Introduction
The Chinese yali pear, Pyrus bretschneideri
Rehd., is one of the main cultivated pear varieties in northern
China and is an important fruit of export earnings. It is not only
popular due to its sweet taste and nutritious value, but also due
to its high water content. The medicinal values of the yali pear
include re-hydration of the body, moistening of the lungs,
hepatoprotective properties and relieving of cough and asthma. In
recent years, with the continuous improvement of the standard of
living in China, the demand for yali pears has increased. In order
to provide yali pears throughout the year, it is required to delay
their ripening, preserve their freshness and extend their
shelf-life, which is currently a major challenge for the yali pear
industry (1). Numerous studies on
the extension of the storage period of yakli pears have been
performed, which indicated that the effects of the external
environment at harvest as well as during storage and processing
affect the aging of yali pears (2,3). A
series of preservation measures were developed; however, these
measures were not able to fundamentally control the aging of pears
(4). Therefore, in order to
overcome the challenge of extending the shelf-life of yali pears,
it is required to generate novel varieties of yali pear by
modifying their genes associated with storage-associated aging
characteristics.
As the pear ripens, its respiratory rate increases,
and in the climacteric stage, increased levels of ethylene, which
has an important regulatory role in its ripening, are released
(5). Therefore, reducing
endogenous synthesis of ethylene in yali is a fundamental way to
extend its storage and preservation period. The biosynthetic
pathway of ethylene in plants is as follows: Methionine is
converted in to S-adenosylmethionine, which is then
transformed into 1-amino-cyclopropane-1-carboxylic acid (ACC),
which is then oxidized by ACC oxidase (ACO) to form ethylene
(6). As ACC is the direct
precursor of ethylene, decreases in the content of ACO in plants
directly affect the in vivo synthesis of ethylene (7). Based on the important regulatory role
of ACO in the endogenous synthesis of ethylene, the aim of the
present study was to inhibit the expression of ACO by anti-sense
RNA technology. For this, complementary DNA (cDNA) fragments of the
ACO gene and anti-sense expression vectors were constructed and
subsequently transfected into pear plantlets using
agrobacterium-mediated genetic transformation technology.
Anti-sense genetic transformation of the ACO gene was performed in
the pear plantlets in order to provide germplasm resources for the
cultivation of novel storage varieties of pears, therefore
providing a reference for further applications of anti-sense RNA
technology in the genetic improvement of pears and other fruit.
Materials and methods
Materials
Fully mature Yali fruit was collected from the Park
of Hebei Agricultural University on the 25th August. Ripe and
disease-free fruits were picked and transported to the lab in ice
boxes (4°C) for cryopreservation. The Escherichia
(E.) coli strain DH5α, the cloning vector pUCm-T, an
UNIQ-10 Column Medi-Preps small-amount plasmid extraction kit
(B511241) and a DNA gel extraction kit (UNIQ-10 Column MicroDNA Gel
Extraction kit; B511139) were purchased from Shanghai Sangon Co.
(Shanghai, China); Oligo (dT) 15, Moloney murine leukemia virus
reverse transcriptase (M-MLV RTase), Taq enzyme, desoxynucleotide
triphosphate (dNTP), restriction endonucleases, T4 ligase, a genome
extraction kit (Takara MiniBEST Plant Genomic DNA Extraction kit;
9768) and DNA markers (1 kb DNA Ladder, DL 2000 Marker, Wide-Range
DNA Ladder and 250 bp DNA Ladder) were purchased from Takara Bio
Inc., (Otsu, Japan); digoxin labeling and detection kits (DIG DNA
Labeling and Detection kit 11093657910) were provided by Roche
Diagnostics (Basel, Switzerland); other conventional chemical
reagents were all obtained from Sangon Biotech Co., Ltd. (Shanghai,
China). The eukaryotic expression vector pBI121 and agrobacterium
LBA4404 were provided by Dr Xiao-Li Liu (Biological Science and
Engineering College, Hebei Economics University, Shijiazhuang,
China) and preserved in our laboratory.
Primer design
According to the conserved sequences of ACO genes
published on GenBank (http://www.ncbi.nlm.nih.gov/genbank), a pair of
polymerase chain reaction (PCR) primers was designed by Oligo6.0
software (Molecular Biology Insights, Inc., Colorado Springs, CO,
USA). Upstream primer, 5′-TTGTGAGAACTGGGGTTTCTTTGAG-3′ and
downstream primer, 5′-TCATAGCTTCAAACCTTGGTTCTTT-3′; primers were
synthesized by Shanghai Sangon Co.
RNA isolation and reverse transcription
(RT)-PCR
Total RNA of yali fruit was extracted and purified
according to the improved cetyltrimethylammonium bromide method
(8). The purified RNA was
subjected to RT with M-MLV RTase with oligo (dT) 15 as the primer.
The obtained RT product was used as a template for PCR
amplification; the reaction was performed in a 25-µl
mixture, which contained the cDNA template (50 ng), 10X PCR buffer
(2.5 µl), Taq enzyme (1.5 units), 30 ng of upstream and
downstream primers each, Mg2+ (2.0 mmol/l) and dNTP
(0.25 mmol/l). PCR was performed in a T Gradient PCR instrument
(Biometra, Göttingen, Germany). The amplification program was as
follows: Pre-denaturation at 94°C for 7 min, denaturation at 94°C
for 50 sec, annealing at 52°C for 1 min and extension at 72°C for 1
min for a total of 35 cycles; subsequently, full extension was
performed at 72°C for 5 min. The PCR products were separated by 1%
agarose gel electrophoresis before the target fragments were
collected using a DNA gel extraction kit. The target fragments were
linked with pUCm-T vector and transformed into DH5α competent E.
coli cells via the heat shock (thermal stimulation conversion)
method for white-blue plaque selection. In brief, selective medium
plates were prepared, the prepared competent cells were thawed on
ice, then ~20 ng of plasmid DNA was added and mixed gently, prior
to placing on ice for 30 min. The cell solution was added to tubes
that were then placed in a 42°C water bath for heat shock for 90
sec, then were rapidly transferred to a centrifuge tube in an ice
bath for 1–2 min. A total of 400 µl Lysogeny Broth medium
(Sangon Biotech Co., Ltd.) was then added with gentle shaking at
37°C for 45 minutes to allow for the recovery of bacteria. The
cells were then cultured for 12–16 h for the observation of white
colonies at 37°C. Positive recombinant colonies were selected and
the plasmid was extracted by a small-amount extraction kit after
amplification and reproduction; after restriction enzyme digestion
(XbaI/SacI), the bacteria were sequenced by Shanghai
Sangon Co.
