Introduction
In the late 1970s, Wasser et al (1) first observed that renal injury became
more severe following renal ischemia reperfusion, thus, the concept
of renal ischemia reperfusion injury (IRI) was introduced (1). The kidney, which is a
hypertransfusion organ, is more sensitive to ischemia and ischemia
reperfusion than other organs. The incidence of renal ischemia
reperfusion worldwide is ~13–38%, of which ~80% is induced by acute
renal tubular injury (2–4). Renal IRI is commonly observed in
clinical settings, including during kidney transplantation, renal
vascular surgery, extracorporeal lithotripsy and shock
resuscitation. Renal IRI is a severe injury, which results in acute
ischemic renal failure. It often induces delayed graft function and
upregulates the expression of major histocompatibility complex
(MHC) I and MHC II antigens, thereby aggravating immunological
rejection and leading to serious graft function loss (5–7).
Inflammation serves an important role in the
occurrence and progression of renal IRI. Previous studies have
demonstrated that neutrophil infiltration in the reperfused renal
tissue, and the levels of tumor necrosis factor (TNF)-α,
transforming growth factor-β, interleukin (IL)-6, IL-1β,
inflammatory cytokines, monocyte chemoattractant protein, IL-8 and
normal T cells, secretory factors and other chemotactic factors
increase in IRI (8–10). Reactive oxygen species (ROS) are
also produced in large quantities under these conditions. High
concentrations of ROS induce apoptosis and necrosis through the
cell oxidative stress response. Therefore, inhibiting the
inflammatory signaling pathway and the production of inflammatory
mediators may effectively reduce kidney damage (11–13).
The mitogen-activated protein kinase (MAPK) pathway is known as the
cellular survival pathway. The MAPK pathway in mammalian cells is
involved in a number of processes affecting the final cellular
response, including cell proliferation, differentiation and
adaptation to environmental stress and apoptosis (14). The c-Jun N-terminal kinase
(JNK)/p38 MAPK signaling pathway, a subclass of MAPKs, is active in
a number of tissues and cells, and serves an important role in
mediating inflammatory reactions. Previous studies have
demonstrated that the JNK/p38 MAPK signaling pathway is activated
in cells following IRI (15,16).
Inflammatory cytokines, including nuclear factor (NF)-κB,
intercellular adhesion molecule (ICAM)-1, TNF-α and IL-6, also
activate this pathway (17,18).
Thus, blocking the JNK/p38 MAPK signaling pathway and inhibiting
the production of ROS may effectively alleviate early organ
dysfunction following renal IRI.
Evodia rutaecarpa, was originally recorded in
‘Shen Nong's Herbal Classic’ (19), and is classified as a traditional
interior-warming herb and a medium-grade drug in traditional
Chinese medicine. The herb is pungent, bitter, heated and slightly
poisonous. In traditional Chinese medicine, it is believed to
affect the liver, spleen, stomach and kidney channels and is
commonly used to treat hypertension, and gastrointestinal and
gynecological diseases. Rutaecarpine (Ru), a quinazoline carboline
alkaloid, is one of the main components of E. rutaecarpa
(Fig. 1) (20). Ru exhibits significant
anti-inflammatory and immunomodulatory effects. Previous studies
have investigated the pharmacological activity of this substance
and have demonstrated its multiple-targeting effects (21–23).
Ru appears to inhibit secretion of inflammatory mediators, release
of ROS, expression of adhesion molecules, activation and
infiltration of neutrophils, as well as generally regulate immunity
(21–23). At present, to the best of our
knowledge, there is no information available on the ability of Ru
to reduce multiple-organ IRI. Therefore, the effect and underlying
mechanism of Ru on early-stage renal IRI were investigated using a
rat renal IRI model. The influence of Ru on inflammatory mediators,
the JNK/p38 MAPK signaling pathway and the oxidative stress
response were also investigated. The present results may provide
information about the potential use of Ru as a new drug to treat
organ IRI.
Materials and methods
Materials
A total of 50 adult male Sprague-Dawley rats (age,
6–8 weeks; weight, 250±10 g) were obtained from the Institute of
Laboratory Animal Sciences, Anhui Medical University (Hefei,
China). All the animals were housed in individual cages with a
constant temperature (18–20°C) and humidity (65–69%) at 12/12 h
light/dark cycle with free access to food and water. All
experimental procedures were approved by the Animal Experimental
Ethics Committee of Anhui Medical University (Hefei, China). Ru was
obtained from the School of Pharmaceutical Sciences, Sun Yat-sen
University (Guangzhou, China). The nuclear magnetic resonance (NMR)
analysis results for Ru were as follows: 1H NMR (500
MHz, d6-DMSO): δ 3.21 (t, J=7.0 Hz, 2H), 4.44 (t,
J=7.0 Hz, 2H), 7.12 (dt, J=7.5, 1.0 Hz, 1H), 7.27
(dt, J=7.5, 1.0 Hz, 1H), 7.47–7.51 (m, 2H), 7.62 (d,
J=8.5 Hz, 1H), 7.70 (d, J=7.5 Hz, 1H), 7.78–7.84 (m,
1H), 8.18 (dd, J=8.0, 1.0 Hz, 1H), 11.88 (s, 1H);
13C NMR (125 MHz, d6-DMSO): 160.5, 146.9, 145.2,
138.8, 134.7, 127.0, 126.4, 126.3, 126.2, 124.8, 124.6, 120.5,
120.1, 119.6, 117.8, 112.5, 40.9, 18.7; HRMS calcd for
C18H13N3O 287.1059; Found:
287.1064. Enzyme-linked immunosorbent assay (ELISA) kits for NF-κB
(cat. no. H202), ICAM-1 (cat. no. KF066), TNF-α (cat. no. H052) and
IL-6 (cat. no. H007) were purchased from Bioval Technologies
(Shanghai, China). Superoxide dismutase activity (SOD; cat. no.
