Introduction
Gliomas are the most common type of primary brain
tumor, accounting for almost 40% of all brain neoplasms (1). They result from the transformation of
glial cells, with astrocytes as the predominant glial type
(2). The current treatment regimen
for gliomas includes surgery, radiotherapy and chemotherapy
(3). However, despite these
treatment modalities, the prognosis for patients with glioma
remains poor. The overall survival rate of patients with
glioblastoma, the most malignant and frequent type of glioma, is
extended only by ~14.6 months (4).
This is predominantly due to the resistance of glioma cells to
chemotherapy (5,6). Traditionally, this resistance has
been attributed to the blood-brain-barrier (BBB) (7,8).
However, clinical and laboratory studies have demonstrated that the
BBB surrounding the gliomas permits leakage (9,10).
Therefore, the BBB is not likely to be the sole mechanism
underlying this resistance.
The organ microenvironment has been implicated in
the progression and survival of tumor cells (11,12).
Astrocytes are the most dominant cell type in the brain, which
connect with each other through gap junctional communication (GJC)
and, thus function as a syncicium (13). Under physiological conditions,
astrocytes function as housekeeping cells and maintain homeostasis
in the brain microenvironment (14–17).
In pathological conditions, astrocytes become activated, via the
upregulation of glial fibrillary acid protein (GFAP), and have been
demonstrated to be capable of protecting neurons from various
injuries, including trauma and ischemic insult (18–21).
GJC, the molecular channel allowing the transmission of critical
survival-associated secondary messengers, has been shown to be one
of the mechanisms underlying this process (22,23).
Previous studies have suggested that there are activated astrocytes
surrounding glioma cells, and that functional GJC exists between
astrocytes and glioma cells (24,25).
These observations raise the possibility that reactive astrocytes
may also protect glioma cells from chemotherapy with a mechanism,
which may be associated with GJC. To confirm this hypothesis, the
present study investigated the sensitivities of glioma cells
treated with various chemotherapeutic drugs, in the presence or
absence of astrocytes. The global gene expression patterns of
glioma cells altered by the co-culture of astrocytes was also
examined using microarray analysis. These investigations were
performed to determine whether astrocytes protect glioma cells from
the apoptosis induced by chemotherapeutic drugs, examine the
expression levels of survival genes in glioma cells and determine
the contribution of GJC between astrocytes and glioma cells. The
findings of the present study provide an insight into resensitizing
glioma cells toward chemotherapy, which may contribute to extending
the limited survival time of patients with glioma.
Materials and methods
Cell lines, cell culture and
reagents
The human glioma cell lines (U87MG, U251 and A172)
and fibroblast cell line (NIH3T3) were purchased from the Cell
Center of Peking Union Medical University (Beijing, China).
Immortal mouse astrocytes were obtained from Dr Fidler's laboratory
(University of Texas, MD Anderson Cancer Center, Houston, Texas,
USA). All cell lines were recovered from frozen stocks and
maintained in Dulbecco's modified Eagle medium supplemented with
10% fetal bovine serum, non-essential amino acids, L-glutamine, a
two-fold vitamin solution (all from Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and a penicillin/streptomycin mixture (Flow
Laboratories, Inc., Rockville, MD, USA). Cultures were maintained
at 37°C in 5% CO2, as previously described (26–28).
Hochest 33342 and the GFAP antibody were purchased from Invitrogen
(Thermo Fisher Scientific, Inc.). Carbenoxolone (CBX) and propidium
iodide (PI) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
The temozolomide (TMZ), paclitaxel and 5-fluo-rouracil (5-FU)
chemotherapeutic agents were purchased from Lunarsun
Pharmaceuticals (Beijing, China). All reagents were dissolved in
dimethyl sulfoxide (ABigen Corporation, Beijing, China), and
reagents were of analytical reagent grade. A plasmid containing
green fluorescent protein (GFP) was purchased from Abigen
Corporation.
Animals
Five athymic Ncr-nu/nu male mice (age, 10 weeks;
weight, 16–18 g) were purchased from the Animal Center of Peking
Union Medical University (Beijing, China) and maintained under
specific pathogen-free conditions with free access to food and
water, at 20–25°C (humidity, 50±5%), under a 12-h light/dark cycle.
Animal protocols were approved and supervised by the Animal Care
and Use Committee of Xuanwu Hospital affiliated with Capital
Medical University (Beijing China) and were in accordance with
institutional guidelines.
Glioma mouse model
The human glioma cells were harvested upon reaching
60–70% confluence and were resuspended in phosphate-buffered saline
(PBS; Abigen Corporation). 1×105 cells (volume, 5
µl) were introduced into the brain parenchyma of the mice
via stereotactic injection, as described previously (29). Following the development of
neurological symptoms in the mice, for example circling, the mice
were sacrificed using CO2 and four brain tissue samples
were harvested for analysis.