Reverse insertion of the pear ACO gene
into the expression vector and agrobacterium transduction
The sequenced fragment of the ACO gene was inversely
inserted into the multiple cloning site of the pUCm-T vector to
obtain a recombinant plasmid, which was double-digested by
XbaI/SacI together with eukaryotic expression vector
pBI121. After electrophoretic separation, the target gene fragments
and linear vector pBI121 without Gus gene were recovered. Following
connecting with 1 µl 10X ligation buffer (Sangon Biotech
Co., Ltd.), 1 µl PEG4000 (Sangon Biotech Co., Ltd.), 1
µl pUCm-T vector, 3 µl PCR Production (Sangon Biotech
Co., Ltd.) and 1 µl T4 ligase overnight, agrobacterium
LBA4404 was transformed by the freeze-thaw method and recombinant
colonies were screened on a Kan/Rif antibiotic tablet (Sangon
Biotech Co., Ltd.). Following amplification and reproduction, the
plasmid was extracted using the small-amount extraction kit, and
identified by XbaI/SacI digestion and PCR. PCR
primers and reaction conditions were the same as mentioned
above.
Genetic transformation of pear
plants
Referring to the re-generation and genetic
transformation system of Li et al (9,10),
genetic transformation of yali was performed. The pear leaf callus
was pre-cultured for two days was used as a starting material for
genetic transformation. It was incubated in a suspension of
agrobacterium grown in agrobacterium culture medium (Sangon Biotech
Co., Ltd.) with a optical density at 600 nm (OD600) of
0.8 for 10 min; after removing excess bacteria with a filter paper,
the leaf was inoculated in regeneration medium [NN69 +
6-benzyl-aminopurine (BA) 3.0 mg/l + indole-3-acetic acid (IAA) 0.5
mg/l; Shanghai Shenggong Co., Ltd., Shanghai, China] and
co-cultured in the dark for two days. Subsequently, 200
µg/ml cefotaxime sodium (Cef) was added as an anti-bacterial
agent. The resistant buds were then transferred into
regeneration-selective medium [NN69 + BA 3.0 mg/l + IAA 0.5 mg/l +
Cef 200 mg/l + kanamycin (Kan) 5 mg/l; Shanghai Shenggong Co.,
Ltd.]; when the length of adventitious buds was 4–6 cm, 1.5–2 cm
fragments with buds or axillary were resected and inoculated in
resistant-seedling selective medium (MS + BA 1.0 mg/l +
1-naphthaleneacetic acid 0.5 mg/l + Cef 200 mg/l + Kan 20 mg/l;
Shanghai Shenggong Co., Ltd.) to screen Kan-resistant regenerated
seedlings.
PCR identification of transgenic
pear
According to the NPT II gene sequence published in
GenBank, a primer pair (upstream primer, 5′-CGTCTGTCGAGAAGTTTC-3′
and downstream primer, 5′-TACTTCTACACAGCCATC-3′) was designed.
According to the method provided in the genome extraction kit of
Takara Bio Inc., the DNA of Kan-resistant transgenic pear was
isolated and amplified by PCR. 10 µl PCR product was mixed
with 2 µl 6X loading buffer and separated on a 1% agarose
gel with a voltage of 5 V/cm for 20 min prior to development of the
1% gel in buffer solution with heating, and capturing of images
with the Nikon D90 microscope (Nikon Corporation, Tokyo,
Japan).
Southern blot analysis of transgenic
pear
According to the 35S promoter sequence, the upstream
primer (5′-AGGCTTACGCAGCAGGTCTCAT-3′) and downstream primer
(5′-GGAAGGGTCTTGCGAAGGAATG-3′) were designed. Taking the pBI121
plasmid as a template, the Southern hybridization probe was
prepared by PCR amplification, and then Southern blot was
hybridized with the EcoRI-digested genome of transgenetic
pear. Probe preparation and Southern hybridization were performed
according to the instructions of the digoxin labeling and detection
kit (Roche Diagnostics) to detect copies of transferred anti-sense
ACO gene in the genome.
Data repetition
All experiments were repeated a total of five times,
in order to verify the results.
