Introduction
Cervical cancer is the third most common type of
malignant tumor and the fourth leading cause of cancer mortality
among females worldwide (1). In
developing countries, ~500,000 females develop cervical cancer and
~270,000 females succumb to this disease, every year (2). Although chemotherapy and radiotherapy
remain the major treatments for invasive cervical cancer, the
five-year survival rate is limited due to the limited efficacy and
high toxicity of numerous anticancer drugs. Therefore, further
studies on the mechanisms of cervical cancer and the identification
of new and effective gene therapy targets are important for the
development of new treatment strategies and the improvement of
patient survival.
The SAM and SH3 domain containing 1 (SASH1) gene, a
member of the SLY-family of signal adapter proteins, encodes a
protein containing sterile α motif (SAM) and Src homology domain 3
(SH3), predominantly observed in signaling molecules, adapters and
scaffold proteins (3). SAM domains
can interact with other protein domains (4) and SH3 domains bind to proline-rich
motifs in proteins (5). These two
domains are frequently found in signal adapter proteins and
scaffolding factors. In previous years, numerous studies have
revealed that SASH1 is downregulated in various types of tumor.
Meng et al reported that the expression of SASH1 in
osteosarcoma tissue was significantly lower than that in normal
bone tissue and that SASH1 significantly reduced osteosarcoma cell
viability, proliferation and invasive ability compared with the
empty vector group and blank control group (6). In addition, the expression of SASH1
was lower in colon cancer compared with the levels found in normal
colon tissue (7). However, the
role of SASH1 in cervical carcinogenesis remains to be elucidated.
Therefore, in the present study, the role of SASH1 in cervical
cancer was investigated. The present findings demonstrated that
SASH1 was downregulated in cervical cancer and that SASH1 inhibited
the proliferation and invasion of cervical cancer cells by
suppressing the focal adhesion kinase (FAK) pathway, indicating
that SASH1 may be a potential therapeutic target in cervical
cancer.
Materials and methods
Human tissue samples
A total of 30 normal cervical and 17 cervical cancer
samples without chemotherapy, immunotherapy or radiotherapy were
obtained via surgery from The Second Affiliated Hospital of the
Medical College of Zhengzhou University (Zhengzhou, China) between
January 2012 and December 2013. The present study was approved by
the Ethics Committee of the Medical College of Zhengzhou University
and informed consent was obtained from the patients prior to sample
collection.
Cell lines and cell culture
Human cervical cancer cell lines (SiHa, C33A and
CaSki) and the normal colonic epithelial cell line (CRL-1831) were
purchased from the American Type Culture Collection (ATCC,
Rockville, MD, USA). All cell lines were cultured in RPMI-1640
medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine
serum (HyClone), 100 U/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich, St. Louis, MO, USA) and grown at 37°C
in an incubator with 5% CO2. The medium was replaced
once every 2–3 days and cells were digested with 0.25% trypsin and
passaged when they reached 80% confluence.
Plasmid construction and stable
transfection
The full-length coding sequence of the human SASH1
gene was amplified by reverse transcription quantitative polymerase
chain reaction (RT-qPCR) and ligated into the pGEM-T vector
(Clontech Laboratories, Inc., Cambridge, UK) at the EcoRI
and KpnI restriction sites. Following sequence confirmation,
positive clones were subcloned into the pcDNA3.1 expression vector
to construct the recombinant expression vector pcDNA3.1-SASH1.
For in vitro transfection, the cells were
seeded in six-well plates at a density of 1.0×105
cells/well and cultured overnight. When the cells grew to 70–80%
confluence, pcDNA3.1-SASH1 and the empty vector were transfected
using Lipofectamine 2000™ (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's
instructions.