Scanning electron microscopy of the
co-cultured glioma cells and astrocytes
The U87MG human glioma cells and murine astrocytes
(1:1 ratio) were seeded onto sterilized glass cover-slips in
24-well plates, at a density of 2.4×104 cells/well.
After 48 h, the co-culture samples were processed as described
previously (30). Briefly, the
samples were fixed for 1 h at room temperature in a solution
containing 3% glutaraldehyde, 2% paraformaldehyde and 7.5% sucrose
in 0.1 M cacodylate buffer (pH 7.3; all Abigen Corporation). The
samples were washed with 0.1 M cacodylate buffer followed by
distilled water and sequentially treated for 30 min in the dark
with Millipore-filtered aqueous 1% tannic acid (Merck Millipore,
Darmstadt, Germany) and washed in distilled water and
Millipore-filtered 1% aqueous uranyl acetate (Merck Millipore) for
1 h in the dark. Samples were mounted onto double-thick carbon tabs
(Ted Pella, Inc., Redding, CA, USA). The samples were then coated
under vacuum using a Balzer MED 010 evaporator (Technotrade
International, Inc., Manchester, NH, USA) with platinum alloy to a
thickness of 25 nm and immediately flash carbon coated under
vacuum. Samples were examined using a JSM-5910 scanning electron
microscope (JEOL USA, Inc., Peabody, MA, USA) at an accelerating
voltage of 5 kV.
Assessment of chemoprotection using flow
cytometric analysis
The GFP expressing astrocytes were established by
transfection with the pLCMV vector containing a GFP expressing
cassette dissolved in Lipofectamin 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Briefly, 4 µg plasmid DNA and 10
µl Lipofectamine 2000 were dissolved in 250 µl
transfection medium (Invitrogen Opti-MEM-I reduced serum medium;
Thermo Fisher Scientific, Inc.) for 20 min. The solution was
combined further by mixing for another 5 min prior to being added
into the culture medium. Eight hours after incubation, the medium
was replaced with fresh antibody-free medium. Forty-eight hours
later, the cells were harvested and GFP-positive cells were sorted
via fluorescence-activated cell sorting (FACS; FACS Elite; BD
Biosciences, Franklin Lakes, NJ, USA). The glioma cells
(1×106; harvested at 60–70% confluence) were cultured in
the presence or absence of GFP-expressing astrocytes at a ratio of
1:1 in the wells (diameter, 35 mm) of the sterile, 6-well tissue
culture plate. Following incubation for 72 h with the
chemotherapeutic agents (TMZ, 5 µM; cisplatin, 1 µM;
5-FU, 2 µM) at 37°C, the floating and adherent cells were
collected and fixed with 70% ethanol. The PI staining procedure was
performed, as described previously (31). Briefly, cells were harvested by
trypsinization (Abigen Corporation) and washed once with PBS. Cells
were centrifuged at 1,000 × g for 3 min at room temperature, and
cell pellets were resuspended in 1% paraformaldehyde for 5 min to
fix the GFP. Subsequently, the cells were centrifuged and resuspend
in 70% ethanol and stored at −20°C in a refrigerator overnight. At
the day of analyzing, cells were centrifuged again and washed once
with PBS. Cells were centrifuged and the PBS was removed; the
resultant pellets were resuspended in PI staining buffer
(containing 50 µg/ml PI in PBS, supplemented with 37.5 units
of RNase enzyme; Abigen Corporation) and placed in an incubator at
37°C for 30 min prior to analysis. FACS analysis was performed
using an EPICS XL cytometer (Beckman Coulter, Inc, Brea, CA, USA).
The fluorescein isothiocyanate (FITC)-PI protocol was used to
analyze the cell cycles of the GFP-expressing FITC-positive
astrocyte population and the FITC-negative glioma cell population
separately. Apoptotic cells were evaluated as the fraction of cells
in the sub-G0 region. Levels of apoptosis in response to
chemotherapeutic drugs in the glioma cells cultured alone or with
astrocytes were assessed, as described above, for all the three
glioma cell lines. To exclude the possibility of the protective
effect being due to the co-culture of mouse cells with human cells,
NIH3T3 mouse fibroblasts were co-cultured with U87MG glioma cells
as a control.
Transwell experiment
To determine whether the protective effect is
dependent on physical contact or mediated by secreted factors, a
6-well plate Boyden chamber (Costar Boyden Chamber; pore size, 0.4
µm; Corning Inc., Corning, NY, USA) was used in the present
study. The transwell membrane pore size facilitates with the
separation of tumor cells from astrocytes while allowing the
passage of secreted factors. Astrocytes (105 cells) were
seeded into the top insert of the Boyden chamber, and glioma cells
(105 cells) were seeded in the bottom insert. Following
overnight incubation at 37°C, the chemotherapeutic agent (TMZ, 5
µM) was added to the medium. 72 h later, the inserts were
removed and the cells (floating and adherent) were harvested and
stained with PI, following the above-mentioned protocol. The cell
cycle profiling was analyzed by FACS, with the sub-G0
and -G1 regions defined as the percentage of cell
death.