Results
Cloning and analysis of cDNA fragments of
the pear ACO gene
According to the conserved sequence of the ACO gene,
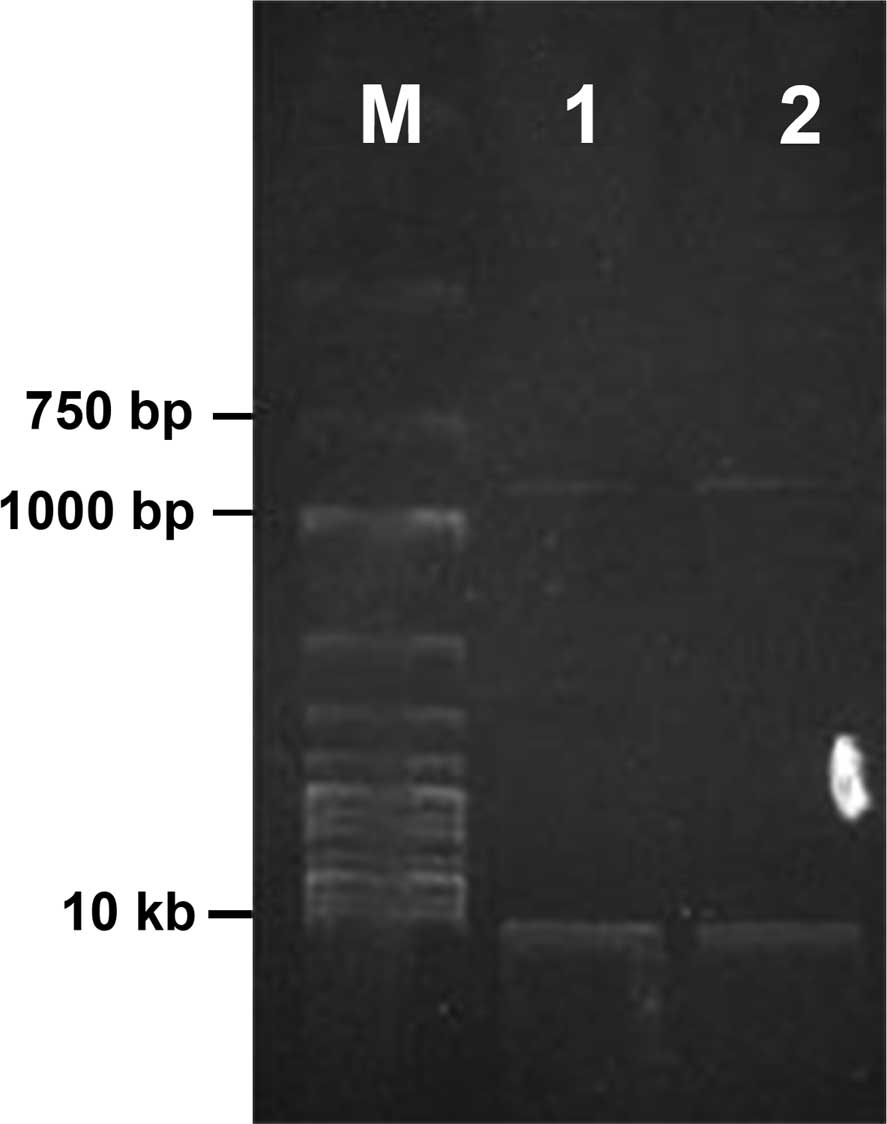
a pair of specific primers were designed; using the total RNA of a
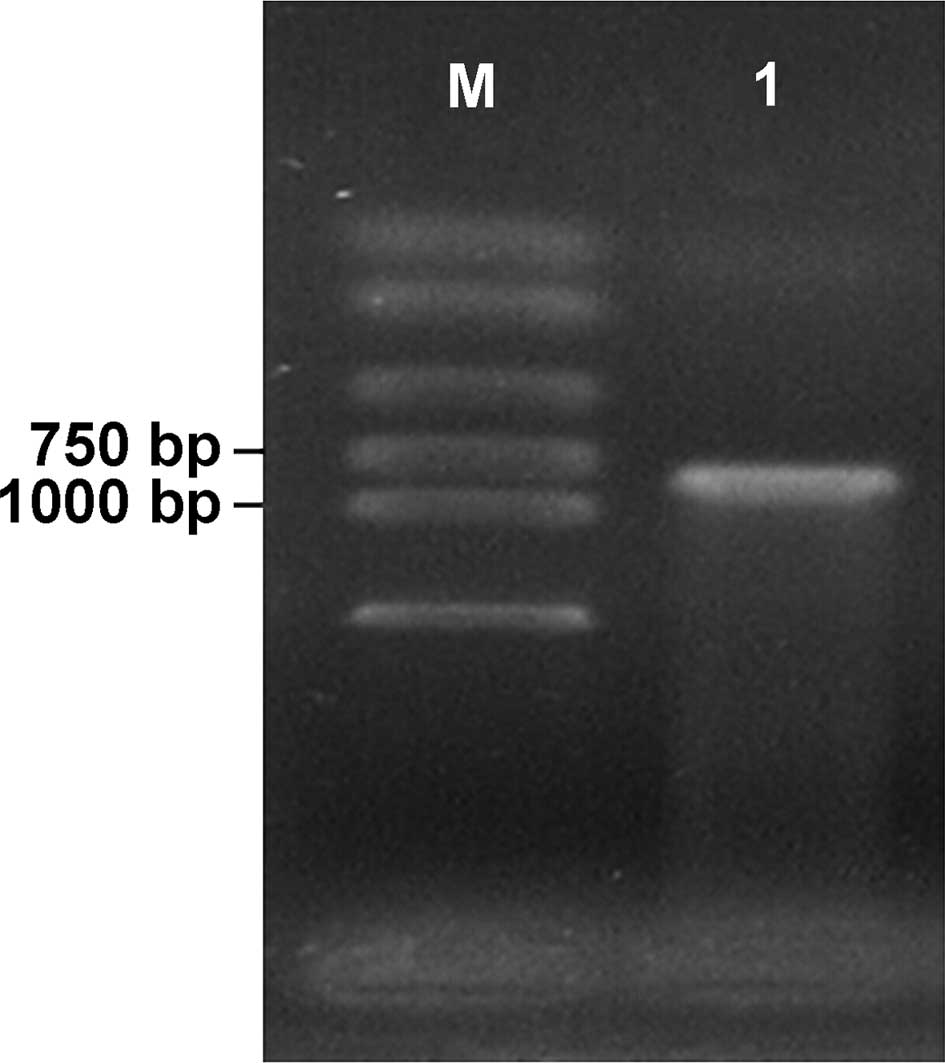
ripe pear as template, a cDNA fragment of 850 bp was obtained by
RT-PCR amplification, as shown in Fig
1. Following being recovered and purified, the fragment was
inserted into a pUCm-T vector and transformed into E. coli
DH5α competent cells; after white-blue plaque selection, positive
recombinant colonies were selected and the plasmid was extracted by
a small-amount extraction kit after amplification and reproduction;
after double digested by EcoRI and XbaI,
electrophoresis was performed; the results are shown in Fig. 2. Digestion of the recombinant
plasmid provided two fragments; apart from the pUCm-T vector
fragment of 2,800 bp, an inserted fragment of ~850 bp was present,
which was consistent with the length of the amplified fragment,
confirming that the amplified fragments had been successfully
inserted into the recombinant plasmid.
