Introduction
Osteoarthritis (OA) is the most common
age-associated degenerative joint disorder, and is a leading cause
of pain and disability among older adults worldwide (1). A key process in OA is loss of the
articular cartilage (1).
Oncostatin M (OSM) is a cytokine of the
interleukin-6 (IL-6) family that is present at elevated levels in
the synovial fluid of OA patients (2), and results in the secretion of
pro-inflammatory cytokines, such as tumor necrosis factor-α, IL-1β
and IL-6, from osteoblasts and synovial cells that degrade the
cartilage in arthritic joints (2).
Previous studies demonstrated that OSM is associated with bone
erosion, synovial inflammation and fibrosis, and cartilage
degeneration (2–4).
Endothelin-1 (ET-1) is a potent vasoconstrictor that
originates from vascular endothelial cells, and functions primarily
through the activation of the ETA and ETB receptors (ETAR and ETBR,
respectively) (5). Previous
studies demonstrated that ET-1 has a role in the regulation of bone
metabolism, stimulating the formation of new bone via osteoblastic
proliferation (6,7). Previous studies demonstrated that
ET-1 is involved in the degradation of osteoarthritic articular
cartilage (5,8), suggesting that ET-1 signaling may
contribute to the destruction of the bone-cartilage unit in the
pathophysiology of OA (5).
A previous study indicated that osteoblasts
participate in the inflammation process in OA (9), and OSM was demonstrated to be
expressed in osteoblasts isolated from the femurs of OA patients
(9,10). In the present study, the effect of
ET-1 on the expression level of OSM in human OA osteoblasts was
investigated, to the best of our knowledge, for the first time.
Identification of this effect may contribute to elucidating the
mechanistic role of ET-1 in the pathophysiology of OA.
Materials and methods
Reagents
Recombinant human ET-1, selective ETAR antagonist,
BQ123, and selective ETBR antagonist, BQ788 were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The human OSM
promoter/luciferase reporter and LightSwitch Luciferase Assay kit
were purchased from SwitchGear Genomics (Shanghai, China). Mutant
Osx promoter/luciferase reporter constructs were generated by
polymerase chain reaction (PCR) and confirmed by sequencing. Life
Technologies Lipofectamine 2000 transfection reagent, TRIzol
reagent and SuperScript II reverse transcriptase were purchased
from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Ets-1 (cat.
no. sc-29309-V) and control (cat. no. sc-108080) shRNA lentiviral
particles, selective phosphatidylinositol 3-kinase (PI3K)
inhibitor, BKM120 (cat. no. sc-364437A), mouse monoclonal anti-OSM
(E-4; cat. no. sc-365136), rabbit polyclonal anti-Ets1 (C-20; cat.
no. sc-350) and mouse monoclonal anti-GAPDH (6C5; cat. no.
sc-32233) antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). The OSM human ELISA kit (cat. no. ab100619)
was purchased from Abcam (Shanghai, China). Putative transcription
factor binding sites in the human OSM gene promoter sequence were
identified using the online software, PROMO (http://alggen.lsi.upc.es) (11,12).
Cell culture and treatment
Primary human OA osteoblasts and growth medium kit
(cat. no. 406OAK-05a) were purchased from Cell Applications Inc.
(San Diego, CA, USA). Cells were treated with 1, 5, 10, 20 or 30 nM
ET-1 for 0.5, 1, 2, 3 or 4 h in the presence or absence of BQ123 (1
µM), BQ788 (1 µM) or BKM120 (10 µM). Cells
were subjected to further analysis at 26–29.5 h after the
treatment, making the total experimental time 30 h. The medium
remained unchanged.
Reverse transcription (RT)-quantitative
(q) PCR
RNA was prepared from cells using the TRIzol
reagent, and cDNAs were synthesized using SuperScript II reverse
transcriptase. RT-qPCR was performed on an ABI PRISM 7700 Sequence
Detection System, with fluorescent dye from the SYBR Green Master
Mix (both Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. PCR amplification
condition were as follows: 20 sec at 95°C followed by 40 cycles of
3 sec at 95°C and 30 sec at 60°C. The primers used in the current
study were as follows: Forward, 5′-AGAGTACCGCGTGCTCCTT-3′ and
reverse, 5′-AGCTTGCGCTGAAAAGCAT-3′ for OSM; forward,
5′-GGGTGACGACTTCTTGTTTG-3′ and reverse, 5′-GTTAATGGAGTCAACCCAGC-3′
for Ets-1; forward, 5′-GACTCATGACCACAGTCCATGC-3′ and reverse,
5′-AGAGGCAGGGATGATGTTCTG-3′ for GAPDH. Relative quantification of
the mRNA level was determined using the 2ΔΔCq method
(13), which normalizes the
expression levels of OSM or Ets-1 against that of GAPDH in the same
samples. The mRNA level of treated cells was demonstrated as the
fold changes to that of untreated control cells (designated as 1).
Each experiment was repeated three times in duplicate.
