Introduction
Annually, ~795,000 individuals experience a new or
recurrent stroke (1). Notably,
stroke survivors exhibit various neurological deficiencies,
including cognitive impairment (2). As the population ages, the incidence
of individuals experiencing neurological damage caused by stroke is
increasing, and cognitive impairment is correlated with an increase
in the mean age at first onset of stroke (3). A recent analysis of a London registry
of stroke patients revealed that 22% were cognitively impaired
following stroke (4). Acupuncture
has been used for treating numerous ailments in China, including
stroke and memory deficiency. Numerous studies have shown the
efficacy of electroacupuncture (EA) in the rehabilitation of
patients with cognitive dysfunction in clinical and experimental
settings, and Baihui (DU20) and Shenting (DU24) are common EA
points selected for this type of treatment (5–8).
In neurons, the Rho GTPases are members of the Ras
superfamily of small (±21 kDa) GTPases. Ras homologous member A
(RhoA), Ras-related C3 botulinum toxin substrate 1 (Rac1) and cell
division cycle 42 (Cdc42) are Rho GTPases that are best known for
their effects on the actin cytoskeleton, which in dendritic spines
are mainly comprised of F-actin (9–10).
At the synapse Rac1, Cdc42 and RhoA have emerged as key regulators
of dendritic spine formation and morphogenesis. These proteins have
recently been implicated in synaptic plasticity, including the
excitatory synapses, which are located on dendritic spines
(11,12). RhoA and Racl/Cdc42 act as mutual
antagonists on dendritic spines. Rac1 and Cdc42 have been shown to
promote the formation, growth and maintenance of spines, whereas
RhoA induces spine retraction and loss (13,14).
Recent evidence indicates that variations in cognitive processes,
in particular learning and memory, are associated with plastic
changes in dendritic spines (15,16).
Together these studies suggest that the regulation of the actin
cytoskeleton by Rho GTPases in synaptic plasticity represents the
cellular basis of cognition.
The precise mechanism of cognitive dysfunction
remains unclear. In the present study, the therapeutic efficacy of
EA against post-stroke cognitive dysfunction was evaluated and the
underlying molecular mechanisms were investigated using a cerebral
ischemia/reperfusion (I/R) rat model.
Materials and methods
Animals and study groups
Healthy adult male Sprague-Dawley rats (weight,
270–310 g; n=54) were purchased from the SLAC Laboratory Animal
Co., Ltd., Shanghai, China [Laboratory Animal Use Certificate no.
SCXK (SH) 2012-0002] and raised in a sterile environment at 22±1°C
and 50% humidity in a 12/12 h light/dark cycle with access to food
and water ad libitum. All experiments were performed
strictly in accordance with the International Ethical Guidelines
and the National Institutes of Health Guide concerning the Care and
Use of Laboratory Animals. The study was approved by the ethics
committee of the College of Rehabilitation Medicine, Fujian
University of Traditional Chinese Medicine (Fuzhou, China; no.:
2013047). Rats were randomly and evenly divided into three groups
(n=18) as follows: i) Sham surgery control group (Sham group); ii)
the middle cerebral artery occlusion (MCAO) model of ischemia
control group (MCAO group); and (iii) the MCAO+EA treatment group
(MCAO+EA group).
Cerebral IR-injury rat model
Longa's suture-occluded method was used to establish
the MCAO and reperfusion model (17). Briefly, rats were anesthetized with
10% chloral hydrate (0.03 ml/100 g body weight; Shanghai Chemical
Reagent Co., Ltd., Shanghai, China) through intraperitoneal
injection. Then, 18–22 mm of nylon surgical thread (Beijing Sunbio
Biotech, Co., Ltd., Beijing, China) was inserted into the left
internal carotid artery to block the MCA when the blunted distal
end met resistance. After 2 h of occlusion, the thread was removed
to allow complete reperfusion of the ischemic area. The rectal
temperatures of the rats were maintained at 37°C throughout the
surgical procedures using heat pads. Following surgery, the rats
were allowed to recover in pre-warmed cages for ~2 h. Sham surgery
was conducted as above without the occlusion of the MCA.