Sequencing of the digested positive
colonies
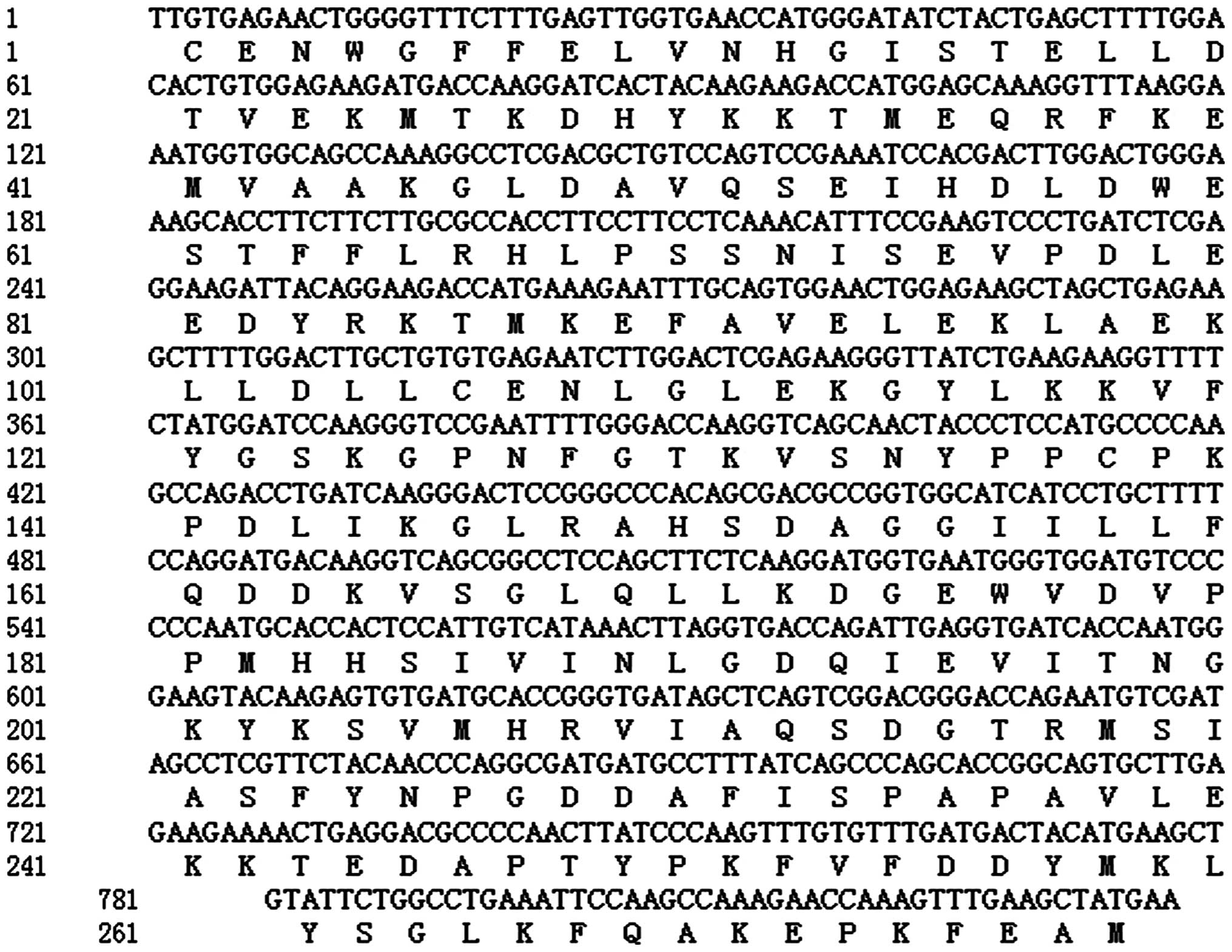
The sequencing results (produced by Shanghai Sangon
Co.) indicated that the cloned cDNA fragment was 831 bp, the
largest reading frame of which was located between 2 and 829 bp
with a total length of 828 bp, encoding 276 amino acid residues
(Fig. 3). In total, nine cDNA
sequences of pear ACO gene were retrieved from GeneBank: EF451060
(Xingshui pear), D67038 (Changshilang pear), EU047708 (snow pear),
AB265797 (Yali), X87097 (Paz - Qassam pear), EU047709 (Golden
Pear), EU047710 (Qieli), AJ504857 (Portugal crisp pear) and
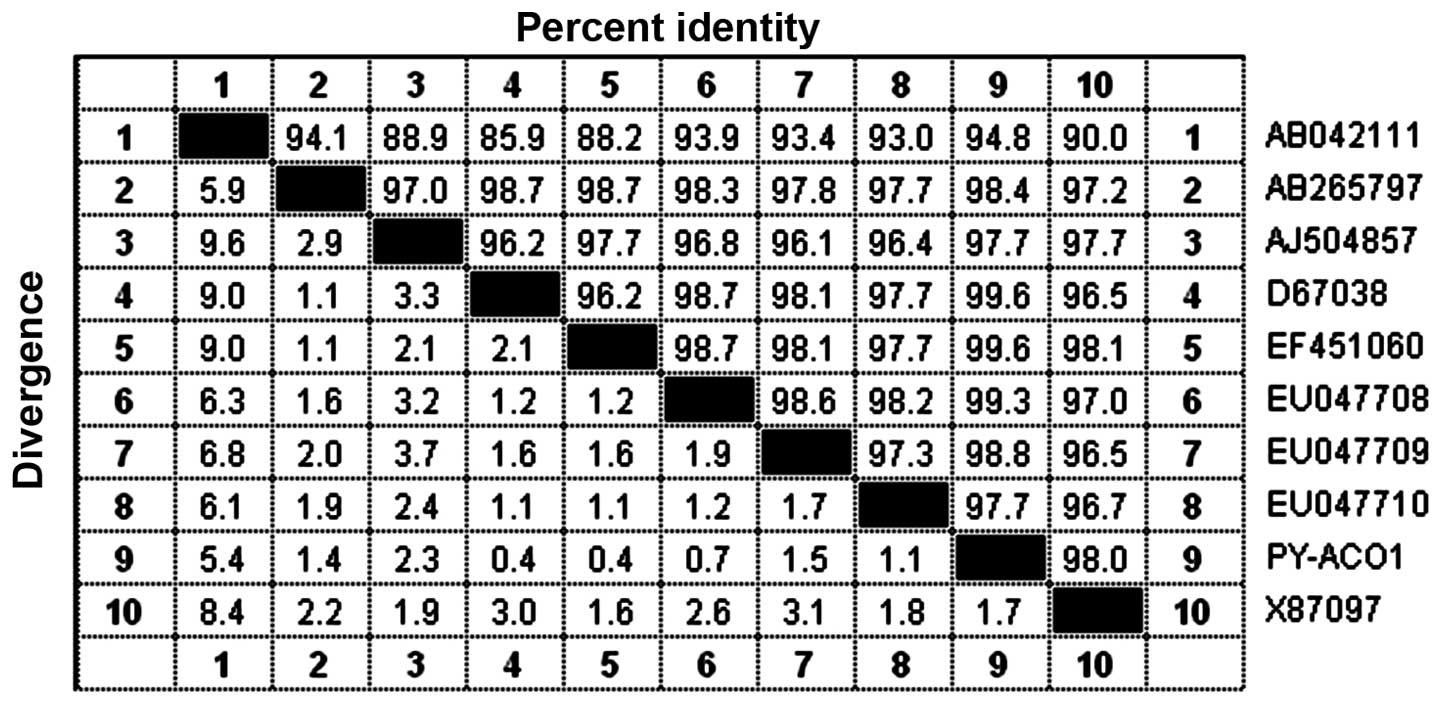
AB042111 (Changshilang pear). Using DNASTAR software, version 7.0
(DNASTAR, Inc., Madison, WI, USA), these sequences were compared
with the cloned fragment (PY-ACO1) in nucleic acid sequence
homology (Fig. 4); the analysis
showed that the cloned gene shared no less than 94% identity with
other published ACO genes of nine varieties of pear, and was ~100%
homologous to that of the Xingshui pear, the Changshilang pear and
the snow pear. This showed that the obtained fragment had a high
identity with other published ACO genes, confirming that the
present fragment was a cDNA fragment of the ACO gene of the Yali
pear. This fragment was named as PY-ACO1 and was given the GenBank
accession number EU333282.
