteins.
Whether the calcium oscillations regulate calcium memory-associated
protein synthesis has remained to be demonstrated, and the present
study aimed to clarify this issue.
The present study assessed the effect of a selective
estrogen receptor modulator, raloxifene, on calcium oscillations
and the expression of calcium memory-associated proteins during
calcium overload.
Materials and methods
Neuron cultures
Primary hippocampal neuron cultures were prepared
from 17–18 day-old Wistar rat embryos as previously described
(14). The neurons were plated on
24-mm round coverslips in six-well plates coated with 20
µg/ml poly-D-lysine (Sigma-Aldrich, St. Louis, MO, USA) and
cultured for 10 days in vitro in neurobasal medium
supplemented with 2% (v/v) B-27 medium (Gibco Life Technologies,
Carlsbad, CA, USA); half of the medium was changed every four days.
One pregnant Wistar rat was ordered from the animal center
affiliated to Wuhan University (Wuhan, China). The present study
was conducted in strict accordance with the recommendations set out
in the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (eighth edition, 2011). The protocol
involving animals was reviewed and approved by the Institutional
Animal Care and Use Committee of the Tongji Medical college of
Huazhong Science and Technology (Wuhan, China).
Calcium measurements
Changes in the cytosolic free calcium concentration,
[Ca2+]i, were measured using the [Ca2+]i
indicator Fura-2 AM (Dojingo Molecular Technologies, Inc.,
Kumamoto, Japan). The neurons were cultured for 10 days on coated
24-mm round glass coverslips at a density of 1×105
cells/cm2 and were incubated in the dark with 5
µM Fura-2 AM for 30 min at 37°C in
Krebs-4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)
buffer [containing: NaCl (137 µM), KCl (4.9 µM),
CaCl2 (2 µM), MgSO4 (1.2 µM),
D-glucose (10 µM) and HEPES (10 µM)]. The coverslips
were then washed, and the cells were maintained for at least 30 min
prior to experimentation in indicator-free Krebs-HEPES buffer. The
emitted Fura-2 fluorescence was recorded from neurons on the
coverslips in a perfusion chamber mounted on the stage of a
modified Olympus inverted epifluorescence microscope (IX-30;
Olympus, Tokyo, Japan) after excitation at 340±10 and 380±10 nm
using a xenon short-arc lamp (Ushio, Tokyo, Japan), corresponding
to the Ca2+-bound and Ca2+-free forms of the
indicator, respectively. Bandpass interference filters (Omega
Optical, Brattleboro, VT, USA) selected wavelength bands of emitted
fluorescence at 510±10 nm. Emitted Fura-2 fluorescence was
collected and measured using a spectrofluorometer (PTI Deltascan;
Photon Technology International, Inc., Monmouth Junction, NJ, USA).
Autofluorescence created by unloaded neurons was generally <5%
of Fura-2-loaded neurons and was subtracted automatically from
Fura-2-fluorescence recordings. The baseline mean ratio value (R
mean) was the mean ratio value after a 3-min recording taken at the
beginning of the experiment. Calcium oscillations, expressed as the
ratio (R) of fluorescence intensities at 340/380 nm, were defined
as variations of 10% from the mean R, occurring synchronously in
several cells of the field.
Western blot analysis
Following the abovementioned treatments, the neurons
were collected after culturing for 10 days and lysed with 1X
loading buffer (containing 1% v/v phenylmethanesulfonylfluoride),
and total protein was extracted. The cell extracts were mixed with
sample buffer containing 50 mM Tris-HCl (pH 7.6), 2% SDS, 10%
glycerol, 10 mM dithiothreitol and 0.2% bromophenol blue and boiled
for 5 min. Boiled samples were subjected to 10% SDS-PAGE
(Sigma-Aldrich) and the separated proteins were transferred onto
nitrocellulose membranes (GE Healthcare Life Sciences, Maidstone,
UK). The membranes were then incubated with primary antibodies (see
Table I) that were detected using
anti-rabbit or anti-mouse immunoglobulin G conjugated to IRDye
(800CW; LI-COR Biosciences, Lincoln, NE, USA) for 1 h at room
temperature and visualized using the Odyssey infrared Imaging
System (no. 9120; LI-COR Biosciences, Lincoln, NE, USA).
 | Table IPrimary antibodies used for western
blot analysis in the present study. |
Table I
Primary antibodies used for western
blot analysis in the present study.
