Introduction
Osteosarcoma (OS) is a rare malignancy deriving from
primitive transformed cells of mesenchymal origin (1). It is ranked among the leading causes
of cancer-associated mortality in the pediatric age group in the
USA (2,3). Following the introduction of
chemotherapy in the 1970s, the 5-year-survival rate has increased
by ~50% (4). However, it is
difficult to obtain meaningful progression in patient survival
rates as a result of the low prevalence and large tumor
heterogeneity of OS (2).
Therefore, in vitro preclinical screening is a vital element
in OS drug investigations.
The canonical Wnt signaling pathway is important in
cancer progression and embryonic development. Mutations, which
promote constitutive activation of the Wnt signaling pathway, lead
to cancer (5). For example,
familial adenomatous polyposis is caused by truncations in
adenomatous polyposis coli, which promotes aberrant activation of
the Wnt pathway (6,7). Mutations in β-catenin have also been
identified in a variety of tumor types (8). Loss-of-function mutations in Axin
have been found in hepatocellular carcinoma (9). Therefore, tight control of the Wnt
signal pathway is essential in preventing cancer.
β-catenin is a pivotal molecule in the Wnt signaling
pathway, which is a dual function protein that regulates the
coordination of cell-cell adhesion and gene transcription (10). Gain-of-function mutations in
β-catenin leads to cancer, owing to the aberrant activation of
target genes of the Wnt signaling pathway (11,12).
The protein level of β-catenin in the cytoplasm is maintained
accurately through phosphorylation/degradation. Mutations in
β-catenin result in amino acid substitution, which affects the
phosphorylation level of β-catenin. Consequently, incorrectly
phosphorylated β-catenin is not recognized by the E3 ubiquitin
ligase, β-Trcp, which targets β-catenin for proteasomal degradation
(13). Dysregulation of Wnt
signaling pathways allows β-catenin to accumulate and translocate
into the nucleus, where it activates oncogenes (14–17).
Therefore, β-catenin is a potential drug target for the treatment
of cancer. It has been demonstrated that β-catenin exhibits higher
levels of expression in mesenchymal tumors (18).
Resveratrol is a natural product derived from grapes
and has been reported to have cancer chemopreventive activity
(19). Previous studies focused on
its anti-tumor activities and have demonstrated that it has potent
antiproliferative effects on tumor cells, causes cell cycle arrest
and promotes apoptosis (20–22).
The potential mechanism was suggested to be associated with the
ERKs/p53 cascade or caspase-3-dependent pathway (22,23).
However, whether the anti-OS effect of resveratrol is associated
with Wnt signaling remains to be elucidated.
In the present study, cellomics high content
screening was performed to identify a novel potential drug for the
treatment of OS.
Materials and methods
Cell culture
The human MG-63 OS cell line (Type Culture
Collection of the Chinese Academy of Sciences, Shanghai, China) was
cultured in Eagle's minimum essential medium (Gibco Life
Technologies, Grand Island, NY, USA), supplemented with 1%
non-essential amino acids, 10% fetal bovine serum, 100 U/ml
penicillin (Sigma-Aldrich, St. Louis, MO, USA) and 100 µg/ml
streptomycin (Sigma-Aldrich), and incubated at 37°C with 5%
CO2 in a humidified incubator.
High content screening
A total of ~5×102 cells were seeded into
each well of a 96-well plate and incubated at 37°C with 5%
CO2 in a humidified incubator. Following incubation for
24 h, the botanical extracts (diosmin, lemon bioflavonoids,
neosperidin dihydrochalcone, Melissa rosemary acid, oleanolic acid,
tartaric acid, ellagic acid, neohesperidin, phillyrin, betaine
anhydrous, panax quinquefolium saponin, paeoniflorin, solanesol and
resveratrol) that were purchased from Dalian Zhuoer Hightechnology
Co., Ltd. (Dalian, China) were added and incubated for 48 h. The
final concentration of each botanical extract was 10 µg/ml.
Finally, the expression of β-catenin in the MG-63 cells treated
with botanical extract was assessed by immunofluorescent staining.
In brief, the cells were fixed with −20°C methyl alcohol for 20
min. Following 10 min of natural drying, the cells were washed with
phosphate-buffered saline (PBS) three times, 0.2% Triton X-100
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) was used
for cell permeabilization (3 min). Following an additional wash
with PBS, the samples were sealed with 5% bovine serum albumin
(BSA; Beyotime Institute of Biotechnology, Shanghai, China) at room
temperature for 30 min. The monoclonal rabbit anti-human
anti-β-catenin primary antibody (1:400; ab32572; Abcam, Cambridge,
MA, USA) was diluted in 1% BSA, added into the samples and
incubated at 4°C overnight. The next day, polyclonal bovine
anti-rabbit aminomethylcoumarin-coupled secondary antibodies (IgG;
1:4,000; Wuhan Amyjet Scientific Co., Ltd., Wuhan, China) were
added for another 30 min of incubation in the dark. The samples
were washed with PBS three times and sealed with 95% glycerinum
(Sinopharm Chemical Reagent Co., Ltd.), then observed and
photographed under a ×100 magnification using a fluorescence
microscope (TFM-680; Shanghai Tuming Optical Instrument Co., Ltd.,
Shanghai, China).