RT-qPCR
RNA was extracted from macrodissected primary tumor
tissues or cultured cells homogenized in TRIzol reagent according
to the manufacturer's instructions (Invitrogen; Thermo Fisher
Scientific, Inc.). The Superscript First-Strand kit was used to
synthesize first strand cDNA (Invitrogen; Thermo Fisher Scientific,
Inc.). RT-qPCR was performed using an ABI Prism 7500 Sequence
Detection System (Applied Biosystems, Foster City, CA, USA) and the
following primers were used: SASH1, sense 5′-CGG GAA AGC GTC AAG
TCG GA-3′ and antisense 5′-ATC TCC TTT CTT GAG CTT GAG-3′; matrix
metalloproteinase (MMP)-2, sense 5′-CCC CAG ACA GGT GAT CTT GAC-3′
and antisense 5′-GCT TGC GAG GGA AGA AGT TG-3′; MMP-9, sense
5′-CGCTGGGCTTAGATCATTCC-3′ and antisense 5′-AGG TTG GAT ACA TCA CTG
CAT TAG G-3′. The levels of individual gene mRNA transcripts were
initially normalized to the control β-actin. Subsequently, the
differential expression of these genes was analyzed using the
2-ΔΔCq method.
Western blot analysis
Equal quantities (30 µg) of protein were
solubilized in Laemmli buffer (62.5 mM Tris/HCl pH 6.8, 10%
glycerol, 2% SDS, 5% mercaptoethanol and 0.00625% bromophenol
blue), boiled for 5 min, separated by 12% polyacrylamide gel
electrophoresis and transferred onto nitrocellulose membranes (EMD
Millipore, Bedford, MA, USA). The membranes were probed with rabbit
anti-SASH1 (cat. no. LS-C285219; LifeSpan Biosciences, Seattle, WA,
USA), rabbit anti-MMP-2 (cat. no. 4022S), rabbit anti-MMP-9 (cat.
no. 3852S), rabbit anti-FAK (cat. no. 3285S) or mouse anti-β-actin
(cat. no. 4967S) (all from Cell Signaling Technology Inc., Beverly,
MA, USA) in Tris-buffered saline with 0.1% Tween 20 (TBST)
containing 5% BSA (Sigma-Aldrich) at 4°C overnight, followed by
three washes in TBST for 5 min per wash. The membranes were
incubated with horseradish peroxidase-conjugated goat anti-rabbit
IgG polyclonal antibody (cat. no. MBS560261; Mybiosource, San
Diego, CA, USA) for 1 h at room temperature. The blotted protein
bands were exposed to and visualized using enhanced
chemiluminescence (ECL) reagent. Developed films were digitized by
scanning, and the optical densities were analyzed using Image J
software 1.61 (National Institutes of Health, Bethesda, MD,
USA).
Cell proliferation assay
Cell viability was determined using an MTT assay as
described previously (8). In
brief, cells were plated in 96-well culture plates at
5×103 cells per well and incubated overnight. Cells were
treated with pcDNA3.1-SASH1 vector or empty vector for 24, 48, 72
and 96 h. Following this, MTT was added to each well. The resulting
formazan was then dissolved in 100 µl of dimethyl sulfoxide
and the absorbance was recorded at 540 nm using a Bio-Rad 3350
microplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
Colony formation assay
Cells were seeded into 10-mm dishes at a density of
2×102 cells/dish and then cultured at 37°C for 10–14
days until the visible cell clones were formed. Following this, the
cells were fixed with methanol. Fixative buffer was discarded and
the cells were stained with Giemsa dye for 10 min. After the
staining solution had been discarded, the number of cell clones was
counted.
Cell invasion assay
Cell invasion was measured using a modified Boyden
chamber (BD Biosciences, Bedford, MA, USA). Cells were treated with
overexpression-SASH1 or the mock for 4 h, and were then seeded in
the upper chamber. DMEM medium with 10% fetal bovine serum was
added into the lower chamber. After 24 h of incubation, cells that
passed through the lower side of the membrane were stained with
hematoxylin and eosin (Sigma-Aldrich) and quantified using a BX51
fluorescence microscope (Olympus Optical Co., Tokyo, Japan) by
counting six high-powered fields in the center of each well.
Statistical analysis
Statistical analysis was performed using SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA). For
comparison among groups, a χ2 test or a one-way analysis
of variance followed by a post hoc Tukey's test was performed.
P<0.05 was considered to indicate a statistically significant
difference. The data are presented as the mean ± standard
deviation.
