Introduction
The aquaporins (AQPs) are a family of membrane
proteins, which form water channels across cell membranes. There
are 13 AQPs in mammals, named AQP0 to AQP12. AQP 1, 2, 4, 5 and 8
appear to function as selective water channels, whereas AQP 3, 7, 9
and 10 can transport water and glycerol. There are up to six AQPs
(AQP 1, 3, 5, 7, 9 and 10) expressed in the skin. AQP1 has been
detected in melanocytes and endothelial cells of the human dermis
(1). Boury-Jamot et al
(2) demonstrated that AQP3 was
expressed in the plasma membrane of human keratinocytes in
vitro and in vivo (2).
AQP5 is expressed in sweat glands (3), AQP7 is expressed in adipocytes
(4), AQP9 is expressed in
preadipocytes and AQP10 is expressed in keratinocytes (2).
AQP3 is the most abundant skin aquaglyceroporin
(5). AQP3 is expressed at high
levels in keratinocyte plasma membranes of the human epidermis and
reconstructed human epidermis (6).
AQP3 is involved in the differentiation and proliferation of
keratinocytes. Using an RNase protection assay, it has been shown
that AQP3 mRNA is expressed in growing and differentiating human
keratinocytes (7). AQP3 is
overexpressed in human skin squamous cell carcinoma (SCC) (8). AQP3-null mice show considerable
resistance to the development of skin tumors following exposure to
tumor initiators (8). Therefore,
AQP3 may be an important determinant in skin tumorigenesis, and a
novel target for tumor prevention and therapy (8).
Phospholipase D (PLD) was first identified in plants
as a distinct phospholipid-specific phosphodiesterase, which
hydrolyses phosphatidylcholine to phosphatidic acid (PA) and
choline (9). PLD is a
phospholipid-degrading enzyme, which generates biologically active
products that are considered to have important functions in cell
regulation (10). The activity of
PLD results in modification of various lipid constituents of the
membrane, and generates one or more messenger molecules, which are
able to recruit or modulate specific target proteins. PA is product
of the PLD enzymatic action and is a major lipid second messenger,
which regulates signaling pathways and cell proliferation. There
are two PLD isoforms in mammals, PLD1 and PLD2. PLD is important in
tumorigenesis, and the elevation of either PLD1 or PLD2 contributes
to cancer progression. Elevated PLD activity and expression have
been reported in several types of cancer (11). PLD provides survival signals and is
involved in the migration, adhesion and invasion of cancer cells
(11).
AQP3 and PLD2 are co-localized in caveolin-rich
membrane microdomains of keratinocytes. AQP3 and PLD2 form a
signaling module in lipid rafts, where AQP3 transports glycerol to
PLD2 for the synthesis of phosphatidylglycerol (PG). PG can mediate
the effects of the AQP3/PLD2 signaling module in the regulation of
keratinocyte proliferation and differentiation (12). AQP3 and PLD2 are abnormally
expressed and/or localized in SCC, basal cell carcinoma and
psoriasis, thus the AQP3/PLD2 signaling module may be involved in,
or serve as surrogate markers for the pathogenesis of these
diseases (13).
To confirm the role of AQP3 and PLD2, and the
AQP3/PLD2 signaling module in SCC, the present study examined the
protein expression and localization of AQP3 and PLD2 in actinic
keratosis (AK), Bowen's disease (BD) and SCC, relative to the
normal epidermis. The anticancer effects of AQP3 small interfering
RNA (siRNA) and PLD2 siRNA on SCC were also examined.
Materials and methods
Skin tissue samples
To analyze the expression of AQP3 and PLD2,
paraffin-embedded tissue sections of skin from patients with
diagnoses of AK, BD and SCC, as determined and verified by two
dermatopathologists, were obtained from the tissue archives of the
Department of Pathology at Hangzhou Hospital of Traditional Chinese
Medicine (Hangzhou, China). Normal skin tissue samples of 10
patients (5 females, 5 males) were obtained from skin biopsies from
October 1 to 31 2013, the remaining patient details are presented
in Table I. The clinical and
histopathological characteristics of the patients are shown in
Table I. The procedures were
approved by the Ethics Committee of Hangzhou Hospital of
Traditional Chinese Medicine and informed consent was obtained from
the patient.
 | Table I.Clinical and histological
characteristics of patients and tissue samples. |
Table I.