Cell counting kit (CCK)-8 assay to
determine cell proliferation
The cells were seeded into a 96-well plate at a
density of 5×102 cells/well in 100 µl culture
medium. The cells were cultured for 24 h at 37°C in 5%
CO2 in a humidified incubator. The botanical extract,
resveratrol, was added to the cells at a final concentration of 10,
20 or 40 µg/ml. Following treatment for 24, 48, 72 or 96 h
at 37°C, 100 µl CCK-8 solution (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was added into the culture
well and the cells were incubated for 4 h at 37°C with 5%
CO2 in a humidified incubator. Following incubation, the
supernatant was discarded and 200 µl dimethyl sulfoxide was
added and incubated for 5 min on an oscillator (HZP-250; Shanghai
Jinghong Laboratory Equipment Co., Ltd.). Finally, the optical
density at 450 nm (OD450) was measured using a
microplate reader (iMark Model 680; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and a cell growth curve was produced.
Flow cytometry for cell apoptosis
analysis
The Annexin V/fluorescein isothiocyanate (FITC)
apoptosis detection kit (Beyotime Institute of Biotechnology) was
used to detect cell apoptosis, as previously described (24). The cells were harvested subsequent
to treatment with resveratrol alone or in combination with the
GSK3β inhibitor chir99021, which was used to investigate whether
resveratrol may target the Wnt signal pathway, and a single cell
suspension was prepared using trypsin (0.25%; Gibco-BRL, Grand
Island, NY, USA). The samples were washed with PBS, and
subsequently, centrifuged at 157 × g and 4°C for 5 min. The cells
were resuspended in binding buffer (BD Biosciences, San Diego, CA,
USA), which consisted of 10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl and
5 mM CaCl2, and the cell density was adjusted to
5×105 cells/ml. A 95-µl aliquot of the cell
suspension was mixed with 5 µl Annexin V-FITC prior to
incubation in the dark at room temperature for 10 min.
Subsequently, the suspension was washed with PBS and resuspended in
190 µl binding buffer, 10 µl propidium iodide (PI;
Sigma-Aldrich) was added. The samples were examined using a flow
cytometer (BD FACSVantage; BD Biosciences) with a 488 nm excitation
source. The test wavelength was set at 515 nm for FITC and 560 nm
for PI. The results were analyzed using CellQuest software (version
5.2.1; BD Biosciences) to determine apoptosis rate.
Western blotting
The total protein from the cells was extracted using
radioimmunoprecipitation lysis buffer, containing 1 mM
phenylmethylsulfonyl fluoride (Sinopharm Chemical Reagent Co.,
Ltd.), and the concentration was determined using the Bradford
Assay kit (Beyotime Institute of Biotechnology), according to the
manufacturer's instructions. The protein sample (20 µg) was
separated on 10% SDS-PAGE gels (Life Technologies, Gaithersburg,
MD, USA) and was subsequently transferred onto nitrocellulose
membrane (Sigma-Aldrich). The membrane was blocked in PBS,
containing Tween-20 (PBST) and 5% non-fat milk for 1 h at 4°C. The
membrane was subsequently incubated in primary monoclonal rabbit
anti-human antibodies from Abcam against c-Myc (1:100; ab32072),
β-catenin (1:400; ab32572) and GAPDH (1:4,000; ab181602), for 12 h
at 4°C. Following antibody incubation, the membrane was washed
three times with PBST and incubated for 30 min at 4°C with the goat
anti-rabbit polyclonal secondary antibody (dilution 1:5,000;
Beijing Boer Xi Technology Co., Ltd., Beijing, China) labeled with
horseradish peroxidase. Finally, the membrane was washed three
times with PBST and the protein bands were visualized with
SuperSignal (Pierce Biotechnology, Inc., Rockford, IL, USA). The
membranes were re-probed with a monoclonal rabbit anti-human
β-actin antibody (1:1,000; A2066; Sigma-Aldrich) as a loading
control at 37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the tissue/cells
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA). The total RNA (0.5 µg) was used as a template to
prepare the cDNA in a 10 µl reaction, which included 1
µl (50 µM) Oligo d (T), 0.5 µl dNTP mixture
(10 mM of each; Takara, Bio, Inc., Otsu, Japan), 0.25 µl
RNase inhibitor (40 U/µl, Takara, Bio, Inc.) and 0.5
µl RTase M-MLV (200 U/µl; Takara, Bio, Inc.). The
reaction conditions were as follows: 42°C for 1 h, followed by 85°C
for 5 sec. The cDNA was diluted five-fold and was used as the PCR
template. A SYBR® Premix Ex Taq™ kit (Takara, Bio, Inc.)
was used to detect gene expression and was performed using a PCR
system (qTOWER 2.2, Analytik Jena, Jena, Germany), according to the
manufacturer's instructions. Briefly, 2 µl cDNA was added to
reaction system with 10 µl SYBR® Premix Ex Taq
(2X), 0.4 µl PCR primer mixture (10 µM each) and 7.6
µl dH2O. The primer sequences from Sangon Biotech
Co., Ltd. (Shanghai, China) used were as follows: β-catenin,
forward 5′-TTCGCACAGTTCTACGTGCT-3′ and reverse 5′-GGTGTGCACGAACAA
GCA AT-3′; c-Myc forward 5′-CTTCTCTCCGTCCTCGGATTCT-3′ and reverse
3′-GAAGGTGATCCAGACTCTGACCTT-3′; β-actin forward
5′-GCACCACACCTTCTACAA-3′ and reverse 5′-TGCTTGCTGATCCACATCTG-3′.