Results
Expression of SASH1 is decreased in
cervical cancer tissues and cells
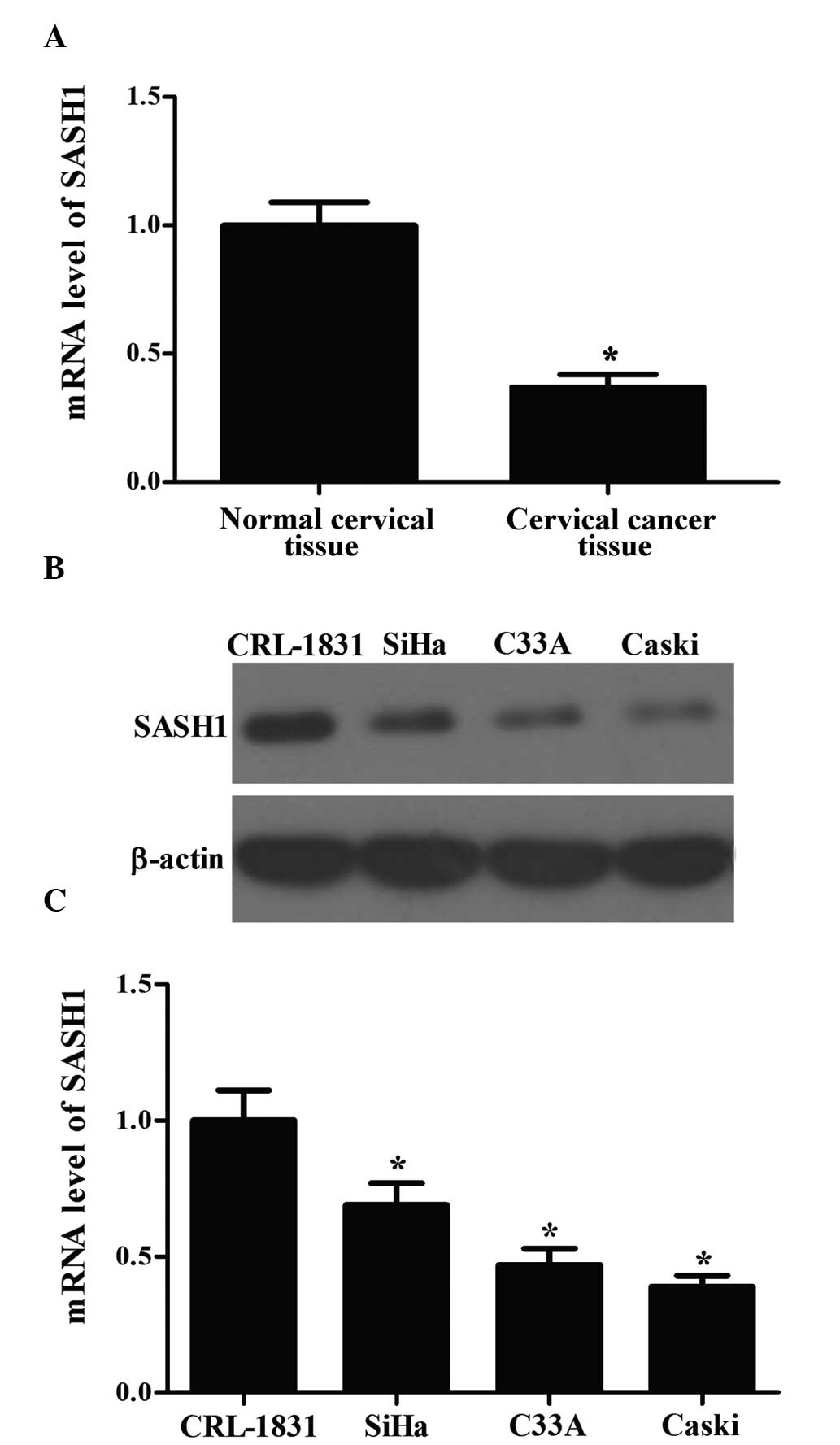
To examine the role of SASH1 in cervical cancer
tumorigenesis, RT-qPCR analyses of cervical cancer tissues and
normal cervical tissues was performed. The results demonstrated
that the mRNA expression of SASH1 was markedly lower in cervical
cancer tissues compared with the normal cervical tissues (Fig. 1A). Similar results were obtained
from the analysis of cervical cancer cell lines. As shown in
Fig. 1B and C, the mRNA and
protein expression levels of SASH1 were also significantly lower in
cervical cancer cell lines compared with the CRL-1831 group.