| Antibody | Dilution | Source |
|---|
| CaMKII (poly) | 1:1,000 | Cell Signaling
Technology, Inc. (Boston, MA, USA) |
| p-CaMKII (mAb) | 1:500 | Cell Signaling
Technology, Inc. |
| NR1 (poly) | 1:500 | Alomone (Jerusalem,
Israel) |
| NR2B (poly) | 1:1,000 | Abcam (Cambridge,
England) |
| Cav1.2 (poly) | 1:200 | Alomone |
| PKC (mAb) | 1:200 | Santa Cruz
Biotechnology (Dallas, TX, USA) |
| PSD95 (mAb) | 1:200 | Santa Cruz
Biotechnology |
| PSD93 (poly) | 1:1,000 | Abcam |
| CREB (poly) | 1:1,000 | Cell Signaling
Technology, Inc. |
| p-CREB (poly) | 1:500 | Cell Signaling
Technology, Inc. |
MTT assay
Hippocampal neurons (1×104 cells/100
µl/well) were plated on 96-well plates, and the neurons were
cultured for 10 days prior to the experiment. After the neurons
matured, they were treated with 10-300 µM glutamate
(Sigma-Aldrich), 300 µM glutamate + raloxifene (300 nM-10
µM; Sigma-Aldrich), or culture media based on the previous
experimental design for 48 h. The cells were treated with 20
µl MTT (5 mg/ml; Sigma-Aldrich) for 4 h, the culture media
were discarded, 150 µl dimethylsulfoxide (Sigma-Aldrich) was
added, and the plates were agitated using a micro-oscillator at a
low speed for 5 min. The optical density (OD) was tested using a
standard ELISA microplate reader (ELX800; BioTek Instruments, Inc.,
Winooski, VT, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation and were analyzed using Graph Pad Prism 5 (Graph Pad
Inc., La Jolla, CA, USA). A one-way analysis of variance procedure
followed by least significant difference post-hoc tests as well as
Student's t-tests were used to determine differences between the
groups. P<0.05 was considered to indicate a statistically
significant difference between values.
Results
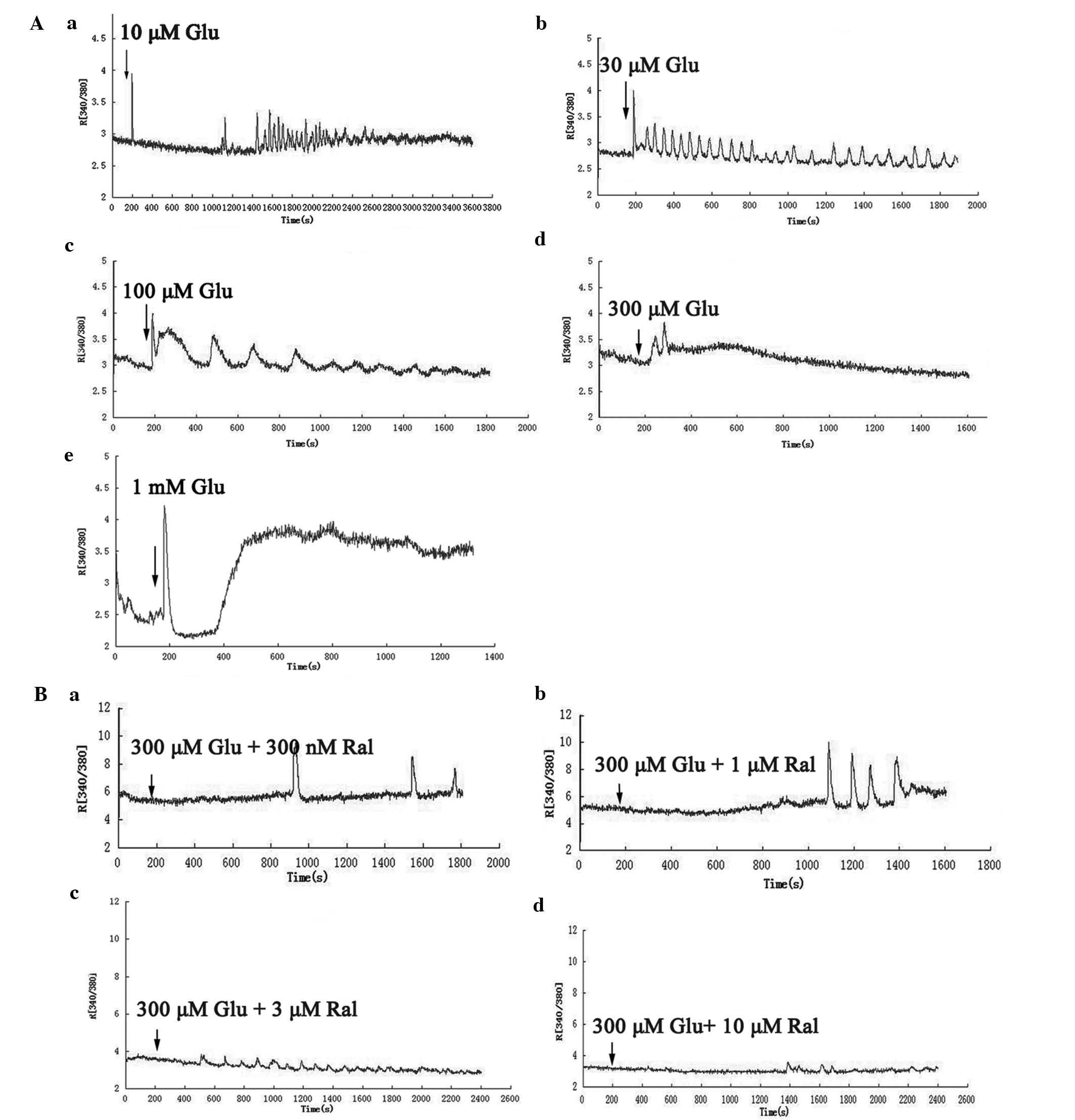
Glutamate regulates calcium oscillations
in a concentration-dependent manner
After the cells were scanned for 3 min, glutamate
was added to the buffer to reach a working concentration of 10
µM to 1 mM (n=30–40 for each calculation; 73–89% of neurons
showed oscillation waves after being stimulated with 30 µM
to 100 µM glutamate; each experiment was repeated three
times) (Fig. 1A). At the 10
µM concentration, the calcium oscillations waves appeared
within 15 min, whereas the oscillation wave frequency was low at
the beginning Fig. 1Aa). At the
28th minute, the frequency increased to 2/min, and the oscillation
waves persisted for 18 sec. For the 30 µM concentration, the
oscillation wave promptly appeared when glutamate was added to the
buffer, the frequency was 1.2/min, and the oscillation waves
persisted for 31 sec at the beginning (Fig. 1Ab). The frequency then declined to