Electroacupuncture treatment
In the MCAO+EA group, rats were administered EA for
30 min daily for 7 days ~4 h after MCAO was conducted. The
acupuncture needles (0.3 mm diameter; Suzhou Medical Supplies
Factory, Co., Ltd., Suzhou, China) were inserted at a depth of 2–3
mm into the Baihui (DU20) and Shenting (DU24) acupoints on the
head. Stimulation was then generated using the EA apparatus (model
G6805; Shanghai Chemical Reagent Co., Ltd.), and the stimulation
parameters were set as disperse waves of 1 and 20 Hz. The Sham and
MCAO groups did not undergo any EA treatment.
Measurement of cerebral infarct
volume
Following completion of the experiment, 7 rats from
each group were sacrificed by overdose of 10% chloral hydrate
(Shanghai Chemical Reagent Co., Ltd.), the injection dosage was
0.03 ml/100 g body weight administered by intraperitoneal
injection. Each rat was perfused transcardially with 0.9% NaCl and
the brain was removed. The brain was sectioned in the coronal plane
into 2-mm thick slices. The slices were stained with 2% tetrazolium
chloride (TTC) solution (Sigma-Aldrich, St. Louis, MO, USA) at 37°C
for 20 min and then fixed with 10% buffered formalin solution. The
normal area of the brain was stained dark red based on intact
mitochondrial function, whereas the infarct area remained
unstained. Stained slices were scanned using a high-resolution
digital camera (Canon SX20; Canon Lexus, Canon International
Trading Shanghai, Co., Ltd., Shanghai, China). The infarct volume
was determined using the Motic Med 6.0 system (Motic, Xiamen,
China), and was expressed as the percentage of the total brain
volume.
Morris water maze
Rats from each group (n=6) were subjected to the
Morris water maze task from the 3rd day following surgery in order
to investigate spatial learning and memory ability. The water maze
(Chinese Academy of Sciences, Beijing, China) was a black circular
pool with a diameter of 120 cm and a height of 50 cm, filled with
26±2°C opaque water (obtained using black ink; Beijing Yidege Ink
Industry, Co., Ltd., Beijing, China) to a depth of 30 cm. The maze
was divided geographically into four equal quadrants, and four
start positions were defined at the cardinal points of the pool. A
MINTRON.1132C video camera (Chinese Academy of Sciences, Beijing,
China) attached to a computer was placed above the center of the
pool to record and analyze each trial. A submerged safe platform
was located in the pool (2 cm below the water surface with a 6 cm
diameter in a fixed position).
Morris water maze tasks predominantly included
orientation navigation and space exploration trials. The
orientation navigation trial consisted of four swims per day for 4
days, performed from days 3–6. During this trial, start positions
were randomly selected each day, and each rat was allowed 90 sec to
swim, find the platform and remain on it for 3 sec. Swimming
duration and distances were measured. If the rat was unable to find
the platform within 90 sec, it was gently placed on it and allowed
to remain there for 10 sec. The average duration and distances
covered by each rat over the four quadrants was assessed every day.
The space exploration trial was performed 24 h after the last swim,
on day 7. The platform was removed and each animal was allowed a
free 90 sec swim. The time it took the rat to cross the location of
the platform within 90 sec was measured, and this assessment tested
their ability to remember the position of the platform. After the
trials, the rats were dried thoroughly with a hair drier and
returned to their cages.
Golgi stain
The FD Rapid GolgiStain kit (FD NeuroTechnologies,
Inc., Columbia, MD, USA) was developed using an improved Golgi-Cox
impregnation method to provide stable, sensitive, and convenient
staining of mature neurons (18).
The Golgi-Cox stain was performed on 150-µm frozen brain
sections (a uniform random sample of sections between +3.7 mm
anterior and -5.8 mm posterior relative to the bregma) obtained
from rats in each group (n=5). The staining process was performed
according to the manufacturer's protocol. The number of dendritic
spines was observed under an optic microscope (Leica DM2500; Leica
Microsystems, Wetzlar, Germany). To measure spine density, 20
pyramidal neurons in the CA1 region of the hippocampus from each
animal were selected. Matching regions of distal branch dendrites
were photographed using a ×1,000 objective. Numbers of spines were
counted in 50 µm segments, with results expressed as the
number of spines/10 µm.