A001-1) and lipid peroxidation malondialdehyde (MDA; cat. no.
A003-1) assay kits were purchased from Nanjing Jian Cheng
Bioengineering Research Institute (Nanjing, China).
Experimental design
The IRI rat model was established by superior
mesenteric artery (SMA) occlusion, as described previously
(24). Rats were anesthetized with
chloral hydrate (100 mg/kg) intraperitoneally and randomly divided
into the following five groups (n=10/group): Sham group (sham
operation; isolation of the SMA without occlusion), normal saline
(NS) group (a group treated with 10 ml/kg saline 10 min prior
reperfusion); IRI group (intestinal ischemia reperfusion), Ru-30
group (30 mg/kg Ru treatment; surgery was performed in the same way
as the IRI group with administration of 10 ml/kg 3% Ru
intraperitoneally 10 min prior to reperfusion), and Ru-60 group (60
mg/kg Ru treatment; surgery was performed in the same way as the
IRI group with administration of 10 ml/kg 6% Ru intraperitoneally
10 min prior to reperfusion). Blood and kidney samples were
obtained for analysis after 2 h and 24 h of reperfusion,
respectively.
Measurement of serum levels of
creatinine (Cr), blood urea nitrogen (BUN) and neutrophil
gelatinase-associated lipocalin (NGAL)
Serum levels of Cr and BUN were measured on an
America Johnson FS 5.1 Biochemistry analyzer (Johnson &
Johnson, New Brunswick, NJ, USA). NGAL levels were measured using
an NGAL Rapid ELISA kit (cat. no. KIT 037; Neobioscience, Shenzhen,
China) according to the manufacturer's instructions.
Renal histopathological
assessment
The left kidney sections were stained with
haematoxylin and eosin (H&E) for the observation of renal
tissues structure (BX50; Olympus Corporation, Tokyo, Japan). Renal
tissues were immersed in 4% paraformaldehyde for 4 h and
transferred to 70% ethanol. Individual lobes of renal tissues
biopsy material were placed in processing cassettes, dehydrated
through a serial alcohol gradient and embedded in paraffin wax
blocks. Prior to staining, 5-µm-thick renal tissue sections were
dewaxed in xylene, rehydrated through decreasing concentrations of
ethanol, and washed in PBS and then stained with H&E. After
staining, sections were dehydrated through increasing
concentrations of ethanol and xylene. Histological assessment of
tubular necrosis was measured semi-quantitatively using the method
by McWhinnie et al (25). A
total of 10 randomly selected high-power fields were observed per
slide. Symptoms were scored from 0 to 3 according to tubular
profiles involving an intersection: 0, normal structures; 1,
tubular epithelial swelling, nuclear condensation, loss of the
brush border with up to one third of the tubular profile exhibiting
nuclear loss; 2, more than one-third though less than two-thirds of
the tubular profile exhibiting nuclear loss; 3, over two-thirds of
the tubular profile exhibiting nuclear loss.
Measurement of NF-κB, TNF-α, IL-6 and
ICAM-1 in renal tissues
Renal tissues were harvested and immediately
homogenized on ice with 5 volumes of normal saline. The homogenates
were centrifuged at 1,200 × g for 10 min at 4°C. NF-κB, TNF-α, IL-6
and ICAM-1 levels were measured in renal tissue supernatants using
commercial ELISA kits (Bioval Technologies, Shanghai, China)).
Reverse transcription-polymerase chain reaction
(RT-PCR) was performed as previously described (26). Briefly, renal tissue RNA was
extracted using the TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in accordance with the
manufacturer's instructions. Any potential DNA contamination was
removed by RNase-free DNaseI (cat. no. M6101, Promega Biotech Co.,
Ltd., Beijing, China) treatment. Then, cDNA was synthesized from
the total RNA using AMV reverse transcriptase (cat. no. M5101,
Promega Biotech Co., Ltd.) according to the manufacturer's
instructions. The primer sequences are listed in Table I. Each PCR reaction (20 µl total)
contained 1X PCR buffer, 1.5 µM of NF-κB, TNF-α, IL-6, ICAM-1 or
0.15 µM GAPDH primers, 500 µM dNTPs, 0.1% RNase-free
H2O, 1 U of Taq polymerase (cat. no. EP0702, Invitrogen;
Thermo Fisher Scientific, Inc.) and 3 µl of the cDNA template. The
reaction was performed in a thermal cycler as follows: 95°C for 5
min then 36 cycles of 95°C for 1 min, 50°C for 1 min and 72°C for 1
min. The amplified products were separated using 1.5% agarose gel
electrophoresis, and images were obtained on a Gel Doc 2000 Imager
System (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
 | Table I.Primer sequences for reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-polymerase chain reaction.
