Introduction
Colorectal cancer is one of the three leading causes
of cancer-associated mortality worldwide, with ~1,200,000 new cases
and 600,000 deaths annually (1).
In China, the incidence and mortality rates of colorectal cancer
have increased rapidly in the past several decades (2), with mortality generally resulting
from tumor recurrence or metastasis (3). Metastasis to regional lymph nodes is
critical in colorectal cancer tumor progression, affects prognosis,
and occurs commonly in early-stage metastasis (4). Additionally, lymph node involvement
often promotes further hematogenous metastasis (5). Following surgery, the 5-year survival
rate of patients with early-stage colorectal cancer is >90%,
however, the 5-year survival rate of advanced-stage patients is
<10% (6). Although several
genes associated with lymph node metastasis have been reported, the
molecular mechanisms of early-stage metastasis in colorectal cancer
remain unclear (7–9). Thus, the identification of
bio-markers associated with lymph node metastasis of colorectal
cancer will benefit clinical evaluation and future treatment
strategies.
Currently, the typical treatment for colorectal
cancer is surgery combined with radiotherapy (10). Radiotherapy is important for the
control of colorectal cancer lymph node metastasis and the
prevention of local recurrence. Tumor-associated macrophages (TAMs)
constitute 30–50% of tumor stromal cells, and secrete a variety of
cytokines to promote tumor growth and progression, including basic
fibroblast growth factor, transforming growth factor-β (TGF-β),
platelet-derived growth factor (PDGF) and epidermal growth factor
(11). The quantity of TAMs in
tumor tissue is considered to be directly associated with poor
disease prognosis (12). Previous
studies in lymphoma and breast cancer have demonstrated that the
growth of tumor cells in vitro was increased by co-culture
with TAMs (13,14). Therefore, to explore the
interactions between TAMs and tumor cells, and elucidate the
mechanism by which TAMs promote growth, it is important to develop
novel processes and targets for the treatment of cancer.
Tumor cell autophagy has been investigated in
numerous studies, however, there are few reports analyzing the
regulation of autophagy in tumor stromal cells and how this impacts
the biological characteristics of tumor cells. Therefore, the
current study used co-culture of TAMs with colorectal cancer cells
to investigate how autophagy of TAMs may influence the
radiosensitivity of colon cancer.
Materials and methods
Cell culture and reagents
The following cell lines were used: LoVo low
differentiated colon adenocarcinoma cell line (American Type
Culture Collection, Manassas, VA, USA); and THP-1 human monocytic
leukemia cell line (Shanghai Institute of Biochemistry and Cell
Biology, Chinese Academy of Sciences, Shanghai, China). The cell
lines were routinely cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
maintained at 37°C in a humidified environment with 5%
CO2. Rapamycin, bafilomycin A1 and
phorbol-12-myristate-13-acetate (PMA) were purchased from Abcam
(Cambridge, UK). Monoclonal mouse anti-human epitope 206 CD206
(cat. no. sc-58986), monoclonal mouse anti-human B-cell lymphoma-2
(Bcl-2; cat. no. sc-7382), polyclonal rabbit anti-human survivin
(cat. no. sc-10811)and monoclonal mouse anti-human second
mitochondria-derived activator of caspase (Smac; cat. no.
sc-393118) antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, Texas, USA). Monoclonal mouse anti-human CD68
antibody (cat. no. sc-20060; Santa Cruz Biotechnology, Inc.),
monoclonal mouse anti-human p53 (cat. no. LS-C43831; LifeSpan
BioSciences, Inc., Seattle, WA, USA), polyclonal rabbit anti-human
LC3B I and II (cat. no. L8918; Sigma-Aldrich) and mouse anti-human
SR-AI/MSR/CD204 (cat. no. 351615; Novus Biologicals, Littleton, CO,
USA) antibodies were also used. Monodansylcadaverine (MDC) was
purchased from Sigma-Aldrich (St. Louis, MO, USA). An Autophagy
Antibody Sampler kit (cat. no. 4445), including the ATG-3, ATG-5,
ATG-7 and Beclin-1 antibodies, was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA) and the human recombinant
interleukin-4 (IL-4; cat. no. 200–04) was obtained from Peprotech
Inc. (Rocky Hill, NJ, USA). The Annexin V/FITC Apoptosis Detection
kit was purchased from Roche Applied Science (Madison, WI, USA) and
a Transwell chamber was purchased from Corning Incorporated
(Corning, NY, USA).
Cell irradiation conditions
Cells were irradiated using a Primus linear
accelerator (Siemens Medical Solutions USA, Inc., Malvern, PA,
USA), 6MV X-ray vertical irradiation at 198 cGy/min and 25°C with a
source skin distance of 100 cm.
Stimulation and identification of
TAMs
THP-1 cells (4×106) were cultured in
medium containing 30 nM PMA for 72 h. Subsequently, 60 ng IL-4 was
added to the medium for a further 24 h. Cells were collected and
immunolabeled with antibodies against CD204, CD206 (1:1,000) and
CD68 (1:1,000) to detect TAM markers. Cells were then stained with
FITC-labeled goat anti-mouse IgG (H+L; cat. no. A0568; Beyotime
Institute of Biotechnology, Shanghai, China) secondary antibody.
Following staining, cells were washed with RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 5% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) twice and
immediately analyzed by flow cytometry (Cytomic FC 500; Beckman
Coulter, Inc., Brea, CA, USA). THP-1 cells treated with
phosphate-buffered saline (PBS) for 72 h, or with only PMA for 72 h
were used as the control groups.
Autophagy of TAM analysis
All groups consisted of TAMs cultured in RPMI 1640
supplemented with PBS and treated as follows: i) The control group,
5% FBS only; ii) the PBS group, PBS + 10% FBS; iii) the autophagy
upregulation group, 200 nM/l rapamycin for 48 h + 10% FBS; and iv)
the autophagy downregulation group, 50nM/l bafilomycin A1 for 48 h
+ 10% FBS. The fluorescent dye MDC (100 nM) was added to all groups
following 2 PBS washes, then incubated for 30 min at 25°C. The
green fluorescence was detected by laser scanning confocal
microscopy (micropublisher 3.3RTV; Olympus, Tokyo, Japan). Western
blot was used to assess the expression of microtubule associated
protein 1 light chain 3 β-I and -II (LC3B I and II), Beclin-1 and
autophagy related-3, -5 and -7 (ATG-3, -5 and -7). Proteins were
extracted using a Total Protein Extraction kit (EMD Millipore,
Billerica, MA, USA) and the protein concentration of lysates was
determined using the bicinchoninic acid method (BCA Protein
Quantification kit; Beyotime Institute of Biotechnology). The
following antibodies were used to develop immunoreactive signals:
LC3B I and II (1:1,000; Sigma-Aldrich), polyclonal rabbit
anti-human ATG-3 (cat. no. 3415; Cell Signaling Technology, Inc.),
monoclonal rabbit anti-human ATG-5 (cat. no. 12994; Cell Signaling
Technology, Inc.), monoclonal rabbit anti-human ATG-7 (cat. no.
8558; Cell Signalling Technology, Inc.), monoclonal rabbit
anti-human Beclin-1 (cat. no. 3495; 1:1,000; Cell Signaling
Technology, Inc.), goat polyclonal GAPDH (cat. no. sc-48167; Santa
Cruz Biotechnology, Inc.) and rabbit polyclonal β-actin (cat. no.
sc-7210; 1:1,000; Santa Cruz Biotechnology, Inc.). Densitometry was
performed using AlphaImager 2200 system (Alpha Innotech, Santa
Clara, CA, USA).