0.8/min, but the oscillation waves persisted for 44 sec. When the
glutamate concentration was 100 µM, the oscillation appeared
when the drug was added to the buffer, the frequency was 0.3/min,
and the oscillation waves persisted for 121 sec (Fig. 1Ac). At a concentration of 300
µM, the single oscillation wave appeared immediately, then
disappeared, and the R[340/380] increased (Fig. 1Ad). When the glutamate
concentration was 1 mM, the R[340/380] declined at first and then
increased to a very high level (Fig.
1Ae).
Raloxifene reverses glutamate-induced
calcium oscillations
10-day-old neurons were pre-treated with 300
µM glutamate for 5 min, the spontaneous calcium oscillations
were inhibited (n=30–40 for each calculation; ~90% of neurons
showed spontaneous calcium oscillations after being cultured for 10
days; each experiment was repeated three times), and raloxifene was
then added to the buffer at working concentrations of 300 nM to 10
µM (Fig. 1B). When the
concentration of raloxifene was 300 nM, the calcium oscillation
waves irregularly re-appeared after 10 min and continued at a low
frequency (Fig. 1Ba). When the
concentration was increased to 1 µM, the oscillation wave
appeared regularly after 15 min, and the frequency was ~0.7/min and
persisted for 52 sec (Fig. 1Bb).
When 3 µM raloxifene was used, the calcium oscillations
appeared after 5–7 min, the frequency was 0.6/min, and the
oscillation waves persisted for 63 sec (Fig. 1Bc). When the concentration of
raloxifene was 10 µM, the oscillation wave appeared
intermittently (Fig. 1Bd).
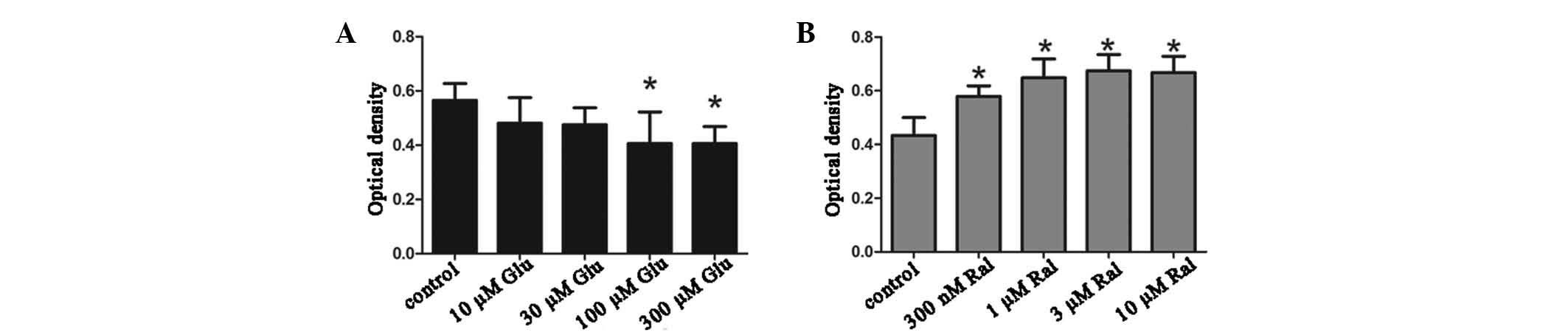
Calcium dyshomeostasis reduces neuronal
survival and is reversed by raloxifene treatment
The present study analyzed neuronal survival using
the MTT assay. Overall, 10–30 µM glutamate did not
significantly affect neuronal survival; however, when the
concentration was increased to 100 µM, the neuronal survival
markedly declined as indicated by the reduced OD values. In
addition, 300 µM glutamate also impaired neuronal survival
to a similar degree to that of 100 µM glutamate. When
raloxifene was added, neuronal survival improved as the
concentration increased, and all groups exhibited increased
neuronal survival compared with that in the control group. When the
neurons were treated with 300 nM and 1 µM raloxifene, the
neuronal survival did not significantly change; however, when the
concentration of raloxifene was increased to 3 µM, neuronal
survival significantly improved. In addition, 10 µM
raloxifene had a similar effect on neuronal survival as that of 3
µM raloxifene (Fig. 2).
