Introduction
Vascular defects are a common occurrence in bone
fractures. The application of orthopedic treatment techniques is
limited owing to technical bottlenecks limiting the
production/regeneration of high-quality blood vessels (1,2).
Following the development of tissue engineering and regenerative
medicine, macrovascular damage repair has generally been
accomplished, however, microvascular damage repair remains a
challenge, which is attributed to the absence of endothelium in
artificial blood vessels, and slow blood flow in the microvascular
predisposing to thrombus formation (3). Currently, the successful tissue
engineering of microvasculature constructs and artificial vessel
endothelialization is urgently required for the reconstruction of
defects and necrotic lesions of the skeleton.
Human umbilical vein endothelial cells (HUVECs), as
a novel cell source for vascular prostheses, have already been
applied in cardiovascular and liver tissue engineering associated
with tissue-engineered microvascular construction (4,5).
Therefore, the preservation of autologous endothelial cells for
prospective use in old age can protect against various diseases and
graft rejection in tissues defects (6). Emerging evidence shows that
cryopreserved/thawed and recultivated endothelial cells are
suitable for endothelialization of autologous allograft veins
(6). However, the use of
cryopreserved HUVECs has also been associated with several
problems, including freezing injury, degeneration of morphology and
decreased endothelial markers (7).
Previous studies have demonstrated that the cryopreservation of
complete vessels results in the loss of endothelial cells (8). It is well known that endothelial
cells are important in mediating normal vascular physiology, and
the partial absence of endothelium leads to the extracellular
matrix being in contact with the circulating blood, which generally
leads to thrombosis and/or restenosis (9,10).
These are the most serious complications observed following
angioplasty, stent deployment and prosthetic graft implantation
(9).
To further investigate the effects of
cryopreservation on HUVECs, the present study compared the
excretory function, cellular adhesion molecules and vessel lumen
formation of HUVECs following different durations of
cryopreservation.
Materials and methods
Cell culture
The HUVECs were prepared from umbilical cord veins
by collagenase digestion, as described previously (11). The human umbilical cords specimens
(n=6) of newborns were obtained from the Taizhou Hospital of
Zhejiang Province, Wenzhou Medical University, (Taizhou, Zhejiang,
China) between June, 2015 and September, 2015. The HUVECs were
maintained in Dulbecco's modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and supplemented with
10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified incubator (Thermo Fisher Scientific, Inc.) with a 5%
CO2, 95% air atmosphere. The medium was replenished
every day. The HUVECs were identified as endothelial in origin by
their cobblestone appearance under phase contrast microscopy. This
present study adhered to the tenets of the Declaration of Helsinki
and ethical approval was provided by the medical ethics committee
of Wenzhou Medical University (Taizhou, China). Informed consent
was obtained from patients.
Cryopreservation and thawing
The HUVECs cells were harvested by exposure to 0.25%
trypsin and 0.02% EDTA (Gibco; Thermo Fisher Scientific, Inc.), and
digestion was terminated with complete medium, following which the
supernatants were removed by centrifugation (150 × g for 7 min at
4°C). A total of 1×107 cells were suspended in 3% w/w
dimethyl sulfoxide (Me2SO; Gibco; Thermo Fisher
Scientific, Inc.) on ice for 10 min, followed by an additional
incubation with 10% Me2SO (final concentration) for a
further 20 min on ice. The cells were then cooled at a rate of
1°C/min in a freezing container (Nalgene, Hereford, UK) to 80°C
and, after 24 h, the cells were stored in liquid nitrogen (−196°C).
After 0, 4, 8, 12 or 24 weeks of cryopreservation in liquid
nitrogen at −196°C, the HUVECs were thawed in a 37°C water bath for
1–2 min. Oscillation of the cryopreserved tube was performed until
the cell suspension had completely thawed (12).
ELISA assay
The HUVECs were plated and treated in 96-well
plates, and were centrifuged to obtain the supernatant. The levels
of endothelin-1 (ET-1), prostaglandin E1 (PGE-1), von Willebrand
factor (vWF) and nitric oxide (NO) were measured in the supernatant
using an ELISA kit (Immutopics, Inc., San Clemente, CA, USA) at 450
nm using an ELISA reader (BioTek Instruments, Inc., Winooski, VT,
USA), according to the manufacturer's protocol.