| Gene target | Primer | Sequence (5′-3′) | Product size
(bp) |
|---|
| NF-κB | Forward |
GAGCCACCAATCCACACAGAGT | 107 |
|
| Reverse |
ATGAGCTTCTGGCGTTTCCTCT |
|
| TNF-α | Forward |
GCAGAAGAGGCACTCCCCCA | 326 |
|
| Reverse |
GATCCATGCCGTTGGCCAGG |
|
| IL-6 | Forward |
AGTTGCCTTCTTGGGACTGA | 191 |
|
| Reverse |
TTCTGCAAGTGCATCATCGT |
|
| ICAM-1 | Forward |
AACCGGAAGGTGTATGAACTG | 390 |
|
| Reverse |
CGAGGTGTTCTCAAACAGCTC |
|
| GAPDH | Forward |
AGAAGGAAATGGCTGCAGAA | 223 |
|
| Reverse |
GCTCGGCTTCCAGTATTGAG |
Phosphorylated (p) and total JNK and
p38 MAPK western blot analyses
Endochylema and cellular nuclear proteins were
extracted from frozen renal tissue with NE-PER Nuclear and
Cytoplasmic Extraction reagent (cat. no. 78833, Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The total protein (20 µg) were separated by 10%
SDS-PAGE, transferred onto polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA) and then assessed by Ponceau S
solution staining. The membranes were blocked with 5% dehydrated
skim milk in TBS/0.1% Tween 20 (TBST) for 1 h at room temperature
and then incubated overnight at 4°C with primary antibodies
specific to p-p38 (cat. no. SAB4301534; 1:500; Sigma-Aldrich, Merck
KGaA, Darmstadt, Germany), p38 (cat. no. SAB1302631; 1:500;
Sigma-Aldrich, Merck KGaA) p-JNK (cat. no. SAB4504449; 1:500;
Sigma-Aldrich, Merck KGaA) and JNK (cat. no. SAB4502398; 1:500;
Sigma-Aldrich, Merck KGaA). The blots were washed thrice in TBST
buffer and subsequently incubated with the horseradish
peroxidase-conjugated anti-rabbit secondary antibody (cat. no.
A4914; 1:1000; Sigma-Aldrich, Merck KGaA) at room temperature for 1
h at room temperature. Specific proteins in the blots were
visualized using an enhanced chemiluminescence reagent (Thermo
Fisher Scientific, Inc.).
SOD and MDA assays
Renal tissues were harvested and immediately
homogenized on ice with 5 volumes of normal saline. The homogenates
were centrifuged at 1,200 × g for 10 min at 4°C. SOD and MDA levels
in the supernatant were determined using SOD and MDA assay kits,
according to the manufacturer's instructions.
Statistical analysis
Each experiment was performed 3 times. Results are
expressed as the mean ± standard deviation. Statistical comparisons
were performed using unpaired Student's t-test and one-way analysis
of variance followed by Dunnett's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
Effect of rutaecarpine on alleviating
renal function injury induced by IRI
Serum Cr and BUN, which are the end products of
nitrogenous organic matter and protein metabolism, serve as
indicators for diagnosing and screening for glomerular filtration
function (27). NGAL can reveal
the occurrence of early-stage acute renal tubular injury with good
sensitivity and specificity (28).
As demonstrated in Table II, the
levels of BUN, Cr and NGAL in the blood significantly increased and
renal function was severely impaired in rats in the IRI and NS
groups when compared with those in the sham group (P<0.05). When
compared with the IRI and NS groups, treatment with 30 mg/kg and 60
mg/kg Ru markedly decreased serum BUN, Cr and NAGL levels (Table II).
 | Table II.Plasma levels of Cr, BUN, and NGAL in
experimental rats. |
Table II.
Plasma levels of Cr, BUN, and NGAL in
experimental rats.
| Factor | Sham | IRI | NS | Ru-30 | Ru-60 |
|---|
| Cr (mmol/l) | 37.82±2.63 |
115.71±4.60a |
109.84±3.72a |