Examination of gene expression profiles
using RNA microarray analysis
The U87MG human glioma cells (105 cells)
were cultured alone, with murine astrocytes or with NIH3T3
fibroblasts in the 35 mm-diameter 6-well plate (BD Falcon; BD
Biosciences, San Jose, CA, USA), in the presence or absence of the
GJC inhibitor, CBX (100 µM). For the cell co-culture, the
ratio of glioma cells to murine astrocytes or NIH3T3 cells was set
as 1:1. Following incubation for 72 h at 37°C, the GFP-labeled
murine astrocytes and fibroblasts were sorted using FACS, and the
glioma cells were harvested for micro-array analyses. For
microarray analyses, total RNA (500 ng) was collected for labeling
and hybridization, in accordance with the manufacturer's protocol
(Illumina, Inc, San Diego, CA, USA), with the use of Illumina's
Sentrix human 6-v2 Expression BeadChips. The BeadChips were scanned
using an Illumina BeadArray Reader (Illumina, Inc). The results of
the microarray data were extracted using Bead Studio 3.7 (Illumina,
Inc.) without any normalization or background subtraction.
Normalization of gene expression data was performed using the
quantile method in LIMMA package in R (www.rproject.org) (32). The expression level of each gene
was transformed into a log2 value prior to further analysis. In
order to select genes, which were differentially expressed in the
two culture groups, a class comparison tool was used in the BRB
array tools (v3.6; Biometrics Research Branch, National Cancer
Institute, Bethesda, MD, USA), as a two-sample t-test with
the estimation of false discovery rate. All experiments were
performed in triplicate to avoid potential false positive genes due
to technical variance.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Student's t-test was performed to compare the
values between the different treatment groups. SPSS version 19 was
used to perform statistical analyses (IBM SPSS, Armonk, NY, USA)
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Interactions between astrocytes and
glioma cells
It has been demonstrated previously that there are
reactive astrocytes surrounding glioma cells in human specimen
(24,25) and, in the present study, similar
observations were made (data not shown). In the glioma in
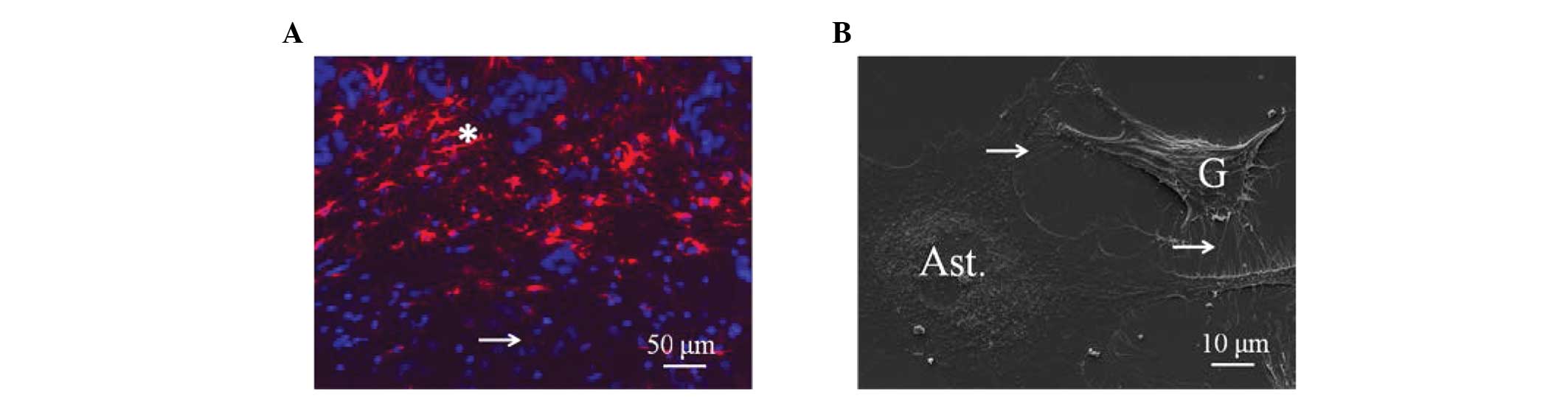
situ xenograft animal model, as shown in Fig. 1A, the U87MG glioma cells were
infiltrated and surrounded by GFAP-positive activated astrocytes
(red fluorescence). In the in vitro co-culture conditions,
astrocytes formed direct contact with the U87MG glioma cells, as
shown by scanning electron microscopy in Fig. 1B. These direct contacts between the
astrocytes and glioma cells were also evident in the in vivo
conditions.