TAMs co-cultured with LoVo cells and
radiation process
The non-contact co-culture group settings were as
follows: i) LoVo cells alone, no radiation; ii) LoVo cells with 6
Gy irradiation; iii) LoVo and TAMs; iv) the autophagy-upregulation
group, LoVo and TAMs with rapamycin treatment; and v) the
autophagy-downregulation group, LoVo and TAMs with bafilomycin A1
treatment. All groups containing TAMs were also exposed to 6 Gy
irradiation. LoVo cells were cultured in the lower chamber, and
TAMs in the upper chamber of a Transwell insert separated by a
polycarbonate membrane, thus, preventing direct cell-cell contact,
but permitting the exchange of soluble factors. LoVo cells
(4×105) were seeded in the lower chamber of a 6-well
plate. TAMs (8×105) were seeded on the micropore
membrane (0.4 µm diameter) in the upper chamber of the
Transwell unit. The cells were co-cultured at 37°C in a 5%
CO2 environment for 48 h. Excluding the control group,
all cells received a dose of 6 Gy irradiation and were then
cultured for another 36 h. LoVo cells were cultured for 2 weeks
prior to colony counting. LoVo cells from the lower chambers were
removed and seeded into 6-aperture plates at a density of 200
cells/well. Colony formation was observed in each group and the
colonies were counted (≥50 cells were considered a standard
cloning). Colony formation rate (%) = cloning efficiency/seeded
cells × 100. Cell survival fraction = cloning efficiency of the
experimental group/control group cloning efficiency (each
containing 10 samples in parallel).
Detection of apoptosis in LoVo cells by
annexin V/propidium iodide (PI) staining
The non-contact co-culture groups were as follows:
i) LoVo cells alone; ii) LoVo and TAMs; iv) the
autophagy-upregulation group, LoVo and TAMs with rapamycin
treatment; and iv) the autophagy-downregulation group, LoVo and
TAMs with bafilomycin A1 treatment. LoVo cells (4×105)
were seeded in the lower chamber of the Transwell insert in 6-well
plates. TAMs (8×105) were seeded on the micropore
membrane (0.4 µm diameter) in the upper chamber. Cells were
co-cultured at 37°C and 5% CO2 environment for 48 h. All
4 groups received a dose of 6 Gy irradiation and were cultured for
a further 24 h. LoVo cells in the lower chamber were collected,
washed with cold PBS buffer twice, and centrifuged at 370 × g for 5
min. Cells were resuspended in 195 µl annexin V-fluorescein
isothiocyanate (FITC) binding buffer, then 5 µl annexin
V-FITC and 10 µl PI were added to the solution and incubated
for 15 min in darkness. The excitation wavelength used for flow
cytometry detection was 488 nm, FITC was detected by a 515 nm
wavelength bandpass filter and PI by a 560 nm filter (Cytomic FC
500; Beckman Coulter, Inc.).
Western blot analysis of Smac, survivin,
Bcl-2 and p53 expression levels
Proteins were extracted using the Total Protein
Extraction kit and the protein concentrations of lysates were
determined using the BCA method as described above. Subsequently,
proteins were separated by 10% SDS-PAGE. At the beginning of
electrophoresis, the voltage was 80 V, then after ~40 min, the
voltage was increased to 120 V for 1.5–2 h. Proteins were then
transferred onto PVDF membranes. When the protein transfer was
completed, the gel was stained with Coomassie Brilliant Blue. PVDF
membranes were washed with 10X TBS for 10 min at room temperature
and then immersed with blocking buffer [100 ml Tris-buffered saline
with Tween 20 (TBST) and 5 g skim milk powder (MeiJi Dairies
Corporation, Tokyo, Japan)] for 1 h. The membranes were incubated
with the following primary antibodies: Mouse anti-human Smac
(1:1,000; Santa Cruz Biotechnology, Inc.), rabbit anti-human
survivin (1:1,000; Santa Cruz Biotechnology, Inc.), mouse
anti-human Bcl-2 (1:1,000; Santa Cruz Biotechnology, Inc.), mouse
anti-human p53 (1:1,000; LifeSpan BioSciences, Inc., Seattle, WA,
USA) and β-actin (1:1,000) for 1 h at room temperature to develop
immunoreactive signals. The membranes were then washed with 10X TBS
for 30 min at room temperature and incubated with HRP-labeled goat
anti-rabbit IgG (cat. no. A0208; Beyotime Institute of
Biotechnology) and goat anti-mouse IgG (cat. no. A0216; Beyotime
Institute of Biotechnology) secondary antibodies. Densitometry was
performed using AlphaImager 2200 system and Quantity software.
Statistical analysis
All experiments were performed a minimum of 4 times.
All results are expressed as the mean ± standard deviation.
Differences among multiple groups were analyzed by one-way analysis
of variance with a Dunnett's multiple comparison post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using GraphPad
Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Induction of THP-1 differentiation and
the identification of TAMs
THP-1 human leukemia cells were treated with PMA for
72 h and subsequently incubated with human recombinant IL-4 for 24
h. The cell morphology was observed (Fig. 1A) and the expression of the TAM
cell surface markers, CD68, CD204 and CD206 were detected by flow
cytometry (Fig. 1B). The combined
effects of PMA and IL-4 led to the differentiation of THP-1 cells
into TAMs. The cells lost their spherical morphology, appeared more
irregular, and became adherent. Cells stimulated with PMA and IL-4
exhibited increased expression of CD68, CD204 and CD206
(67.32±1.58, 33.49±2.11 and 45.42±2.65%) compared with control
THP-1 cells (39.69±1.59, 8.56±1.25 and 7.93±0.53%) (Fig. 1B; P<0.01). The differentiated
TAM group demonstrated the highest expression levels of CD68, CD204
and CD206 cell surface markers among the 3 groups, consistent with
previous studies (15–17) (Fig.
1C).