Western blotting analysis
The left cerebral hippocampal tissues were collected
and triturated in a radioimmunoprecipitation assay buffer (Fans
Bio, Guangdong, China), and the proteins were quantified using a
bicinchoninic acid assay (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Equal quantities of protein (50 µg),
obtained from the hippocampus tissue in the left side of brain of
rats in each group (n=6), were loaded onto 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gels (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). After electrophoresis,
proteins were transferred onto polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). The blots were blocked with 5%
non-fat milk for 2 h at room temperature and then incubated with
the following mouse monoclonal primary antibodies overnight at 4°C:
Anti-Cdc42 (cat. no. ab41429), anti-Rac1 (cat. no. ab33186),
anti-RhoA (cat. no. ab86297), anti-F-actin (cat. no. ab205),
anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; cat. no.
ab9485) and anti-β-actin (cat. no. ab189073) (1:1,000; Abcam,
Cambridge, MA, USA). The membranes were washed in TBST three times
for 5 min per wash. Subsequently, the blots were incubated with a
horseradish peroxidase-conjugated secondary antibody (cat. no.
ab131368; 1:5,000; Cell Signaling Technology, Inc.) for 60 min.
After washing again in TBST, the blots were detected with Clarity
Western Enhanced Chemiluminescence Substrate (Bio-Rad Laboratories,
Inc.) for 1 min using a camera along with the ChemiDoc
XRS+ system (Bio-Rad Laboratories, Inc.). The pixel
intensities of the immunoreactive bands were quantified using the
percentage adjusted volume feature of Image Lab 3.0 software
(Bio-Rad Laboratories, Inc.). β-actin served as an internal
control.
Statistical analysis
All data were analyzed using the SPSS 18.0 (SPSS,
Inc., Chicago, IL, USA) software package. Data are expressed as the
mean ± standard deviation. Student's t-test, the Mann-Whitney U
test and one-way analysis of variance were used to assess
statistical differences among groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of EA treatment on infarct volume
in rats with cerebral IR injury
The effect of EA treatment at the Baihui (DU20) and
Shenting (DU24) acupoints after cerebral infarction was
investigated using TTC staining. As shown in Fig. 1, EA treatment reduced the cerebral
infarct volumes. The total infarct volumes were 26.53±3.32 and
17.36±7.04% of the total brain volume in the MCAO and MCAO+EA
groups, respectively (P<0.05).
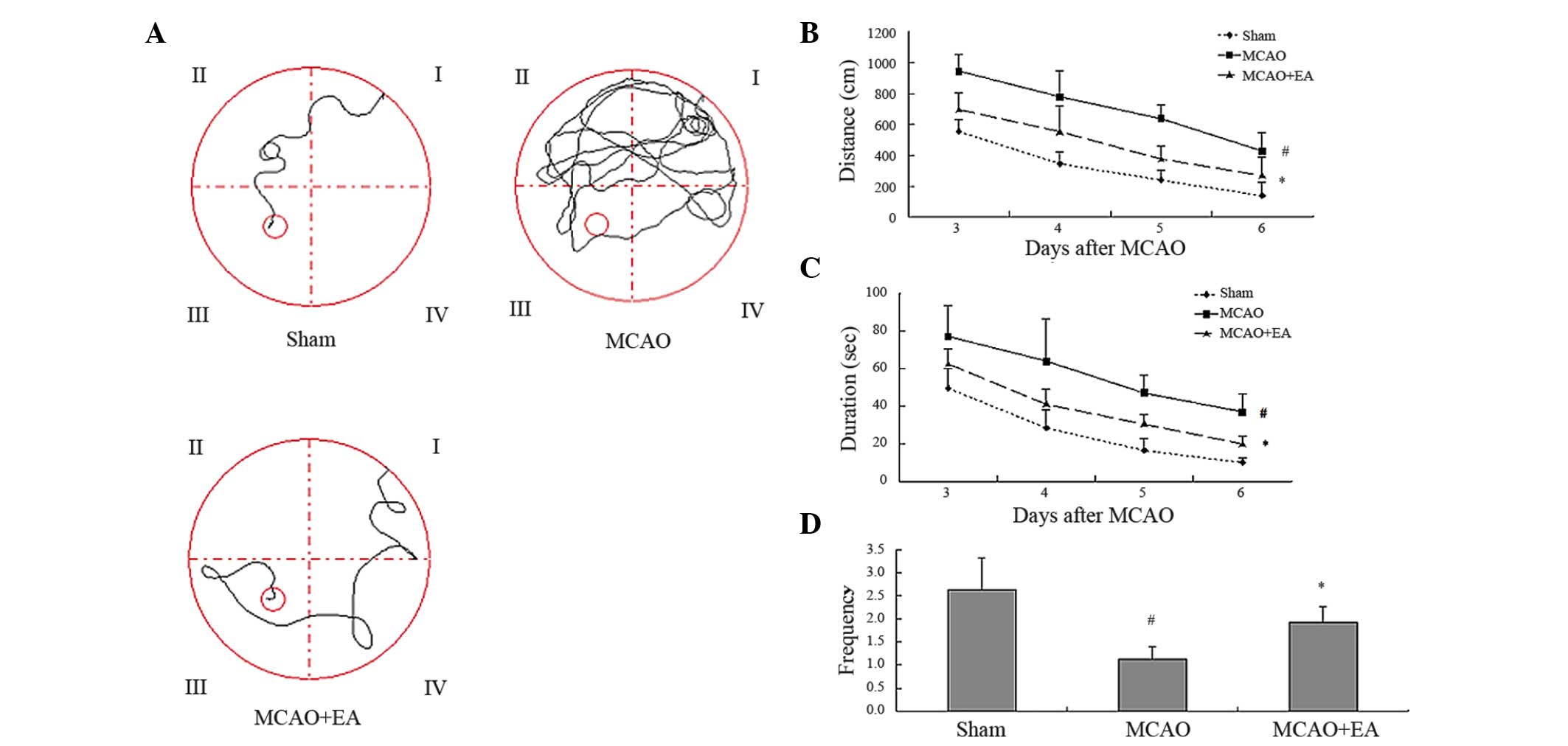
Effect of EA treatment on cognitive
impairment in rats with cerebral IR-injury
In order to investigate the effect of EA treatment
on cognitive impairment, a Morris water maze test was performed