Astrocytes protect glioma cells from
cytotoxicity caused by chemotherapeutic agents
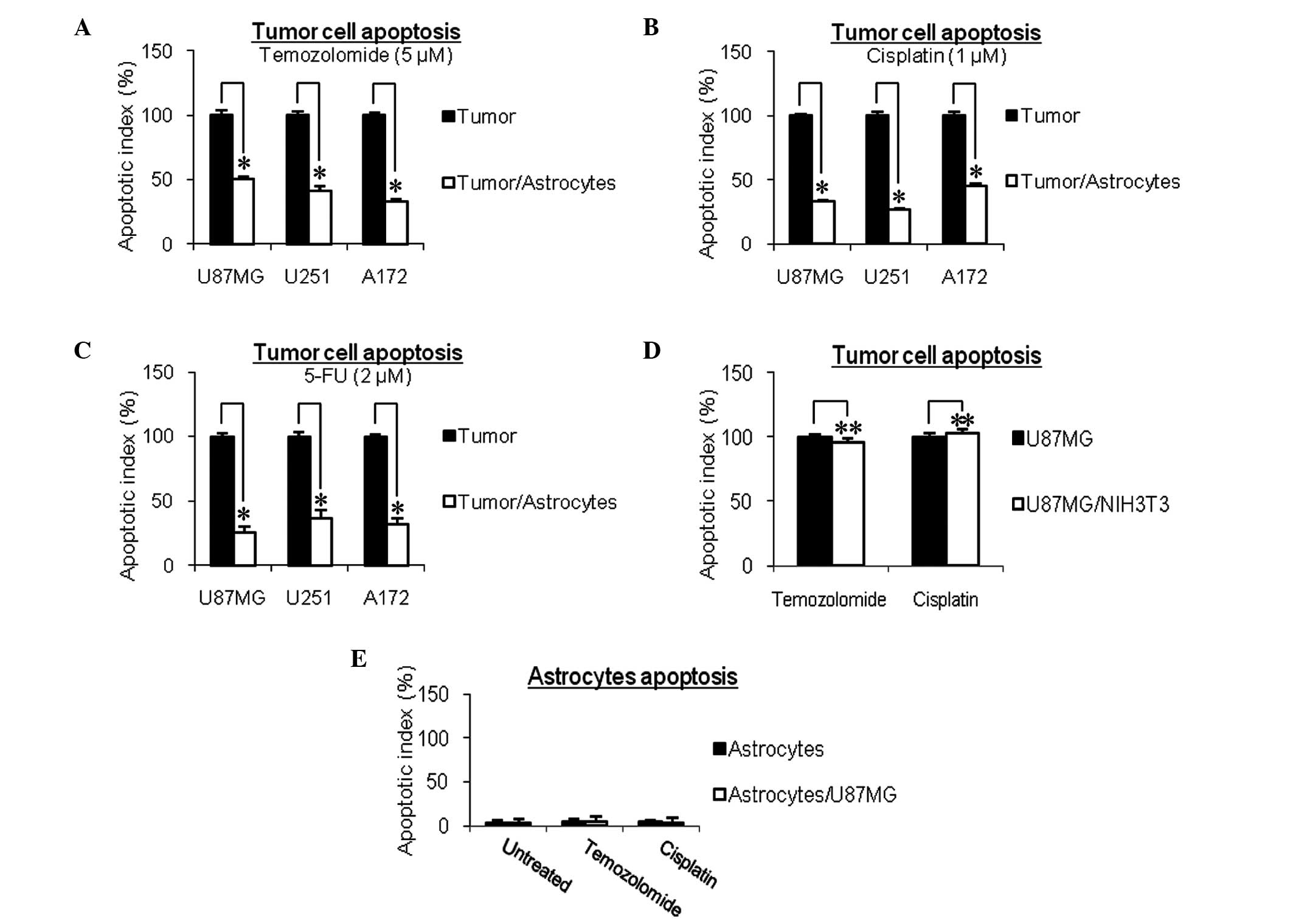
Following co-culture with astrocytes, the apoptotic
rates of the U87MG, U251 and A172 glioma cells induced by the TMZ
(5 µM) chemotherapeutic drug were significantly reduced, by
49.5±1.5% (P<0.05), 58.8±4.2% (P<0.05) and 67.1±1.6%
(P<0.05), respectively (Fig.
2A). This protection against the chemotherapeutic drug was not
specific to TMZ, as the similar reductions in the apoptosis of
U87MG, U251 and A172 glioma cells were observed in the astrocytes
exposed to cisplatin (66.6±1.4, 73.2±0.7 and 54.2±1.8%,
respectively; Fig. 2B) and 5-FU
(74.5±4.2, 63.8±6.6 and 68.7±5.3%, respectively; Fig. 2C). To rule out the possibility that
this protection was due to the co-culture of human cells (glioma
cells) with murine cells (astrocytes), murine NIH3T3 fibroblasts
were included as a control. As shown in Fig. 2D, the murine NIH3T3 fibroblasts
failed to protect glioma cells from the chemotherapeutic agents.
The astrocytes, when cultured either alone or with glioma cells,
did not undergo apoptosis when treated with the same
chemotherapeutic drugs (Fig.
2E).