Glutamate regulates expression of calcium
memory-associated proteins in a concentration-dependent manner
After the intracellular calcium concentrations were
measured, the neurons were collected and subjected to western blot
analysis of calcium memory-associated proteins. The present study
selectively assessed the following proteins: Calcium channel,
voltage-dependent, L type (L-VGCC), alpha 1C subunit (Cav1.2),
which is the primary subunit of L-VGCC in the brain (15); the NMDA receptor (the important
post-synaptic calcium channel) subunits NR1 and NR2B; two important
proteins distributed in postsynaptic density, postsynaptic density
protein (PSD) 95 and PSD 93; the calcium-sensitized proteins CaMKII
and phosphorylated (p) CaMKII; protein kinase C (PKC), which
mediates the conversion of short-term memory into permanent memory
(16,17); and key molecules that control
transcription, CREB and pCREB (18,19).
The results showed that 30 µM and 100 µM glutamate
significantly increased the expression of Cav1.2, NR1, NR2B, PSD95
and pCREB. 10 µM glutamate non-significantly increased the
expression of Cav1.2, NR1, NR2B, PKC and CREB. However, none of the
test concentrations of glutamate significantly affected the
expression of pCaMKII, PKC and CREB. 300 µM and 1 mM
glutamate significantly decreased the expression of PSD93 and
pCREB, and 1 mM glutamate significantly decreased the expression of
CaMKII. These results suggested that low concentrations of
glutamate (10–100 µM) stimulated the release of calcium and
increased the expression of several calcium memory-associated
proteins, while high concentrations (300 µM or higher) of
glutamate decreased the expression of several calcium
memory-associated proteins (Fig.
3).
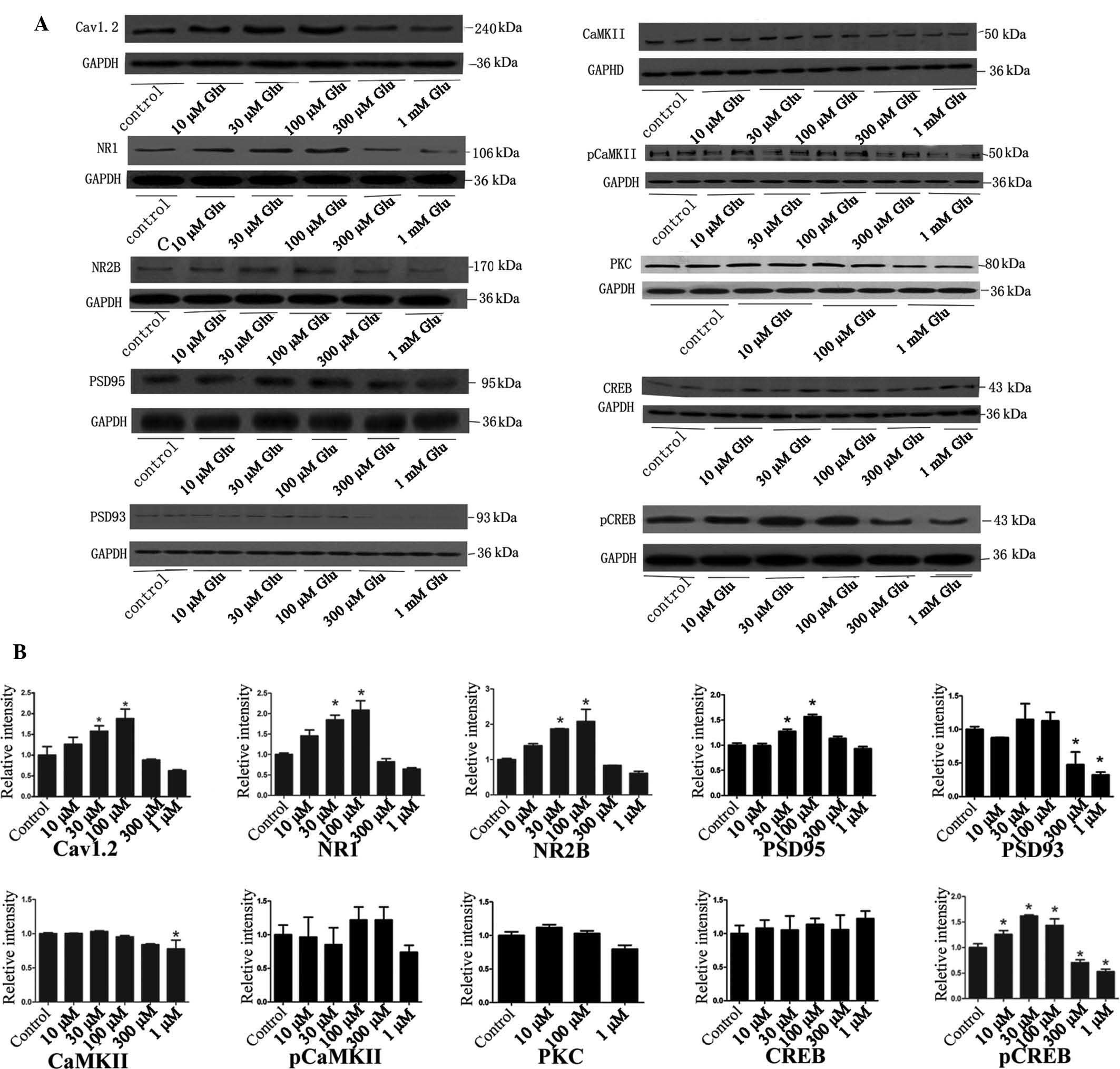 | Figure 3Western blot analysis of calcium
memory-associated proteins following stimulation of neurons with
various concentrations of Glu. (A) Representative western blots and
(B) column diagrams showing the quantified protein levels obtained