77.49±3.52b |
66.72±2.14b |
| BUN (µmol/l) | 7.94±0.62 |
36.82±1.46a |
35.24±0.92a |
11.47±1.18b |
8.80±1.35b |
| NGAL (ng/ml) | 56.38±4.57 |
123.72±5.20a |
119.42±3.84a |
99.75±5.34b |
81.36±6.17b |
Rutaecarpine treatment improves the
necrotic degree of proximal tubules in renal tissue
Renal tissue pathology can directly reveal the
degree of renal injury induced by renal ischemia reperfusion
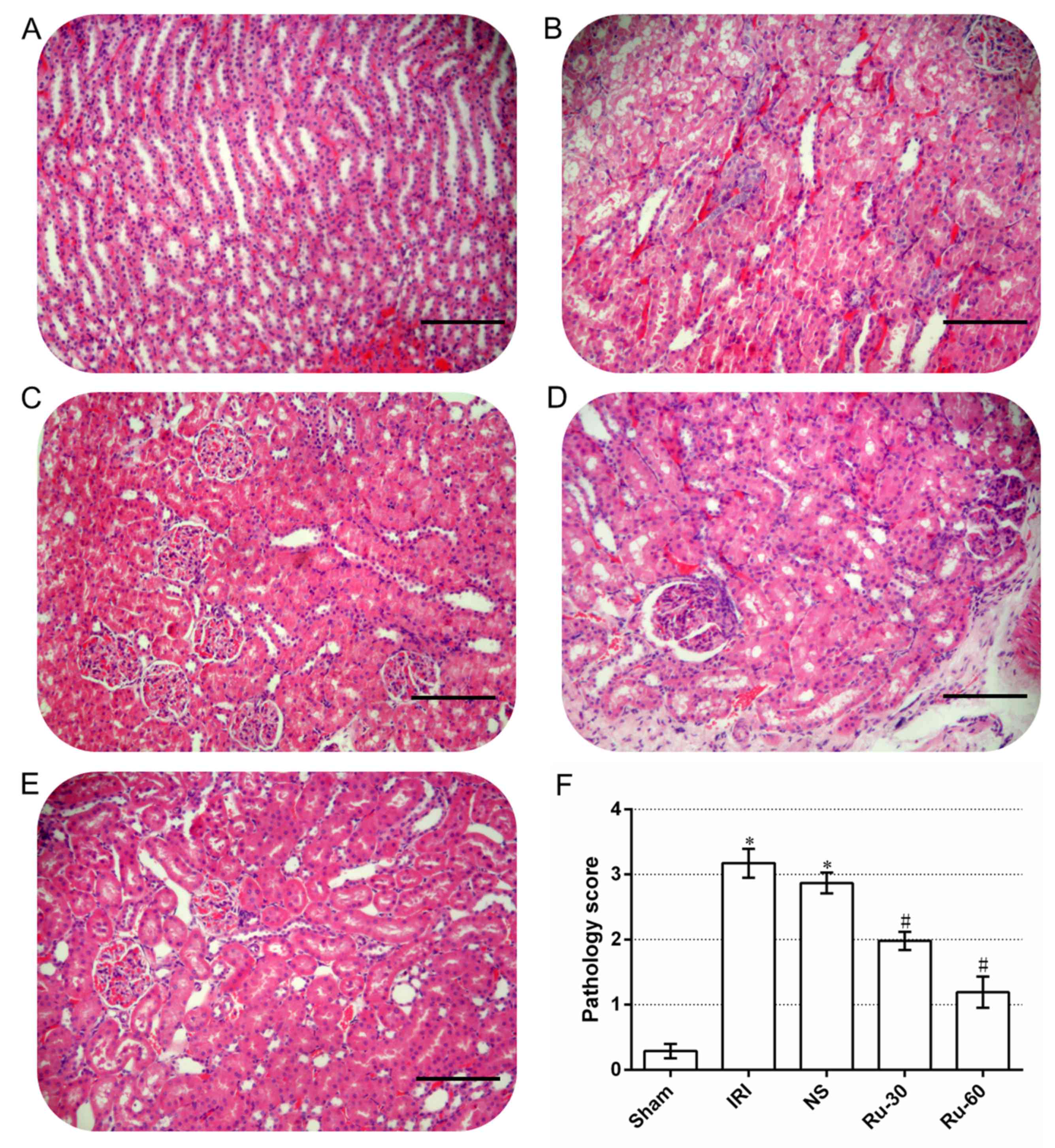
(29). Under a light microscope,
no noticeable necrosis was observed in the renal tissue of the sham
group after 24 h (Fig. 2A). The
cellular morphology of the renal tissue from this group was normal
and no vacuoles were observed in the cytoplasm (Fig. 2A). The nuclei were round or
elliptical, staining was uniform and no necrotic exfoliative cells
were observed in the renal tubules (Fig. 2A). Alterations in the renal tubules
were similar in all experimental groups. No significant change was
observed in the glomeruli. In the majority of the renal tubular
epithelial cells in the IRI and NS groups, significantly swollen
vacuolar degeneration and necrosis were observed (Fig. 2B and C). Karyopyknosis,
karyorrhexis, cytoplasmic shrinkage, apoptotic body formation and
other features of apoptosis were also observed (Fig. 2B and C). The renal tubules were
narrow and a large number of necrotic exfoliative cells were
observed (Fig. 2B and C). Cellular
and protein casts were observed in some of the renal tubules. Renal
interstitial edema, congestion and inflammatory cell infiltration
were also observed (Fig. 2B and
C). While cellular and protein casts and apoptosis were
detected in the renal tubules of the 30 mg/kg (Fig. 2D) and 60 mg/kg (Fig. 2E) Ru groups, edema and necrosis of
renal tubular epithelial cells were significantly alleviated, and
cast and leukocyte infiltration decreased.
The pathology scores of the sham, IRI and NS groups
were 0.29±0.11, 3.14±0.27 and 2.87±0.16, respectively (Fig. 2F). The scores for the latter groups
were significantly higher than that of the sham group (P<0.05).
The pathology scores of the Ru groups, 1.98±0.14 for the Ru-30
group and 1.19±0.24 for the Ru-60 group, were significantly lower
than those of the other groups and the decrease observed was
dose-dependent (P<0.05).
Rutaecarpine treatment inhibits the
production of inflammatory cytokines in renal tissue
The inflammatory cytokines secreted by renal
endothelial cells and parenchymal cells are important markers of
the inflammatory response following IRI (30). As shown in Table III, when compared with the sham
group, levels of the inflammatory cytokines NF-κB, TNF-α, IL-6 and
ICAM-1 increased in the renal tissues of rats in the IRI, NS, Ru-30
and Ru-60 groups (P<0.05) compared with the sham group,
indicating that inflammatory factors were released from the renal
tissue during the early stage of IRI. When compared with the IRI
group, the contents of inflammatory cytokines in the Ru-30 and
Ru-60 groups decreased with increasing Ru treatment (Table III). The effect of Ru on mRNA
expression levels of NF-κB, TNF-α, IL-6 and ICAM-1 was also
examined by RT-PCR. As demonstrated in Fig. 3, Ru treatment markedly inhibited
the mRNA expression of inflammatory factors. These findings
combined with the results of the renal biopsy and renal function
diagnosis, indicate that Ru may alleviate renal injury caused by
ischemia reperfusion in rats.