Protection of glioma cells by astrocytes
is dependent on physical contact GJC
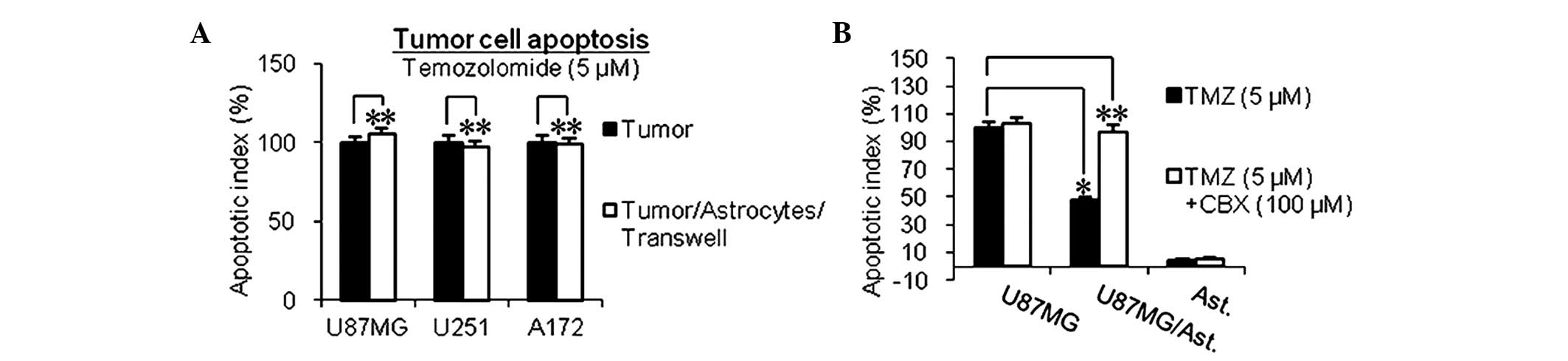
To investigate whether the protection provided by
the astrocytes is dependent on direct physical contact or mediated
by secreted factors, the present study used a semi-permeable
Transwell membrane (Transwell-Boyden Chamber; 0.4-µm pore
size membrane), which ensures physical separation of the glioma
cells from the astrocytes, while allowing the transmission of
secreted factors. Under this condition, astrocytes failed to
protect the glioma cells from the chemotherapeutic drugs (Fig. 3A), which suggested that the
protection was dependent upon physical contact.
GJC, which directly connects the cytoplasm of
neighboring cells, has been shown to transmit critical secondary
survival and apoptotic factors (22). Connexin 43, the major component of
GJC, has been demonstrated to be expressed by astrocytes and the
above-mentioned glioma cells (33,34).
Furthermore, functional GJC exists between astrocytes and glioma
cells, as shown in in vitro and in vivo experiments
(25). The present study made
similar observations (data not shown). To determine whether GJC was
involved in this chemoprotection, CBX, a well-documented specific
inhibitor of GJC (35,36) was used. As shown in Fig. 3B, the ability of the astrocytes to
protect glioma cells from chemotherapeutic drugs was lost when the
GJC between the astrocytes and glioma cells was inhibited.
Gene expression patterns in glioma cells
are altered by astrocytes through GJC
As GJC was involved in the contact-mediated
protection of glioma cells toward chemotherapeutic drugs, the
present study aimed to determine the alterations in gene expression
patterns in glioma cells by astrocytes through GJC. The human U87MG
glioma cells were incubated for 72 h, in the presence or absence of
astrocytes, with or without the GJC inhibitor, CBX (100 µM).
NIH3T3 fibroblasts were used as a control. Changes in gene
expression levels in the glioma cells were determined using the two
sample t-test (P<0.001) in microarray analysis. By
applying this procedure, co-culture with astrocytes was found to
alter the expression of 3,216 genes in the U87MG glioma cells,
whereas co-culture with fibroblasts altered the expression of 640
genes in the U87MG glioma cells. By applying cross comparison of
the genes expressed, a total of 299 genes were found to be commonly
altered by the astrocytes and fibroblasts. Therefore, these 299
genes were excluded, narrowing the list of gene, which were altered
specifically by astrocytes to 2,917 (Fig. 4A and B). The present study then
aimed to identify those genes that were changed via GJC. The
addition of CBX (100 µM) resulted in the altered expression
of 1,334 genes. By comparing this list of 1,334 genes to the list
of 2,917 genes, 699 genes were identified in the U87MG glioma
cells, which were exclusively altered by astrocytes through GJC
(Fig. 4C and D). Among these 699
genes, several genes were identified, which are known to be
associated with drug resistance, anti-apoptosis and survival,
including mitogen-activated protein kinase, tyrosine-protiein
kinase and B cell lymphoma-2. These data demonstrated the effect of
the brain microenvironment on glioma cells, with the unique feature
of astrocytes as an example.