Collectively, these data suggest that SASH1 is downregulated in
cervical cancer.
Expression of SASH1 is increased
following transfection with pcDNA3.1-SASH1
To further investigate the effect of SASH1 on
cervical cancer tumorigenesis, the recombinant expression vector
pcDNA3.1-SASH1 was constructed, and then RT-qPCR and western blot
analysis were employed to determine the efficiency of SASH1
transfection. As shown in Fig. 2A,
the mRNA expression of SASH1 was significantly higher in the
pcDNA3.1-SASH1-transfected group relative to the control group. No
significant difference in the mRNA expression level of SASH1
between the control group and the empty vector group was
identified. Consistent with the results of RT-qPCR, western blot
analysis demonstrated that the expression of SASH1 protein was also
significantly increased in the pcDNA3.1-SASH1-transfected group
(Fig. 2B). Collectively, these
data show that the expression of SASH1 was successfully elevated in
the CaSki cell line.
SASH1 inhibits cervical cancer cell
proliferation
Subsequently, the effect of SASH1 on cervical cancer
cell proliferation was investigated. As shown in Fig. 3A, SASH1 significantly inhibited the
proliferation of cervical cancer cells, in a time-independent
manner. In addition, the effect of SASH1 on colony formation in
soft agar was investigated. SASH1 also reduced the colony formation
in soft agar relative to the control (lentiviral vector)-transduced
cells (Fig. 3B). These data
indicate that SASH1 inhibits cervical cancer cell
proliferation.
SASH1 inhibits cervical cancer cell
invasion
The invasive behavior of cervical cancer cells was
determined using a Boyden chamber assay. Cervical cancer cells
demonstrated a reduced capacity for invasion when transfected with
pcDNA3.1-SASH1 (Fig. 4A).
Furthermore, since the upregulation of MMP-2 and MMP-9 expression
contributes to the invasiveness of cancer cells (9,10),
the effects of SASH1 on MMP-2 and MMP-9 was investigated in
cervical cancer cells. As shown in Fig. 4B and C, SASH1 significantly
decreased the expression levels of MMP-2 and MMP-9.
SASH1 inhibits cervical cancer cell
proliferation and invasion through the FAK pathway
The FAK pathway is involved in cell proliferation
and invasion. The present study thus examined whether SASH1 affects
the level of FAK. As shown in Fig.
5, SASH1 overexpression significantly inhibited the expression
level of FAK. This indicated that SASH1 may be involved in the
regulation of the FAK pathway as an upstream molecule, suggesting
that SASH1 regulates cell proliferation and invasion via the FAK
pathway.
Discussion
Previous studies have demonstrated that SASH1 is
important in tumorigenesis and tumor development. However, the role
of SASH1 in cervical cancer remains to be elucidated. The present
study demonstrated that SASH1 expression was down-regulated in
cervical cancer tissues and cell lines. SASH1 overexpression
inhibited cell proliferation and invasion. Furthermore, SASH1
inhibited cervical cancer cell proliferation and invasion through
the FAK pathway.
Several studies have demonstrated that SASH1 is
down-regulated in tumors and that this expression is correlated
with tumor grade and prognosis. For example, in breast cancer cell
lines, SASH1 is only expressed at low levels and is downregulated
in the majority (74%) of breast tumors compared with corresponding
normal breast epithelial tissues (3). The expression of SASH1 is also
downregulated in tumors of the lung (11). In addition, the expression levels
of SASH1 were significantly reduced in colon cancer at Union for
International Cancer Control stage II, III and IV, as well as in
liver metastases, and the expression of SASH1 in colon tumors was
lower compared with the levels found in normal colon tissue
(7). Consistent with the
abovementioned results, in the present study, SASH1 expression was
examined in clinical tissue samples and cell lines, and it was
found that SASH1 mRNA and protein expression was decreased in tumor
tissues, suggesting that SASH1 may be a candidate tumor suppressor
gene in cervical cancer.