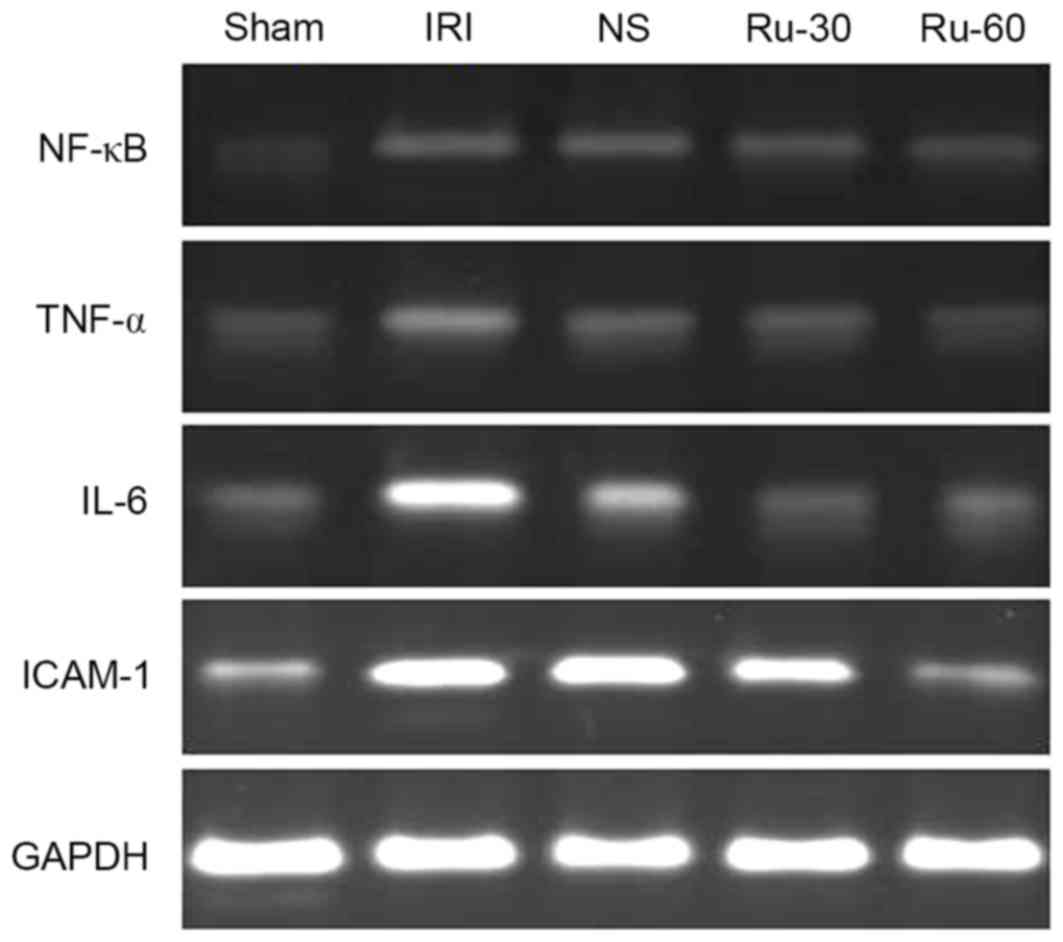 | Figure 3.mRNA expression levels of NF-κB,
TNF-α, IL-6 and ICAM-1 were measured in the five experimental
groups by reverse transcription-polymerase chain reaction.
Representative images from the gel electrophoresis are shown, with
GAPDH used as an internal reference control. NF-kB, nuclear
factor-κB; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6;
ICAM-1, intercellular adhesion molecule-1; Sham, sham operation
group; IRI, ischemia reperfusion injury; NS, normal saline; Ru-30,
30 mg/kg Ru treatment prior to IRI; Ru-60, 60 mg/kg Ru treatment
prior to IRI. |
 | Table III.Comparison of NF-κB, TNF-α, IL-6 and
ICAM-1 levels in rat kidney tissues. |
Table III.
Comparison of NF-κB, TNF-α, IL-6 and
ICAM-1 levels in rat kidney tissues.
| Group | NF-κB | TNF-α | IL-6 | ICAM-1 |
|---|
| Sham | 0.57±0.13 | 0.61±0.16 | 1.01±1.06 | 10.42±1.63 |
| IRI |
1.31±0.09a |
1.18±0.14a |
6.08±1.42a |
36.54±3.16a |
| NS |
1.42±0.11a |
0.98±0.13a |
6.21±1.06a |
36.87±2.71a |
| Ru-30 |
1.02±0.21b |
0.78±0.17b |
4.91±1.37b |
30.57±3.29b |
| Ru-60 |
0.73±0.15b |
0.67±0.09b |
4.32±1.16b |
17.38±2.62b |
Rutaecarpine treatment inhibits
phosphorylation of JNK and p38 in renal tissue
The MAPK signaling pathway participates in multiple
cell processes, including inflammation, proliferation,
differentiation and apoptosis. Activation of the MAPK signaling
pathway induces the expression of cytokines, including IL-6, IL-8
and TNF-α, which in turn enhance the inflammatory and immune
responses (15–17). Therefore, the effects of Ru on the
phosphorylation of JNK and p38 MAPK in renal tissue were
investigated by western blotting. The experimental results are
demonstrated in Fig. 4. Protein
expression levels of p-JNK and p-p38 MAPK in the renal tissues from
rats treated with different concentrations (30 or 60 mg/kg) of Ru
were lower than those in the sham and NS groups. Among the groups
tested, the decrease in p-JNK and p-p38 MAPK was obvious in the
Ru-60 treated group. Total levels of p38 and JNK MAPKs were not
affected by IRI or by IRI in combination with Ru. These results
suggest that Ru treatment effectively suppressed IRI-induced
phosphorylation of JNK and p38MAPK, which may be involved in
reducing cellular damage in renal tissue following IRI.
