Introduction
Global cancer statistics suggest that ~455,800 new
cases of esophageal cancer (EC) were diagnosed in 2012, and
~400,200 people succumbed to this disease worldwide (1). EC is ~3–4 times more common in males
than females. EC is more malignant than other gastrointestinal
cancers and is characterized by a variable geographic distribution
of incidence and histological subtypes, including esophageal
squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC)
(1). ESCC is the most common
subtype and accounts for over 90% of all EC cases in Asian
countries, including China and Japan (2).
Cisplatin, a widely used platinum-containing
chemotherapeutic drug, has been employed in the treatment of
various types of cancer, and its primary function is induction of
cell death by apoptosis (3). It is
hypothesized that three different stages are involved in the
apoptosis process: The initiation phase, the effector phase and the
execution phase (4). The Bcl-2
protein family is a key regulator of the execution phase. There are
>30 members of the Bcl-2 family of proteins reported in the
literature, that are divided into the Bcl-2-like proteins, the
Bcl2-associated X (Bax)-like proteins, and the Bcl-2 homology
domain (BH3)-only proteins, according to their structure and
function (5). When exposed to
cytotoxic stimuli, BH3-only proteins initiate apoptosis by binding
and inhibiting the anti-apoptotic Bcl-2-like proteins. This leads
to the pro-apoptotic Bax-like proteins forming oligomers that
permeabilize the mitochondrial outer membrane, resulting in the
release of apoptogenic factors and activating the effector caspases
to cause apoptosis (6).
Bcl-2-interacting mediator of cell death (Bim), one of the BH3-only
protein members, has been confirmed to have at least 6 isoforms
(7). Among the various isoforms,
BimEL (extra long form), BimL (long form) and
BimS (short form) are the most well-characterized
(5,8). Although all three isoforms promote
apoptosis, they differ from each other in cytotoxicity and tissue
expression distribution; BimS is the most cytotoxic but
not universally expressed, while BimEL and
BimL are expressed in various tissues and cell types
(9).
Basic helix-loop-helix transcription factors
differentiated embryonic chondrocyte expressed gene 1 [DEC1;
officially known as basic helix-loop-helix family member e40
(BHLHE40)] and differentiated embryonic chondrocyte expressed gene
2 [DEC2; officially known as basic helix-loop-helix family member
e41 (BHLHE41)] are regulators of circadian rhythms, response to
hypoxia, mediators of epithelial-mesenchymal transition, and
apoptosis (10–13). The function of DEC1 in apoptosis is
controversial, however previous studies have revealed that DEC2
functions as an anti-apoptotic factor in several cancer cells, such
as breast cancer MCF-7 cells and oral cancer HSC-3 cells (13–15).
In the present study, the role of DEC1 and DEC2 in
cisplatin-induced apoptosis was assessed in human esophageal TE-11
cancer cells.
Materials and methods
Cell culture and treatment
The human ESCC cell line TE-11 was purchased from
the RIKEN BioResource Center (Tsukuba, Japan) through the National
Bio-Resource Project of the Ministry of Education, Culture, Sports,
Science and Technology, Japan. The cells were cultured in RPMI
Medium 1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere of 95% air and
5% CO2. Where noted, the cells were incubated with
cisplatin (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) at
various concentrations for 24 h.
DEC1 and DEC2 overexpression
The expression plasmids for human DEC1 and DEC2 were
donated by Dr Katusmi Fujimoto (Hiroshima University, Japan)
(11). TE-11 cells were seeded at
5×104 cells per 35 mm well. DEC1 or DEC2 plasmid was
transfected into the cells using Lipofectamine® LTX
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Following transfection, the cells were
incubated for 24 h and then treated with cisplatin at 20 or 40 µM
for an additional 24 h. The cells were then used in the western
blot analysis or the MTS assay.