from the 3rd day after MCAO. As shown in Fig. 2, the duration and distances of
swimming achieved by rats in the MCAO group increased as they
sought the hidden platform during the trial, whereas the frequency
of rats actually crossing the location of the platform was
significantly decreased compared with rats in the Sham group
(P<0.05). These results indicated that cerebral I/R injury led
to cognitive impairment in the MCAO group. EA treatment
significantly decreased the duration and distances swum by rats in
the MCAO+EA group, as well as increased their frequency of crossing
the location of the platform, when compared with rats in the MCAO
group (P<0.05). These results suggest that EA treatment at the
Baihui and Shenting acupoints protects cognitive function against
impairment from cerebral IR-injury to a certain extent.
Effects of EA on dendritic spines in rats
with cerebral IR-injury
Images of Golgi-stained neurons are shown in
Fig. 3. In the Sham, MCAO and
MCAO+EA groups, the majority of the dendritic spines were
mushroom-shaped. The dendritic spines were sparse in the MCAO
group. Compared with the Sham group, the MCAO group had a lower
spine density (P<0.05). The density of dendritic spines in the
MCAO+EA group was significantly increased compared with that in the
MCAO group on day 7 (P<0.05).
Effect of EA treatment on the expression
of Cdc42, Rac1, RhoA and F-actin
In order to investigate the mechanism underlying the
protective effect of EA treatment on cognitive function, the
expression of Cdc42, Rac1, RhoA and F-actin was examined in the
hippocampus of the left brain using western blotting. Figure 4 shows that MCAO injury decreased
the expression of Cdc42, Rac1 and F-actin expression, but increased
the expression of RhoA. By contrast, EA treatment significantly
increased the expression of Cdc42, Rac1 and F-actin (P<0.05),
and decreased the expression of RhoA at the protein level compared
with the MCAO group. According to these results, EA treatment may
protect cognitive function in MCAO rats through a mechanism that is
closely associated with the regulation of Cdc42, Rac1, RhoA and
F-actin expression.
 | Figure 4Effect of EA on Rho GTPases and
F-actin. Western blot analysis of levels of Cdc42, Rac1, RhoA and
F-actin in hippocampus tissue of Sham, MCAO and MCAO+EA groups
(n=6). β-actin and GAPDH were used as internal controls. EA,
electroacupuncture; MCAO, middle cerebral artery occlusion; RhoA,
Ras homologous member A, Rac1, Ras-related C3 botulinum toxin
substrate 1; Cdc42, cell division cycle 42; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
Discussion
Stroke is associated with a higher risk of dementia
and cognitive impairment without dementia, and ischemic stroke
accounts for 80% of stroke cases (19,20).