The cycling conditions were as follows: 95°C for 30 sec; 40 cycles
of 95°C for 5 sec and 60°C for 30 sec; followed by a melting curve
stage in which the temperature increased from 60–95°C at a rate of
5°C/sec. The actin gene was used as a control to normalize the
relative expression levels of the target gene. The
2−ΔΔCt method was performed to calculate the relative
expression of the target gene (25).
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis of the data was performed using
Student's t-test with SPSS software, version 19.0 (IBM SPSS,
Armonk, NY, USA). P<0.01 was considered to indicate a
statistically significant difference.
Results
High content screening
β-catenin is one of the members of the canonical Wnt
signaling pathway. The dysregulation of the canonical Wnt signaling
pathway has been demonstrated in a variety of tumors. In order to
screen for drugs, which effectively inhibit the expression of
β-catenin, high content screening was performed in the human MG-63
OS cell line. The present study assessed 14 botanical extracts. The
results demonstrated that, of the 14 botanical extracts,
resveratrol markedly inhibited the protein expression of β-catenin,
whereas no pronounced changes were observed in the remaining 13
botanical extracts (Fig. 1).
Therefore, it was hypothesized that resveratrol may be used as a
potential drug for the treatment of OS.
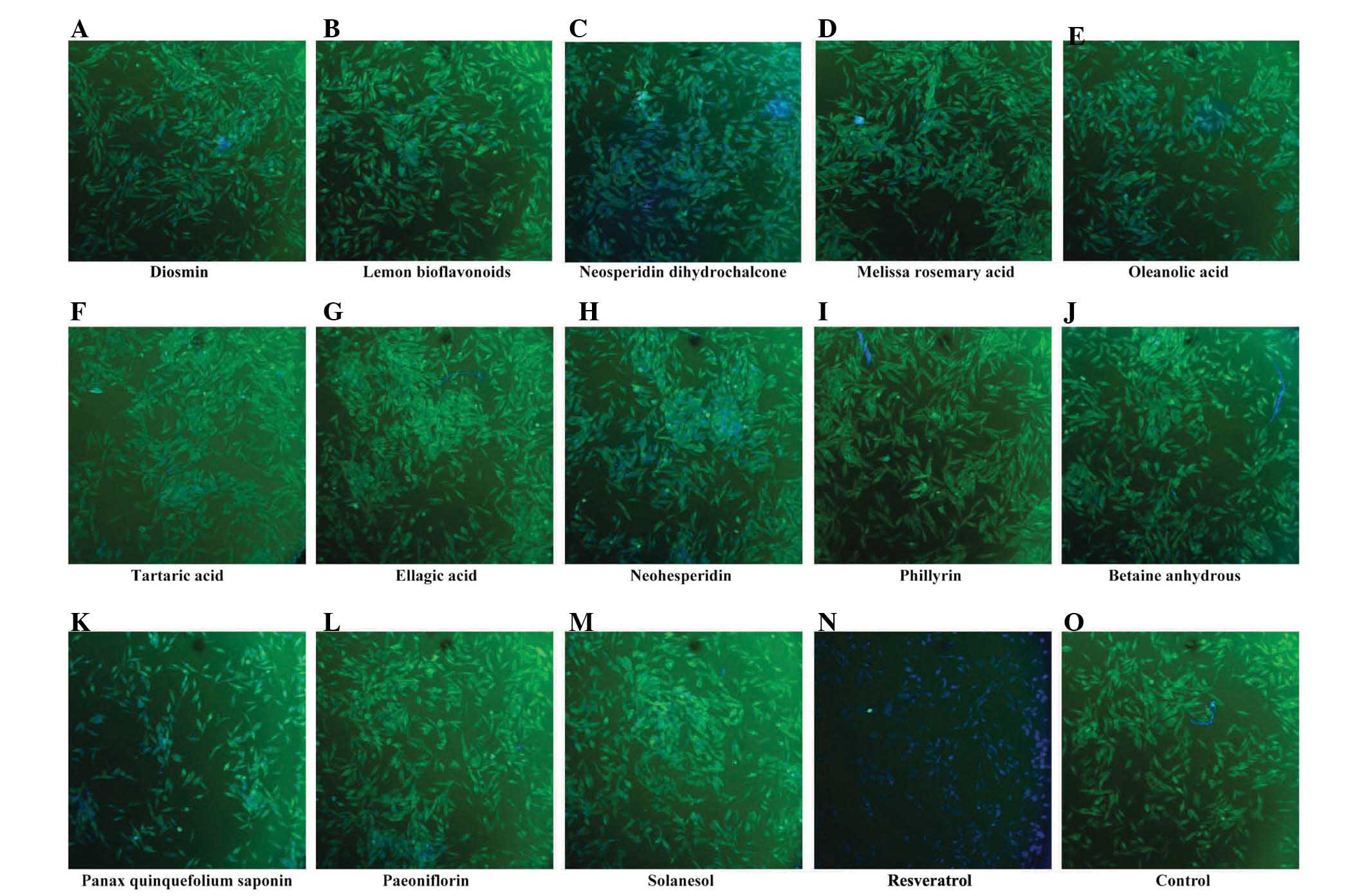 | Figure 1Resveratrol downregulates the protein