Antibodies
Primary antibodies to DEC1 (cat. no. NB100-1800;
Novus Biologicals, Ltd. Littleton, CO, USA; 1:5,000 dilution), DEC2
(H-72; cat. no. sc-32853; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA; 1:10,000 dilution), Bax (N-20; cat. no. sc-493; Santa Cruz
Biotechnology, Inc.) and β-actin (cat. no. A5060; Sigma-Aldrich;
Merck Millipore; 1:10,000 dilution) were used. Primary antibodies
to poly ADP-ribose polymerase (PARP; cat. no. 9542; 1:5,000
dilution), cleaved caspase-3 (Asp175; cat. no. 9961; 1:1,000
dilution), cleaved caspase-8 (Asp391; cat. no. 9496; 1:1,5000
dilution), Bim (cat. no. 2819; 1:2,000 dilution), Bcl-2 (50E3; cat.
no. 2870; 1:10,000 dilution) and Bcl-2-like 1 protein extra large
(Bcl-xL; 54H6; cat. no. 2764; 1:10,000 dilution) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Anti-rabbit IgG (cat. no. 17502) and anti-mouse IgG (cat. no.
17601) secondary antibodies were purchased from Immuno-Biological
Laboratories Ltd. (Fujioka, Japan). Can Get Signal Immunoreaction
Enhancer Solution (Toyobo Co., Ltd. Osaka, Japan) or Immunoshot
Immunoreaction Enhancer Solution (Cosmobio Co., Ltd., Tokyo, Japan)
were used to dilute the primary antibodies.
Western blot analysis
M-PER lysis buffer (Thermo Fisher Scientific, Inc.)
was added to the cells cultured in a 6-well plate, and cells were
incubated for 5 min at room temperature, with gentle agitation. The
lysate was collected and transferred to a microcentrifuge tube and
centrifuged at 12,000 × g for 10 min at 4°C. The supernatant
was collected and transferred to a new tube for analysis. Protein
concentration was determined using the bicinchoninic acid (BCA)
assay. The purified protein (10 µg per lane) were subjected to 10%
SDS-PAGE, and the proteins were transferred onto polyvinylidene
difluoride membranes (Immobilon P; Merck Millipore), which were
then probed with primary antibodies at 4°C overnight. The membranes
were subsequently washed with TBS containing Tween 20, and were
incubated with secondary antibodies for 1 h at room temperature
with agitation. Proteins of interest were visualized with enhanced
chemiluminescence (ECL) reagents using the ECL, ECL-Prime, or
ECL-Select Western Blotting Detection system (Amersham; GE
Healthcare Life Sciences, Chalfont, UK). Densitometry was performed
using ImageJ version 1.48 (National Institutes of Health, Bethesda,
MD, USA). Each experiment was repeated 3 times.
Cell viability assay
TE-11 cells were seeded at a density of
2.5×103 into 96-well plates. The cells were transfected
with an empty plasmid (pcDNA) or the expression plasmids for DEC1
or DEC2 (DEC1 pcDNA or DEC2 pcDNA, respectively). Following 18 h of
transfection, the cells were cultured with or without 40 µM
cisplatin for another 24 h. Cell viability was assessed with the
MTS assay, as previously described (16).
Hematoxylin and eosin (H&E)
staining
Apoptosis was evaluated by H&E staining.
Briefly, TE-11 cells at 70% confluency were transfected with DEC2
plasmid DNA for 18 h, followed by treatment with 40 µM of cisplatin
for 24 h. The cells were then fixed with 4% paraformaldehyde (Wako
Pure Chemical Industries, Ltd., Osaka, Japan) in phosphate-buffered
saline (PBS) for 15 min, permeabilized with 0.25% Triton X-100
(Sigma-Aldrich; Merck Millipore) in PBS for 20 min and finally
stained by H&E.