Flow cytometry
The HUVECs (5×105) were collected in cold
PBS and EDTA (5 mM) followed by incubation with anti-ICAM-1 (cat.
no. HA58; 1:1,000; BD Biosciences, San Jose, CA, USA) for 1 h at
37°C. The LSR II system (BD Biosciences) was used for fluorescence
acquisition and data were analyzed with FACSDiva software (version
6.1.3; BD Biosciences). The samples were gated using a forward
scatter and side scatter gate eliminating debris. The fluorescent
parameters were set based on the unstained controls.
Immunofluorescence staining
The HUVECs (5×105) were stained with anti-CD31
antibody (ab28364; 1:100; Abcam, Cambridge, UK), anti-CD34 antibody
(ab8536; 1:100; Abcam) and anti-factor VIII antibody (sc-27,649;
1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C
overnight, as described previously (13). The CD34-/CD31+ cells were isolated
from the HUVEC suspension using a two-step sorting method with
immunomagnetic beads, as described previously (14). Negative control staining was
performed in parallel with the omission of primary antibodies. All
images were obtained using an inverted fluorescence microscope
(Leica DM5500B; Leica Microsystems, Inc., Wetzlar, Germany). The
uptake of acetylated low-density lipoprotein (LDL) was visualized
by immunofluorescence.
Mean vessel density analysis
According to modified version of a previously
described protocol (15),
equipment for an angiogenesis assay (BD Pharmingen, Franklin Lakes,
NJ, USA) was used to determine the angiogeneic capabilities of the
thawed HUVECs. In addition, the angiogenic function of the thawed
HUVECs stained with calcein (BD Biosciences) was recorded using a