Construction of ACO anti-sense expression
vector
Two modes (positive and negative) were available for
PY-ACO1 insertion into the pUCm-T vector; the sequenced plasmid
with reverse insertion was selected and double-digested by
XbaI/SacI; small pieces were recovered by
electrophoresis, and directionally linked with the same digested
linear vector pBI121 in order to construct the anti-sense
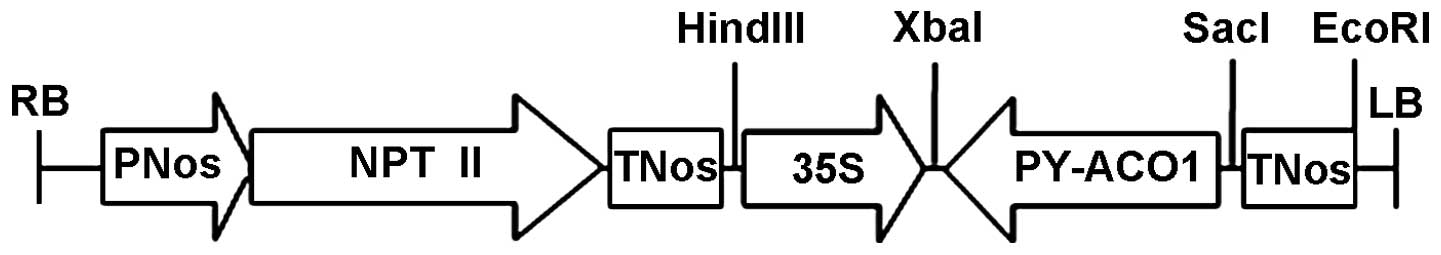
expression vector for pear ACO. The vector map is shown in Fig. 5.
The obtained anti-sense expression vector was used
to transform agrobacterium LBA4404; single colonies were selected
using a Kan/Rif antibiotic tablet for propagation; the recovered
plasmids were double-digested by XbaI/SacI. Analysis
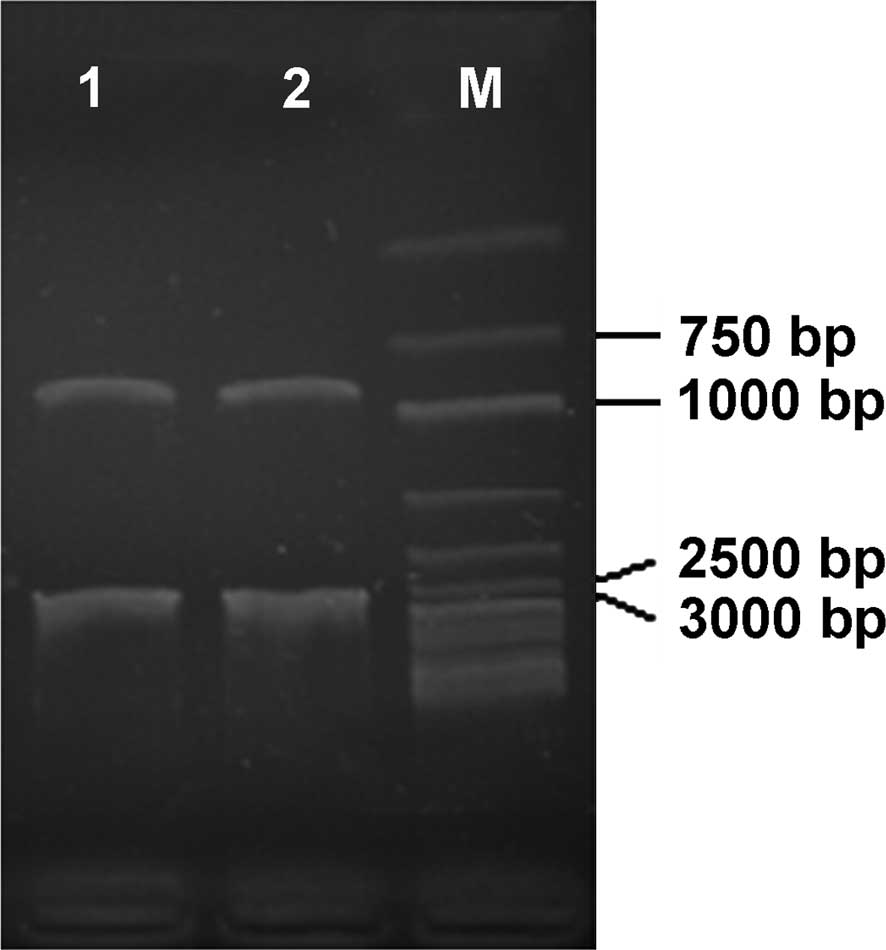
of the products showed that digestion provided a fragment of ~850
bp (Fig. 6), consistent with the
length of the PY-ACO1 fragment. The extracted plasmid was further
identified by PCR using PY-ACO1 as the primer, and the
amplification result is shown in Fig.