by grey value analysis of A. Grey values are expressed as the mean
± standard deviation. *P<0.05 vs. control. Glu,
glutamate; p, phosphorylated; poly, rabbit polyclonal antibody;
CaMK, calcium/calmodulin-dependent protein kinase; CREB, cyclic
adenosine monophosphate response element binding protein; NR2B,
N-methylD-aspartate receptor subtype 2B; NR1, NR1 subunit of
the N-methyl-D-aspartate receptor; PKC, protein kinase C;
Cav1.2, calcium channel, voltage-dependent, L type, alpha 1C
subunit; PSD95, postsynaptic density protein 95. |
Raloxifene increases the expression of
calcium memory-associated proteins in cells pre-treated with 300 µM
glutamate
To examine the reversal effect of raloxifene on the
expression of calcium memory-associated proteins after
pre-treatment with 300 µM glutamate, the neuronal cells were
subjected to western blot analysis after the calcium oscillation
tests. The results showed that 100 µM raloxifene
significantly increased the expression of Cav1.2. The expression of
NR2B was significantly elevated in cells treated with raloxifene at
100 nM-10 µM, but not at 3 µM. The PKC levels
significantly increased when the concentration of raloxifene was 1
µM, and raloxifene concentrations ranging from 1–10
µM significantly promoted the expression of CREB. 100 nM-1
µM raloxifene significantly increased the expression of
pCREB in a concentration-dependent manner, while 3 and 10 µM
pCREB significantly decreased its expression. The expression levels
of NR1, PSD95, PSD93, CaMKII, pCaMKII were not significantly
altered at the tested concentrations (Fig. 4).
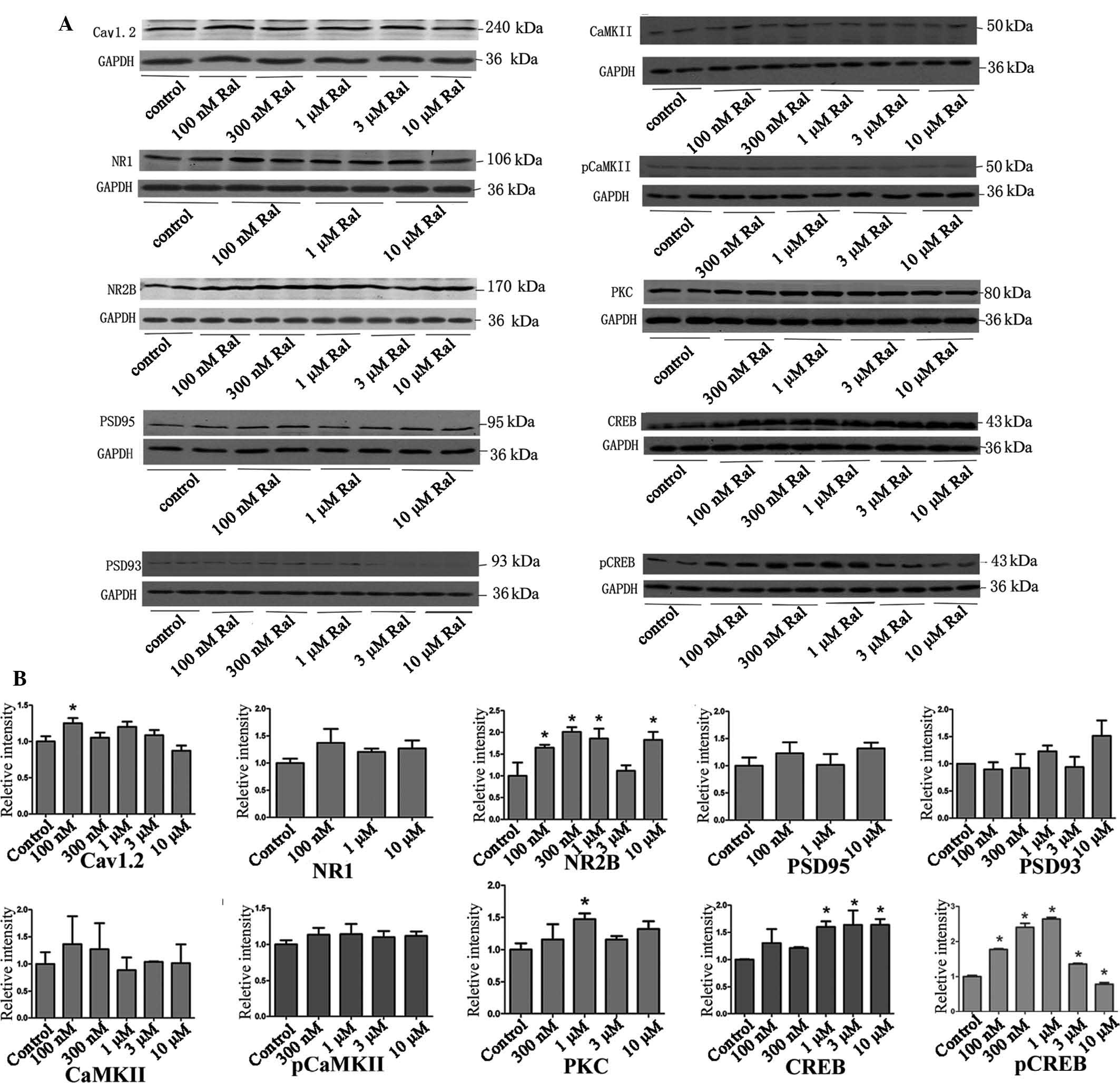 | Figure 4Neutralizing effect of Ral treatment
after pre-treatment with 300 µM Glu. (A) Representative
western blots and (B) column diagrams showing the quantified
protein levels obtained by grey value analysis of A. Grey values
are expressed as the mean ± standard deviation.
*P<0.05 vs. control. Ral, raloxifene, Glu, glutamate;
p, phosphorylated; poly, rabbit polyclonal antibody; CaMK,
calcium/calmodulin-dependent protein kinase; CREB, cyclic adenosine