ELISA and western blot analyses
The secreted OSM levels in cell culture supernatants
were determined using an ELISA kit according to the manufacturer's
instructions, normalized against cell number (per 105
cells), and demonstrated as the fold changes to that of the
untreated control cells (designated as 1). Each ELISA experiment
was repeated three times in duplicate. For western blot analysis,
human osteoblasts were lysed by three rounds of 3-sec sonication on
ice, with a hypotonic buffer, containing 2% Nonidet-P and a
protease inhibitor cocktail (Sigma-Aldrich). The supernatant
obtained following centrifugation at 2,000 × g for 15 min at 4°C
was used to determine the protein concentration with the Coomassie
Blue staining (Thermo Fisher Scientific, Inc.). Equal quantities of
proteins were separated by 10% SDS-polyacrylamide gel (Thermo
Fisher Scientific, Inc.) at 100 V for 2 h and blotted onto a
polyvinylidene difluoride microporous membrane (EMD Millipore,
Billerica, MA, USA). Membranes were incubated for 1 h with a
1:1,000 dilution of the primary antibodies, washed thrice for 5 min
and incubated for 1 h at room temperature with 1:5,000 dilution of
secondary antibodies, including bovine anti-rabbit immunoglobulin G
(IgG)-horseradish peroxidase (HRP; cat. no. sc-2370) or bovine
anti-mouse IgG-HRP (cat. no. sc-2371; both Santa Cruz
Biotechnology, Inc.). Peroxidase activity was revealed using an ECL
kit (GE Healthcare Life Sciences, Shanghai, China). Three
independent experiments were performed.
Transient transfection and luciferase
assay
Cells were transfected with human OSM
promoter/luciferase reporter plasmids using Lipofectamine 2000
transfection reagent. The luciferase assays were performed 30 h
after transfection using the LightSwitch Luciferase Assay kit
according to the manufacturer's instructions. The pRL-CMV plasmid
(Promega Corporation, Madison, WI, USA) encoding Renilla
reniformis luciferase (at one-fifth molar ratio to test
plasmids) was co-transfected with test plasmids in each
transfection as an internal control for data normalization. Each
experiment was repeated three times in duplicate.
Electrophoretic mobility shift assay
(EMSA)
Nuclear extracts were prepared as previously
described (14). EMSA was
performed with 32P-labeled double-stranded oligonucleotides that
were incubated with nuclear extract in EMSA buffer [10 mM Tris (pH
7.5), 5% glycerol, 1 mM EDTA (pH 7.1), 50 mM NaCl, 1 mM DTT, 1 mM
EDTA and 0.1 mg/ml poly(dI-dC); Sigma-Aldrich]. For the
oligonucleotide competition analysis, a 100-fold molar excess of
unlabeled competitor oligonucleotide was added to the mixture and
incubated at room temperature for 30 min. For the antibody
supershift assays, monoclonal Ets-1 antibody (1 µl) was
added to the mixture. The reaction was then incubated on ice for 1
h. Protein-DNA complexes and free DNA were fractionated on 5%
polyacrylamide gels in 1X Tris-glycine EDTA buffer (both
Sigma-Aldrich) at 4°C and were visualized with a Cyclone Plus
Phosphor Imager (C431200; Perkin Elmer, Inc., Waltham, MA,
USA).
Lentiviral transduction
The Ets-1 shRNA lentiviral particles contained
expression constructs encoding target-specific 19–25 nt (plus
hairpin) shRNA designed to specifically knockdown the Ets-1 gene
expression. The control shRNA lentiviral particles contained a
scrambled shRNA sequence that would not lead to degradation of any
cellular mRNA, and served as a negative control for the Ets-1 shRNA
lentiviral particles. Lentiviral transduction was performed in the
cells 24 h prior to western blotting and EMSAs, according to the
manufacturer's protocol.
Statistical analysis
Statistical analyses were performed with SPSS
software for Windows, version 19.0 (IBM SPSS, Armonk, NY, USA).
Data are expressed as the mean ± standard deviation. Comparisons of
means among multiple groups were performed with one-way analysis of
variance, followed by post-hoc pairwise comparisons using Tukey's
test. Tests were two-tailed and P<0.05 was considered to
indicate a statistically significant difference.
Results
ET-1 elevates the secreted OSM level of
OA osteoblasts by inducing OSM mRNA expression levels
To investigate the potential effect of ET-1 on OSM
expression in OA osteoblasts, primary human OA osteoblasts were
treated with ET-1 (1, 5, 10, 20 or 30 nM) for 0.5, 1, 2, 3 and 4 h.
The OSM mRNA and secreted protein levels were determined 26–29.5 h
subsequent to treatment, making the total experimental time 30 h.