Statistical analysis
Each experiment was repeated a minimum of three
times. GraphPad Prism software version 7.02 (GraphPad Software,
Inc., La Jolla, CA, USA) was used to perform one-way or two-way
analyses of variance, followed by Dunnett's or Šidák's tests. Data
are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of cisplatin on the expression
of DEC1 and DEC2 in TE-11 cells
Cisplatin treatment resulted in different outcomes
on the endogenous expression of DEC1 and DEC2 (Fig. 1A). Expression of DEC2 was decreased
with 20 and 50 µM cisplatin, whereas expression of DEC1 was
increased in the same conditions (Fig.
1A). Treatment with 10 µM cisplatin induced expression of
cleaved PARP, cleaved caspase-8, BimEL, BimL
and BimS (Fig. 1A).
Treatment with 20 and 50 µM cisplatin further increased the amounts
of cleaved PARP, cleaved caspase-8, cleaved caspase-3, Bim and Bax,
whereas it decreased the expression of Bcl-2 and Bcl-xL (Fig. 1A). In addition, the ratio of
Bax/Bcl-2 protein expression was strongly increased with 50 µM
cisplatin (Fig. 1A). Treatment of
TE-11 cells with 10, 20 and 50 µM cisplatin was demonstrated to
significantly reduce cell viability (Fig. 1B).
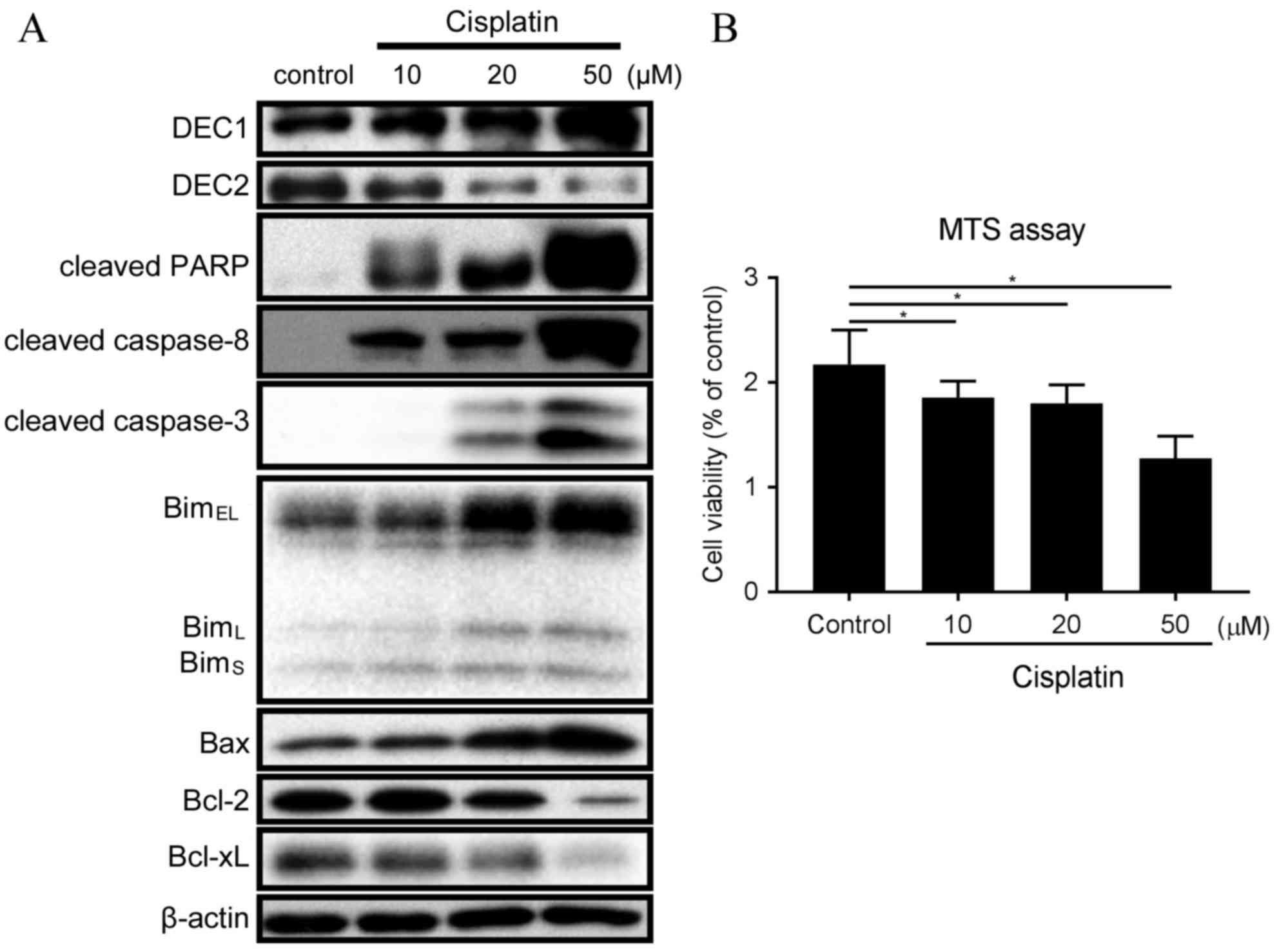 | Figure 1.Cisplatin treatment affects expression
of DEC1, DEC2 and apoptotic markers in TE-11 cells. (A) TE-11 cells
were treated with 10, 20 and 50 µM cisplatin for 24 h. Cell lysates
were subjected to western blot analysis for protein expression of
DEC1, DEC2, cleaved PARP, cleaved caspase-8, cleaved caspase-3, Bim