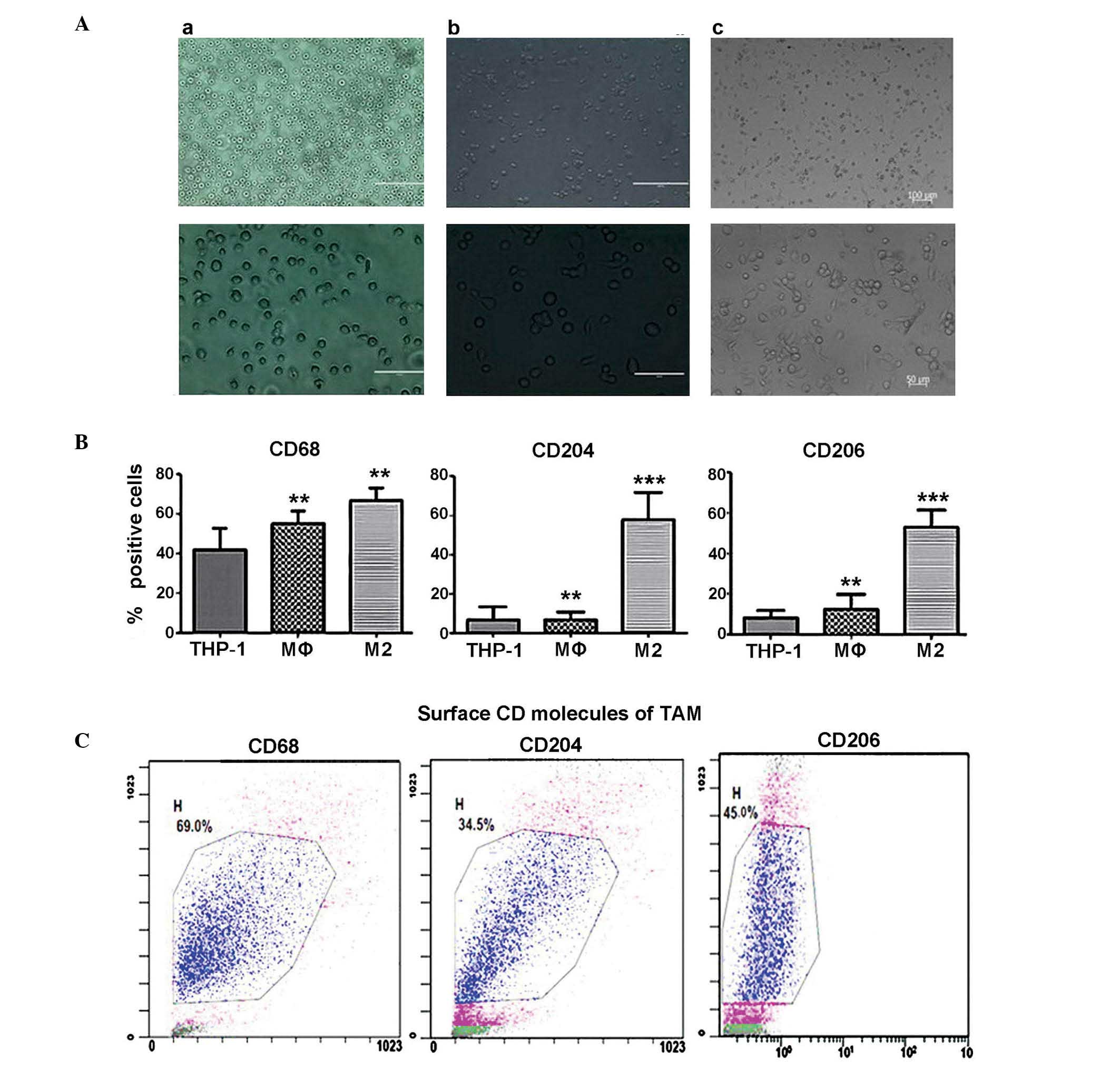 | Figure 1Induction of human monocytic leukemia
THP-1 cells to TAMs. (Aa) Phosphate-buffered saline treatment; (Ab)
PMA treatment (30 nM) for 72 h (M0); or (Ac) PMA treatment + 60 ng
IL-4 for another 24 h (M2). Top panel, low magnification (scale
bar=100 µM); lower panel, high magnification (scale bar=50
µM). (B) Flow cytometry detection of surface CD molecules as
markers of THP-1, M0 and M2 cells, with THP-1 cells as the control
group. CD68, CD204 and CD206 expression were 39.69±1.59, 8.56±1.25
and 7.93±0.53% in THP-1 cells, 49.72±1.82, 7.90±1.19 and
14.39±0.85% in M0 cells and 67.32±1.58, 33.49±2.11 and 52.42±2.65%
in TAMs. **P<0.01 and ***P<0.001 vs.
the THP-1 group. (C) Surface CD molecule expression in TAMs.
Processed flow cytometry detection was performed four times, and
representative results are presented. TAM, tumor-associated
macrophages; CD, cluster of differentiation. |
TAM autophagy analysis
MDC-positive cells were almost undetectable in the
control group (3.6±0.64%; Fig.
2Aa; Table I) and in the
untreated group, the rate of MDC-positive cells was 38.3±1.07%
(Fig. 2Ab; Table I). By contrast, following 48-h
treatment with rapamycin or bafilomycin A1 for 48 h, the rate of
MDC-positive cells in the autophagy-upregulation group was
76.4±1.14% (Fig. 2Ac; Table I) and in the
autophagy-downregulation group it was 21.3±1.21% (Fig. 2Ad; Table I). Western blot analysis was
performed to detect the expression of LC3B I and II in the 4
groups, and the highest relative expression level of the LC3Bs was
observed in the autophagy-upregulation group, in which a
significant increase was observed compared with the control group
(P<0.001; Fig. 2B). Expression
levels of ATGs and Beclin-1 were highest in the
autophagy-upregulation group, demonstrating that rapamycin
treatment successfully stimulated autophagy of TAMs (Fig. 2C). The relative expression level of
ATGs and Beclin-1 in the autophagy-downregulation group was the
lowest of the 3 co-culture groups, demonstrating that bafilomycin
A1 suppressed autophagy (Fig. 2C).
Although MDC-positive cells were almost undetectable in the control
group, western blot detected ATG-3, -5 and -7 and Beclin-1
expression (0.74±0.01, 2.38±0.02, 2.43±0.03 and 1.01±0.03; Fig. 2Ca). This may be due to the cells
being cultured in a closed environment, with changes of pH and
nutrient consumption spontaneously stimulating autophagy, resulting
in detection at the protein level.
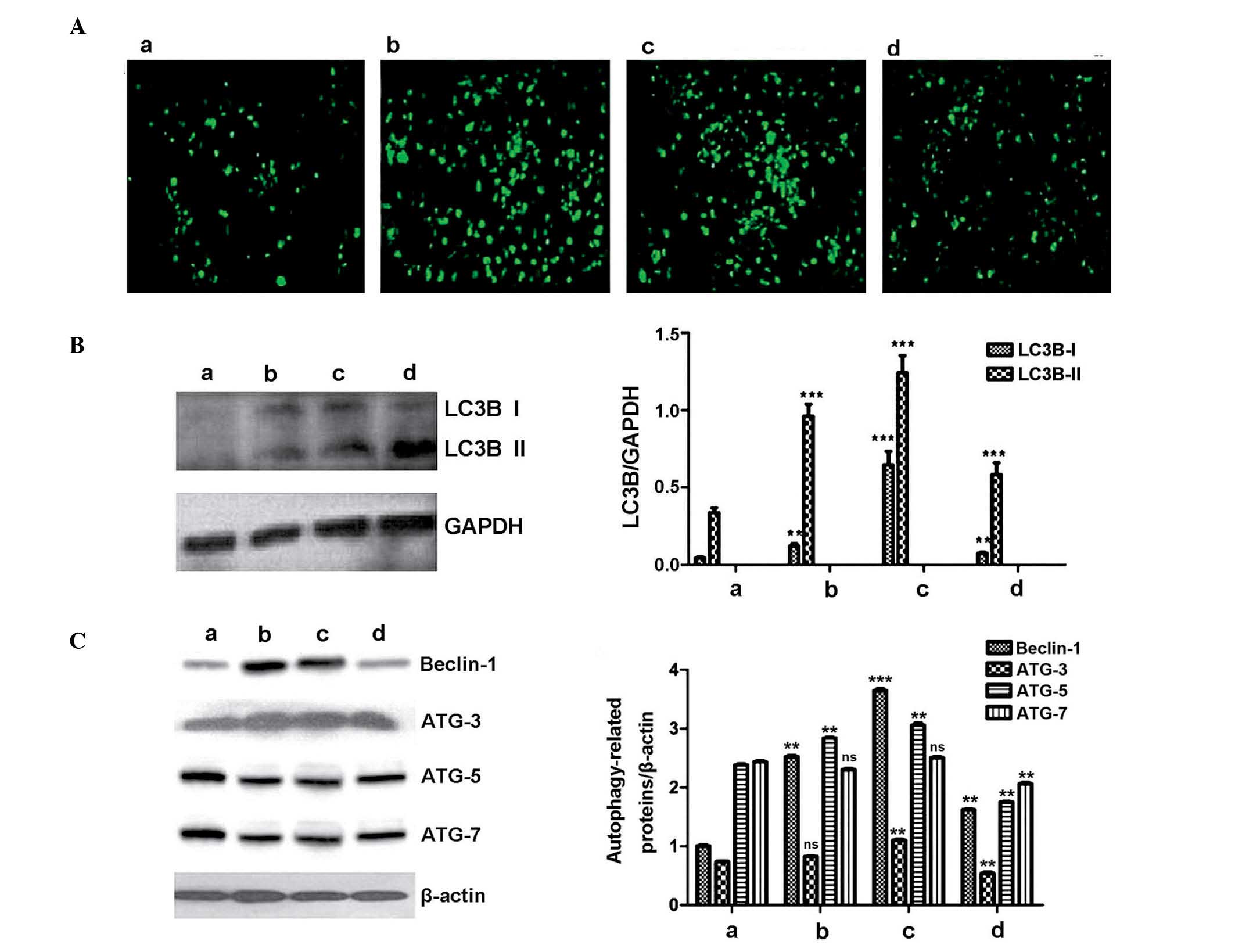 | Figure 2TAM autophagy analysis. The treatment
groups were as follows: a, 5% FBS; b, PBS + 10% FBS; c, rapamycin +
10% FBS; and d, bafilomycin A1 + 10% FBS. (A) MDC-positive
autophagy capsules in TAMs. MDC is absorbed by autophagosomes and
accumulates in the cytoplasm of TAMs, the green fluorescence
represents MDC-positive autophagy capsules in TAMs. The frequency
of green cells indicates degree of autophagy. (B) Western blot
analysis detected the expression of LC3B I and II. (C) Western blot