As demonstrated in Table I, 1–20
nM ET-1 treatment significantly increased the OSM mRNA levels
within 3 h in a dose- and time-dependent manner, and this effect
was suppressed by the ETAR antagonist, BQ123 and PI3K inhibitor,
BKM120, however not the ETBR antagonist, BQ788. ELISA assays
demonstrated that the data trend for the secreted OSM level of the
cell culture supernatants was similar to that of the OSM mRNA
expression level (Fig. 1).
 | Table IOncostatin M mRNA levels in human OA
osteoblasts treated with ET-1 and ET receptor antagonists. |
Table I
Oncostatin M mRNA levels in human OA
osteoblasts treated with ET-1 and ET receptor antagonists.
| ET-1 (nM) | Time (h)
|
|---|
| 0.5 | 1 | 2 | 3 | 4 |
|---|
| 1 | 1.02±0.03 | 1.03±0.04 | 1.05±0.05 | 1.07±0.05 | 1.08±0.07 |
| 5 | 1.07±0.05 | 1.20±0.07a,d | 1.59±0.1a,d,e | 2.13±0.14a,d–f | 2.42±0.17a,d–f |
| 10 | 1.15±0.06a | 1.59±0.11a,b,d | 2.36±0.15a,b,d,e | 2.94±0.19a,b,d–f | 3.33±0.21a,b,d–f |
| 20 | 1.34±0.08a,b | 2.41±0.15a–d | 3.20±0.20a–e | 3.71±0.23a–f | 3.88±0.21a–f |
| 30 | 1.51±0.12a–c | 2.67±0.18a–d | 3.26±0.21a–e | 3.78±0.22a–f | 3.90±0.20a–f |
| 30 + BQ123 (1
µM) | 0.95±0.05 | 0.97±0.06 | 1.02±0.09 | 1.04±0.1 | 1.05±0.12 |
| 30 + BQ788 (1
µM) | 1.45±0.12 | 2.56±0.19a–d | 3.21±0.23a–e | 3.75±0.25a–f | 3.89±0.28a–f |
| 30 + BKM120 (10
µM) | 1.06±0.1 | 1.09±0.12 | 1.14±0.13 | 1.17±0.15 | 1.19±0.16 |
As demonstrated in Fig.
2, western blot analysis verified that intracellular protein
levels of OSM in OA osteoblasts treated with ET-1 (1, 5, 10, 20 or
30 nM) for 4 h were consistent with the corresponding OSM mRNA
levels (Table I) and secreted
protein levels (Fig. 1). The
results suggested that ET-1 may elevate the secreted OSM levels of
OA osteoblasts by inducing OSM mRNA expression in an ETAR- and
PI3K-dependent manner.
ET-1 trans-activates the OSM gene
promoter by increasing the specificity of Ets-1 binding to the
promoter in OA osteoblasts
To examine whether ET-1 induced OSM mRNA expression
by trans-activating the OSM gene promoter, the human OSM gene
promoter activities were investigated using a commercial human OSM
promoter/luciferase reporter, which contained 1,064 bp of
5′-untranslated region directly upstream of the OSM gene
translation start codon (Fig. 3A).
This gene promoter sequence was inserted in frame with the
luciferase cDNA and termed pOSM1064. The luciferase reporter
without the inserted promoter sequence served as a basic luciferase
activity control. Luciferase assays demonstrated that compared with
the basic control, the OSM promoter was constitutively active in OA
osteoblasts. ET-1 treatment markedly increased the OSM promoter
activity, which was suppressed by BQ123 and BKM120, but not BQ788
(Fig. 3B). The results indicate
that ET-1 trans-activates the OSM promoter in OA osteoblasts in an
ETAR- and PI3K-dependent manner.
 | Figure 3OSM gene promoter activities in human
OA osteoblasts treated with ET-1 in the presence and absence of ET
receptor antagonists (BQ123, BQ788 and BKM120). (A) A 1,064-bp
human OSM gene promoter sequence. The ATG translation start codon
is marked as +1, and an Ets-1 binding site at -494/-488 and the
start sites of 5′-end deletion constructs of the OSM
promoter/luciferase reporter (pOSM1064) are underlined in bold. (B)
pOSM1064 was used to assess the basic luciferase activity control.
The constructs were transfected into human OA osteoblasts, and
treated with 30 nM ET-1 and BQ123 (1 µM), BQ788 (1
µM) and BKM120 (10 µM) for 4 h. Luciferase activity
was measured 26 h later and expressed as the fold change of that of
pOSM1064 in the untreated cells (designated as 1).
*P<0.05 vs. the untreated group. ET-1, endothelin-1;
OSM, oncostatin M; OA, osteoarthritis; BQ123, endothelin receptor
type A antagonist; BQ788, endothelin receptor type B antagonist;
BKM120, phosphatidylinositol 3-kinase inhibitor; basic, luciferase
reporter without the inserted promoter sequence. |
To identify the potential ET1-responsive cis-DNA
element in the OSM promoter, a deletional analysis of the OSM
promoter activity was conducted. As demonstrated in Fig. 4A, a series of 5′-end deletion
constructs of the human OSM promoter/luciferase reporter were
established. As demonstrated in Fig.
4B, the luciferase assays suggested that the OSM promoter
region between -664 and -464 bp contained potential ET1-responsive
cis-DNA elements. Further luciferase assays suggested that the
cis-DNA element was between -514 and -464 bp in the OSM promoter
(Fig. 4C). In addition, a
mutational analysis of the OSM promoter activity was performed. A
series of mutant OSM promoter reporters were generated by
introducing mutations into the OSM promoter region between -514 and
-464 bp in pOSM1064 with an EcoRI linker (GAATTC) at 6-bp
intervals. As demonstrated in Fig.