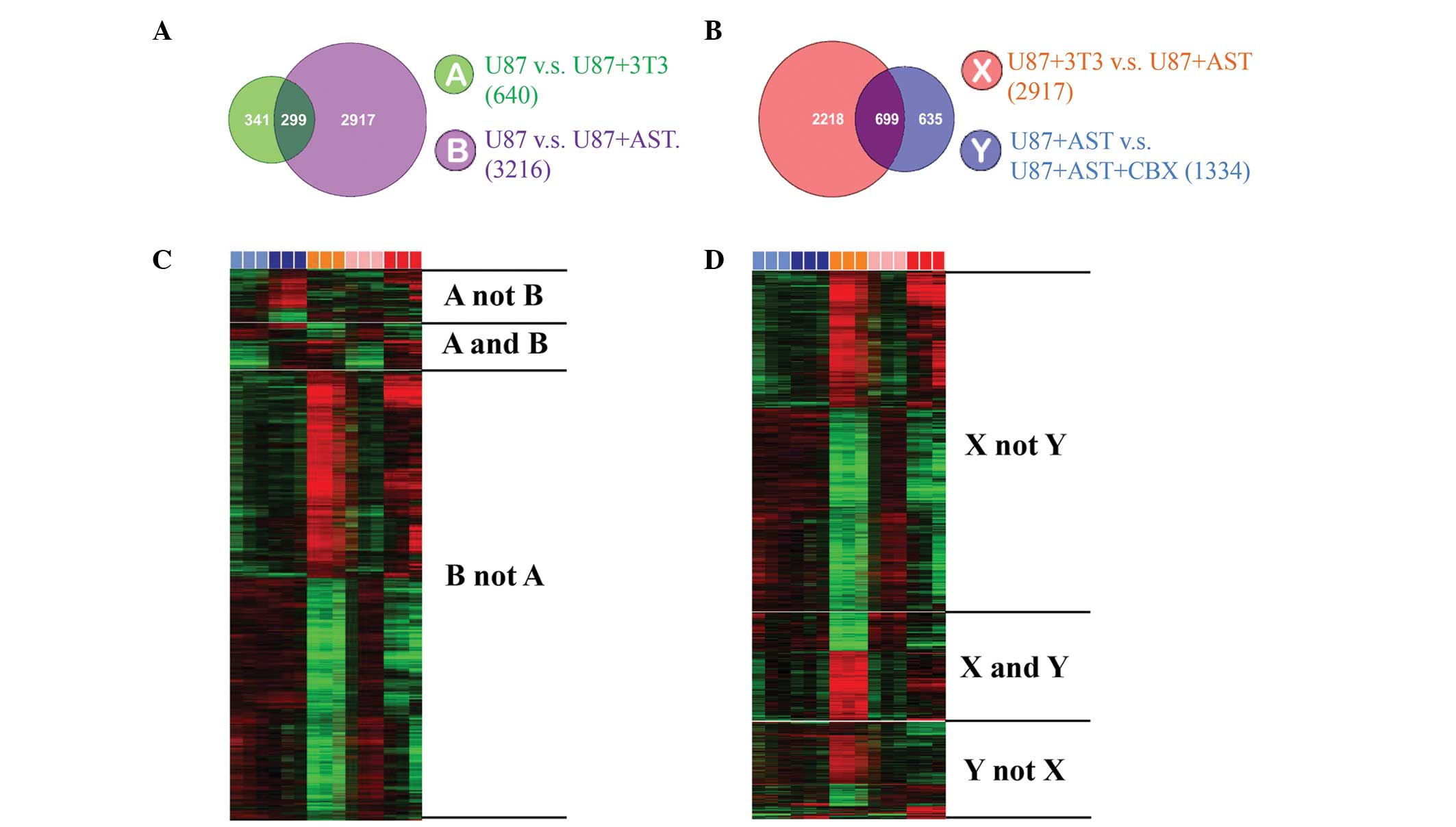 | Figure 4Gene expression patterns in U87MG
glioma cells are altered by astrocytes via GJC. Cross comparison of
gene lists from two independent statistical tests (P<0.001) was
applied. A matrix format in heat map was used to present the data,
in which rows represent individual genes and columns represent each
tested hybridization event (in triplicate), with the intensity of
color indicating the magnitude of expression (log2-transformed
scale). Of note, different colors represent different cultures
(light blue, U87MG alone; pink, U87MG with CBX; dark blue, U87MG
with NIH3T3; orange, U87MG with astrocytes; red, U87MG with
astrocytes and CBX). (A and B) Co-culture with astrocytes altered
the expression of 3,216 genes in the U87MG glioma cells (group
'B'), whereas co-culture with NIH3T3 fibroblasts altered the
expression of 640 genes in the glioma cells (group 'A'). The
expression of 299 genes was altered by the two co-cultures, which
were excluded from further analysis (group 'A and B'), with the
remaining 2,917 genes regarded as the genes altered specifically by
astrocytes (group 'B not A'). (C and D) Application of CBX altered
the expression of 1,334 genes (group 'Y'), of which 699 genes were
shared with the previous analysis (group 'X') and thus regarded as
genes that were altered by astrocytes through GJC. Several
well-known genes associated with cell survival and drug resistance
were among these 699 genes (group 'X and Y'). GJC, gap junctional
communication; CBX, carbenoxolone; AST, astrocytes. |
Discussion
As the most common type of primary brain tumor,
gliomas remain one of the few types of cancer with a poor
prognosis, which is primarily due to their drug resistance
properties. This resistance cannot be completely attributed to the
BBB, as evidence has revealed that the BBB surrounding gliomas
permits leakage. The data obtained in the present study present a
novel mechanism underlying this resistance, which is due to the
protection from astrocytes via GJC and the upregulated expression
levels of survival genes. Astrocytes maintain the homeostasis of
the brain microenvironment and protect neurons from various
injuries, with GJC as one of the underlying mechanisms. However,
this neuroprotective feature of astrocytes is harnessed by
metastatic cells in the brain (37), as has been reported previously. In
the present study, it was further demonstrated that glioma cells
were also able to 'exploit' this unique feature of astrocytes for
their own survival benefits, to avoid apoptosis caused by
chemotherapeutic drugs. In addition, the gene expression data
obtained in the present study revealed the extend to which the
organ microenvironment (astrocytes) affected the tumor cells
(glioma) at the molecular level, and revealed genes, which were
altered via GJC by neighboring cells. Taken together, these data
reinforce the importance of the organ microenvironment for the
behavior of tumor cells, and indicate that successful treatment of
glioma in the future requires consideration of tumor cells and
astrocytes, with GJC as a potential promising target.