analysis indicated the expression levels of Beclin-1 and ATG-3, -5
and -7 in the different TAM treatment groups.**P<0.01
and ***P<0.001 vs. TAMs cultured in RPMI-1640 medium
supplemented with 5% FBS. LC3B, microtubule associated protein 1
light chain 3β; TAM, tumor-associated macrophage; MDC,
monodansylcadaverine; PBS, phosphate-buffered saline; ATG,
autophagy related; ns, not significant. |
 | Table IPercentage of
monodansylcadaverine-activated TAMs. |
Table I
Percentage of
monodansylcadaverine-activated TAMs.
| Group | Fluorescent cells
(%) |
|---|
| TAM (5% FBS) | 3.6±0.64 |
| TAM+PBS (10%
FBS) | 38.3±1.07 |
| TAM+rapamycin (10%
FBS) | 76.4±1.14 |
| TAM+bafilomycin A1
(10% FBS) | 21.3±1.21 |
Colony formation and western blot
analysis of Smac, survivin, Bcl-2 and p53 expression in LoVo
cells
The colony formation rate of LoVo cells cultured
alone, without irradiation, was 10.01±0.48%, and the cell survival
fraction was 100% (Fig. 3A and B;
Table II). When LoVo cells
received 6 Gy irradiation, the colony number was reduced
significantly compared with the control group (P<0.01), the
colony formation rate was 3.98±0.73% and the cell survival fraction
was 39.7±1.14% (Fig. 3Ab and B;
Table II). When LoVo cells were
co-cultured with TAMs and received 6 Gy irradiation, the colony
number increased significantly compared with the LoVo cells alone
with 6 Gy radiation, and the colony formation rate was increased to
7.12±0.14% (Fig. 3Ac and B;
Table II). This demonstrated that
TAMs promote the proliferation of LoVo cells and reduce the cell
death caused by radiation. When the autophagy status of TAM was
altered by treatment with rapamycin or bafilomycin A1, the colony
formation rate of the LoVo cells was affected. In the
autophagy-upregulation group, the colony formation rate was
4.21±0.18%, which was reduced compared with the downregulation
group (5.28±0.23%) and untreated group (7.12±0.14%) (Fig. 3B; Table II). Western blot analysis was
performed to measure the expression levels of Bcl-2, Smac, survivin
and p53. Relative expression of Bcl-2 in the untreated TAM group
was 0.24±0.02, in the autophagy-upregulation group it was
0.08±0.01, whilst in the autophagy-downregulation group it was
0.42±0.02 (Fig. 3C). In the
control group of LoVo cells cultured alone, the relative Bcl-2
expression level was 0.61±0.05. Relative expression levels of Smac
in the TAM co-culture untreated, autophagy-upregulated and
-downregulated groups were 1.26±0.03, 1.49±0.24 and 0.85±0.03,
respectively, and 0.68±0.03 in the control group (Fig. 3C). Expression levels of survivin in
the TAM co-culture untreated, autophagy-upregulated and
-downregulated groups were 0.48±0.01, 0.23±0.02 and 0.55±0.05,
respectively, and 0.80±0.05 in the control group. Expression levels
of p53 in the TAM co-culture untreated, autophagy-upregulated and
-downregulated groups were 0.14±0.006, 0.10±0.008 and 0.07±0.012,
respectively, and 0.12±0.011 in the control group (Fig. 3C).