mono-phosphate response element binding protein; NR2B,
N-methylD-aspartate receptor subtype 2B; NR1, NR1 subunit of
the N-methyl-D-aspartate receptor; PKC, protein kinase C;
Cav1.2, calcium channel, voltage-dependent, L type, alpha 1C
subunit; PSD95, postsynaptic density protein 95. |
Discussion
Calcium signaling in the cell is highly regulated
and is generated by an ion influx through voltage and/or
ligand-gated calcium-permeable ion channels, or ion release from
internal stores. Furthermore, calcium signaling is sequestered or
cleared by calcium pumps and exchangers (1). Numerous pathogenic changes influence
calcium oscillations. For example, the APPswe mutation was shown to
increase the frequency of spontaneous calcium oscillations in rat
hippocampal neurons (20).
Furthermore, the endogenous activation of metabotropic glutamate
receptors during neocortical development causes neuronal calcium
oscillations (21). The
phosphorylation of T668, the expression of the human amyloid
precursor protein, treatment with Aβ25-35 or isoflurane-induced
ischemic tolerance all inhibit calcium oscillations (22–24).
Glutamate is an important excitatory amino acid in
the brain. In the present study, glutamate was used to induce
calcium oscillations. It is a ligand of the NMDA receptor and
induces calcium influx. If the glutamate concentration is too high,
calcium flows into the cell and cannot be eliminated in a timely
manner, which results in excess calcium buildup in the cell. This
accumulation inversely inhibits calcium oscillations. The present
study found that low concentrations of glutamate (10–100 µM)
increased the duration of a single peak (18–44 sec) but decreased
the frequency of the waves (2-0.8/min). Furthermore, high
concentrations of glutamate (300 µM or higher) inhibited
calcium oscillations. These results suggested that glutamate is
necessary to maintain neural excitation at specific concentrations,
but it terminates signal transduction if the concentration is too
high.