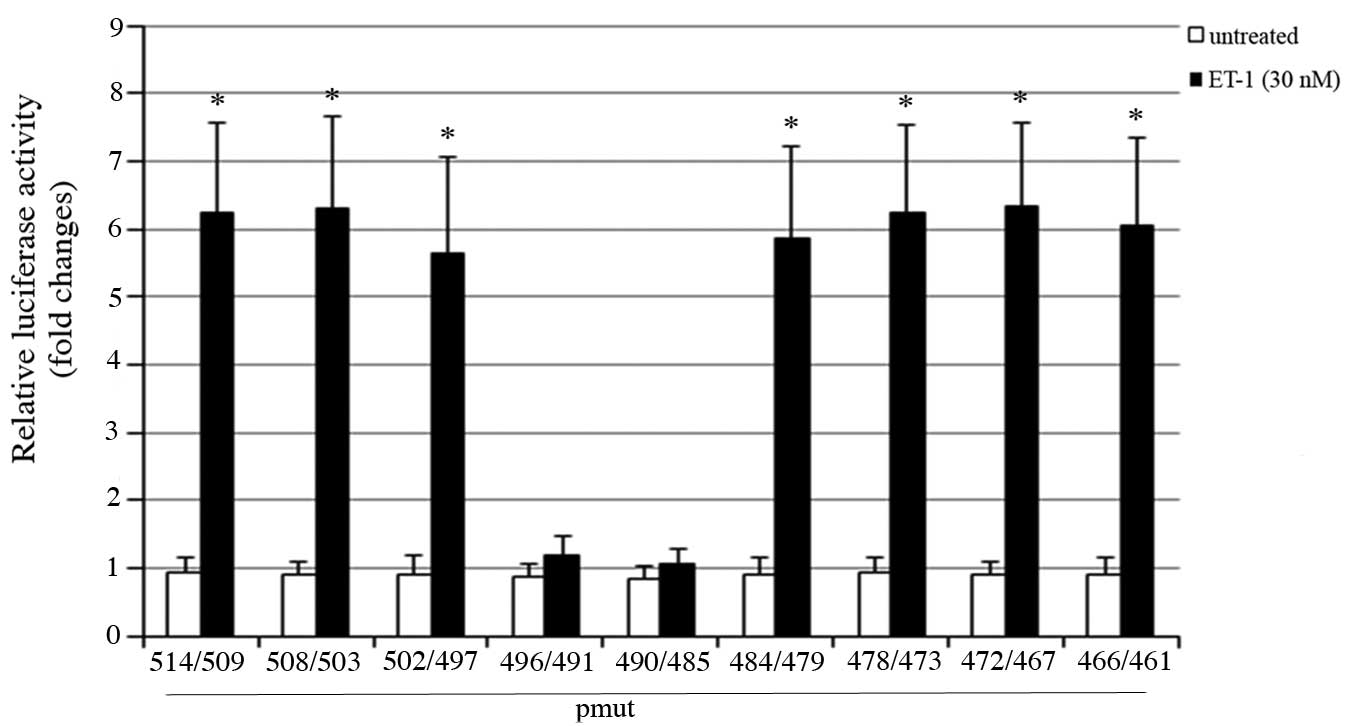
5, mutations in the promoter region between -496 and -485 bp
suppressed the trans-activating effect of ET-1 on the OSM promoter.
Analysis of this region with the online software, PROMO (11,12)
revealed a consensus Ets-1 binding site between -494 and -488 bp
(Fig. 6A).
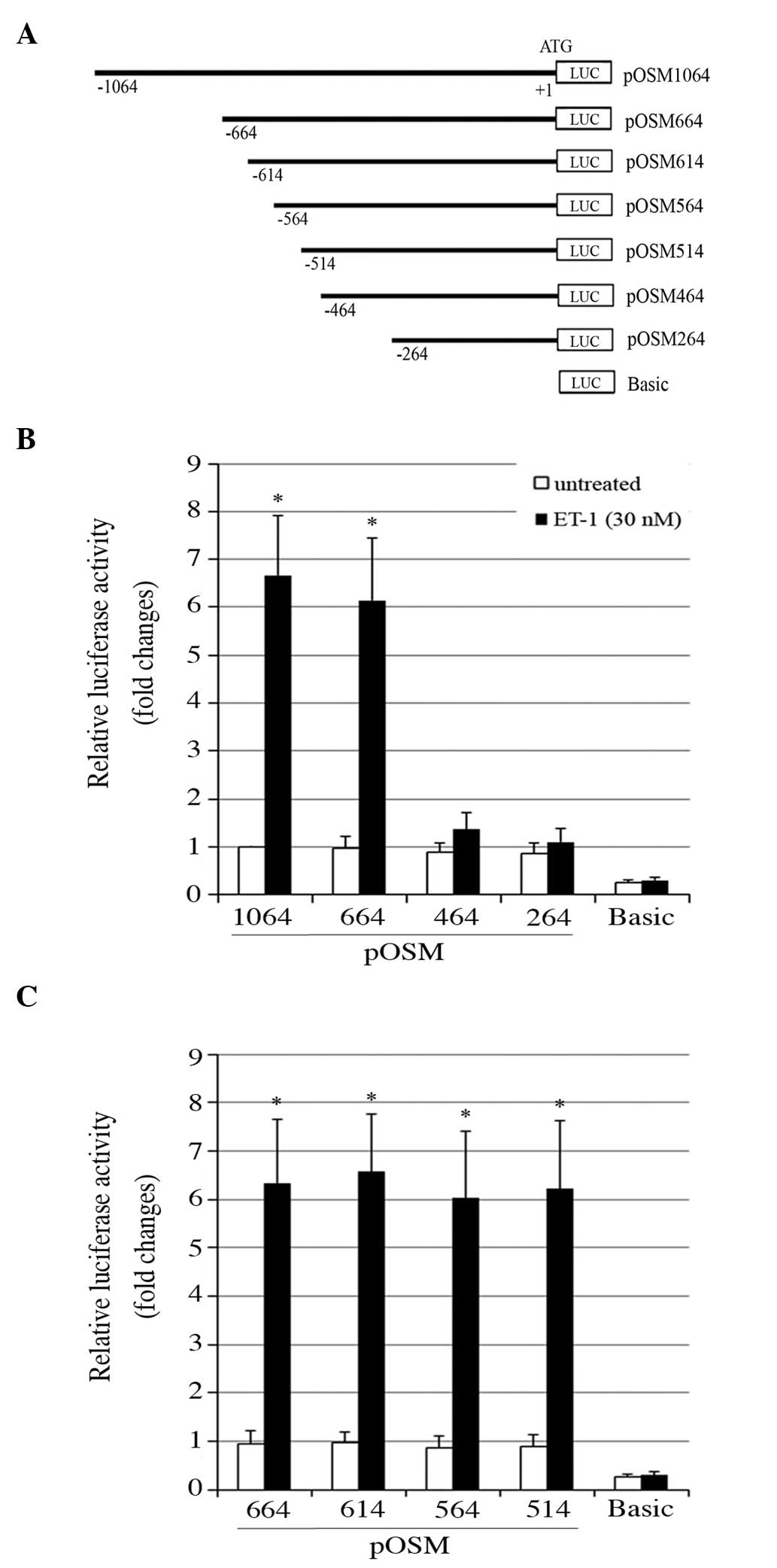 | Figure 4Deletional analysis of the human OSM
gene promoter activity in ET-1-treated OA osteoblasts. The cells
were untreated or treated with 30 nM ET-1 for 4 h. (A) Schematic
representation of the human OSM gene promoter regions that were
included in a series of 5′-end deletion constructs of the human OSM
promoter/luciferase reporter. Each construct was designated by the
relative position of the 5′-end nucleotide of the inserted OSM
promoter region, with the ATG translation start codon marked as +1.
(B) 5′-end deletion constructs pOSM1064, -664, -464, -264 and (C)
pOSM664, -614, -564, -514 and Basic were transfected into human OA
osteoblasts. The luciferase activity assays were performed 26 h
later and results are expressed as the fold change of that of
pOSM1064 in the untreated cells (designated as 1).
*P<0.05 vs. the untreated groups. ET-1, endothelin-1;
OSM, oncostatin M; OA, osteoarthritis; basic, luciferase reporter
without the inserted promoter sequence. |
 | Figure 6Specific protein-binding activity at
the putative Ets-1 binding site in the human OSM gene promoter. (A)
Oligonucleotide WT504/475 contained the human OSM gene promoter
sequence from -504 to -475 encompassing the -494/-488 putative