expression of β-catenin in human MG-63 osteosarcoma cells. A total
of 14 botanical extracts (20 µg/ml) were examined by
immunofluorescent staining and a fluorescence microscope, at a
magnification of ×100: (A) Diosmin, (B) Lemon bioflavonoids, (C)
Neosperidin dihydrochalcone, (D) Melissa rosemary acid, (E)
Oleanolic acid, (F) Tartaric acid, (G) Ellagic acid, (H)
Neohesperidin, (I) Phillyrin, (J) Betaine anhydrous, (K) Panax
quinquefolium saponin, (L) Paeoniflorin, (M) Solanesol, (N)
Resveratrol and an (O) untreated control. Following drug treatment
for 48 h, immuno-fluorescence staining was performed to determine
changes in the expression of β-catenin in the MG-63 cells. |
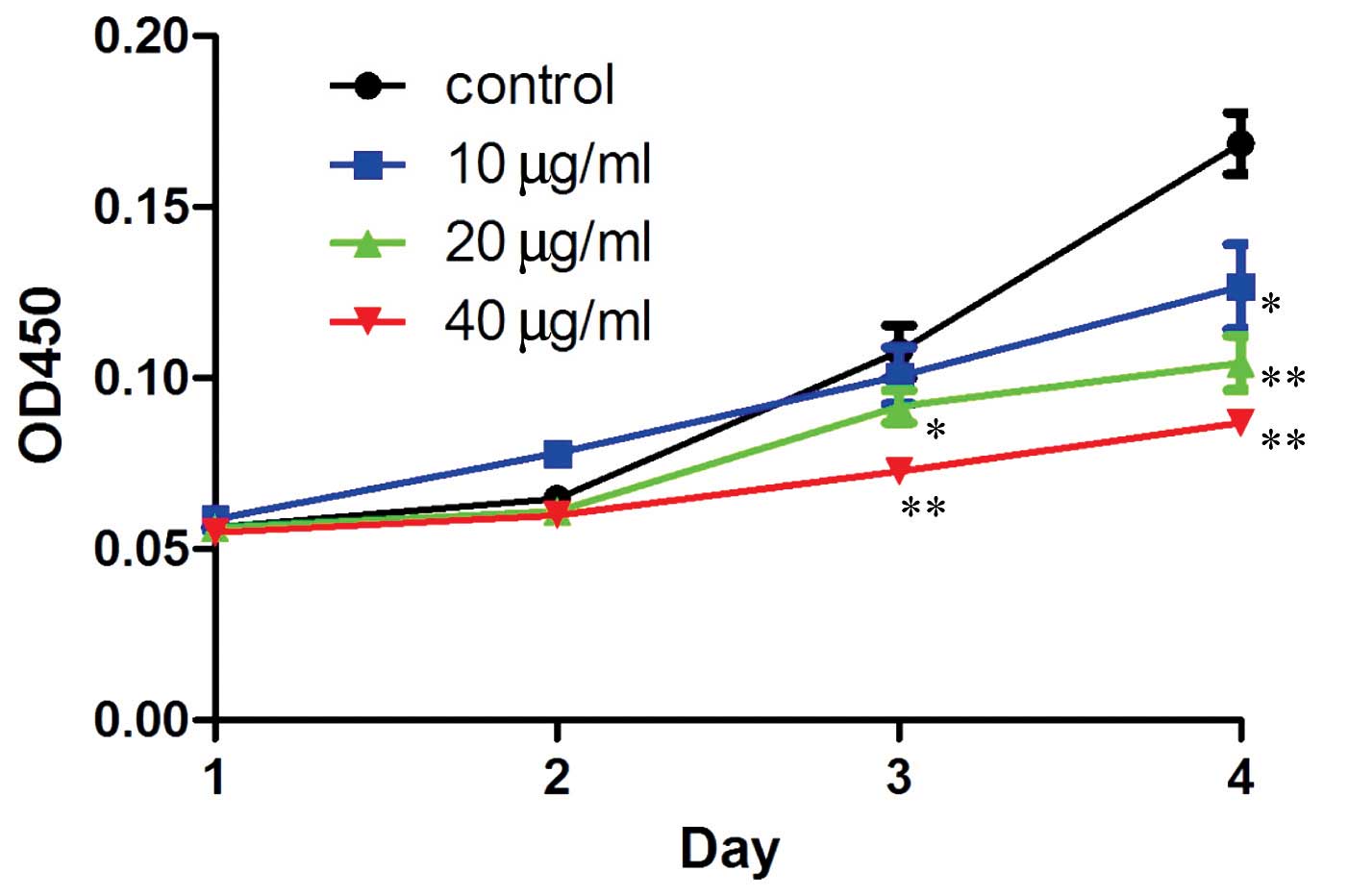
Resveratrol inhibits the proliferation of
human MG-63 OS cells
In order to confirm the hypothesis that resveratrol
may benefit OS treatment, a CCK-8 assay was performed to detect the
proliferation rate of the human MG-63 OS cells treated with
resveratrol. The results demonstrated that resveratrol
significantly (P=0.043, 20 µg/ml treatment group, 3 days;
P=0.006, 40 µg/ml treatment group, 3 days; P=0.037, 10
µg/ml treatment group, 4 days; P=0.002, 20 µg/ml
treatment group, 4 days; P=0.0009, 40 µg/ml treatment group,
4 days; treatment v.s. control). inhibited the proliferation of the
human MG-63 OS cells. Furthermore, the inhibition rate was dose-and
time-dependent (Fig. 2).