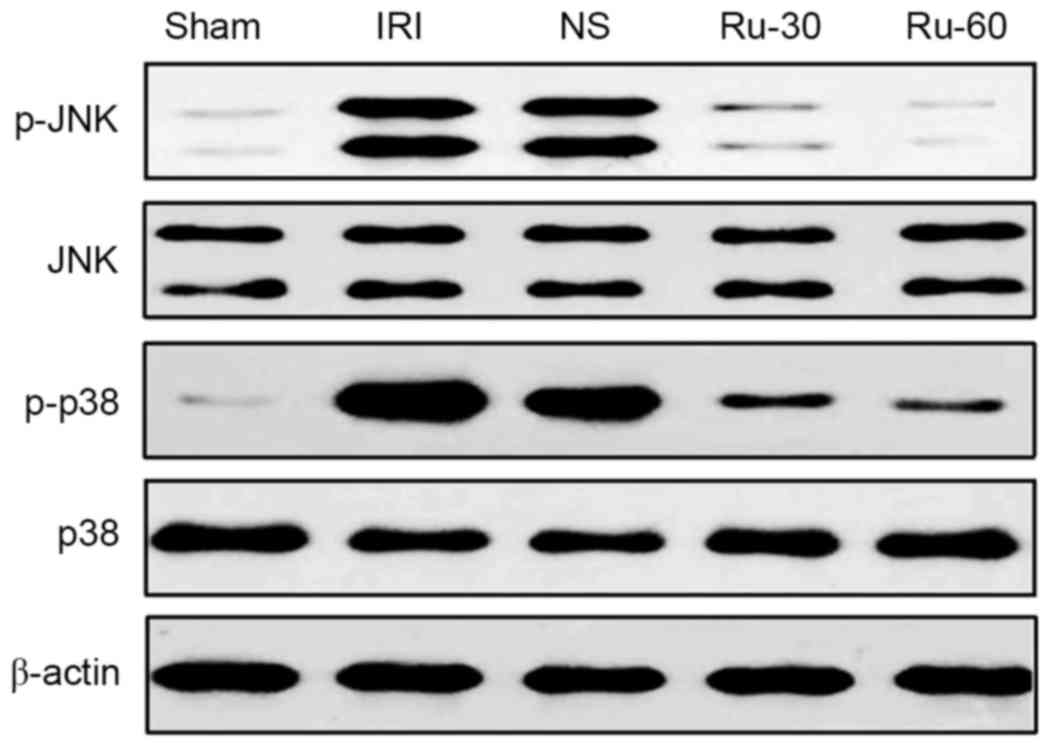 | Figure 4.Effect of Ru treatment on IRI-induced
p38 and JNK mitogen-activated protein kinase phosphorylation in
renal tissues. Total tissue extracts were subjected to western blot
analysis for protein expression levels of p-JNK, total JNK, p-p38
and total p38. β-actin was used as a loading control. Ru,
rutaecarpine; JNK, c-Jun N-terminal kinase; p, phosphorylated;
Sham, sham operation group; IRI, ischemia reperfusion injury; NS,
normal saline; Ru-30, 30 mg/kg Ru treatment prior to IRI; Ru-60, 60
mg/kg Ru treatment prior to IRI. |
Rutaecarpine treatment significantly
decreases MDA contents and increases SOD activity in renal
tissue
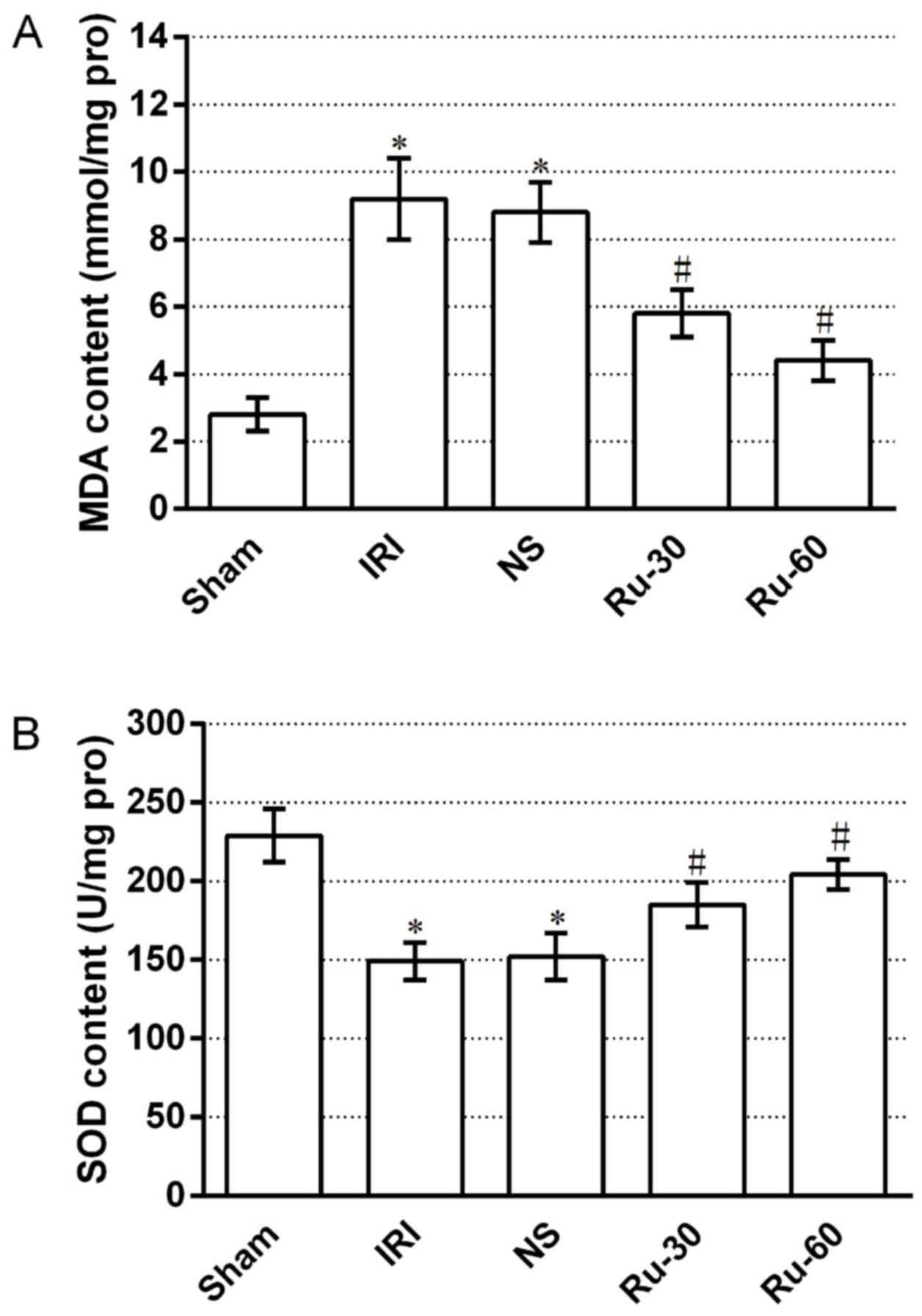
The oxidative stress response is an important
indicator of the early stage of renal tissue injury following IRI
(31). MDA, a degradation product
of lipid peroxidation, can disturb protein, glucose and nucleic
acid metabolism, decrease enzyme activity, induce nucleic acid
template dysfunction and tissue structure damage, and exacerbate
IRI (32). Thus, MDA levels
reflect the content of ROS in cells. SOD is a major macromolecule
antioxidant that eliminates ROS in vivo and can
disproportionate oxygen into hydrogen peroxide to protect cells
from ROS damage. SOD activity thus represents the ability of an
organ to eliminate ROS (33).
Consistent with the observed changes in renal function, the MDA
contents (Fig. 5A) increased and
SOD activity (Fig. 5B) decreased
in renal tissue following IRI, compared with the sham group. These
alterations were consistent with the deterioration of renal
function, indicating that renal IRI induced renal ROS production,
increased endogenous antioxidant consumption, significantly
decreased the ability of cells to eliminate ROS and exacerbated
renal damage. Ru treatment 10 min prior to ischemia markedly
reduced MDA contents and enhanced SOD activity in renal tissues,
compared with the IRI group. Thus, Ru treatment may have increased
ROS scavenging by decreasing ROS generation and inhibiting lipid
peroxidation to relieve renal damage.
The present study confirmed that rutaecarpine
exerted extensive anti-IRI effects. Ru treatment was demonstrated