1, confirming once again that the anti-sense expression vector
of pear ACO was successfully constructed and had been transformed
into agrobacterium LBA4404, which were then used in studies of
genetic transformation.
Transformation of yali by
agrobacterium-mediated genetic anti-sense expression vector
Genetic transformation of yali was performed
referring to the regeneration and genetic transformation system of
Li et al (10). The pear
leaf callus pre-cultured for two days was used as a starting
material for genetic transformation. It was incubated in the
agrobacterium suspension with an OD600 of 0.8 for 10 min
and then inoculated in regeneration medium and co-cultured in the
dark for two days; subsequently, 200 µg/ml cefotaxime sodium
was added as an anti-bacterial agent. 412 resistant buds were
obtained within a week. The resistant buds were then transferred
into regeneration-selective medium; when the length of the
adventitious buds was 4–6 cm, 1.5–2-cm fragments with buds or
axillary were resected and inoculated in resistant-seedling
selective medium; the medium was changed once intermediately, and
ultimately, a total of 29 Kan-resistant Yali seedlings were
obtained, accounting for 7.03% of the total resistant buds.
PCR identification of transgenic
yali
The total DNA of Kan-resistant transgenic yali was
extracted; a pair of specific primers were designed based on the
NPT II gene sequence published in GenBank; transgenic plants of
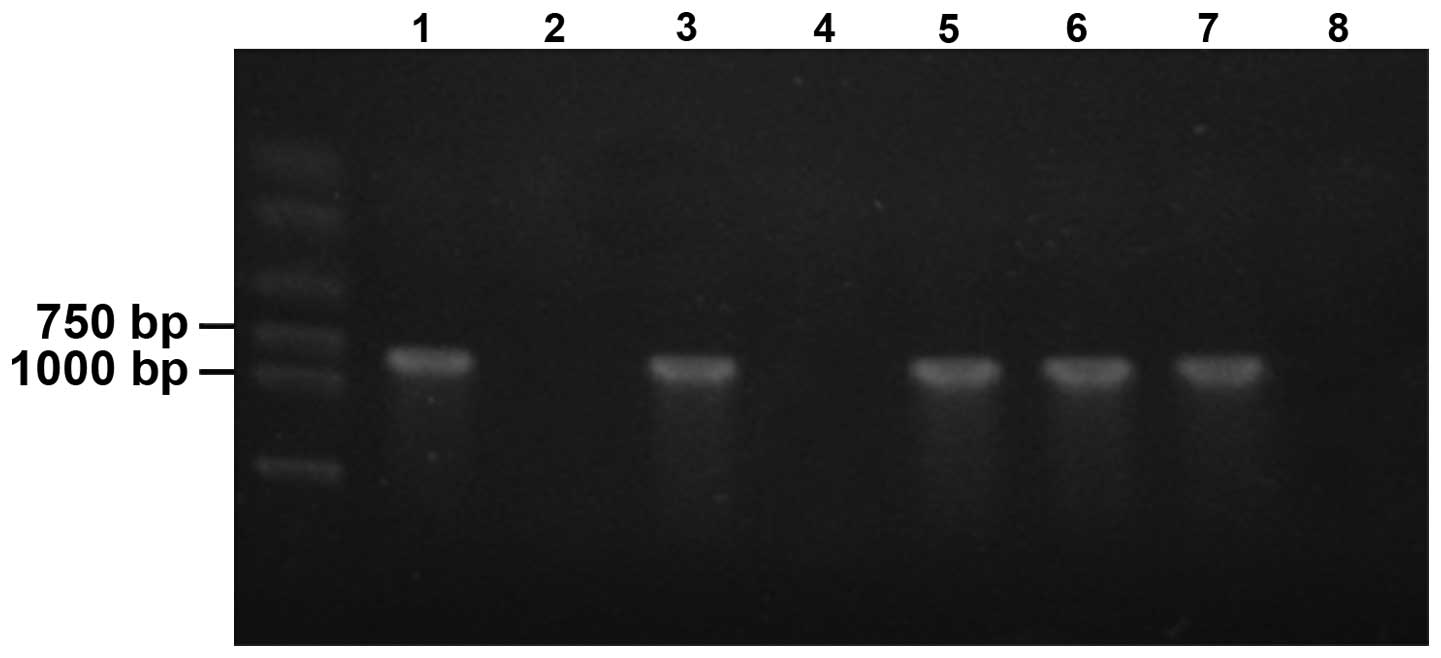
yali were identified by PCR. The results shown in Fig. 7 indicated that there were no
amplification products in the negative controls (lane 2) using DNA
of non-transfected yali plants as a template, while the target
fragments of 800 bp were contained in the positive control (lane
1), in which anti-sense expression vector was used as a template,
and in four transgenic plants, consistent with the design length,
initially confirming that the anti-sense ACO gene had been
successfully transformed in four yali plants. The other 25 plants
failed to amplify the target fragment and appeared as a false
positive, accounting for 86.21% of all Kan-resistant plants.