Analysis of apoptosis
The apoptotic changes in the human MG-63 OS cells
treated with resveratrol were determined using flow cytometry. The
results revealed that the apoptotic rate of the cells increased
significantly (P=0.046) following 72 h drug treatment, and the
apoptotic rate reached 27.5% in the group treated with 40
µg/ml resveratrol (Fig.
3D). In the Wnt signaling pathway, GSK3β is a negative
regulator, which is involved in the degradation of β-catenin
(26). In the present study,
chir99021, an inhibitor of GSK3β, was used to inhibit GSK3β.
Following treatment with resveratrol and chir99021, cell apoptosis
was detected using flow cytometry. As shown in Fig. 3, the apoptotic rate decreased
markedly, compared with the control groups. These results suggested
that resveratrol inhibited the proliferation of the human MG-63 OS
cells through the apoptotic pathway.
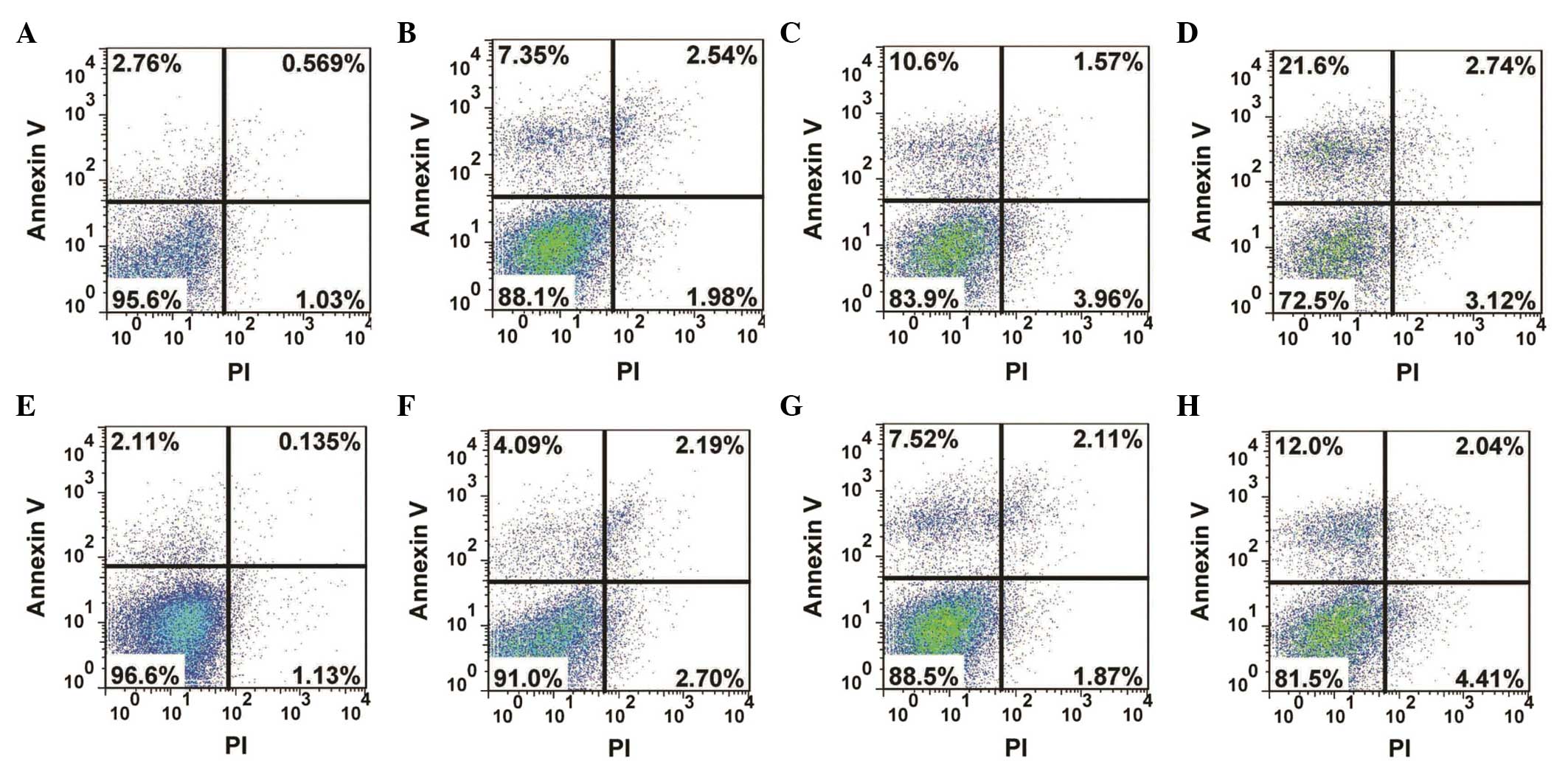 | Figure 3Analysis of apoptosis in the human
MG-63 osteosarcoma cell line following treatment with resveratrol
alone or in combination with the GSK3β inhibitor, chir99021.