Clinical and histological
characteristics of patients and tissue samples.
| Characteristic | Control | Actinic
keratosis | Bowen's
disease | Squamous cell
carcinoma |
|---|
| Cases (n) | 10 | 12 | 15 | 20 |
| Age
(years)a | 73.43±8.49 | 79.86±8.09 | 71±6.51 | 67.67±14.04 |
| Site (n) |
|
|
|
|
|
Head | 4 | 9 | 0 | 7 |
|
Trunk | 3 | 0 | 9 | 3 |
|
Limbs | 3 | 3 | 6 | 10 |
| siRNA | Sense (5′-3′) | Antisense
(5′-3′) |
| Tumor stage
(n)b |
|
|
|
|
| I |
|
|
| 10 |
| II |
|
|
| 4 |
|
III |
|
|
| 4 |
| IV |
|
|
| 2 |
AQP3 and PLD2
immunohistochemistry
Immunohisto-chemistry was performed as previously
described (14). The slides used
for AQP3 staining were deparaffinized, washed twice for 5 min in
phosphate-buffered saline (PBS), incubated for 10 min at 37°C in 3%
hydrogen peroxide, washed three times for 5 min in PBS, incubated
for 30 min in 0.3% goat serum (Maixin Biotech Co., Ltd., Fuzhou,
China), and then incubated overnight with the following primary
antibodies: Rabbit anti-AQP3 (cat. no. BS3671; Bioworld Technology,
Inc., St. Louis Park, MN, USA; 1:200 dilution); rabbit-anti-PLD2
(cat. no. AP14669a; Abgent, Inc., San Diego, CA, USA; 1:100
dilution), in a humidified chamber at 4°C. Following secondary
antibody (cat. no. AP40467a; Maixin Biotech Co., Ltd.; 1:100
dilution) incubation for 15 min at 37°C, an ABC staining kit (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was used to
visualize immunoreactivity with Olympus CX21FS1C microscope and
development with the chromogen 3,30-diaminobenzidine (Maixin
Biotech Co., Ltd.) for 3 min.
The expression levels of AQP3 and PLD2 on each slide
were graded according to a previously described scoring system
(14). Each slide was scored
according to staining intensity and the proportion of positive
cells. The scores of staining intensity were: 0, negative staining;
1, mild staining; 2, moderate staining; 3, severe staining. The
scores for the proportion of positive cells were: 1, <33%
positive cells; 2, 33–66% positive cells; 3, >66% positive
cells. The final score of each slide was the multiplication result
of the two above scores. The results of the immunohistochemical
staining were graded according to the final score in a
semiquantitative manner on a four-point scale:-, negative
expression; +, low expression (score 1–3); ++, moderate expression
(score 4–6); and +++, high expression (score 7–9).
Cell culture
The human A431 SCC cell line was purchased from the
China Center for Type Culture Collection (Wuhan, China). The A431
cells were maintained at 37°C in a humidified atmosphere (95% air
and 5% CO2) and grown in plastic tissue-culture flasks
containing DMEM (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.).
Transfection of siRNAs
The three specific siRNAs (AQP3 siRNA, PLD2 siRNA
and negative control siRNA) were designed by GenePharma (Shanghai,
China). The sequences are shown in Table II. Transfection of the A431 cells
with AQP3 siRNA and PLD2 siRNA was performed according to the
manufacturer's protocol and as previously described (15). Briefly, 50,000 cells/cm2 were
plated into 6-well plates and allowed to adhere for 24 h.