isoforms, Bax, Bcl-2, Bcl-xL and β-actin. (B) TE-11 cells were
treated with 10, 20 and 50 µM cisplatin for 24 h, and then cell
viability was measured using the MTS-assay. Data are presented as
the mean ± standard deviation, from three independent experiments
(*P<0.05). DEC, differentiated embryonic chondrocyte expressed
gene; PARP, poly ADP-ribose polymerase; Bim, Bcl-2-interacting
mediator of cell death; EL, extra long; L, long; S, short; Bax,
Bcl-2 associated protein X apoptosis regulator; Bcl-2, B-cell
lymphoma 2 apoptosis regulator; BcL-xL, Bcl-2-like 1 protein extra
large. |
DEC2 inhibits Bim expression and
apoptosis induced by cisplatin in TE-11 cells
The effect of DEC1 or DEC2 overexpression on
apoptosis was examined by transient transfection of expression
plasmids into TE-11 cells. DEC2 overexpression in the presence of
20 µM cisplatin visibly decreased the amounts of cleaved PARP,
cleaved caspase-3, cleaved caspase-8 and Bim (EL,
L, and S) in TE-11 cells, compared with cells
transfected with empty vector control (Fig. 2A). Conversely, DEC2 overexpression
slightly increased the expression of Bcl-xL compared with control,
but had little effect on the expression of Bcl-2 (Fig. 2A). Cisplatin augmented DEC1
expression in a dose dependent manner (Fig. 1A); however, DEC1 overexpression had
no significant effect on cleaved PARP (1.24-fold those of
pcDNA-trasnsfected group) or cleaved caspase-8 (1.28-fold those of
pcDNA-transfected group) protein expression levels under cisplatin
treatment in TE-11 cells (Fig.
2B).