Following drug treatment for 72 h, the cells were labeled with
annexin V and PI and the apoptosis was detected using flow
cytometry. (A) 0 µg/ml, (B) 10 µg/ml, (C) 20
µg/ml and (D) 40 µg/ml resveratrol, (E) dimethyl
sulfoxide, (F) 3 µM chir99021+10 µg/ml, (G) 3
µM chir99021+20 µg/ml, (H) 3 µM chir99021+40
µg/ml resveratrol. Upper left quadrant, apoptotic cells;
upper right, dead and late apoptotic cells; lower left, living
cells; lower right, mechanically injured cells. PI, propidium
iodide. |
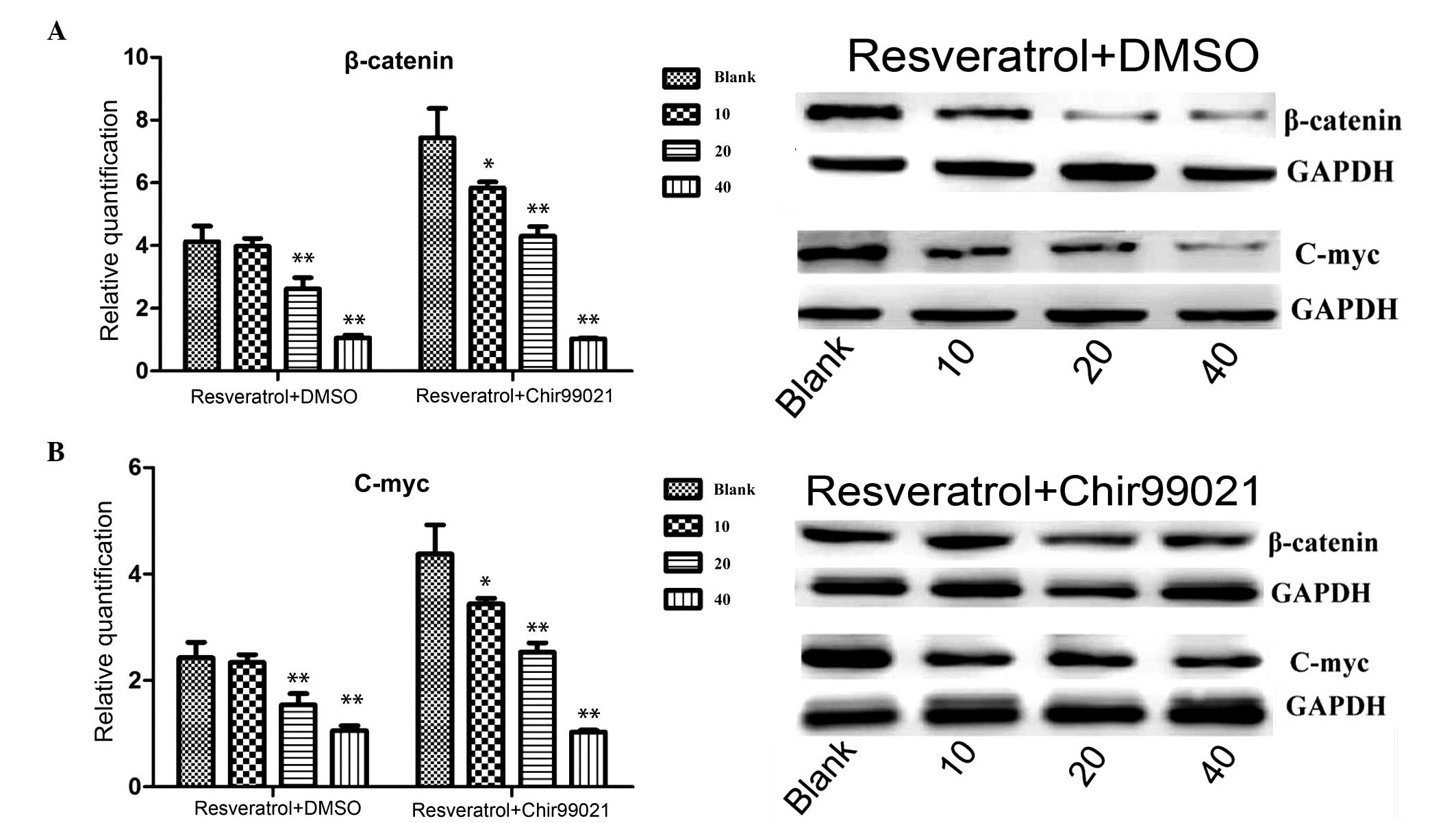
Immunoblotting and RT-qPCR
To further understand the mechanism underlying the
effects of resveratrol, western blotting and RT-qPCR were performed
following treatment with resveratrol alone or combined with
chir99021. As shown in Fig. 4,
β-catenin was markedly (mRNA level: P=0.003, 20 µg/ml
treatment group; P=0.0017, 40 µg/ml treatment group;
resveratrol + DMSO, treatment v.s. control) downregulated at the
protein and mRNA expression levels following treatment with
resveratrol, however, the expression of β-catenin increased
following treatment with resveratrol and chir99021. In addition,
c-Myc, one of target genes of the Wnt signaling pathway, was also
detected. c-Myc was markedly (mRNA level: P=0.003, 20 µg/ml
treatment group; P=0.0023, 40 µg/ml treatment group;
resveratrol + DMSO treatment v.s. control) downregulated at the
protein and mRNA expression levels following treatment with
resveratrol. When the inhibitor, chir99021, was added, the
expression of c-Myc increased.