Acupuncture is based on traditional Chinese medicine, with proven
efficacy in numerous health conditions, such as stroke and memory
deficiency (21,22). According to the theory of
traditional Chinese medicine, Baihui (GV20) and Shenting (DU24)
acupoints belong to the Du Meridian, which is considered to affect
the diseases of the nervous system. It has been established that
electroacupuncture at the Baihui and Shenting acupoints exerts a
therapeutic effect on post-stroke cognitive impairment (5). Therefore, the MCAO model was used to
test the efficacy of EA. In the present study, EA treatment at
Baihui and Shenting reduced infarct volume, and improved learning
ability and memory in MCAO+EA rats compared with the MCAO group as
determined by the Morris water maze test.
Learning and memory have a certain functional
location in the brain. The hippocampus is an important storage
structure for learning and memory. It has been extensively
investigated in terms its physiological function as well as its
involvement in pathological models (23,24).
Dendritic spines are small mushroom-like protrusions arising from
neurons where the majority of excitatory synapses reside. Dendritic
spines are critical in cognitive and motor function, as well as
memory formation. Memory formation is hypothesized to lead to an
increase in dendritic spine density, and an increased spine density
has been proposed to enhance learning ability after exposure to an
enriched environment (25,26). The cytoskeleton of the dendritic
spines predominantly consists of F-actin. It serves as a structural
framework and as the principal regulator of protein and vesicular
trafficking. In the present study, it was demonstrated that
cerebral I/R injury decreased dendritic density, and treatment with
EA was shown to significantly increase spine density and the
expression of F-actin in the hippocampus.
Cognitive impairment is strongly associated with
synaptic plasticity, which is regulated by various intracellular
mechanisms, such as fluctuating expression levels of Rho GTPases.
Over the past few years, it has become clear that Rho GTPases and
related molecules are important in various aspects of neuronal
development, including neurite outgrowth and differentiation, and
the formation and maintenance of dendritic spines through its
effect on the actin cytoskeleton (27–29).
A recent imaging study detected persistent activation of Rho
GTPases in the dendritic spine following long-term potentiation
induction. The pharmacological blockade affected several downstream
signaling pathways of these proteins, including p21-activated
kinase (PAK) and Rho-associated, coiled-coil containing protein
kinase (ROCK), and this effectively inhibited spine enlargement
(30).
Rho GTPases act on several downstream effectors
involved in the stabilization, contraction, polymerization and
capture of cytoskeletal building blocks. Protein assemblies
required for actin polymerization are induced by several Rho
GTPases. For example, RhoA binds to mDia; Rac1 binds to WAVE; and
Cdc42 binds to N-WASP (31).
Microtubule stabilization is regulated by RhoA, Rac1 and Cdc42
through the actions of mDia, PAK or PAR6 (32). Regulation of the actin cytoskeleton
by Rac and Cdc42 controls local dendritic spine growth, and its
stability is associated with the Rac or Cdc42/PAK3/LIMK1 pathways.
PAK3 is a downstream effector for Rac/Cdc42 Rho GTPases. Activation
of PAK3 by Rac1 or Cdc42 leads to the phosphorylation of LIMK. In
turn, activated LIMK phosphorylates and inactivates cofilin,
resulting in actin depolymerization (33,34).
RhoA activates several other effector proteins,
among them the Rho-associated coiled-coil-containing protein
kinases, ROCKI and ROCKII. These proteins phosphorylate the myosin
light chain and myosin phosphatase-targeting subunit 1, resulting
in enhanced actomyosin-based contractility (35,36).
The introduction of constitutively active RhoA decreases spine
density and length, indicating that RhoA has a negative effect on
spine formation and maintenance (37,38).
Conversely, inhibition of Rho using C3 exoenzyme has been shown to
increase the density and length of spines of certain mouse cortical
and hippocampal pyramidal neurons (39). It is hypothesized that the
extension of the dendritic spines and the maintenance of several
pathways of the nervous system require the positive effects of
Racl/Cdc42 and a negative effect of RhoA. In the present study, it
was demonstrated that EA treatment upregulated the levels of Rac1
and Cdc42, and downregulated the expression of RhoA, compared with
the MCAO group.
In conclusion, these results demonstrate the
positive effect of EA on Baihui and Shenting acupoints, which lead
to the improvement of cognitive function following cerebral I/R
injury. In addition, the possible mechanisms through which the Rho
family of GTPases act to enhance dendritic spine plasticity during
EA analgesia was investigated. These data suggest that EA is a
promising solution for the treatment of cognitive impairment after
stroke. However, the long-term effects of EA on Baihui and Shenting
are yet to be determined.