fluorescent microscope (Leica DM5500B, Leica Microsystems, Inc.),
as described previously (16,17).
Images from the fluorescent immunohistochemical analysis were
imported into ImageJ analysis software (version 2.1; NIH, Bethesda,
MD, USA; http://www.rsbweb.nih.gov/ij/).
Statistical analysis
The data from all experiments are reported as the
mean ± standard deviation for each group. All statistical analyses
were performed using PRISM version 4.0 (GraphPad Software, Inc., La
Jolla, CA, USA). Intergroup differences were analyzed using one-way
analysis of variance, and followed by Tukey's multiple comparison
test as a post hoc test to compare group means if significant.
P<0.05 was considered to indicate a statistically significant
difference.
Results
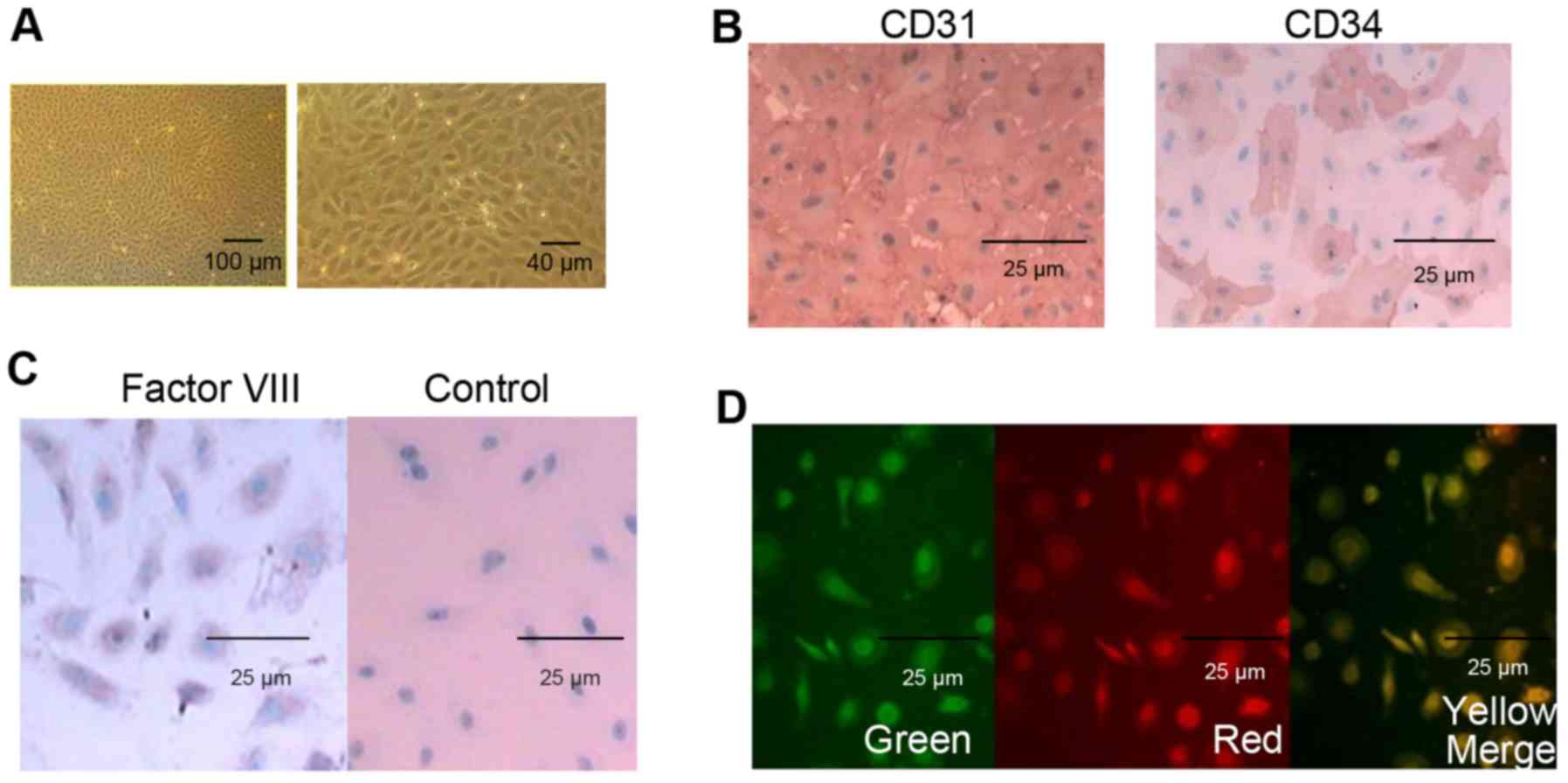
Morphological observation and
identification of HUVECs
The high purity HUVECs were successfully harvested
from fetal umbilical cords, and morphological observation of HUVECs
was performed using an inverted microscope following culture for 24
h. As shown in Fig. 1A, the
purified HUVECs exhibited a typical cobblestone-like endothelial
morphology and had high multiplicative ability. In addition, the
HUVECs were defined by the expression of CD31, CD34 and factor
VIII. The pre-cryopreserved HUVECs were almost 100% positive for
CD31 and factor VIII, and ~30% positive for CD34, as demonstrated
by immunohistochemistry (Fig. 1B and
C). The pre-cryopreserved HUVECs were also examined for
endothelial cell phenotype by the uptake of acetylated LDL. As
shown in Fig. 1D,
immunofluorescence microscopy showed positive staining for