The migration and invasion of cancer cells involves
a host of processes and the interaction of multiple genes (12–14).
MMPs are a major group of enzymes that regulate cellular matrix
composition, and are zinc- and calcium-dependent endopeptidases.
Among the MMPs, MMP-2 and MMP-9 have a pivotal role in the
degradation of laminin, type IV collagen and gelatin, components of
the extracellular matrix (ECM) and basement membrane (15). In addition, high levels of MMP-2
and MMP-9 expression have been demonstrated to be associated with
cancer progression, invasion and metastasis (16). Therefore, inhibiting MMP-9
expression may be critical for treating malignant tumors. The
present study found that SASH1 inhibits the levels of MMP-2 and
MMP-9 in cervical cancer cells, resulting in a reduction in
invasive ability.
FAK, an intracellular tyrosine kinase, localizes at
focal adhesions and is a major regulator of signals from the ECM.
It can also mediate the signaling of various growth factor
receptors, including epidermal growth factor receptor,
platelet-derived growth factor receptor, G-protein coupled
receptors and the hepatocyte growth factor receptor c-MET (17). It is involved in the regulation of
cancer cell metastasis and survival and is associated with
aggressive tumor behavior (18–20).
Certain studies have demonstrated a direct role of FAK in promoting
tumor growth. Breast tumors of higher histological grades and of a
triple-negative sub-type express elevated levels of FAK (21). A small molecule FAK inhibitor
(PF-228) prevented the migration of melanoma cells and the invasion
of the highly invasive 1205Lu and WM9 melanoma cell lines (22). Inhibition of FAK signaling has been
demonstrated to suppress the proliferation of Hep3 human carcinoma
cells and leads to tumor dormancy in vivo, and this dormancy
can be reversed by the expression of an active mutant of MEK1
(MAPK/ERK kinase 1) (23). The
present study found that SASH1 overexpression reduced the level of
FAK. This indicated that SASH1 could be an upstream regulator of
FAK. These data strongly support the role of SASH1 in regulating
cell migration and invasion via the FAK pathway.
In conclusion, the present study demonstrated that
SASH1 inhibits cervical cancer cell proliferation and invasion.
Therefore, SASH1 may be important in cervical cancer and may
represent a novel therapeutic target for cervical cancer
treatment.
References
|
1
|
Greenlee RT, Murray T, Bolden S and Wingo
PA: Cancer statistics, 2000. CA Cancer J Clin. 50:7–33. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saavedra KP, Brebi PM and Roa JC:
Epigenetic alterations in preneoplastic and neoplastic lesions of
the cervix. Clin Epigenetics. 4:132012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeller C, Hinzmann B, Seitz S, Prokoph H,
Burkhard-Goettges E, Fischer J, Jandrig B, Schwarz LE, Rosenthal A
and Scherneck S: SASH1: A candidate tumor suppressor gene on
chromosome 6q24.3 is downregulated in breast cancer. Oncogene.
22:2972–2983. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim CA, Gingery M, Pilpa RM and Bowie JU:
The SAM domain of polyhomeotic forms a helical polymer. Nat Struct
Biol. 9:453–457. 2002.PubMed/NCBI
|
|
5
|
Pawson T: Protein modules and signalling
networks. Nature. 373:573–580. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng Q, Zheng M, Liu H, Song C, Zhang W,
Yan J, Qin L and Liu X: SASH1 regulates proliferation, apoptosis,
and invasion of osteosarcoma cell. Mol Cell Biochem. 373:201–210.