Acknowledgments
This study was partially supported by grants from
the National Nature Science Foundation (grant no. 81101664) and the
Beijing Nature Science Foundation (grant no. 7132079) to Dr
Qingtang Lin, and the National Science and Technology Supporting
Project (grant no. 2014BAI04B00).
Abbreviations:
|
BBB
|
blood-brain-barrier
|
|
CBX
|
carbenoxolone
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
FITC
|
fluorescein isothiocyanate
|
|
5-FU
|
5-fluorouracil
|
|
GFAP
|
glial fibrillary acidic protein
|
|
GFP
|
green fluorescent protein
|
|
GJC
|
gap junctional communication
|
|
PI
|
propidium iodide
|
|
TMZ
|
temozolomide
|
References
|
1
|
Wrensch M, Minn Y, Chew T, Bondy M and
Berger MS: Epidemiology of primary brain tumors: Current concepts
and review of the literature. Neuro Oncol. 4:278–299.
2002.PubMed/NCBI
|
|
2
|
Fisher JL, Schwartzbaum JA, Wrensch M and
Wiemels JL: Epidemiology of brain tumors. Neurol Clin. 25:867–890.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lévy S, Chapet S and Mazeron JJ:
Management of gliomas. Cancer Radiother. 18:461–467. 2014.
View Article : Google Scholar
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ashmore SM, Thomas DG and Darling JL: Does
P-glycoprotein play a role in clinical resistance of malignant
astrocytoma? Anticancer Drugs. 10:861–872. 1999. View Article : Google Scholar
|
|
6
|
Jennings MT and Iyengar S: The molecular
genetics of therapeutic resistance in malignant astrocytomas. Am J
Pharmacogenomics. 1:93–99. 2001. View Article : Google Scholar
|
|
7
|
Shapiro WR and Shapiro JR: Principles of
brain tumor chemotherapy. Semin Oncol. 13:56–69. 1986.PubMed/NCBI
|
|
8
|
Mousseau M: Chemotherapy of brain tumors:
Biological basis of its limited efficacy. Bull Cancer. 81:414–424.
1994.In French. PubMed/NCBI
|
|
9
|
Stewart DJ: A critique of the role of the
blood-brain barrier in the chemotherapy of human brain tumors. J
Neurooncol. 20:121–139. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang RD, Price JE, Fujimaki T, Bucana CD
and Fidler IJ: Differential permeability of the blood-brain barrier
in experimental brain metastases produced by human neoplasms
implanted into nude mice. Am J Pathol. 141:1115–1124.
1992.PubMed/NCBI
|
|
11
|
Hoelzinger DB, Demuth T and Berens ME:
Autocrine factors that sustain glioma invasion and paracrine
biology in the brain microenvironment. J Natl Cancer Inst.
99:1583–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fidler IJ: The pathogenesis of cancer
metastasis: The 'seed and soil' hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abbott NJ, Rönnbäck L and Hansson E:
Astrocyte-endothelial interactions at the blood-brain barrier. Nat
Rev Neurosci. 7:41–53. 2006. View
Article : Google Scholar
|
|
14
|
Fields RD and Stevens-Graham B: New
insights into neuron-glia communication. Science. 298:556–562.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bullock TH, Bennett MV, Johnston D,
Josephson R, Marder E and Fields RD: Neuroscience: The neuron
doctrine, redux. Science. 310:791–793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miller G: Neuroscience: The dark side of
glia. Science. 308:778–781. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allen NJ and Barres BA: Neuroscience:
Glia-more than just brain glue. Nature. 457:675–677. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sofroniew MV: Reactive astrocytes in
neural repair and protection. Neuroscientist. 11:400–407. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crooks DA, Scholtz CL, Vowles G, Greenwald
S and Evans S: The glial reaction in closed head injuries.