Dil-labeled acetylated LDL in the HUVECs.
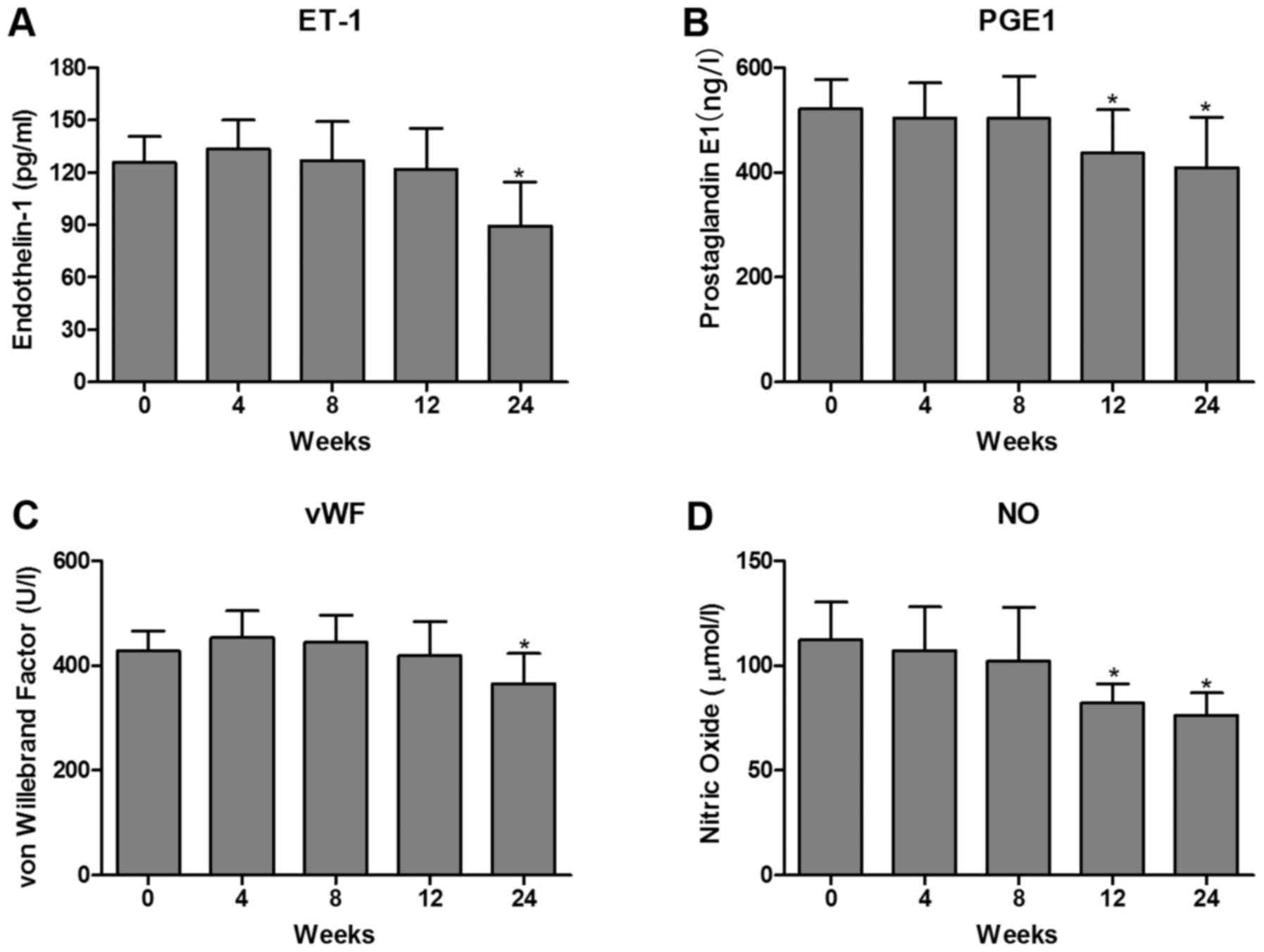
Effects of cryopreservation on the
excretory function of HUVECs
The vascular endothelium is involved in the
production of several important substances, which are involved in
endothelial dysfunction. ET-1 is characterized as a potent
vasoconstrictor and is regulated in the endothelium. NO is the most
important vascular relaxing factor, which is also regulated in the
endothelium. Alterations in the endothelial production of NO and
ET-1 are known to correlate with endothelial dysfunction (18). In addition, PGE-1 can effectively
protect endothelial cells against oxidative stress induced by
hydrogen peroxide, which may depend on the regulation of the
expression of NO (19). One such
substance, which is synthesized by, and stored in, endothelial
cells is vWF. High vWF levels have been shown to be of prognostic
value in patients with ischemic heart disease, peripheral vascular
disease and inflammatory vascular disease (20). However, there is limited
information regarding whether ET-1, PGE-1, vWF and NO are involved
in the effects of cryopreservation on the excretory function in
HUVECs. In the present study, the levels of ET-1, PGE-1, vWF and NO
were measured using an ELISA assay. The results showed that the
levels of ET-1, PGE-1, vWF and NO were significantly decreased in
the post-cryopreserved HUVECs at 24 weeks. However, no differences
were observed in these markers in the post-cryopreserved HUVECs in
the first 8 weeks (Fig. 2A-D).
These results suggested that prolonged cryopreservation may lead to
excretory dysfunction in post-thawed HUVECs.