 | Figure 2.DEC2 inhibits expression of apoptotic
markers following cisplatin treatment. TE-11 cells were transfected
for 24 h with pcDNA, (A) DEC2 pcDNA or (B) DEC1 pcDNA. Transfected
cells were incubated with 20 µM cisplatin for an additional 24 h,
and then lysed. Cell lysates were subjected to western blot
analysis for protein expression of DEC1, DEC2, cleaved PARP,
cleaved caspase-8, cleaved caspase-3, Bim isoforms, Bax, Bcl-2,
Bcl-xL and β-actin. Data are presented as the mean ± standard
deviation, from three independent experiments. DEC, differentiated
embryonic chondrocyte expressed gene; pcDNA, empty vector control;
DEC2 pcDNA, DEC2 overexpression vector; DEC1 pcDNA, DEC1
overexpression vector; PARP, poly ADP-ribose polymerase; Bim,
Bcl-2-interacting mediator of cell death; EL, extra long; L, long;
S, short; Bax, Bcl-2 associated protein X apoptosis regulator;
Bcl-2, B-cell lymphoma 2 apoptosis regulator; BcL-xL, Bcl-2-like 1
protein extra large. |
DEC2 overexpression inhibits
cisplatin-induced cell death in TE-11 cells
Overexpression of DEC1 or DEC2 and cisplatin
treatment were combined in TE-11 cells to investigate whether DEC
proteins affect cell viability (Fig.
3). DEC2 overexpression significantly increased the number of
live cells in the absence or presence of cisplatin compared with
pcDNA control (Fig. 3A), whereas
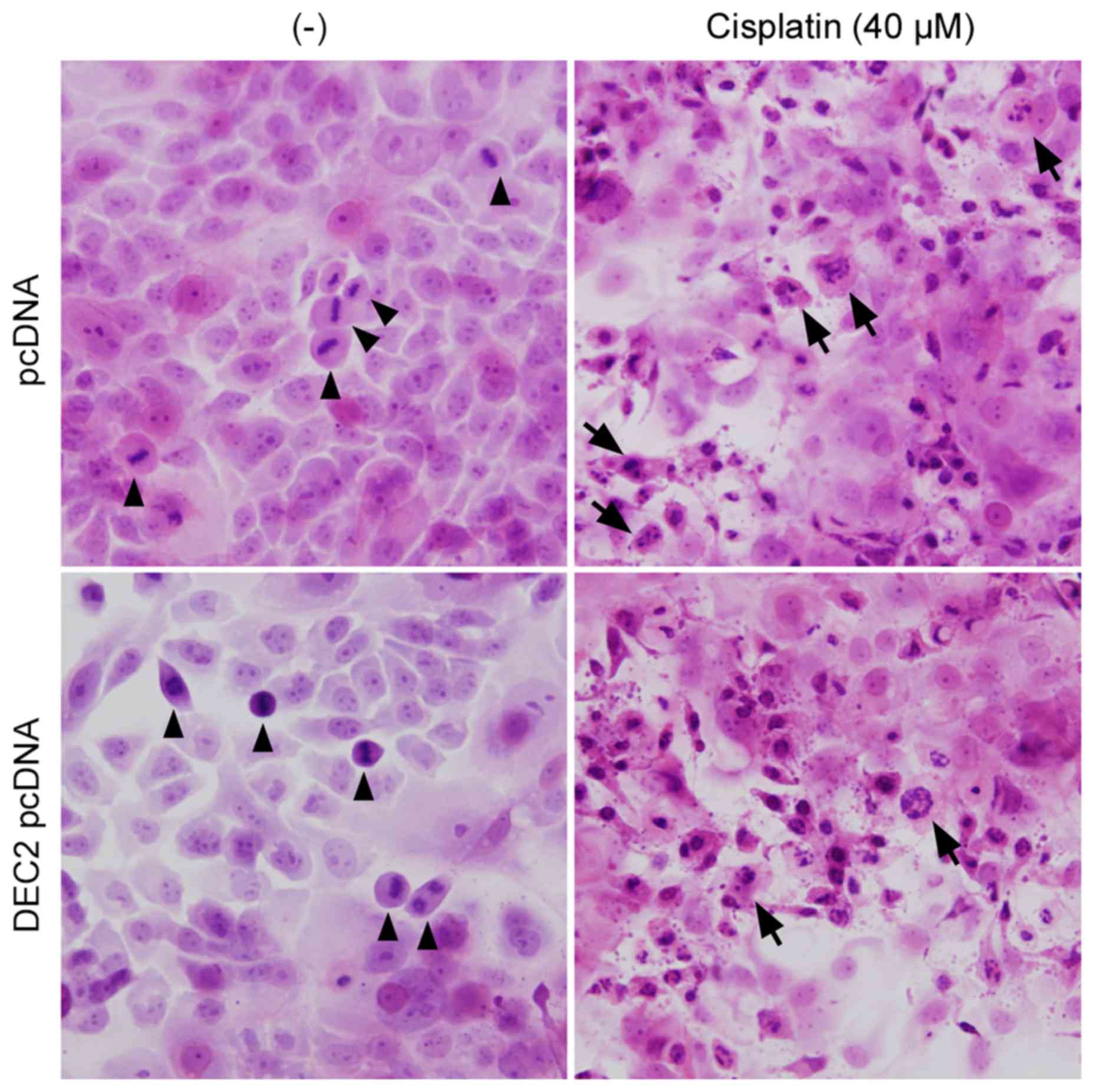
DEC1 overexpression had little effect (Fig. 3B). Finally, the apoptotic changes
in TE-11 cells were examined by H&E staining (Fig. 4). Cell mitoses were observed in the
pcDNA- or DEC2 pcDNA-transfected cells without cisplatin treatment
(Fig. 4, arrowheads), however,
cisplatin significantly increased the number of apoptotic TE-11
cells (Fig. 4, arrows) and
decreased the number of cell mitoses.
Discussion
In the present study, the roles of DEC1 and DEC2 in
the process of cisplatin-induced apoptosis of human esophageal
carcinoma cells were analyzed. Cisplatin treatment increased
expression of DEC1, but decreased expression of DEC2 in TE-11
cells. DEC2 overexpression in the presence of cisplatin markedly
inhibited multiple apoptotic markers, including all three splice
variants of Bim, BimEL, BimL and