2013. View Article : Google Scholar
|
|
7
|
Rimkus C, Martini M, Friederichs J,
Rosenberg R, Doll D, Siewert JR, Holzmann B and Janssen KP:
Prognostic significance of downregulated expression of the
candidate tumour suppressor gene SASH1 in colon cancer. Brit J
Cancer. 95:1419–1423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu GS, Song YL, Yin ZQ, Guo JJ, Wang SP,
Zhao WW, Chen XP, Zhang QW, Lu JJ and Wang YT: Ganoderiol
A-enriched extract suppresses migration and adhesion of MDA-MB-231
cells by inhibiting FAK-SRC-paxillin cascade pathway. PLoS One.
8:e766202013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: Multifunctional contributors to tumor
progression. Mol Med Today. 6:149–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen EG, Chen Y, Dong LL and Zhang JS:
Effects of SASH1 on lung cancer cell proliferation, apoptosis, and
invasion in vitro. Tumor Biol. 33:1393–1401. 2012. View Article : Google Scholar
|
|
12
|
Beshir AB, Ren G, Magpusao AN, Barone LM,
Yeung KC and Fenteany G: Raf kinase inhibitor protein suppresses
nuclear factor-κB-dependent cancer cell invasion through negative
regulation of matrix metalloproteinase expression. Cancer Lett.
299:137–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Li Y, Banerjee S, Kong D, Ahmad A,
Nogueira V, Hay N and Sarkar FH: Down-regulation of Notch-1 and
Jagged-1 inhibits prostate cancer cell growth, migration and
invasion and induces apoptosis via inactivation of Akt, mTOR, and
NF-kappaB signaling pathways. J Cell Biochem. 109:726–736.
2010.PubMed/NCBI
|
|
14
|
Arozarena I, Sanchez-Laorden B, Packer L,
Hidalgo-Carcedo C, Hayward R, Viros A, Sahai E and Marais R:
Oncogenic BRAF induces melanoma cell invasion by downregulating the
cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 19:45–57. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai WC, Zhou M, Shankavaram U, Peng G and
Wahl LM: Differential regulation of lipopolysaccharide-induced
monocyte matrix metalloproteinase (MMP)-1 and MMP-9 by p38 and
extracellular signal-regulated kinase 1/2 mitogen-activated protein
kinases. J Immunol. 170:6244–6249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.
|
|
17
|
Luo M and Guan JL: Focal adhesion kinase:
A prominent determinant in breast cancer initiation, progression
and metastasis. Cancer Lett. 289:127–139. 2010. View Article : Google Scholar :
|
|
18
|
Lark AL, Livasy CA, Calvo B, Caskey L,
Moore DT, Yang X and Cance WG: Overexpression of focal adhesion
kinase in primary colorectal carcinomas and colorectal liver
metastases: Immunohistochemistry and real-time PCR analyses. Clin
Cancer Res. 9:215–222. 2003.PubMed/NCBI
|
|
19
|
Zhao J and Guan JL: Signal transduction by
focal adhesion kinase in cancer. Cancer Metastasis Rev. 28:35–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ward KK, Tancioni I, Lawson C, Miller NL,
Jean C, Chen XL, Uryu S, Kim J, Tarin D, Stupack DG, et al:
Inhibition of focal adhesion kinase (FAK) activity prevents
anchorage-independent ovarian carcinoma cell growth and tumor
progression. Clin Exp Metastasis. 30:579–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yom CK, Noh DY, Kim WH and Kim HS:
Clinical significance of high focal adhesion kinase gene copy
number and overexpression in invasive breast cancer. Breast Cancer
Res Treat. 128:647–655. 2011. View Article : Google Scholar
|
|
22
|
John JK, Paraiso KH, Rebecca VW, Cantini
LP, Abel EV, Pagano N, Meggers E, Mathew R, Krepler C, Izumi V, et
al: GSK3β inhibition blocks melanoma cell/host interactions by
downregulating N-cadherin expression and decreasing FAK
phosphorylation. J Invest Dermatol. 132:2818–2827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aguirre Ghiso JA: Inhibition of FAK
signaling activated by urokinase receptor induces dormancy in human
carcinoma cells in vivo. Oncogene. 21:2513–2524. 2002. View Article : Google Scholar : PubMed/NCBI
|