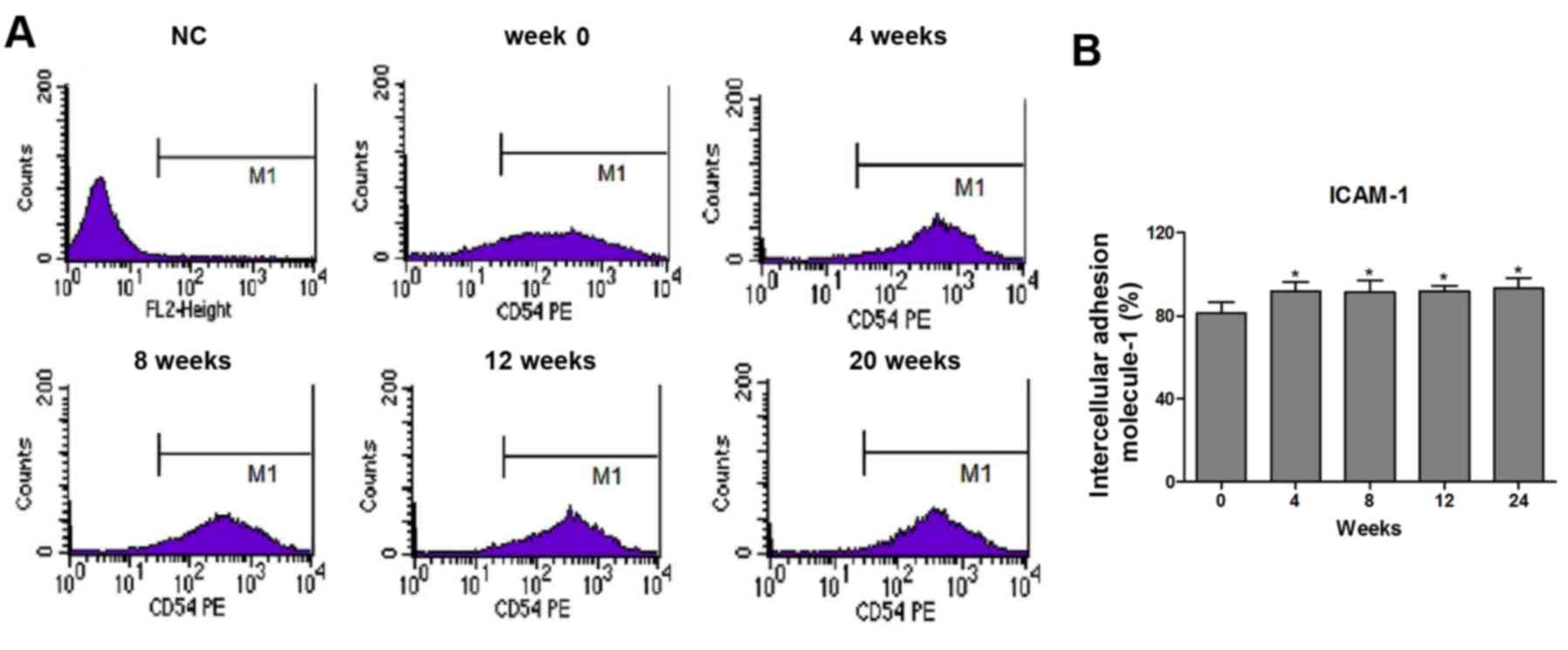
Effects of cryopreservation on the
expression of intercellular adhesion molecule-1 (ICAM-1) in
HUVECs
A previous study has demonstrated that interactions
between ICAM-1 expressed on endothelial cells and circulating
monocytes may be critical for the adhesion of these cells on the
vascular endothelium (21). In the
present study, the effect of cryopreservation on the expression of
ICAM-1 was also examined. Using flow cytometry, it was demonstrated
that the post-cryopreserved HUVECs exhibited marked upregulation in
the expression of ICAM-1 (Fig. 3A and
B). The cryopreservation-mediated induction of ICAM-1 in the
HUVECs was significantly upregulated as early as 4 weeks following
treatment, with no differences in the time course of ICAM-1
induction among the cryopreserved groups (Fig. 3B).