Discussion
Although OS is a rare type of primary malignancy, it
is ranked among the leading causes of cancer-associated mortality
in the pediatric age group (2,27).
With the introduction of chemotherapy, the 5-year-survival rate has
increased to 60–70%, however, ~30 years have passed since the
method was introduced, and the development of novel therapeutic
drugs for the treatment of OS has been slow (28). Therefore, the development of novel
drugs for the treatment of OS is urgently required to overcome this
bottleneck. The present study aimed to screen for a novel
therapeutic drug for the treatment of OS using high content
screening and reveal its functional mechanism.
A mutation in β-catenin, one of the key members of
the Wnt signaling pathway, has been identified in a variety of
types of cancer (11,29–31).
Therefore, the present study used β-catenin as a drug target and
performed high content screening to identify novel drugs against
the human MG-63 OS cell line. As shown in Fig. 1, the botanical extract,
resveratrol, markedly downregulated the expression of β-catenin at
the protein level. A previous study demonstrated that the aberrant
expression of β-catenin activates its numerous downstream targets,
a number of which are associated with cancer progression (32). Therefore, the present study
hypothesized that resveratrol may inhibit the proliferation of
human MG-63 OS cells. Based on this hypothesis, the proliferation
rate of human MG-63 OS cells treated with resveratrol was assessed.
The results were consistent with the hypothesis that resveratrol
can significantly (P<0.05) inhibit proliferation of the human
MG-63 OS cell line. The present study subsequently aimed to further
understand the underlying mechanism. Aberrant activation of the Wnt
signaling pathway can initiate the expression of numerous
downstream genes, including c-Myc (33), cyclin D1 (34) and survivin (35). Survivin is an inhibitor of
apoptosis, therefore, the apoptosis of human MG-63 OS cells treated
with resveratrol was assessed using flow cytometry. As shown in
Fig. 3, the apoptotic ratio
markedly increased following treatment with resveratrol. In
parallel experiments, resveratrol and chir99021, an inhibitor of
GSK3β that can activate the Wnt signaling pathway, were added and
the apoptotic response of the cells was analyzed. The results
demonstrated that the apoptotic ratio was markedly reduced,
compared with resveratrol-only group. These data suggested that
resveratrol inhibited the proliferation of human MG-63 OS cell by
inhibiting the canonical Wnt signaling pathway. To further confirm
this conclusion, western blotting and RT-qPCR were performed to
determine the expression levels of β-catenin and its target gene,
c-Myc. As shown in Fig. 4, the
mRNA and protein expression levels of β-catenin and c-Myc were
markedly downregulated. Following the addition of chir99021, the
expression levels were increased. This data suggested that
resveratrol inhibited the Wnt signaling pathway by downregulating
the expression of β-catenin.
In conclusion, the present study identified
resveratrol as a novel botanical extract, which markedly inhibited
the proliferation of human MG-63 OS cells by downregulating the
expression of β-catenin in the Wnt signaling pathway. Although the
present study was preliminary, it indicates potential for the
treatment of OS in the future. Subseqeunt experiments in animal
models are required to determine the inhibitory efficiency for OS
cells.
Acknowledgments
This study was supported by the National Natural
Foundation of China (grant. no. 60171009).
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Pediatric and Adolescent Osteosarcoma. Jaffe N,
Bruland OS and Bielack S: Springer US; pp. 3–13. 2010
|
|
2
|
Botter SM, Neri D and Fuchs B: Recent
advances in osteosarcoma. Curr Opin Pharmacol. 16:15–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Linabery AM and Ross JA: Trends in
childhood cancer incidence in the U.S.(1992–2004). Cancer.
112:416–432. 2008. View Article : Google Scholar
|
|
4
|
Allison DC, Carney SC, Ahlmann ER,
Hendifar A, Chawla S, Fedenko A, Angeles C and Menendez LR: A
meta-analysis of osteosarcoma outcomes in the modern medical era.