BimS, as well as cleaved caspase-3, cleaved caspase-8,
and cleaved PARP. DEC2 had been demonstrated to inhibit apoptosis
in the breast adenocarcinoma cell line MCF-7 (13) and the squamous cell carcinoma cell
line HSC-3 (14). However, in
another squamous cell carcinoma cell line, CA9-22, which
demonstrates high endogenous expression of epidermal growth factor
receptor (EGFR), DEC2 failed to affect cisplatin-induced apoptosis
(14). In order to address this
discrepancy, EGFR expression was analyzed in TE-11 cells. A431
cells, which are known to express high levels of EGFR, were used as
a positive control. TE-11 cells exhibited significantly lower
amount of EGFR expression than A431 cells (data not shown), which
might explain why DEC2 functions as an inhibitor of apoptosis in
TE-11 cells, but not in the previously reported CA9-22 cells.
DEC protein expression is modulated in normal and
cancerous cells by several types of stimulation, such as cytokines
(15), anticancer reagents
(13), and hypoxia (12). DEC2 has also been identified as one
of the members of the circadian genes, which exhibit rhythmic
expression during the day, not only in normal cells but also in
cancer cells (11,17). As a result, scientists and
clinicians are beginning to take chronochemotherapy into
consideration, which is a time-dependent manner of treatment
administration to cancer patients. Cisplatin is one of the most
commonly used chemotherapeutic drugs for the treatment of a variety
of tumors. However, side effects including tumor resistance and
nephrotoxicity greatly limit its use. It has been reported that
cisplatin transporter molecules exhibit varying activity levels
over a 24-h period, with greater expression in the evening compared
with the morning (18). Future
studies are required into the association between DEC2 and these
transporter molecules, and the functions and underlying mechanisms
of DEC2 in regulating apoptosis, to evaluate its full potential as
a target in esophageal carcinoma chemotherapy.
Acknowledgements
This work was supported by JSPS KAKENHI,
Grants-in-Aid for Young Scientists from the Ministry of Education,
Culture, Sports, Science, and Technology of Japan (grant no.