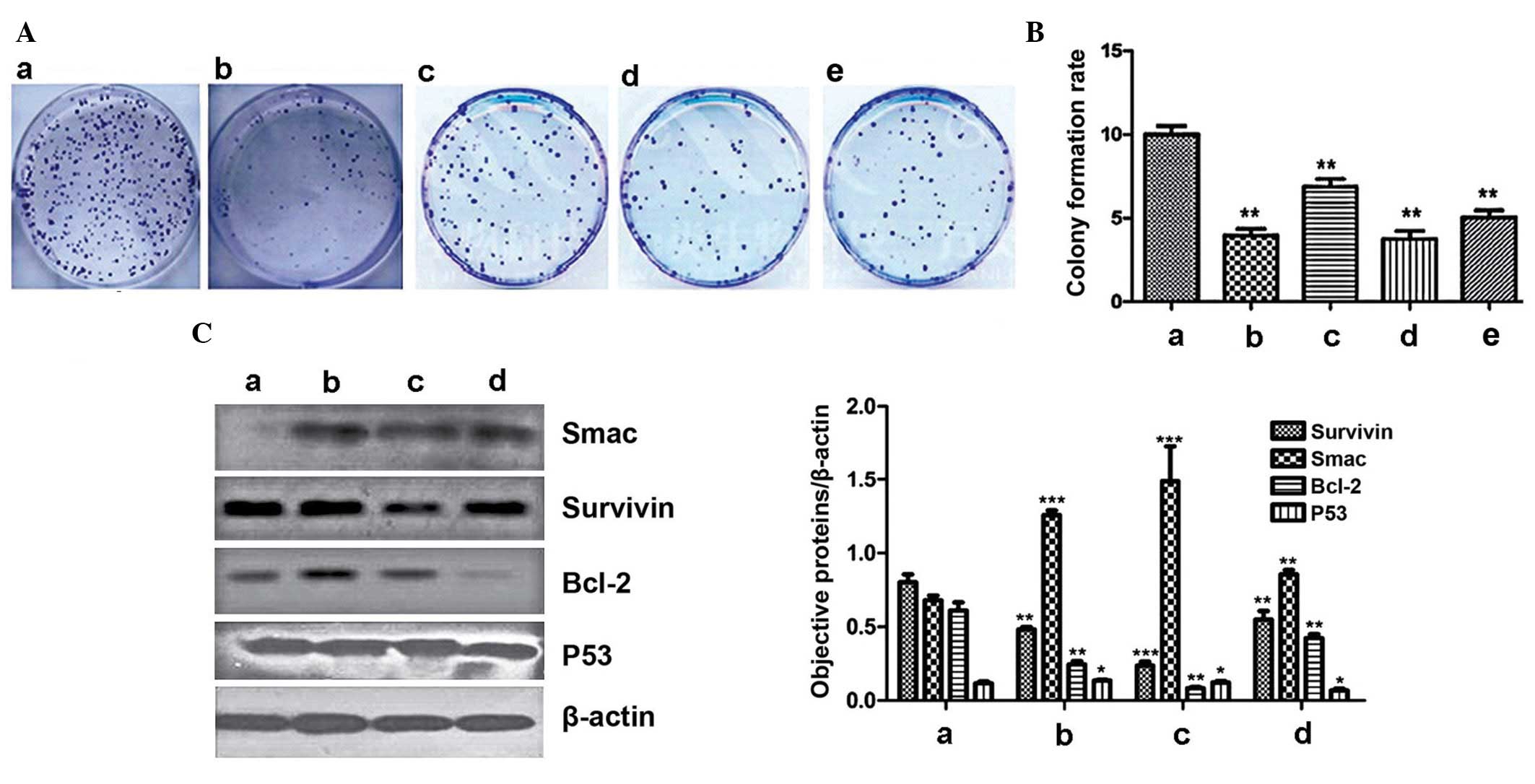 | Figure 3Colony formation and western blot
analysis of the expression of Smac, survivin, Bcl-2 and p53 in LoVo
cells. (A) Clonogenesis of LoVo cells. Treatment groups were as
follows: a, LoVo cells + no radiation; b, LoVo cells + 6 Gy
radiation; c, LoVo cells + TAM co-culture with 6 Gy radiation; d,
LoVo + autophagy-upregulation TAM co-culture (rapamycin) with 6 Gy
radiation; and e, LoVo + autophagy-downregulation TAM co-culture
(bafilomycin A1) with 6 Gy radiation. (B) Colony formation rate of
LoVo cells in the 5 groups. **P<0.01 and
***P<0.001 vs. LoVo cells cultured alone without
irradiation. (C) Expression of Bcl-2, Smac, survivin and p53.
Treatment groups were as follows: a, LoVo cells alone; b, LoVo
cells + untreated TAM co-culture; c, LoVo + autophagy-upregulated
TAM co-culture (rapamycin); and d, LoVo + autophagy-downregulated
TAM co-culture (bafilomycin A1). *P<0.05,
**P<0.01 and ***P<0.001 vs. LoVo cells
cultured alone without irradiation. TAM, tumor-associated
macrophage; Smac, second mitochondria-derived activator of
caspase. |
 | Table IIColony formation rate and survival
fraction of LoVo cells. |
Table II
Colony formation rate and survival
fraction of LoVo cells.
| Group | Colony formation
rate (%) | Survival fraction
(%) |
|---|
| LoVo (6 Gy
irradiation) | 3.98±0.73 | 39.70±1.14 |
| LoVo+TAM (PBS + 6
Gy irradiation) | 7.12±0.14 | 71.12±0.34 |
| LoVo+TAM (rapamycin
+ 6 Gy irradiation) | 4.21±0.18 | 42.05±0.27 |
| LoVo+TAM
(bafilomycin A1 + 6 Gy irradiation) | 5.28±0.23 | 52.74±0.42 |
| LoVo (without
irradiation) | 10.01±0.48 | 100 |
When LoVo cells were co-cultured with TAMs, their
colony-forming ability was improved and resistance to radiotherapy
was enhanced. The results of the current study indicate that the
autophagy status of TAMs may influence the proliferation and
radiosensitivity-associated protein expression in LoVo cells.
Therefore, the upregulation of autophagy in TAMs may reduce colony
formation and enhance the anticancer effects of radiotherapy on
colorectal cancer cells.
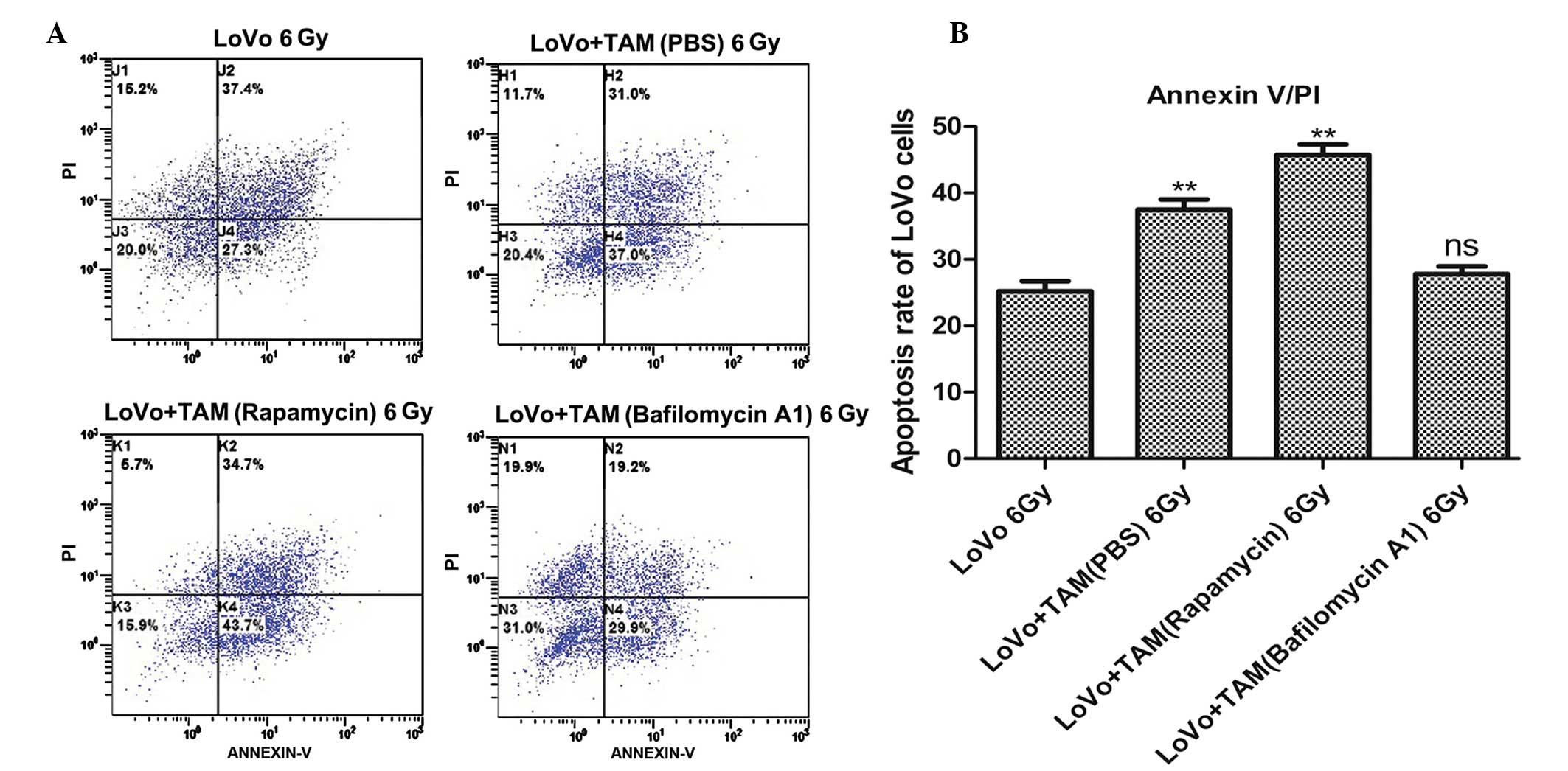
Annexin V/PI double staining flow
cytometry as detection of apoptosis
Apoptosis of LoVo cells was measured by annexin V/PI
double staining and flow cytometry. The percentages of apoptotic
cells in the untreated, autophagy-upregulation and -downregulation
groups were 37.49±3.42, 45.73±3.49 and 27.79±2.59%, respectively.