Acknowledgments
This study was sponsored by the Mechanism of
Acupuncture to Improve Cognitive Function (grant no.
X2012004-collaborative), the National Natural Science Foundation of
China (grant nos. 81273835 and 81373778). The authors would like to
thank Clarity Manuscript Consultants LLC for assistance with
editing the manuscript.
References
|
1
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al:
Heart disease and stroke statistics-2013 update: A report from the
American heart association. Circulation. 127:e6–e245. 2013.
View Article : Google Scholar
|
|
2
|
Shim H: Vascular cognitive impairment and
post-stroke cognitive deficits. Curr Neurol Neurosci Rep.
14:4182014. View Article : Google Scholar
|
|
3
|
Béjot Y and Giroud M: Mean age at stroke
onset: An instructive tool from epidemiological studies. Eur J
Neurol. 16:e32009. View Article : Google Scholar
|
|
4
|
Douiri A, Rudd AG and Wolfe CD: Prevalence
of poststroke cognitive impairment: South London stroke register
1995–2010. Stroke. 44:138–145. 2013. View Article : Google Scholar
|
|
5
|
Feng X, Yang S, Liu J, Huang J, Peng J,
Lin J, Tao J and Chen L: Electroacupuncture ameliorates cognitive
impairment through inhibition of NF-κ-mediated neuronal cell
apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med
Rep. 7:1516–1522. 2013.PubMed/NCBI
|
|
6
|
Zhang H, Zhao L, Yang S, Chen Z, Li Y,
Peng X, Yang Y and Zhu M: Clinical observation on effect of scalp
electroacupuncture for mild cognitive impairment. J Tradit Chin
Med. 33:46–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao L, Zhang H, Zheng Z and Huang J:
Electroacupuncture on the head points for improving gnosia in
patients with vascular dementia. J Tradit Chin Med. 29:29–34. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Zhou J, Li J, Yang SB, Mo LQ, Hu
JH and Yuan WL: Electroacupuncture pretreatment prevents cognitive
impairment induced by limb ischemia-reperfusion via inhibition of
microglial activation and attenuation of oxidative stress in rats.
Brain Res. 1432:36–45. 2012. View Article : Google Scholar
|
|
9
|
Govek EE, Hatten ME and Van Aelst L: The
role of Rho GTPase proteins in CNS neuronal migration. Dev
Neurobiol. 71:528–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matus A: Actin-based plasticity in
dendritic spines. Science. 290:754–758. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Newey SE, Velamoor V, Govek EE and Van
Aelst L: Rho GTPases, dendritic structure and mental retardation. J
Neurobiol. 64:58–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bosch M and Hayashi Y: Structural
plasticity of dendritic spines. Curr Opin Neurobiol. 22:383–388.
2012. View Article : Google Scholar
|
|
13
|
Vadodaria KC, Brakebusch C, Suter U and
Jessberger S: Stage-specific functions of the small Rho GTPases
Cdc42 and Rac1 for adult hippocampal neurogenesis. J Neurosci.
33:1179–1189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Georges PC, Hadzimichalis NM, Sweet ES and
Firestein BL: The yin-yang of dendrite morphology: Unity of actin
and microtubules. Mol Neurobiol. 38:270–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kasai H, Fukuda M, Watanabe S,
Hayashi-Takagi A and Noguchi J: Structural dynamics of dendritic
spines in memory and cognition. Trends Neurosci. 33:121–129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodriguez GA, Burns MP, Weeber EJ and
Rebeck GW: Young APOE4 targeted replacement mice exhibit poor
spatial learning and memory, with reduced dendritic spine density
in the medial entorhinal cortex. Learn Mem. 20:256–266. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koyama Y and Tohyama M: A modified and
highly sensitive Golgi-Cox method to enable complete and stable
impregnation of embryonic neurons. J Neurosci Methods. 209:58–61.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jacquin A, Binquet C, Rouaud O,
Graule-Petot A, Daubail B, Osseby GV, Bonithon-Kopp C, Giroud M and
Béjot Y: Post-stroke cognitive impairment: High prevalence and
determining factors in a cohort of mild stroke. J Alzheimers Dis.