Ets-1 binding site (underlined in bold). Oligonucleotides
Mut496/491 and Mut490/485 contained the same sequence as WT504/475
except for the EcoRI linker (GAATTC) mutations at -496/-491
and -490/-485 (underlined in bold), respectively. (B)
Electrophoretic mobility shift assays were performed using
WT504/475 as the radiolabeled probe in the presence of equal
quantities of nuclear extract from human OA osteoblasts. Lane 1,
radiolabeled probe only; lanes 2–3, control reaction; lane 4,
100-fold molar excess of unlabeled oligonucleotide WT504/475 as a
competitor; lane 5, mixture of 100-fold molar excess of each of
unlabeled oligonucleotides, Mut496/491 and Mut490/485 as
competitors; lane 6, control serum; lane 7, anti-Ets-1 antibody;
lane 8, cells transduced with lentiviral scramble control shRNA;
lane 9, cells transduced with lentiviral Ets-1 shRNA. Major
protein-DNA and supershifted complexes are indicated by arrows.
ET-1, endothelin-1; shRNA, small hairpin RNA; OSM, oncostatin M;
OA, osteoarthritis; WT, wild-type; Mut, mutant. |
EMSAs were conducted to examine whether Ets-1
specifically binds to the -494/-488 putative Ets-1 binding site.
Oligonucleotide WT504/475 corresponding to the human OSM promoter
sequence between -504 and -475 bp was radiolabeled and used as the
probe to incubate with OA osteoblast cell nuclear extract in the
EMSAs (Fig. 6A). Unlabeled
WT504/475, and Mut496/491 and Mut490/485, two oligonucleotides with
the same sequence as WT504/475 except for the EcoRI linker
(GAATTC) mutations at -496/-491 and -490/-485, respectively, served
as competitors to the probe (Fig.