26870028) and a Grant for Hirosaki University Institutional
Research (grant no. 5310139303).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eastman A: The Mechanism of Action of
Cisplatin: From Adducts to Apoptosis. Cisplatin Verlag Helvetica
Chimica Acta. 111–134. 2006.
|
|
5
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marani M, Tenev T, Hancock D, Downward J
and Lemoine NR: Identification of novel isoforms of the bh3 domain
protein bim which directly activate bax to trigger apoptosis. Mol
Cell Biol. 22:3577–3589. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Connor L, Strasser A, O'Reilly LA,
Hausmann G, Adams JM, Cory S and Huang DC: Bim: A novel member of
the Bcl-2 family that promotes apoptosis. EMBO J. 17:384–395. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Reilly LA, Cullen L, Visvader J,
Lindeman GJ, Print C, Bath ML, Huang DC and Strasser A: The
Proapoptotic BH3-only protein bim is expressed in hematopoietic,
epithelial, neuronal, and germ cells. Am J Pathol. 157:449–461.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu Y, Sato F, Yamada T, Bhawal UK,
Kawamoto T, Fujimoto K, Noshiro M, Seino H, Morohashi S, Hakamada
K, et al: The BHLH transcription factor DEC1 plays an important
role in the epithelial-mesenchymal transition of pancreatic cancer.
Int J Oncol. 41:1337–1346. 2012.PubMed/NCBI
|
|
11
|
Honma S, Kawamoto T, Takagi Y, Fujimoto K,
Sato F, Noshiro M, Kato Y and Honma K: Dec1 and Dec2 are regulators
of the mammalian molecular clock. Nature. 419:841–844. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyazaki K, Kawamoto T, Tanimoto K,
Nishiyama M, Honda H and Kato Y: Identification of functional
hypoxia response elements in the promoter region of the DEC1 and
DEC2 genes. J Biol Chem. 277:47014–47021. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Sato F, Bhawal UK, Kawamoto T,
Fujimoto K, Noshiro M, Morohashi S, Kato Y and Kijima H: Basic
helix-loop-helix transcription factors DEC1 and DEC2 regulate the
paclitaxel-induced apoptotic pathway of MCF-7 human breast cancer
cells. Int J Mol Med. 27:491–495. 2011.PubMed/NCBI
|
|
14
|
Wu Y, Sato F, Bhawal UK, Kawamoto T,
Fujimoto K, Noshiro M, Seino H, Morohashi S, Kato Y and Kijima H:
BHLH transcription factor DEC2 regulates pro-apoptotic factor Bim
in human oral cancer HSC-3 cells. Biomed Res. 33:75–82. 2011.
View Article : Google Scholar
|
|
15
|
Liu Y, Sato F, Kawamoto T, Fujimoto K,
Morohashi S, Akasaka H, Kondo J, Wu Y, Noshiro M, Kato Y and Kijima
H: Anti-apoptotic effect of the basic helix-loop-helix (bHLH)
transcription factor DEC2 in human breast cancer cells. Genes
Cells. 15:315–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Sato H, Suzuki T, Yoshizawa T,
Morohashi S, Seino H, Kawamoto T, Fujimoto K, Kato Y and Kijima H:
Involvement of c-Myc in the proliferation of MCF-7 human breast
cancer cells induced by bHLH transcription factor DEC2. Int J Mol
Med. 35:815–820. 2015.PubMed/NCBI
|
|
17
|
Sato F, Bhawal UK, Kawamoto T, Fujimoto K,
Imaizumi T, Imanaka T, Kondo J, Koyanagi S, Noshiro M, Yoshida H,
et al: Basic-helix-loop-helix (bHLH) transcription factor DEC2
negatively regulates vascular endothelial growth factor expression.
Genes Cells. 13:131–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sorensen DN, Dakup PP and Gaddameedhi S:
Chronopharmacology of cisplatin: Role of the circadian rhythm in
modulating the cisplatin transporters levels. The FASEB Journal.
30:(1 Suppl). S9352016.
|