40:1029–1038. 2014.PubMed/NCBI
|
|
20
|
Thrift AG, Dewey HM, Macdonell RA, McNeil
JJ and Donnan GA: Incidence of the major stroke subtypes: Initial
findings from the north east Melbourne stroke incidence study
(NEMESIS). Stroke. 32:1732–1738. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen L, Fang J, Ma R, Froym R, Gu X, Li J,
Chen L, Xu S and Ji C: Acupuncture for acute stroke: Study protocol
for a multi-center, randomized, controlled trial. Trials.
15:2142014. View Article : Google Scholar
|
|
22
|
Liu F, Li ZM, Jiang YJ and Chen LD: A
meta-analysis of acupuncture use in the treatment of cognitive
impairment after stroke. J Alternat Complement Med. 20:535–544.
2014. View Article : Google Scholar
|
|
23
|
Deng W, Aimone JB and Gage FH: New neurons
and new memories: How does adult hippocampal neurogenesis affect
learning and memory? Nat Rev Neurosci. 11:339–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manns JR and Eichenbaum H: A cognitive map
for object memory in the hippocampus. Learn Mem. 16:616–624. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eyre MD, Richter-Levin G, Avital A and
Stewart MG: Morphological changes in hippocampal dentate gyrus
synapses following spatial learning in rats are transient. Eur J
Neurosci. 17:1973–1980. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Halpain S, Spencer K and Graber S:
Dynamics and pathology of dendritic spines. Prog Brain Res.
147:29–37. 2005. View Article : Google Scholar
|
|
27
|
Govek EE, Newey SE and Van Aelst L: The
role of the Rho GTPases in neuronal development. Genes Dev.
19:1–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Babayan AH and Kramár EA: Rapid effects of
oestrogen on synaptic plasticity: Interactions with actin and its
signaling proteins. J Neuroendocrinol. 25:1163–1172. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Santos Da Silva J, Schubert V and Dotti
CG: RhoA, Rac1 and cdc42 intracellular distribution shift during
hippocampal neuron development. Mol Cell Neurosci. 27:1–7. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murakoshi H, Wang H and Yasuda R: Local,
persistent activation of Rho GTPases during plasticity of single
dendritic spines. Nature. 472:100–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Auer M, Hausott B and Klimaschewski L: Rho
GTPases as regulators of morphological neuroplasticity. Ann Anat.
193:259–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iden S and Collard JG: Crosstalk between
small GTPases and polarity proteins in cell polarization. Nat Rev
Mol Cell Biol. 9:846–859. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Edwards DC, Sanders LC, Bokoch GM and Gill
GN: Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase
signalling to actin cytoskeletal dynamics. Nat Cell Biol.
1:253–259. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng Y, Zhang Y, Tregoubov V, Janus C,
Cruz L, Jackson M, Lu WY, MacDonald JF, Wang JY, Falls DL and Jia
Z: Abnormal spine morphology and enhanced LTP in LIMK-1 knockout
mice. Neuron. 35:121–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun ico CR, González-Forero D, Dom ínguez
G, García-Verdugo JM and Moreno-López B: Nitric oxide induces
pathological synapse loss by a protein kinase G-, Rho
kinase-dependent mechanism preceded by myosin light chain
phosphorylation. J Neurosci. 30:973–984. 2010. View Article : Google Scholar
|
|
36
|
Aburima A, Wraith KS, Raslan Z, Law R,
Magwenzi S and Naseem KM: cAMP signaling regulates platelet myosin
light chain (MLC) phosphorylation and shape change through
targeting the RhoA-Rho kinase-MLC phosphatase signaling pathway.
Blood. 122:3533–3545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pilpel Y and Segal M: Activation of PKC
induces rapid morphological plasticity in dendrites of hippocampal
neurons via Rac and Rho-dependent mechanisms. Eur J Neurosci.
19:3151–3164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin X, Ogiya M, Takahara M, Yamaguchi W,
Furuyama T, Tanaka H, Tohyama M and Inagaki S: Sema4D-plexin-B1
implicated in regulation of dendritic spine density through
RhoA/ROCK pathway. Neurosci Lett. 428:1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tashiro A, Minden A and Yuste R:
Regulation of dendritic spine morphology by the rho family of small
GTPases: Antagonistic roles of Rac and Rho. Cereb Cortex.
10:927–938. 2000. View Article : Google Scholar : PubMed/NCBI
|