Calcium oscillations increase the efficiency and
specificity of gene expression. CaMKII is sensitive to the
frequency of oscillations modulated by several factors, such as the
amplitude and duration of individual peaks (25). Calcium oscillations at a specific
periodicity of 12 min were found to affect gene expression in
target epithelial cells. For example, calcium oscillations
specifically induced the pro-inflammatory cytokine interleukin
(IL)-6 and chemokine IL-8 (26).
Trophic factor-induced intracellular calcium oscillations are
required for the expression of post-synaptic acetylcholine
receptors during synapse formation between Lymnaea neurons
(13). The results of the present
study showed that an increase in the duration of calcium
oscillations promotes the expression of several calcium
memory-associated proteins, and inhibition of calcium oscillations
decreased the expression of several calcium memory-associated
proteins. The MTT analysis showed that glutamate at 100 µM
(moderate concentration) and 300 µM (high concentration)
decreased neuronal survival. These results suggested that memory
formation and consolidation were enhanced when the concentration of
intracellular calcium was suitable but weakened due to the decline
of calcium memory-associated proteins induced by calcium
overload.
Several medicines have been used to regulate calcium
dyshomeostasis, including nimodipine and memantine, inhibit calcium
flux (27,28). Of note, the inhibition of calcium
oscillations, neuronal survival and calcium memory-associated
protein synthesis were reversed in the present study when the
neurons were treated with raloxifene, which is a selective estrogen
receptor modulator that induces calcium oscillations when the
calcium concentration is very high or low. There are three
mechanisms to explain the restorative effects of raloxifene on
calcium oscillations: Its activity agains oxidative stress
(29), up-regulation of telomerase
activity (30) and activation of
gene transcription and expression (31). Raloxifene may decrease the risk of
mild cognitive impairment by 33% and slightly lowers the risk of
Alzheimer's disease (32).
Therefore, this drug has the potential to be used clinically to
regulate calcium dyshomeostasis.
In conclusion, glutamate regulates calcium
oscillations or the expression of calcium memory-associated
proteins and neuronal survival in a dose-dependent manner, which
may be an important mechanism of memory impairment. Raloxifene,
which is a selective estrogen receptor modulator, effectively
reversed these effects, and it may therefore be used as an
alternative drug to regulate calcium dyshomeostasis for treating
memory impairment diseases such as Alzheimer's disease.
Acknowledgments
This study was funded by the National Natural
Science Fund of China (grant nos. 30971008 and 81260172).
References
|
1
|
Kawamoto EM, Vivar C and Camandola S:
Physiology and pathology of calcium signaling in the brain. Front
Pharmacol. 3:612012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oliveira AM and Bading H: Calcium
signaling in cognition and aging-dependent cognitive decline.
Biofactors. 37:168–174. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carew TJ: Molecular enhancement of memory
formation. Neuron. 16:5–8. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lucchesi W, Mizuno K and Giese KP: Novel
insights into CaMKII function and regulation during memory
formation. Brain Res Bull. 85:2–8. 2011. View Article : Google Scholar
|
|
5
|
Berridge MJ: Calcium signalling and
Alzheimer's disease. Neurochem Res. 36:1149–1156. 2011. View Article : Google Scholar
|
|
6
|
Garwood C, Faizullabhoy A, Wharton SB,
Ince PG, Heath PR, Shaw PJ, Baxter L, Gelsthorpe C, Forster G,
Matthews FE, et al: MRC Cognitive Function and Ageing
Neuropathology Study Group: Calcium dysregulation in relation to
Alzheimer-type pathology in the ageing brain. Neuropathol Appl
Neurobiol. 39:788–799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pelizzoni I, Macco R, Morini MF, Zacchetti
D, Grohovaz F and Codazzi F: Iron handling in hippocampal neurons:
Activity-dependent iron entry and mitochondria-mediated
neurotoxicity. Aging Cell. 10:172–183. 2011. View Article : Google Scholar
|
|
8
|
Huang Y, Huang YL, Lai B, Zheng P, Zhu YC
and Yao T: Raloxifene acutely reduces glutamate-induced
intracellular calcium increase in cultured rat cortical neurons via
inhibition of high-voltage-activated calcium current. Neuroscience.
147:334–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Dykens JA, Perez E, Liu R, Yang S,
Covey DF and Simpkins JW: Neuroprotective effects of
17beta-estradiol and nonfeminizing estrogens against H2O2 toxicity
in human neuroblastoma SK-N-SH cells. Mol Pharmacol. 70:395–404.
2006.PubMed/NCBI
|
|
10
|
Zhu L, Luo Y, Chen T, Chen F, Wang T and
Hu Q: Ca2+ oscillation frequency regulates
agonist-stimulated gene expression in vascular endothelial cells. J
Cell Sci. 121:2511–2518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Q, Deshpande S, Irani K and Ziegelstein
RC: [Ca2+](i) oscillation frequency regulates
agonist-stimulated NF-kappaB transcriptional activity. J Biol Chem.