Sarcoma. 2012:7048722012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kinzler KW, Nilbert MC, Su LK, Vogelstein
B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P and
McKechnie D: Identification of FAP locus genes from chromosome
5q21. Science. 253:661–665. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishisho I, Nakamura Y, Miyoshi Y, Miki Y,
Ando H, Horii A, Koyama K, Utsunomiya J, Baba S and Hedge P:
Mutations of chromosome 5q21 genes in FAP and colorectal cancer
patients. Science. 253:665–669. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giles RH, van Es JH and Clevers H: Caught
up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.PubMed/NCBI
|
|
9
|
Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa
N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al:
AXIN1 mutations in hepatocellular carcinomas, and growth
suppression in cancer cells by virus-mediated transfer of AXIN1.
Nat Genet. 24:245–250. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Korinek V, Barker N, Morin PJ, van Wichen
D, de Weger R, Kinzler KW, Vogelstein B and Clevers H: Constitutive
transcriptional activation by a beta-catenin-Tcf complex in
APC−/−colon carcinoma. Science. 275:1784–1787. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He TC, Sparks AB, Rago C, Hermeking H,
Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tetsu O and McCormick F: Beta-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roose J, Huls G, van Beest M, Moerer P,
van der Horn K, Goldschmeding R, Logtenberg T and Clevers H:
Synergy between tumor suppressor APC and the beta-catenin-Tcf4
target Tcf1. Science. 285:1923–1926. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jho EH, Zhang T, Domon C, Joo CK, Freund
JN and Costantini F: Wnt/beta-catenin/Tcf signaling induces the
transcription of Axin2, a negative regulator of the signaling
pathway. Mol Cell Biol. 22:1172–1183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ng TL, Gown AM, Barry TS, Cheang MC, Chan
AK, Turbin DA, Hsu FD, West RB and Nielsen TO: Nuclear beta-catenin
in mesenchymal tumors. Mod Pathol. 18:68–74. 2005. View Article : Google Scholar
|
|
19
|
Jang M, Cai L, Udeani GO, et al: Cancer
chemopreventive activity of resveratrol, a natural product derived
from grapes. Science. 275:218–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Joe AK, Liu H, Suzui M, Vural ME, Xiao D
and Weinstein IB: Resveratrol induces growth inhibition, S-phase
arrest, apoptosis, and changes in biomarker expression in several
human cancer cell lines. Clin Cancer Res. 8:893–903.
2002.PubMed/NCBI
|
|
21
|
Athar M, Back JH, Tang X, et al:
Resveratrol: A review of preclinical studies for human cancer
prevention. Toxicol Appl Pharmacol. 224:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alkhalaf M and Jaffal S: Potent
antiproliferative effects of resveratrol on human osteosarcoma
SJSA1 cells: Novel cellular mechanisms involving the ERKs/p53
cascade. Free Radic Biol Med. 41:318–325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alkhalaf M, El-Mowafy A, Renno W, Rachid
O, Ali A and Al-Attyiah R: Resveratrol-induced apoptosis in human
breast cancer cells is mediated primarily through the
caspase-3-dependent pathway. Arch Med Res. 39:162–168. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He N and Zhang Z: Baicalein suppresses the
viability of MG-63 osteosarcoma cells through inhibiting c-MYC
expression via Wnt signaling pathway. Mol Cell Biochem.
405:187–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Nakamura T, Hamada F, Ishidate T, Ishidate
T, Anai K, Kawahara K, Toyoshima K and Akiyama T: Axin, an
inhibitor of the Wnt signalling pathway, interacts with
beta-catenin, GSK-3beta and APC and reduces the beta-catenin level.
Genes Cells. 3:395–403. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-Where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar
|
|
28
|
Yamamoto N and Tsuchiya H: Chemotherapy
for osteosarcoma-where does it come from? What is it? Where is it
going? Expert Opin Pharmacother. 14:2183–2193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyaki M, Iijima T, Kimura J, Yasuno M,
Mori T, Hayashi Y, Koike M, Shitara N, Iwama T and Kuroki T:
Frequent mutation of beta-catenin and APC genes in primary
colorectal tumors from patients with hereditary nonpolyposis
colorectal cancer. Cancer Res. 59:4506–4509. 1999.PubMed/NCBI
|
|
30
|
Morin PJ: β-catenin signaling and cancer.
Bioessays. 21:1021–1030. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
32
|
Polakis P: The oncogenic activation of
beta-catenin. Curr Opin Genet Dev. 9:15–21. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He T-C, Sparks AB, Rago C, Hermeking H,
Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hedberg Y, Ljungberg B, Roos G and
Landberg G: Expression of cyclin D1, D3, E and p27 in human renal
cell carcinoma analysed by tissue microarray. Br J Cancer.
88:1417–1423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang LQ, Fang DC, Wang RQ and Yang SM:
Effect of NF-kappaB, survivin, Bcl-2 and Caspase3 on apoptosis of
gastric cancer cells induced by tumor necrosis factor related
apoptosis inducing ligand. World J Gastroenterol. 10:22–25.
2004.
|