to block activation of the JNK/p38 MAPK signaling pathway, inhibit
transcription of inflammatory cytokines, resist lipid peroxidation
and eliminate free radicals, all of which may help to alleviate
renal ischemia reperfusion-induced apoptosis and oxidative stress
injury. Therefore, rutaecarpine may be an effective compound to
prevent or treat renal IRI.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81370856 and
81272092).
References
|
1
|
Wasser WG, Krakoff LR, Haimov M, Glabman S
and Mitty HA: Restoration of renal function after bilateral renal
artery occlusion. Arch Intern Med. 141:1647–1651. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Devarajan P: Update on mechanisms of
ischemic acute kidney injury. J Am Soc Nephrol. 17:1503–1520. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharfuddin AA and Molitoris BA:
Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol.
7:189–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ali T, Khan I, Simpson W, Prescott G,
Townend J, Smith W and Macleod A: Incidence and outcomes in acute
kidney injury: A comprehensive population-based study. J Am Soc
Nephrol. 18:1292–1298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lenaerts K, Ceulemans LJ, Hundscheid IH,
Grootjans J, Dejong CH and Damink Olde SW: New insights in
intestinal ischemia-reperfusion injury: Implications for intestinal
transplantation. Curr Opin Organ Transplant. 18:298–303. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salvadori M, Rosso G and Bertoni E: Update
on ischemia-reperfusion injury in kidney transplantation:
Pathogenesis and treatment. World J Transplant. 5:52–67.
2015.PubMed/NCBI
|
|
7
|
Menke J, Sollinger D, Schamberger B,
Heemann U and Lutz J: The effect of ischemia/reperfusion on the
kidney graft. Curr Opin Organ Transplant. 19:395–400. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Friedewald JJ and Rabb H: Inflammatory
cells in ischemic acute renal failure. Kidney Int. 66:486–491.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ysebaert DK, De Greef KE, De Beuf A, Van
Rompay AR, Vercauteren S, Persy VP and De Broe ME: T cells as
mediators in renal ischemia/reperfusion injury. Kidney Int.