The percentage of apoptotic cells detected when LoVo cells were
cultured alone was 25.19±3.46% (Fig.
4A; Table III). The highest
degree of apoptosis occurred in the autophagy-upregulated TAM group
(Fig. 4A; Table III). Therefore, the upregulation
of TAM autophagy promoted the apoptosis of LoVo cells. The
proportion of dead cells in the control group, including necrotic
and apoptotic cells (62.13%), was higher than that in the
autophagy-downregulation group (47.44%) as presented in Fig. 4A and Table III. The group with the highest
number of necrotic cells was the control group, 36.94±0.48%,
compared with 19.65±0.96% in the autophagy-downregulation group.
Therefore, when LoVo cells were co-cultured with TAMs,
radiation-induced apoptosis was enhanced, and the upregulation of
TAM autophagy significantly promoted the apoptosis of LoVo cells,
improving the radiosensitivity of colorectal cancer cells.
 | Table IIIProportion of apoptotic and necrotic
LoVo cells. |
Table III
Proportion of apoptotic and necrotic
LoVo cells.
| Group | Apoptotic cells
(%) | Necrotic cells
(%) |
|---|
| LoVo (6 Gy
irradiation) | 25.19±3.46 | 36.94±0.48 |
| LoVo+TAM (PBS + 6
Gy irradiation) | 37.49±3.42 | 30.78±0.69 |
| LoVo+TAM (rapamycin
+ 6 Gy irradiation) | 45.73±3.49 | 34.12±0.89 |
| LoVo+TAM
(bafilomycin A1 + 6 Gy irradiation) | 27.79±2.59 | 19.65±1.10 |
Discussion
It has long been recognized that cancer cells are
not the only component of solid tumors. Tumor growth does not occur
independently, it has a complicated and close association with
tumor stromal cells and the extracellular matrix within the tumor
microenvironment (18,19). Stromal cells interact with tumors
through a variety of signaling factors, contributing to tumor
proliferation, invasion, infiltration and metastasis to distant
sites (20).
TAMs are an integral part of the tumor
microenvironment and are important in numerous processes, including
tumor proliferation, invasion and metastasis (21,22).
TAMs in the tumor microenvironment have an M2 phenotype. Compared
with normal macrophages, their antigen-presenting function and
stimulation of type I adaptive immune responses are weak (21). Additionally, TAMs inhibit T cell
proliferation and cytotoxic activity, induce tumor angiogenesis,
promote malignant proliferation and aid tumor tissue repair
(22,23). Wyckoff et al (13) observed that greater TAM
infiltration of tumor tissue was associated with worse disease
prognosis in patients. Studies have also confirmed that TAMs
secrete TGF-β, PDGF, tumor necrosis factor-α, matrix
metalloproteinase-9 and other factors, to promote tumor
angiogenesis and accelerate tumor growth (21,24).
Previous studies have demonstrated that TAMs secrete PDGF, IL-1 and
-6 to aid tumor cell survival and reduce tumor radiosensitivity
(15,16,25).
Thus, the study of TAMs may uncover novel methods to improve the
results of radiotherapy.
Autophagy is the degradation system of cells. By
forming an autophagic-palade, cell fractions, including cellular
protein and organelles, are passed to the lysosome for degradation,
maintaining the metabolic balance of the cell (17). It has been observed that defects in
autophagy can promote tumor development. When a tumor is
progressing, cancer cells are under hypoxic stress and
nutrient-limited conditions; this activates autophagy to degrade
denatured proteins and damaged organelles, providing nutrients and
energy to enable tumor cells to survive (26). The regulatory mechanisms of
autophagy are complex. The mechanistic target of rapamycin (mTOR)
kinase, is an important regulator of cell growth and development,
and negatively regulates autophagy. The anti-fungal drug,
rapamycin, can inhibit the activity of mTOR leading to autophagy
activation (27). The current
study used a rapamycin derivative, RAD001, to upregulate cellular
autophagy. In the autophagy process, autophagic vesicles combine
with lysosomes. The enzyme H+-ATPase is required for the
acidification of vesicles, enabling mature lysosomal autophagy and
autophagic degradation function. Bafilomycin A1 is a macro-cyclic
antibiotic that inhibits vesicle-type H+-ATPase, and
therefore, acts as an inhibitor of autophagy (28).
In the current study, using previously described
methods (29–31), PMA and IL-4 were used to induce
human monocytic leukemia THP-1 cells to differentiate into TAMs.
Differentiation was identified by observing changes to cell
morphology and the expression of cell surface markers.
Subsequently, rapamycin and bafilomycin A1 were used to stimulate
and inhibit the autophagy of TAM.
MDC is a specific marker of autophagy that is
absorbed by cells and accumulates in autophagic vesicles. The
current study used fluorescence microscopy to observe MDC-positive
autophagic vesicles. Autophagic vesicles have a punctate structure,
and are located in the cytoplasm and nucleus. LC3 proteins are
widely recognized as molecular markers of autophagy. LC3s are
similar to the yeast proteins Apg8/Aut7/Atg8 and are important in
the autophagy process. LC3 proteins are cleaved immediately by Atg4
at their carboxy-terminus following synthesis, producing LC3-I,
which is localized in the cytoplasm. During the process of
autophagy, LC3-I is modified by a ubiquitin-like system, involving
the proteins ATG-3 and -7, resulting in a 14-kD protein, LC3-II,
which localizes to the autophagic body. Thus, the presence of
autophagic bodies and the detection of LC3-I and LC3-II are treated
as molecular markers of autophagy. There are three types of LC3
expressed in mammalian cells, including LC3A, B and C. They are
required for the post-translational modifications associated with
autophagy. However, only LC3B is expressed in autophagic
structures, therefore, the expression of LC3B was used as a marker
of autophagy in the current study. The number of MDC-stained
autophagic vesicles (Fig. 2A) and
the expression of LC3B (Fig. 2B)
demonstrated that autophagy of TAMs was up- and downregulated by
rapamycin and bafilomycin A1, respectively. The expression levels
of ATGs and Beclin-1 were also altered following drug treatment
(Fig. 2C).
Tumor radiosensitivity indicators include apoptosis,
proliferation, cell survival fraction and expression of the tumor
suppressor gene, p53 and its downstream targets. In the current
study, apoptosis, proliferation, cell survival and the expression
levels of p53, Smac, survivin and Bcl-2 were selected as an
evaluation index of LoVo cell radiosensitivity.