274:33995–33998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dolmetsch RE, Xu K and Lewis RS: Calcium
oscillations increase the efficiency and specificity of gene
expression. Nature. 392:933–936. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu F, Hennessy DA, Lee TK and Syed NI:
Trophic factor-induced intracellular calcium oscillations are
required for the expression of postsynaptic acetylcholine receptors
during synapse formation between Lymnaea neurons. J Neurosci.
29:2167–2176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaech S and Banker G: Culturing
hippocampal neurons. Nat Protoc. 1:2406–2415. 2006. View Article : Google Scholar
|
|
15
|
Tsuruta F, Green EM, Rousset M and
Dolmetsch RE: PIKfyve regulates CaV1.2 degradation and prevents
excitotoxic cell death. J Cell Biol. 187:279–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosenegger D and Lukowiak K: The
participation of NMDA receptors, PKC, and MAPK in the formation of
memory following operant conditioning in Lymnaea. Mol Brain.
3:242010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Michel M, Green CL and Lyons LC: PKA and
PKC are required for long-term but not short-term in vivo operant
memory in Aplysia. Learn Mem. 18:19–23. 2011. View Article : Google Scholar :
|
|
18
|
Kornhauser JM, Cowan CW, Shaywitz AJ,
Dolmetsch RE, Griffith EC, Hu LS, Haddad C, Xia Z and Greenberg ME:
CREB transcriptional activity in neurons is regulated by multiple,
calcium-specific phosphorylation events. Neuron. 34:221–233. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bito H, Deisseroth K and Tsien RW: CREB
phosphorylation and dephosphorylation: A Ca(2+) and stimulus
duration-dependent switch for hippocampal gene expression. Cell.
87:1203–1214. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kloskowska E, Malkiewicz K, Winblad B,
Benedikz E and Bruton JD: APPswe mutation increases the frequency
of spontaneous Ca2+ oscillations in rat hippocampal
neurons. Neurosci Lett. 436:250–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Flint AC, Dammerman RS and Kriegstein AR:
Endogenous activation of metabotropic glutamate receptors in
neocortical development causes neuronal calcium oscillations. Proc
Natl Acad Sci USA. 96:12144–12149. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santos SF, Tasiaux B, Sindic C and Octave
JN: Inhibition of neuronal calcium oscillations by cell surface APP
phosphorylated on T668. Neurobiol Aging. 32:2308–2313. 2011.
View Article : Google Scholar
|
|
23
|
Santos SF, Pierrot N, Morel N, Gailly P,
Sindic C and Octave JN: Expression of human amyloid precursor
protein in rat cortical neurons inhibits calcium oscillations. J
Neurosci. 29:4708–4718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rui Y, Li R, Liu Y, Zhu S, Yu X, Sheng Z
and Xie Z: Acute effect of beta amyloid on synchronized spontaneous
Ca2+ oscillations in cultured hippocampal networks. Cell
Biol Int. 30:733–740. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Koninck P and Schulman H: Sensitivity
of CaM kinase II to the frequency of Ca2+ oscillations.
Science. 279:227–230. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Söderblom T, Laestadius A, Oxhamre C,
Aperia A and Richter-Dahlfors A: Toxin-induced calcium
oscillations: A novel strategy to affect gene regulation in target
cells. Int J Med Microbiol. 291:511–515. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Doody RS, Tariot PN, Pfeiffer E, Olin JT
and Graham SM: Meta-analysis of six-month memantine trials in
Alzheimer's disease. Alzheimers Dement. 3:7–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liljelund P, Netzeband JG and Gruol DL:
L-type calcium channels mediate calcium oscillations in early
postnatal purkinje neurons. J Neurosci. 20:7394–7403.
2000.PubMed/NCBI
|
|
29
|
Biewenga E, Cabell L and Audesirk T:
Estradiol and raloxifene protect cultured SN4741 neurons against
oxidative stress. Neurosci Lett. 373:179–183. 2005. View Article : Google Scholar
|
|
30
|
Kawagoe J, Ohmichi M, Takahashi T, Ohshima
C, Mabuchi S, Takahashi K, Igarashi H, Mori-Abe A, Saitoh M, Du B,
et al: Raloxifene inhibits estrogen-induced up-regulation of
telomerase activity in a human breast cancer cell line. J Biol
Chem. 278:43363–43372. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Engdahl C, Jochems C, Gustafsson JA, van
der Saag PT, Carlsten H and Lagerquist MK: In vivo activation of
gene transcription via oestrogen response elements by a raloxifene
analogue. J Endocrinol. 203:349–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Legault C, Maki PM, Resnick SM, Coker L,
Hogan P, Bevers TB and Shumaker SA: Effects of tamoxifen and
raloxifene on memory and other cognitive abilities: Cognition in
the study of tamoxifen and raloxifene. J Clin Oncol. 27:5144–5152.
2009. View Article : Google Scholar : PubMed/NCBI
|