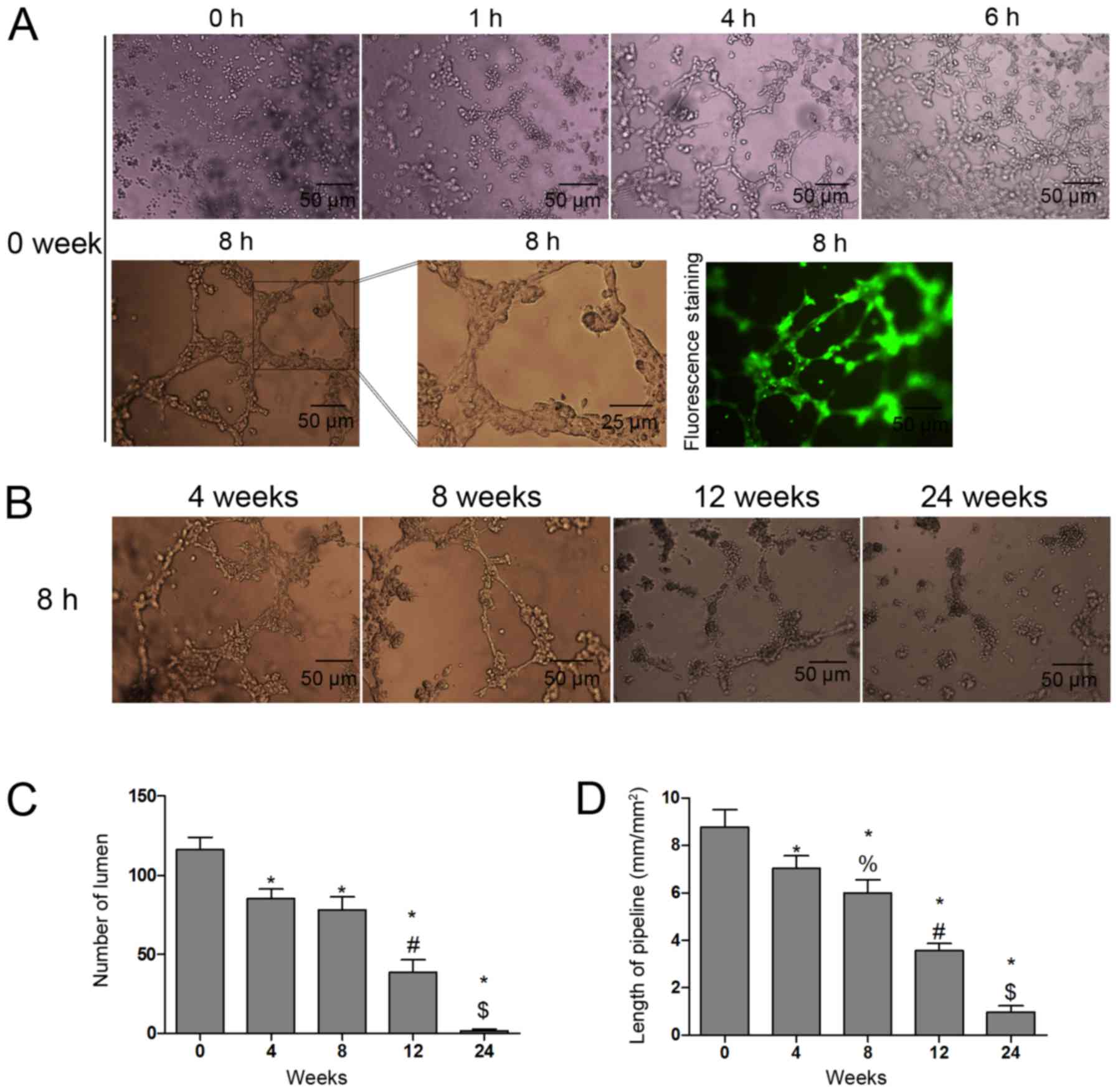
Effects of cryopreservation on vessel
lumen formation in HUVECs
The numbers of lumen and the length of the pipeline
were assessed to indicate the angiogenic function of the frozen and
the fresh HUVECs in the second passage. The results showed that the
tube-like structures were found in fresh HUVECs with increasing
incubation duration and were fully formed at 8 h (Fig. 4A). In addition, the tube-like
structure-forming potential was weakened with increasing
cryopreservation duration (Fig.
4B). The numbers of lumen (Fig.
4C) and the length of the pipeline (Fig. 4D) were decreased in the thawed
HUVECs in a time-dependent manner.
Discussion
Cryopreservation is a valuable technique for
preserving cells and tissue materials for regenerative medicine and
autologous tissue regeneration, depending on improving technology
(6). The present study aimed to
investigate the effect of cryopreservation on excretory function,
cellular adhesion molecules and vessel lumen formation in HUVECs,
which were cryopreserved following a standard protocol. The results
demonstrated that cryopreserved/thawed and recultivated HUVECs were
unsuitable for tissue engineered microvascular construction or
artificial vessel endothelialization. Specifically, the excretory
function of HUVECs was significantly decreased in the
post-cryopreserved HUVECs at 24 weeks. In addition, the
cryopreservation-mediated induction of ICAM-1 in the HUVECs was
significantly upregulated from week 4. Furthermore, the potential
to form tube-like structures was weakened with increasing
cryopreservation duration, and the number of lumen and pipeline
length were decreased in the thawed HUVECs in a time-dependent
manner.