Neuropathol Appl Neurobiol. 17:407–411. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mahesh VB, Dhandapani KM and Brann DW:
Role of astrocytes in reproduction and neuroprotection. Mol Cell
Endocrinol. 246:1–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen LW, Yung KL and Chan YS: Reactive
astrocytes as potential manipulation targets in novel cell
replacement therapy of Parkinson's disease. Curr Drug Targets.
6:821–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krysko DV, Leybaert L, Vandenabeele P and
D'Herde K: Gap junctions and the propagation of cell survival and
cell death signals. Apoptosis. 10:459–469. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin JH, Weigel H, Cotrina ML, Liu S, Bueno
E, Hansen AJ, Hansen TW, Goldman S and Nedergaard M:
Gap-junction-mediated propagation and amplification of cell injury.
Nat Neurosci. 1:494–500. 1998. View
Article : Google Scholar
|
|
24
|
Rouach N, Avignone E, Même W, Koulakoff A,
Venance L, Blomstrand F and Giaume C: Gap junctions and connexin
expression in the normal and pathological central nervous system.
Biol Cell. 94:457–475. 2002. View Article : Google Scholar
|
|
25
|
Zhang W, Couldwell WT, Simard MF, Song H,
Lin JH and Nedergaard M: Direct gap junction communication between
malignant glioma cells and astrocytes. Cancer Res. 59:1994–2003.
1999.PubMed/NCBI
|
|
26
|
Zhou B, Bu G, Zhou Y, Zhao Y, Li W and Li
M: Knockdown of CDC2 expression inhibits proliferation, enhances
apoptosis and increases chemosensitivity to temozolomide in
glioblastoma cells. Med Oncol. 32:3782015. View Article : Google Scholar
|
|
27
|
Chai KM, Wang CY, Liaw HJ, Fang KM, Yang
CS and Tzeng SF: Downregulation of BRCA1-BRCA2-containing complex
subunit 3 sensitizes glioma cells to temozolomide. Oncotarget.
5:10901–10915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Langley RR, Fan D, Guo L, Zhang C, Lin Q,
Brantley EC, McCarty JH and Fidler IJ: Generation of an
immortalized astrocyte cell line from H-2Kb-tsA58 mice to study the
role of astrocytes in brain metastasis. Int J Oncol. 35:665–672.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grossman R, Brastianos H, Blakeley JO,
Mangraviti A, Lal B, Zadnik P, Hwang L, Wicks RT, Goodwin RC, Brem
H and Tyler B: Combination of anti-VEGF therapy and temozolomide in
two experimental human glioma models. J Neurooncol. 116:59–65.
2014. View Article : Google Scholar :
|
|
30
|
Peng Q, Moan J, Ma LW and Nesland JM:
Uptake, localization and photodynamic effect of meso-tetra
(hydroxyphenyl) porphine and its corresponding chlorin in normal
and tumor tissues of mice bearing mammary carcinoma. Cancer Res.
55:2620–2626. 1995.PubMed/NCBI
|
|
31
|
Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH,
Fidler IJ and Hung MC: Survival of cancer cells is maintained by
EGFR independent of its kinase activity. Cancer Cell. 13:385–393.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bolstad BM, Irizarry RA, Astrand M and
Speed TP: A comparison of normalization methods for high density
oligonucleotide array data based on variance and bias.
Bioinformatics. 19:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hinkerohe D, Wolfkühler D, Haghikia A,
Meier C, Faustmann PM and Schlegel U: Dexamethasone differentially
regulates functional membrane properties in glioma cell lines and
primary astrocytes in vitro. J Neurooncol. 103:479–489. 2011.
View Article : Google Scholar
|
|
34
|
Zhang B, Feng X, Wang J, Xu X, Liu H and
Lin N: Adenovirus-mediated delivery of bFGF small interfering RNA
increases levels of connexin 43 in the glioma cell line, U251. J
Exp Clin Cancer Res. 29:32010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goldberg GS, Moreno AP, Bechberger JF,
Hearn SS, Shivers RR, MacPhee DJ, Zhang YC and Naus CC: Evidence
that disruption of connexin particle arrangements in gap junction
plaques is associated with inhibition of gap junctional
communication by a glycyrrhetinic acid derivative. Exp Cell Res.
222:48–53. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guan X, Wilson S, Schlender KK and Ruch
RJ: Gap-junction disassembly and connexin 43 dephosphorylation
induced by 18 beta-glycyrrhetinic acid. Mol Carcinog. 16:157–164.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fidler IJ, Balasubramanian K, Lin Q, Kim
SW and Kim SJ: The brain microenvironment and cancer metastasis.
Mol Cells. 30:93–98. 2010. View Article : Google Scholar : PubMed/NCBI
|