Smac is a mitochondrial apoptotic regulatory
protein. The N-terminal region of Smac associates with the BIR
domains of survivin and inhibitor of apoptosis protein (IAP) family
members, suppressing their anti-apoptotic activity. The inhibition
of X-linked IAP (XIAP) is an important process. Smac and XIAP are
important regulatory factors in the apoptotic pathway, which is
closely associated with tumor development and radiation-induced
apoptosis. During radiation-induced apoptosis, the BIR3 domain of
XIAP binds with Smac, so it cannot associate with the initiation
factor caspase 9 to stimulate the apoptotic cascade and induce
apoptosis. Zheng et al (32) observed that the upregulation of
Smac expression can enhance ionizing radiation-induced apoptosis
and increase the sensitivity of HeLa cells to ionizing radiation.
Giagkousiklidis et al (33)
demonstrated that Smacdx molecule analogs can enhance
radiation-induced apoptosis by antagonizing IAP and increasing
caspase activity. These results suggest that therapeutically
targeting Smac is a potential method to improve radiosensitivity in
oncology. The IAP family is a group of anti-apoptotic proteins. The
family have homologous sequences at the BIR and the RING finger
domains, and can inhibit apoptosis and promote tumorigenesis. Smac
is known to exist in mammalian cells and can directly inhibit IAP
proteins. Studies have demonstrated that Smac expression is reduced
in colon cancer cells (32,34),
suggesting that enhanced expression of Smac may improve the
radiosensitivity of colorectal cancer.
Survivin is a member of the IAP family. It is not
strongly expressed in differentiated mature tissue, however,
survivin is highly expressed in embryonic tissue and stem cells.
Analysis of fresh specimens identified that survivin is highly
expressed in colon cancer, pancreatic cancer and other malignant
tumors (35). Therefore, the
inhibition of survivin expression may promote tumor cell apoptosis.
Currently, studies have confirmed that reduced expression of
survivin can induce apoptosis in various tumor cell lines in
vitro, can slow tumor growth in experimental animal models and
increase the sensitivity of tumors to various chemotherapeutic
drugs and radiation (36). Rödel
et al (37) detected the
expression of survivin in several colon cancer cell lines (SW480,
LoVo, SW48 and CT215), and observed that survivin was negatively
correlated with spontaneous and radiation-induced apoptosis. Higher
survivin expression levels were associated with decreased
spontaneous apoptosis and improved resistance to radiation.
In the current study, the expression levels of Smac
were increased, and survivin levels decreased in the TAM/LoVo
co-culture groups compared with the LoVo alone control group
(P<0.01). Downregulation of TAM autophagy increased survivin
expression in LoVo cells and inhibited the expression of Smac
compared with the control group (P<0.01). Upregulation of TAM
autophagy inhibited survivin expression in LoVo cells and increased
the expression of Smac compared with the LoVo cells alone.
Following irradiation, apoptosis in the TAM autophagy-upregulation
group was higher than all other groups. The results indicated that
apoptosis is closely associated with the expression of Smac and
survivin. Upregulation of TAM autophagy may inhibit survivin
expression and increase Smac expression to promote the caspase
cascade pathway, inducing apoptosis and increasing the
radio-sensitivity of LoVo cells.
The Bcl-2 family includes pro-apoptotic and
anti-apoptotic proteins. Bcl-2, Bcl-xL and Bcl-w are anti-apoptotic
family members. In the present study, western blot demonstrated
that Bcl-2 expression in the autophagy-upregulation group was the
lowest of all groups, whilst the rate of apoptosis was highest in
this group. By contrast, the autophagy-downregulation group
exhibited the highest Bcl-2 expression and the lowest LoVo
apoptosis rate of the 3 co-culture groups. The current study
demonstrated that co-culture with TAMs increased the expression of
p53 in LoVo cells, and that the expression of p53 was influenced by
the autophagy status of TAMs (P<0.05 vs. the control group).
The cell survival fraction and colony formation of
LoVo cells were also analyzed. Following irradiation, the colony
formation rate in the untreated TAM co-culture group was increased
compared with the LoVo cell only group, and its surviving fraction
was the highest of all groups. Compared with when the colon cancer
cells were cultured alone, the co-culture with TAM promoted the
proliferation of LoVo cells, and proliferation was altered by the
autophagy status of the TAMs. The colony formation rate of LoVo
cells in the autophagy-upregulation group was lower than the
untreated and autophagy-downregulation co-culture groups. When LoVo
cells received 6 Gy irradiation, the survival fraction of LoVo
cells was significantly decreased compared with LoVo cells cultured
alone without irradiation. This indicates that the upregulation of
TAM autophagy may suppress the effect of TAM on the promotion of
tumor proliferation. Correlational studies have demonstrated that
TAMs secrete IL-10, resulting in reduced activity of NF-κB and
inhibition of transcriptional control of cell division (38). Therefore, the present study
hypothesized that TAMs may act via the secretion of cytokines such
as IL-10, to suppress NF-κB activity, improving the efficiency of
gene transcription to promote cell proliferation.
Drug intervention by rapamycin and bafilomycin Al
was used to change the autophagy status of TAMs. This directly
affected the biological behavior of TAMs, and altered the levels of
proliferation, apoptosis and expression of
radiosensitivity-associated proteins in tumor cells. Thus, the
regulation of TAM autophagy changes the biological behavior of LoVo
cells. Upregulation of TAM autophagy can inhibit the proliferation
and promote apoptosis of colorectal cancer cells, thus, improving
the efficacy of radiotherapy.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81172348), the
Jiangsu Provincial Key Laboratory of Radiation Medicine and
Protection (no. KJS1334) and Suzhou Science and Education Guardian
Youth Science and Technology Project (no. 2011010).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang Z, Huang D, Ni S, Peng Z, Sheng W
and Du X: Plasma microRNAs are promising novel biomarkers for early
detection of colorectal cancer. Int J Cancer. 127:118–126. 2010.
View Article : Google Scholar
|
|
3
|
Gutman M and Fidler IJ: Biology of human
colon cancer metastasis. World J Surg. 19:226–234. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sasaki H, Miura K, Horii A, Kaneko N,
Fujibuchi W, Kiseleva L, Gu Z, Murata Y, Karasawa H, Mizoi T, et
al: Orthotopic implantation mouse model and cDNA microarray
analysis indicates several genes potentially involved in lymph node
metastasis of colorectal cancer. Cancer Sci. 99:711–719. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sleeman JP: The lymph node as a bridgehead
in the metastatic dissemination of tumors. Cancer Res. 157:55–81.
2000.
|
|
6
|
Xu N, Qiu H and Ding Y: The relation
between DNA replication error and clinicopathological features of
colorectal carcinoma. Zhonghua Bing Li Xue Za Zhi. 27:359–361.
1998.In Chinese.
|
|
7
|
Lin Y, Buckhaults PJ, Lee JR, Xiong H,
Farrell C, Podolsky RH, Schade RR and Dynan WS: Association of the
actin-binding protein transgelin with lymph node metastasis in
human colorectal cancer. Neoplasia. 11:864–873. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akagi T, Hijiya N, Inomata M, Shiraishi N,
Moriyama M and Kitano S: Visinin-like protein-1 overexpression is
an indicator of lymph node metastasis and poor prognosis in
colorectal cancer patients. Int J Cancer. 131:1307–1317. 2012.