It has been demonstrated that the cryopreservation
of endothelial cells from human umbilical cord vessels offers the
opportunity to provide seeding cells for tissue engineering
(22). However, certain studies
have suggested that the cryopreservation of human saphenous veins
is accompanied by endothelial desquamation with loss of
anticoagulant function and endothelial healing in vivo
(8). Current cryopreservation
methods universally involve the use of additives and
cytoprotectants, which may trigger osmotic damage during the
addition and removal of cryoprotectants (17,23,24).
As the results of the present study showed, the anabiosis HUVECs
lost partial function, which was consistent with previous
investigations. However, there is evidence that
cryopreserved/thawed and recultivated endothelial cells are
suitable for the endothelialization of autologous allograft veins
(6). In porcine endothelial
progenitor cells, no significant differences in cell viability were
found between storage durations of 1, 3, 6, 12 or 18 months
following cryopreservation, and no significant differences in cell
proliferation or migration were detected between fresh cells and
cells cryopreserved for up to 18 months (17).
The aims of the present study were to elucidate the
potential of HUVECs as a tool, in terms of the feasibility of
creating stable cell lines, maintaining them over a long time
period and reusing them following cryopreservation. The expression
of the adhesion marker, ICAM-1, and vessel luminal formation
potential in cell culture over time (continuous cultivation time of
6 months) were evaluated following cryopreservation. Prolonged
cryopreservation did not create stable cell lines for
tissue-engineered microvascular construction and artificial vessel
endothelialization. ICAM-1 in the HUVECs was significantly
upregulated following cryopreservation. ICAM-1 is known to mediate
leukocyte and platelet-endothelial cell interactions in the
vasculature under physiological and pathological conditions
(25). In addition, ICAM-1 has
been shown to be vital in the development of vascular inflammation
and endothelial dysfunction, which is associated with the
pathogenesis of multiple cardiovascular diseases (26,27).
The results suggested that prolonged cryopreservation may lead to
HUVEC dysfunction. However, the tube-like structure-forming
potential was weakened with increasing cryopreservation duration.
The numbers of lumen and pipeline length were decreased in the
thawed HUVECs in a time-dependent manner.
In conclusion, the results of the present study
revealed that prolonged cryopreservation may lead to HUVEC
dysfunction and did not create stable cell lines for
tissue-engineered microvascular construction. However, it has
previously been documented that minimal cell damage occurs during
the storage period when using optimal cryopreservation methods
(28). Therefore, the
establishment of an optimal condition for cryopreservation is an
urgent requirement of future investigations.
References
|
1
|
Tabbaa SM, Horton CO, Jeray KJ and Burg
KJ: Role of vascularity for successful bone formation and repair.
Crit Rev Biomed Eng. 42:319–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson EO, Troupis T and Soucacos PN:
Tissue-engineered vascularized bone grafts: Basic science and
clinical relevance to trauma and reconstructive microsurgery.
Microsurgery. 31:176–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishii M, Shibata R, Kondo K, Kambara T,
Shimizu Y, Tanigawa T, Bando YK, Nishimura M, Ouchi N and Murohara
T: Vildagliptin stimulates endothelial cell network formation and
ischemia-induced revascularization via an endothelial nitric-oxide
synthase-dependent mechanism. J Biol Chem. 289:27235–27245. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kadner A, Hoerstrup SP, Tracy J, Breymann
C, Maurus CF, Melnitchouk S, Kadner G, Zund G and Turina M: Human
umbilical cord cells: A new cell source for cardiovascular tissue
engineering. Ann Thorac Surg. 74:S1422–S1428. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Risbud MV, Karamuk E, Moser R and Mayer J:
Hydrogel-coated textile scaffolds as three-dimensional growth
support for human umbilical vein endothelial cells (HUVECs):
Possibilities as coculture system in liver tissue engineering. Cell
Transplant. 11:369–377. 2002.PubMed/NCBI
|
|
6
|
Lehle K, Hoenicka M, Jacobs VR, Schmid FX
and Birnbaum DE: Cryopreservation of human endothelial cells for
vascular tissue engineering. Cryobiology. 50:154–161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bellón JM, Buján J, Honduvilla NG,
Hernando A and Navlet J: Behavior of cryopreserved endothelial
cells in different phases: Their application in the seeding of
vascular prostheses. Ann Vasc Surg. 9:266–273. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bambang LS, Mazzucotelli JP, Moczar M,
Beaujean F and Loisance D: Effects of cryopreservation on the
proliferation and anticoagulant activity of human saphenous vein
endothelial cells. J Thorac Cardiovasc Surg. 110:998–1004. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pascual G, Escudero C, Rodriguez M,
Corrales C, Serrano N, Bellón JM and Buján J: Restoring the
endothelium of cryopreserved arterial grafts: Co-culture of venous
and arterial endothelial cells. Cryobiology. 49:272–285. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Komori K, Inoguchi H, Kume M, Shoji T and
Furuyama T: Differences in endothelial function and morphologic
modulation between canine autogenous venous and arterial grafts:
Endothelium and intimal thickening. Surgery. 131:(1 Suppl).