66:491–496. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thurman JM: Triggers of inflammation after
renal ischemia/reperfusion. Clin. Immunol. 123:7–13. 2007.
|
|
11
|
Garcia-Criado FJ, Eleno N, Santos-Benito
F, Valdunciel JJ, Reverte M, Lozano-Sánchez FS, Ludeña MD,
Gomez-Alonso A and López-Novoa JM: Protective effect of exogenous
nitric oxide on the renal function and inflammatory response in a
model of ischemia-reperfusion. Transplantation. 66:982–990. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shingu C, Koga H, Hagiwara S, Matsumoto S,
Goto K, Yokoi I and Noguchi T: Hydrogen-rich saline solution
attenuates renal ischemia-reperfusion injury. J Anesth. 24:569–574.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang F, Yu G, Liu SY, Li JB, Wang JF, Bo
LL, Qian LR, Sun XJ and Deng XM: Hydrogen-rich saline protects
against renal ischemia/reperfusion injury in rats. J Surg Res.
167:e339–e344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pearson G, Robinson F, Gibson Beers T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Terada Y, Inoshita S, Kuwana H, Kobayashi
T, Okado T, Ichijo H and Sasaki S: Important role of apoptosis
signal-regulating kinase 1 in ischemic acute kidney injury. Biochem
Biophys Res Commun. 364:1043–1049. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El-Mas MM, El-Gowelli HM, Ghazal AR,
Harraz OF and El-Din Mohy MM: Facilitation of central imidazoline
I(1)-site/extracellular signal-regulated kinase/p38
mitogen-activated protein kinase signalling mediates the
hypotensive effect of ethanol in rats with acute renal failure. Br
J Pharmacol. 158:1629–1640. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Irving EA and Bamford M: Role of mitogen-
and stress-activated kinases in ischemic injury. J Cereb Blood Flow
Metab. 22:631–647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy-from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guining Wei: Evodia rutaecarpa. Shen
Nong's Herbal Classic Beijing: Military Medical Science Press; pp.
902015
|
|
20
|
Hou X, Yu Z, Xu Z, Wang Y and Dai F:
Determination of the Contents of Evodiamine and Rutaecarpine in 34
Species of Evodia Rutaecarpa by HPLC. J Shenyang Pharm Univ.
17:334–337. 2000.
|
|
21
|
Yu Q, Guo C and Cheng Z: Current advances
in the study on rutaecarpine. Yaoxue Shijian Zazhi. 25:353–357.
2007.
|
|
22
|
Hu CP and Li YJ: Research progress in
pharmacological actions of evodiamine and rutaecarpine. Zhongguo
Yaolixue Tongbao. 19:1084–1087. 2003.
|
|
23
|
Lee SH, Son JK, Jeong BS, Jeong TC, Chang
HW, Lee ES and Jahng Y: Progress in the studies on rutaecarpine.
Molecules. 13:272–300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mutlu G, Abbasoğlu L, Doğru-Abbasoğlu S,
Solakoğlu S and Bulut M: Morphologic changes and lipid peroxidation
in renal tissues of young rats following intestinal
ischemia-reperfusion. Pediatr Surg Int. 18:337–340. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McWhinnie DL, Thompson JF, Taylor HM,
Chapman JR, Bolton EM, Carter NP, Wood RF and Morris PJ:
Morphometric analysis of cellular infiltration assessed by
monoclonal antibody labeling in sequential human renal allograft
biopsies. Transplantation. 42:352–358. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang S, Chou WP and Pei L: Effects of
propofol on renal ischemia/reperfusion injury in rats. Exp Ther
Med. 6:1177–1183. 2013.PubMed/NCBI
|
|
27
|
Trof RJ, Di Maggio F, Leemreis J and
Groeneveld AB: Biomarkers of acute renal injury and renal failure.
Shock. 26:245–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Di Grande A, Giuffrida C, Carpinteri G,
Narbone G, Pirrone G, Di Mauro A, Calandra S, Noto P, Le Moli C,
Alongi B and Nigro F: Neutrophil gelatinase-associated lipocalin: A
novel biomarker for the early diagnosis of acute kidney injury in
the emergency department. Eur Rev Med Pharmacol Sci. 13:197–200.
2009.PubMed/NCBI
|
|
29
|
Makris K and Spanou L: Acute kidney
injury: Definition, pathophysiology and clinical phenotypes. Clin
Biochem Rev. 37:85–98. 2016.PubMed/NCBI
|
|
30
|
Oguz E, Yilmaz Z, Ozbilge H, Baba F, Tabur
S, Yerer MB and Hekimoglu A: Effects of melatonin on the serum
levels of pro-inflammatory cytokines and tissue injury after renal
ischemia reperfusion in rats. Ren Fail. 37:318–322. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McCullough JW, Renner B and Thurman JM:
The role of the complement system in acute kidney injury. Semin
Nephrol. 33:543–556. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gutteridge JM and Halliwell B: The
measurement and mechanism of lipid peroxidation in biological
systems. Trends Biochem Sci. 15:129–135. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tábara LC, Poveda J, Martin-Cleary C,
Selgas R, Ortiz A and Sanchez-Niño MD: Mitochondria-targeted
therapies for acute kidney injury. Expert Rev Mol Med. 16:e132014.
View Article : Google Scholar : PubMed/NCBI
|