Southern blot analysis of transgenic
yali
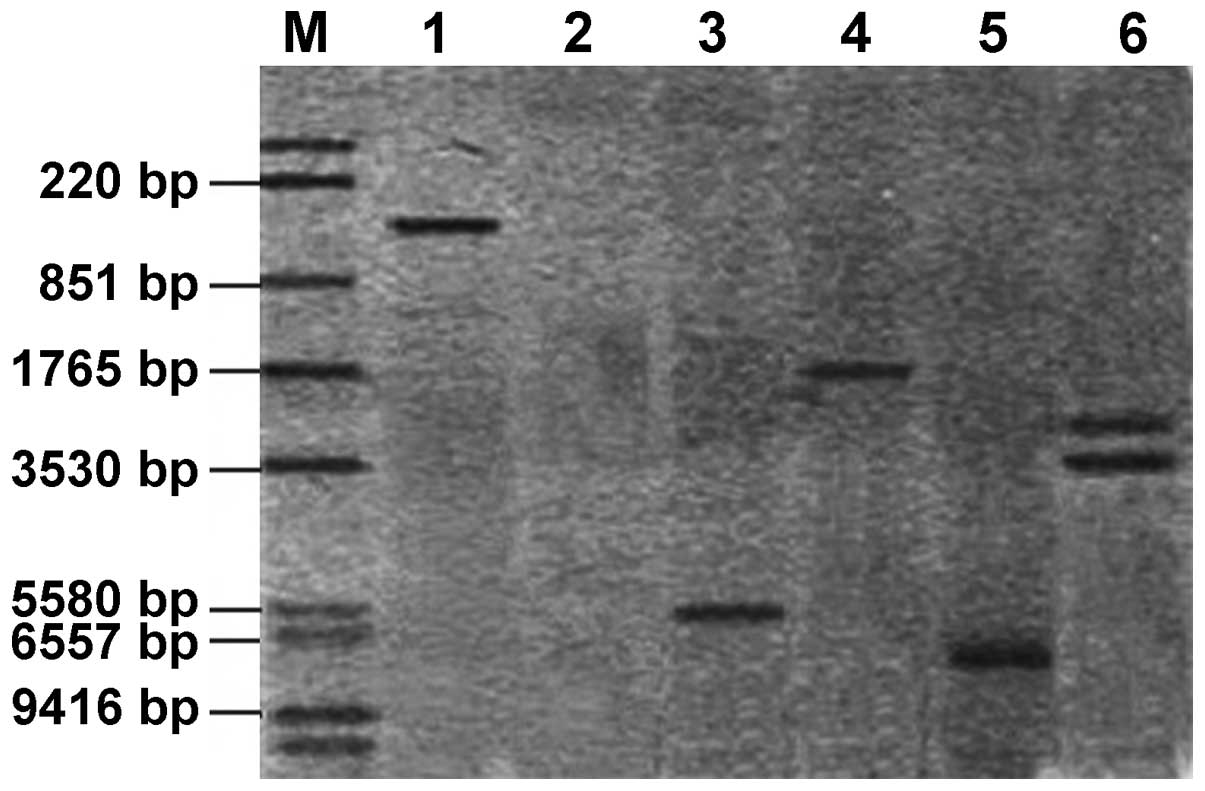
The total DNA of the positive transgenic plants
identified by PCR was digested with EcoRI; after
electrophoresis, the fragment of the 35S promoter was used as a
probe to perform Southern hybridization for further identification
of transgenic plants. Southern blot analysis (Fig. 8) showed that the hybridization
signal was detected in four PCR-positive transgenic plants, out of
which three (lanes 3–5) contained single copies and one (lane 6)
contained double copies. Non-transformed control plants had no
hybridization signal, confirming once again that pear ACO
anti-sense gene had been successfully transformed into the four
plants.
Discussion
The pear tree is a perennial fruit tree. Its long
juvenile period and highly heterozygous genetic characteristics
obstruct the use of traditional breeding methods. Anti-sense RNA
technology provides novel methods for pear breeding with the
advantage of its high specificity and short breeding cycle
(11,12). The present study used anti-sense
RNA technology, to reversely connect the ACO gene fragment to a
promoter and transfected the construct into pear plants, which
greatly accelerated the pace of pear cultivation and provided
possible novel varieties of pear, which are likely to display
delayed aging and a longer shelf life. In anti-sense RNA
technology, it is crucial to construct an expression vector
containing the correct anti-sense gene fragment. In the present
study, primers were designed according to the conserved regions of
published ACO gene sequences, and yali cDNA was cloned using
RT-PCR; the sequencing analysis showed that the cloned fragment
shared high homology with ACO gene sequences of other pear
varieties, which fully confirmed that the fragment was the
component of the yali ACO gene. The anti-sense expression vector
containing this fragment specifically inhibited the endogenous
expression of the yali ACO gene. Based on the high homology of the
fragment and other ACO gene sequences, the anti-sense expression
vector constructed in the present study can be applied to the gene
transformation of pears of any other variety, which is convenient
for the application of anti-sense RNA technology in other pear
varieties for extending their storage life.
Li et al (10) constructed a regeneration and
genetic transformation system for yali, which was adopted in the
present study. However, taking into account the different types of
agrobacterium and ablastin [Li et al (10) used agrobacterium EHA105 and
ablastin carbenicillin], the factors affecting the genetic
transformation of yali were re-assessed in the present study
(results not shown). These preliminary experiments by our group
demonstrated that, apart from the concentration of agrobacterium
and the infection time requiring adjustment, the other genetic
transformation conditions were consistent with those of Li et
al (10). Therefore, compared
with the plant genotype, the impact of the agrobacterium type on
the genetic transformation system was low, only differing in the
incubation conditions. When applying different strains of
agrobacterium on the same species for genetic transformation, the
existing genetic transformation system should be appropriately
adjusted according to the type of agrobacterium in order to achieve
effective transformation of the exogenous gene.
In general, identification of genes on the molecular
level is the most direct and simple way to assess successful
transfection of plants (13). PCR
amplification as well as Southern and northern blot hybridization
are commonly used as molecular-level detection methods. In the
present study, since the transformation of the pear plants was the
insertion of the ACO anti-sense gene fragment and the plants'
genome itself contained the ACO gene, the direct identification of
the transferred anti-sense fragment would not have provided any
useful information. Therefore, PCR amplification an Southern blot
analysis of the NPT II gene and the 35S promoter, which were
transfected together with the anti-sense fragment, was performed in
order to assess successful transfection. Although this
molecular-level validation was indirect, the NPT II gene, 35S
promoter and the target gene were connected in the inserted
expression vector. Thus, the presence of NPT II and 35S implies the
insertion of the target gene into the recipient's genome, which has
been confirmed in other anti-sense genetic transformation studies
(14). Due to restricted
resources, northern hybridization detection of gene expression
levels for the detection of the inhibitory effects of anti-sense
RNA transfection in pear trees was not performed.
In transgenic studies, successfully transfected
species are selected by screening with antibiotics, while the
detection of false-positives is common on the molecular level
(15,16). In the present study, a total of 29
kanamycin-resistant seedlings were gained; however, PCR analysis
identified only four strains of transformed resistant seedlings.