S249–S255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jaffe EA, Nachman RL, Becker CG and Minick
CR: Culture of human endothelial cells derived from umbilical
veins. Identification by morphologic and immunologic criteria. J
Clin Invest. 52:2745–2756. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nicoud IB, Clarke DM, Taber G, Stolowski
KM, Roberge SE, Song MK, Mathew AJ and Reems JA: Cryopreservation
of umbilical cord blood with a novel freezing solution that mimics
intracellular ionic composition. Transfusion. 52:2055–2062. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Engler C, Kelliher C, Chang S, Meng H and
Jun AS: Cryopreservation and long-term culture of transformed
murine corneal endothelial cells. Graefes Arch Clin Exp Ophthalmol.
250:103–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu X, Jiang Z and Liu N: A novel approach
for harvesting lymphatic endothelial cells from human foreskin
dermis. Lymphat Res Biol. 4:191–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu Z, Hofman FM and Zlokovic BV: A simple
method for isolation and characterization of mouse brain
microvascular endothelial cells. J Neurosci Methods. 130:53–63.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Russell JS and Brown JM: Circulating mouse
Flk1+/c-Kit+/CD45- cells function as endothelial progenitors cells
(EPCs) and stimulate the growth of human tumor xenografts. Mol
Cancer. 13:1772014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu J, Lu Z, Nie M, Zhou H, Sun X, Xue X,
Bi J and Fang G: Optimization of cryopreservation procedures for
porcine endothelial progenitor cells. J Biosci Bioeng. 113:117–123.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee CC, Chen PR, Lin S, Tsai SC, Wang BW,
Chen WW, Tsai CE and Shyu KG: Sesamin induces nitric oxide and
decreases endothelin-1 production in HUVECs: Possible implications
for its antihypertensive effect. J Hypertens. 22:2329–2338. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang WT, Li HJ and Zhou LS: Protective
effects of prostaglandin E1 on human umbilical vein endothelial
cell injury induced by hydrogen peroxide. Acta Pharmacol Sin.
31:485–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lip GY and Blann A: von Willebrand factor:
A marker of endothelial dysfunction in vascular disorders?
Cardiovasc Res. 34:255–265. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meerschaert J and Furie MB: The adhesion
molecules used by monocytes for migration across endothelium
include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1,
VCAM-1, and other ligands on endothelium. J Immunol. 154:4099–4112.
1995.PubMed/NCBI
|
|
22
|
Jiang Z, Hu X, Kretlow JD and Liu N:
Harvesting and cryopreservation of lymphatic endothelial cells for
lymphatic tissue engineering. Cryobiology. 60:177–183. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pegg DE: Cryopreservation of vascular
endothelial cells as isolated cells and as monolayers. Cryobiology.
44:46–53. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Campbell LH and Brockbank KG: Culturing
with trehalose produces viable endothelial cells after
cryopreservation. Cryobiology. 64:240–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hope SA and Meredith IT: Cellular adhesion
molecules and cardiovascular disease. Part I. Their expression and
role in atherogenesis. Intern Med J. 33:380–386. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duan M, Yao H, Hu G, Chen X, Lund AK and
Buch S: HIV Tat induces expression of ICAM-1 in HUVECs:
Implications for miR-221/−222 in HIV-associated cardiomyopathy.
PLoS One. 8:e601702013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krieglstein CF and Granger DN: Adhesion
molecules and their role in vascular disease. Am J Hypertens.
14:44S–54S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Richards M, Fong CY, Tan S, Chan WK and
Bongso A: An efficient and safe xeno-free cryopreservation method
for the storage of human embryonic stem cells. Stem Cells.
22:779–789. 2004. View Article : Google Scholar : PubMed/NCBI
|