6A). As demonstrated in Fig.
6B, nuclear extract from ET-1-treated cells indicated a
markedly stronger binding activity with the probe compared with
that of the untreated control cells. A 100-fold molar excess of
unlabeled WT504/475 (but not the mixture of 100-fold molar excess
of each of Mut496/491 and Mut490/485) suppressed the binding
activity (Fig. 6B), suggesting
specific protein binding at the -494/-488 putative Ets-1 binding
site. Furthermore, the control serum exerted no effect, whereas the
anti-Ets1 antibody supershifted the major protein-DNA complex to a
higher position (Fig. 6B). The
results indicate that ET-1 may markedly increase specific binding
of Ets-1 to the -494/-488 region in the OSM promoter in OA
osteoblasts. In addition, the Ets1-DNA complex was abolished by
Ets-1 shRNA, and not the scramble control shRNA, further confirming
the specificity of the Ets1-DNA complex.
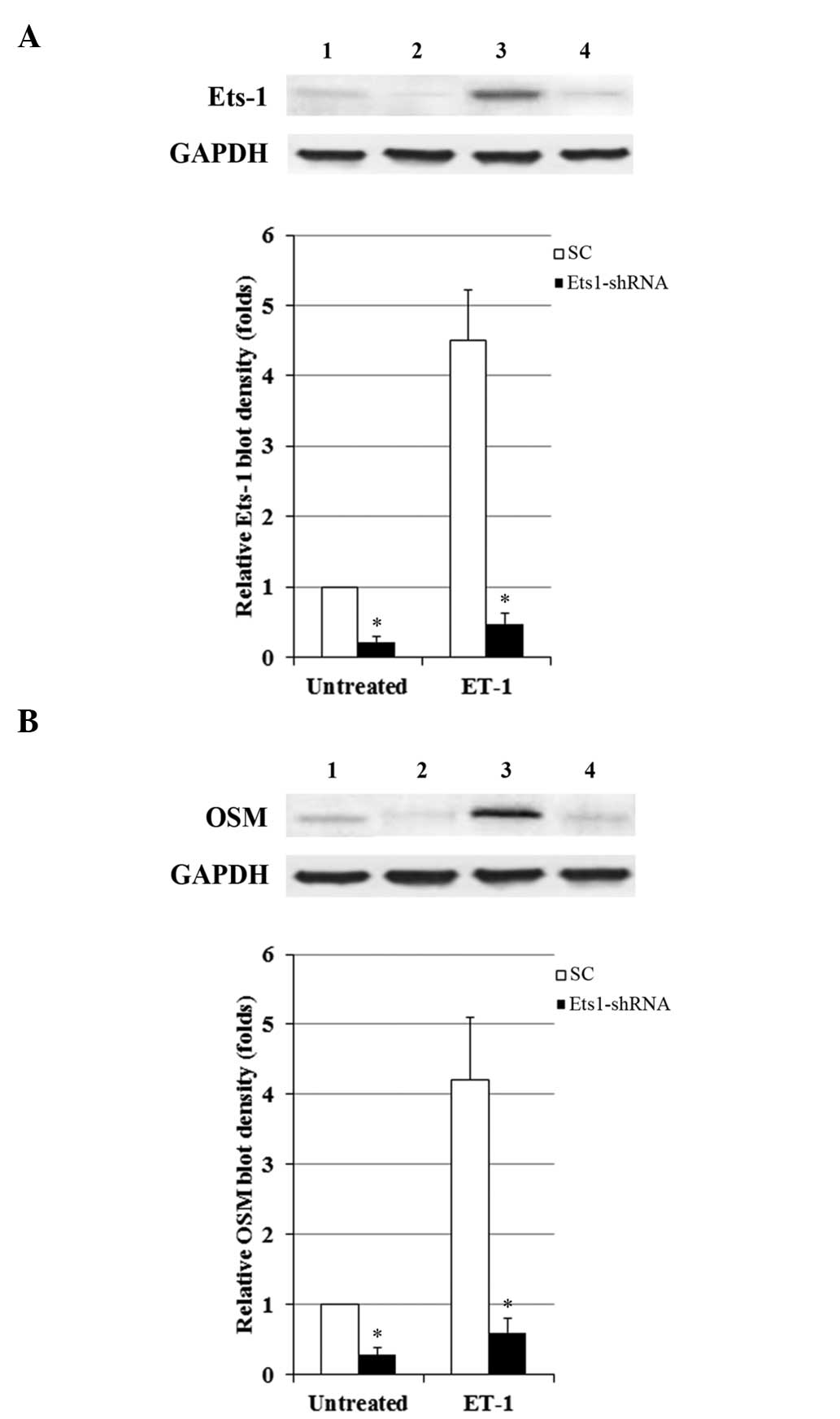
Ets-1 mediates the inducing effect of
ET-1 on OSM expression in OA osteoblasts
To determine the functional role of Ets-1 in the
ET1-induced expression of OSM, the activity of Ets-1 in OA
osteoblasts was knocked down with lentiviral shRNA. As demonstrated
in Fig. 7A, the protein levels of
Ets-1 were markedly increased by ET-1, compared with the
Ets-1-shRNA group. However, transduction of Ets-1-shRNA
significantly decreased the Ets-1 protein level by ~80%, in the
presence and absence of ET-1 (P<0.05). As demonstrated in
Fig. 7B, Ets-1 knockdown
suppressed the ET1-induced expression of OSM. The results indicate
that Ets-1 is an important mediator of the inducing effect of ET-1
on OSM expression in OA osteoblasts.