The positive rate of PCR was 13.79%. The reason for the high rate
of false positives, in the present study may have been the fact
that only two generations of seedlings were screened; furthermore,
the concentrations of kanamycin may have been too low and the
screening pressure may have been insufficient; furthermore,
residual agrobacteria on the seedlings may have reduced the
kanamycin concentration in the medium. The suppression of the
occurrence of false-positives would save large amounts of time and
effort. In order to reduce the amount of false-positive
identification of seedlings, it is recommended to appropriately
increase the concentration of kanamycin, tighten the screening
criteria and sufficiently remove any residual agrobacteria in
future experiments.
Numerous studies have confirmed that isoenzymes of
ACO exist in plants. The gene coding for ACO constitutes a
multi-gene family (17,18). The gene family members have a range
of different functions and temporal expression patterns, which
affects the endogenous ethylene synthesis in the type 1 (low rate;
regulation of plant organs, growth and aging) and 2 (rapid
synthesis; plant reproductive organ growth regulation) climacteric
phases (6,19). When pear ACO anti-sense gene
fragments from pear fruit were previously transfected into pear
plants, ACO was expressed in the flowers and fruit only
(unpublished results by our group). Therefore, it is speculated
that the ACO gene is responsible for the climacteric type 2
synthesis of ethylene in the reproductive parts of the pear tree.
By contrast, the generation of basal levels of ethylene in the
remaining parts of the pear tree was low, while ACO was absent,
indicating that the type 1 synthesis of ethylene was regulated by
other ACO gene family members.
In conclusion, anti-sense genetic transformation of
the ACO gene was performed in pear plantlets in order to provide
germplasm resources for the cultivation of novel storage varieties
of pears, therefore providing a reference for further applications
of anti-sense RNA technology in the genetic improvement of pears
and other fruit. In future studies, ACO should be detected in the
transgenic pear species generated in the present study, which can
only be performed after the flowering and fruiting of the
respective trees.
Acknowledgments
The present study was supported by the Science and
Technology plan of Hebei province in China (no. 11220103D-9).
References
|
1
|
Zhang H and Zhang S, Qin G, Wang L, Wu T,
Qi K and Zhang S: Molecular cloning and expression analysis of a
gene for sucrose transporter from pear (Pyrus bretschneideri Rehd)
fruit. Plant Physiol Biochem. 73:63–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu L, Li JK, Zhang J, et al: Effects of
1-MCP treatment on Ya pear storage of different harvest. North
Hortic. 5:222–224. 2009.
|
|
3
|
Li JZ, Yang WD, Ma J, et al: Effects of
high carbon dioxide permeability film packaging on postharvest
physiology and storage properties of Yali pear fruit. Storage
Process. 12:16–19. 2012.
|
|
4
|
Liu XY, Wuyun TN and Zeng HY: Cloning,
characterization and promoter analysis of S-RNase gene promoter
from Chinese pear (Pyrus pyrifolia). Gene. 505:246–253. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang XS and Yan XX: Progress in fruit
ripening by controlling ethylene. J Shandong Agric Univ.
25:487–490. 1994.
|
|
6
|
Yang SF and Hoffman NE: Ethylene
biosynthesis and its regulation in high plants. Annu Rev Plant
Physiol. 35:155–189. 1984. View Article : Google Scholar
|
|
7
|
Adams DO and Yang SF: Ethylene
biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic
acid as an intermediate in the conversion of methionine to
ethylene. Proc Natl Acad Sci USA. 76:170–174. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong Z: The study on the ACC oxidase genes
clone of pear fruit (unpublished PhD thesis). Baoding: Agricultural
University of Hebei province; 2007
|
|
9
|
Li GQ, Zhang YX, Gao ZH, et al:
Establishment of shoot regeneration system from leaf Yali pear. J
Fruit Sci. 3:436–440. 2010.
|
|
10
|
Li GQ, Qi J, Gao ZH, et al: Construction
and transformation for antisense expression vector of polyphenol
oxidase gene in Yali pear (Pyrus bretschneideri Rehd.). J Plant
Genet Resour. 5:635–639. 2010.
|
|
11
|
Ding Y, Li S and Huang H: Applications of
antisense-RNA technology in filamentous fungal metabolic
engineering - a review. Sheng Wu Gong Cheng Xue Bao. 25:1316–1320.
2009.In Chinese. PubMed/NCBI
|
|
12
|
Temple SJ, Bagga S and Sengupta-Gopalan C:
Down-regulation of specific members of the glutamine synthetase
gene family in alfalfa by antisense RNA technology. Plant Mol Biol.
37:535–547. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carannante A, De Carolis E, Vacca P, Vella
A, Vocale C, De Francesco MA, Cusini M, Del Re S, Dal Conte I,
Cristaudo A, et al: Evaluation of matrix-assisted laser desorption
ionization-time of flight mass spectrometry (MALDI-TOF MS) for
identification and clustering of Neisseria gonorrhoeae. BMC
Microbiol. 15:1422015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye ZB: Genetic transformation with
antisense cDNA of ethylene-forming enzyme and the breeding of
longer shelf-life cultivar in tomato (unpublished PhD thesis).
Huazhong Agricultural University; Wuhan: 2000
|
|
15
|
Urban LA, Sherman JM, Moyer JW and Daub
ME: High frequency shoot regeneration and Agrobacterium-mediated
transformation of chrysanthemum (Dendranthema grandiflora). Plant
Sci. 98:69–79. 1994. View Article : Google Scholar
|
|
16
|
Mitiouchkina TY and Dolgov SV:
Modifieation of chrysanthemum plant and flower architecture by rolC
gene from Agrobacterium rhizogenes introduction. Acta Hortic.
508:163–169. 2000. View Article : Google Scholar
|
|
17
|
Kim WT and Yang SF: Structure and
expression of cDNAs encoding 1-aminocyclopropane-1-carboxylate
oxidase homologs isolated from excised mung bean hypocotyls.
Planta. 194:223–229. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kahana A, Silberstein L, Kessler N,
Goldstein RS and Perl-Treves R: Expression of ACC oxidase genes
differs among sex genotypes and sex phases in cucumber. Plant Mol
Biol. 41:517–528. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McMurchie EJ, McGlasson WB and Eaks IL:
Treatment of fruit with propylene gives information about the
biogenesis of ethylene. Nature. 237:235–236. 1972. View Article : Google Scholar : PubMed/NCBI
|