View Article : Google Scholar
|
|
9
|
Toiyama Y, Yasuda H, Saigusa S, Tanaka K,
Inoue Y, Goel A and Kusunoki M: Increased expression of Slug and
Vimentin as novel predictive biomarkers for lymph node metastasis
and poor prognosis in colorectal cancer. Carcinogenesis.
34:2548–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yue ZQ, Lui YP, Ruan JS, Zhou L and Lu Y:
Tumor-associated macrophages: A novel potential target for cancer
treatment. Chin Med J (Engl). 125:3305–3311. 2012.
|
|
12
|
Rigo A, Gottardi M, Zamò A, Mauri P,
Bonifacio M, Krampera M, Damiani E, Pizzolo G and Vinante F:
Macrophages may promote cancer growth via a GM-CSF/HB-EGF paracrine
loop that is enhanced by CXCL12. Mol Cancer. 9:273–279. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley
F, Stanley ER, Graf T, Pollard JW, Segall J and Condeelis J: A
paracine loop between tumor cells and macrophages is required for
tumor cell migration in mammary tumors. Cancer Res. 64:7022–7029.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Canioni D, Salles G, Mounier N, Brousse N,
Keuppens M, Morchhauser F, Lamy T, Sonet A, Rousselet MC, Foussard
C, et al: High numbers of tumor-associated macrophages have an
adverse prognostic value that can be circumvented by rituximab in
patients with follicular lymphoma enrolled onto the GELA-COELAMS
FL-2000 trial. J Clin Oncol. 26:440–446. 2008. View Article : Google Scholar
|
|
15
|
Kuwahara Y, Oikawa T, Ochiai Y, Roudkenar
MH, Fukumoto M, Shimura T, Ohtake Y, Ohkubo Y, Mori S, Uchiyama Y,
et al: Enhancement of autophagy is a potential modality for tumors
refractory to radiotherapy. Cell Death Dis. 2:e1772011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barker HE, Paget JT, Khan AA and
Harrington KJ: The tumour microenvironment after radiotherapy:
Mechanisms of resistance and recurrence. Nat Rev Cancer.
15:409–425. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allavena P, Sica A, Solinas G, Porta C and
Mantovani A: The inflammatory micro-environment in tumor
progression: The role of tumor-associated macrophages. Crit Rev
Oncol Hematol. 66:1–9. 2008. View Article : Google Scholar
|
|
19
|
Stout RD, Jiang C, Matta B, Tietzel I,
Watkins SK and Suttles J: Macrophages sequentially change their
functional phenotype in response to changes in microenvironment
influences. J Immunol. 175:342–349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sica A, Schioppa T, Mantovani A and
Allavena P: Tumor-associated macrophages are a distinct M2
polarised population promoting tumor progression: Potential targets
of anti-cancer therapy. Eur J Cancer. 42:717–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gocheva V, Wang H-W, Gadea BB, Shree T,
Hunter KE, Garfall AL, Berman T and Joyce JA: IL-4 induces
cathepsin protease activity in tumor-associated macrophages to
promote cancer growth and invasion. Genes Dev. 24:241–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pander J, Heusinkveld M, der Straaten TV,
Jordanova ES, Baak-Pablo R, Gelderblom H, Morreau H, van der Burg
SH, Guchelaar HJ and van Hall T: Activation of tumor-promoting type
2 macrophages by EGFR-targeting antibody Cetuximab. Clin Cancer
Res. 17:5668–5673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coffelt SB, Hughes R and Lewis CE:
Tumor-associated macrophages: Effectors of angiogenesis and tumor
progression. Biochim Biophys Acta. 1796:11–18. 2011.
|
|
24
|
Bingle L, Lewis CE, Corke KP, Reed MW and
Brown NJ: Macrophages promote angiogenesis in human breast tumour
spheroids in vivo. Br J Cancer. 94:101–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karar J and Maity A: Modulating the tumor
microenvironment to increase radiation responsiveness. Cancer Biol
Ther. 8:1994–2001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amaravadi R, Lippincott Schwartz J, Yin X,
Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT and White E:
Principles and current strategies for targeting autophagy for
cancer treatment. Clin Cancer Res. 17:654–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan SH, Shui GH, Zhou J, Li JJ, Bay BH,
Wenk MR and Shen HM: Induction of autophagy by palmitic acid via
protein Kinase C-mediated signaling pathway independent of mTOR. J
Biol Chem. 287:14364–14376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanematsu S, Uehara N, Miki H, Yoshizawa
K, Kawanaka A, Yuri T and Tsubura A: Autophagy inhibition enhances
sulforaphane-induced apoptosis in human breast cancer cells.
Anticancer Res. 30:3381–3390. 2010.PubMed/NCBI
|
|
29
|
Essafi-Benkhadir K, Refai A, Riahi I,
Fattouch S, Karoui H and Essafi M: Quince (Cydonia oblonga Miller)
peel polyphenols modulate LPS-induced inflammation in human
THP-1-derived macrophages through NF-κB, p38MAPK and Akt
inhibition. Biochem Biophys Res Commun. 418:180–185. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spencer M, Yao-Borengasser A, Unal R,
Rasouli N, Gurley CM, Zhu B, Peterson CA and Kern PA: Adipose
tissue macrophages in insulin-resistant subjects are associated
with collagen VI and fibrosis and demonstrate alternative
activation. Am J Physiol Endocrinol Metab. 299:E1016–E1027. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qin Z: The use of THP-1 cells as a model
for mimicking the function and regulation of monocytes and
macrophages in the vasculature atherosclerosis. Proc Natl Acad Sci
USA. 221:2–11. 2012.
|
|
32
|
Zheng LD, Xiong ZF, Zhu JW and Wang ZH:
Effects of Smac gene over-expression on the radiotherapeutic
sensitivities of cervical cancer cellline HeLa. Chin Med J (Engl).
8:226–30. 2008.
|
|
33
|
Giagkousiklidis S, Vogler M, Westhoff MA,
Kasperczyk H, Debati KM and Fulda S: Sensitization for
gamma-irradiation-induced apoptosis by second mitochondria-derived
activator of caspase. Cancer Res. 65:10502–10513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kohli M, Yu J, Seaman C, Bardelli A,
Kinzler KW, Vogelstein B, Lengauer C and Zhang L:
SMAC/Diablo-dependent apoptosis induced by nonsteroidal
antiinflammatory drugs (NSAIDs) in colon cancer cells. Proc Natl
Acad Sci USA. 101:16897–16902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sreevalsan S, Jutooru I, Chadalapaka G,
Walker M and Safe S: 1,1-Bis(3′-indolyl)-1-(p-bromophenyl)methane
and related compounds repress survivin and decrease γ-radiation
induced survivin in colon and pancreatic cancer cells. Int J Oncol.
35:1191–1199. 2009.PubMed/NCBI
|
|
36
|
Yu L-W and Ma X-T: Stat3 signaling pathway
regulates the expression of Survivin and promotes apoptosis of
human colon cancer cells. Zhong Hua Shi Yan Wai Ke Za Zhi She.
3:291–293. 2008.In Chinese.
|
|
37
|
Rödel C, Haas J, Groth A, Grabenbauer GG,
Sauer R and Rödel F: Spontaneous and radiation-induced apoptosis in
colorectal carcinoma cells with different intrinsic
radiosensitivities: Survivin as a radioresistance factor. Int J
Radiat Oncol Biol Phys. 55:1341–1347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Teng Z, Yu L and Zhang J:
Tumor-associated macrophages affect biological behavior of SW480
cell-line. Acad Med. 33:71–75. 2011.
|