Effect of ET-1 on the protein expression
of Ets-1
OA osteoblasts were treated with ET-1 (10 and 30 nM)
for 4 h to assess the effect of ET-1 on the Ets-1 mRNA and protein
expression levels. As demonstrated in Fig. 8, ET-1 treatment (10 and 30 nM)
significantly increased the expression of Ets-1 at the mRNA and
protein levels compared with the control group (P<0.05). This
effect was significantly suppressed following co-treatment with
BQ123 or BKM120, compared with the control and ET-1 only groups
(Fig. 8; P<0.05). However,
co-treatment with BQ788 significantly increased the expression of
Ets-1 compared with the control and ET-1 only groups (Fig. 8; P<0.05). These findings
indicate that ET-1 may increase Ets-1 expression levels in OA
osteoblasts in an ETAR- and PI3K-dependent manner.
Discussion
OSM is an established contributor to cartilage
degeneration in OA (2–4) and is expressed in OA osteoblasts
(9,10). ET-1 is implicated in the
degradation of OA articular cartilage (6,7), and
involved in osteoblast proliferation and bone development (5,8). The
present study demonstrated that ET-1 induced the expression of OSM
in OA osteoblasts.
The ET family consists of ET-1, -2, and -3 and the
endothelin-converting enzymes (ECEs) that catalyze the generation
of biologically active ETs (15).
The roles of ET-2 and -3, and ECE-1 in the expression of OSM and
Ets-1 in OA osteoblasts remain unclear. In addition, the effect of
ET-1 on the expression of OSM in normal osteoblasts is yet to be
determined.
ET-1 treatment (5–20 nM) for ≥1 h dose-dependently
induced the expression of OSM in OA osteoblasts. In particular, 20
or 30 nM ET-1 induced mRNA and secreted protein levels of OSM by
4-fold within 30 h, indicating that ET-1 is an inducer of OSM in OA
osteoblasts. As the important role of OSM in the pathogenesis and
progression of OA is well known (2–4), the
results of the present study revealed, to the best of our
knowledge, the first evidence of the mechanistic role of ET-1 in
the pathophysiology of OA. Furthermore, ETAR antagonist, BQ123
significantly suppressed the ET-1-induced effect on the expression
of OSM in OA osteoblasts, thus ETAR antagonists may be beneficial
for patients with OA. The effects of ETAR antagonists on OA require
further investigation in vivo.
The present study demonstrated that specific binding
of Ets-1 to a -494/-488 cis-DNA element in the OSM gene promoter
was required for the ET1-induced expression of OSM in OA
osteoblasts, and that ET-1 dose-dependently increased the
expression level of Ets-1 in OA osteoblasts. The results suggested
that ET-1 induced the expression of OSM in OA osteoblasts primarily
via inducing Ets-1 expression and Ets-1-dependent trans-activation
of the OSM gene promoter. Furthermore, ET-1 induced the expression
of Ets-1 in an ETAR- and PI3K-dependent manner, which explains why
ET-1 induced OSM expression in OA osteoblasts. Future studies are
required to investigate how the administration of ET-1 results in
the increased expression of Ets-1 in OA osteoblasts via the PI3K
signaling pathway.
A previous study demonstrated that ET-1 induced the
expression of Ets-1 in primary proximal tubule cells (16), which is consistent with the
observations of the OA osteoblasts in the present study. However,
in the current study, the inducing effect of ET-1 on the expression
of Ets-1 was via an ETAR, compared with the previous study, which
demonstrated that the effect of ET-1 was via an ETAR and, more
markedly, an ETBR (16). This
discrepancy may be due to the different cell models used in the
studies.
ET-1 was demonstrated to induce the production of
matrix metalloproteinase (MMP)-1 by articular chondrocytes and
synoviocytes, by which it may trigger the enzymatic degradation of
articular cartilage (5). Hence,
ET-1 may be a target of novel therapeutic approaches for OA
(5). Ets-1 is a transcription
factor that regulates the gene expression of proteases, such as
urokinase-type plasminogen activator and MMP-1 (17,18).
The current study demonstrated that ET-1 induced the expression of
Ets-1 in OA osteoblasts, thus whether ET-1 may induce the
production of MMP-1 in OA osteoblasts via Ets-1 may be of interest
for a future study. In addition to the above-mentioned finding, the
ET1-induced expression of OSM in OA osteoblasts, and the MMP-1 in
articular chondrocytes and synoviocytes, indicates that the role of
ET-1 in contributing to the pathogenesis and progression of OA may
be diverse.
In conclusion, the present study has demonstrated
that ET-1 induces the expression of OSM in OA osteoblasts via
trans-activation of the OSM gene promoter. ET-1 increased the
expression/specific binding of Ets-1 to an Ets-1 binding site (-494
to -488) in the OSM promoter in an ETAR- and PI3K-dependent manner.
It therefore provides a novel insight into the mechanistic role of
ET-1 in the pathophysiology of OA.
References
|
1
|
Sin A, Tang W, Wen CY, Chung SK and Chiu
KY: The emerging role of endothelin-1 in the pathogenesis of
subchondral bone disturbance and osteoarthritis. Osteoarthritis
Cartilage. 23:516–524. 2015. View Article : Google Scholar
|
|
2
|
Chen CY, Su CM, Huang YL, Tsai CH, Fuh LJ
and Tang CH: CCN1 induces oncostatin M production in osteoblasts
via integrin-dependent signal pathways. PLoS One. 9:e1066322014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sims NA and Walsh NC: GP130 cytokines and
bone remodelling in health and disease. BMB Rep. 43:513–523. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walker EC, McGregor NE, Poulton IJ, Solano
M, Pompolo S, Fernandes TJ, Constable MJ, Nicholson GC, Zhang JG,
Nicola NA, et al: Oncostatin M promotes bone formation
independently of resorption when signaling through leukemia
inhibitory factor receptor in mice. J Clin Invest. 120:582–592.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roy-Beaudry M, Martel-Pelletier J,
Pelletier JP, M'Barek KN, Christgau S, Shipkolye F and Moldovan F:
Endothelin 1 promotes osteoarthritic cartilage degradation via
matrix metalloprotease 1 and matrix metalloprotease 13 induction.
Arthritis Rheum. 48:2855–2864. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tatrai A, Foster S, Lakatos P, Shankar G
and Stern PH: Endothelin-1 actions on resorption, collagen and
noncollagen protein synthesis, and phosphatidylinositol turnover in
bone organ cultures. Endocrinology. 131:603–607. 1992.PubMed/NCBI
|
|
7
|
Tatrai A, Lakatos P, Thompson S and Stern
PH: Effects of endothelin-1 on signal transduction in UMR-106
osteoblastic cells. J Bone Miner Res. 7:1201–1209. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Manacu CA, Martel-Pelletier J, Roy-Beaudry
M, Pelletier JP, Fernandes JC, Shipkolye FS, Mitrovic DR and
Moldovan F: Endothelin-1 in osteoarthritic chondrocytes triggers
nitric oxide production and upregulates collagenase production.
Arthritis Res Ther. 7:R324–332. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lisignoli G, Piacentini A, Toneguzzi S,
Grassi F, Cocchini B, Ferruzzi A, Gualtieri G and Facchini A:
Osteoblasts and stromal cells isolated from femora in rheumatoid
arthritis (RA) and osteoarthritis (OA) patients express IL-11,
leukaemia inhibitory factor and oncostatin M. Clin Exp Immunol.
119:346–353. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lisignoli G, Toneguzzi S, Pozzi C,
Piacentini A, Riccio M, Ferruzzi A, Gualtieri G and Facchini A:
Proinflammatory cytokines and chemokine production and expression
by human osteoblasts isolated from patients with rheumatoid
arthritis and osteoarthritis. J Rheumatol. 26:791–799.
1999.PubMed/NCBI
|
|
11
|
Messeguer X, Escudero R, Farré D, Núñez O,
Martínez J and Albà MM: PROMO: Detection of known transcription
regulatory elements using species-tailored searches.
Bioinformatics. 18:333–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farré D, Roset R, Huerta M, Adsuara JE,
Roselló L, Albà MM and Messeguer X: Identification of patterns in
biological sequences at the ALGGEN server: PROMO and MALGEN.
Nucleic Acids Res. 31:3651–3653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
14
|
Johnson DR, Levanat S and Bale AE: Direct
molecular analysis of archival tumor tissue for loss of
heterozygosity. Biotechniques. 19:190–192. 1995.PubMed/NCBI
|
|
15
|
Nelson JB, Hedican SP, George DJ, Reddi
AH, Piantadosi S, Eisenberger MA and Simons JW: Identification of
endothelin-1 in the pathophysiology of metastatic adenocarcinoma of
the prostate. Nat Med. 1:944–949. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Von Brandenstein M, Schlosser M, Richter
C, Depping R and Fries JW: ETS-dependent p16INK4a and p21waf1/cip1
gene expression upon endothelin-1 stimulation in malignant versus
and non-malignant proximal tubule cells. Life Sci. 91:562–571.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iwasaka C, Tanaka K, Abe M and Sato Y:
Ets-1 regulates angiogenesis by inducing the expression of
urokinase-type plasminogen activator and matrix metalloproteinase-1
and the migration of vascular endothelial cells. J Cell Physiol.
169:522–531. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oda N, Abe M and Sato Y: ETS-1 converts
endothelial cells to the angiogenic phenotype by inducing the
expression of matrix metalloproteinases and integrin beta3. J Cell
Physiol. 178:121–132. 1999. View Article : Google Scholar : PubMed/NCBI
|