Subsequently, 5 µl of siRNA was added to 250 µl of Opti-MEM (Gibco;
Thermo Fisher Scientific, Inc.) thoroughly mixed, and incubated at
room temperature for 5 min. Lipofectamine™ 2000 (5 µl; Gibco;
Thermo Fisher Scientific, Inc.) was added to 250 µl of Opti-MEM,
thoroughly mixed and incubated at room temperature for 5 min. The
diluted siRNA and diluted Lipofectamine™ 2000 were mixed and
incubated at room temperature for 20 min. The siRNA/Lipofectamine
mixture was transferred into 6-well plates at 500 µl/well. The
cells were maintained for 6 h at 37°C. Following replacement of the
culture medium, the cells were incubated for an additional 24–72 h.
AQP3- and PLD2-knockdown were verified using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analyses.
 | Table II.Sequences of siRNA. |
Table II.
Sequences of siRNA.
| siRNA | Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| AQP3-homo-612 |
CCCUUAUCGUGUGUGUGCUTT |
AGCACACACACGAUAAGCGTT |
| AQP3-homo-363 |
CCUUUGCCAUGUGCUUCCUTT |
AGGAAGCACAUGGCAAAGGTT |
| AQP3-homo-360 |
GGGCUGUAUUAUGAUGCAATT |
UUGCAUCAUAAUACAGCCCTT |
| PLD2-homo-602 |
CAGCCAGCAAACAGAAAUATT |
UAUUUCUGUUUGCUGGCUGTT |
| PLD2-homo-1352 |
GGCAUCAACAGUGGCUAUATT |
UAUAGCCACUGUUGAUGCCTT |
| PLD2-homo-5338 |
GGCACCGAAAGAUAUACCATT |
UGGUAUAUCUUUCGGUGCCTT |
| Negative
control |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
RT-qPCR analysis
Total RNA was extracted from A431 cells using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) at 24 h post-siRNA
transfection. cDNA was synthesized from the isolated RNA using a
RevertAid First Strand cDNA synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
The qPCR assay was performed on a CFX Connect
Real-Time PCR detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to the manufacturer's protocol. The
qPCR analysis was performed with 25 µl (final volume) reaction
mixture, containing 10.5 µl of cDNA, 12.5 µl of IQ SYBR Green
Supermix (Bio-Rad Laboratories, Inc.) and 1 µl each of 10 µM
forward and reverse primers (Takara Bio, Inc., Kusatsu, Otsu,
Japan). The following primers were used for amplification of AQP3,
PLD2 and the internal control (GAPDH): AQP3, forward
5′-CCCCTCTGGACACTTGGAT-3′ and reverse 5′-CACGAAGACACCCGCAAT-3′.
PLD2, forward 5′-GCCTTGGGCATCAACAGT-3′ and reverse
5′-AGGTCAGTCAGTCGGTAGTG-3′. GAP DH, forward
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse 5′-AGGGGCCATCCACAGTCTTC-3′.
Thermal cycling was initiated with an initial denaturation step at
50°C for 3 min, 95°C for 3 min, followed by 40 cycles of 95°C for
10 sec, 61°C for 20 sec and 72°C for 20 sec. The 2−ΔΔCq
method (16) was used for data
analysis, with results representative of three independent
experiments.
Western blot analysis
Total protein was extracted using a protein
extraction kit (Active Motif, Carlsbad, CA, USA) and protein
concentration was determined using the Bradford method (Active
Motif) according to manufacturer's protocol at 24 h following siRNA
transfection and western blot analysis was performed as previously
described (17) with the following
primary and secondary antibodies: Rabbit-anti-AQP3 cat. no.
sc-9885; Santa Cruz Biotechnology, Inc.; 1:1,000 dilution),
rabbit-anti-PLD2 (cat. no. AP14669a; Abgent, Inc.; 1:1,000
dilution), rabbit-anti-GAPDH (cat. no. AP0063; Bioworld Technology,
Inc.; 1:1,000 dilution) and horseradish peroxidase-conjugated goat
anti-rabbit IgG (cat. no. BS13278; Bioworld Technology, Inc.;
1:1,000 dilution). Briefly, 25 µg of protein was electrophoresed on
a 10% sodium dodecyl sulfate-polyacrylamide gel. The protein was
then transferred onto a polyvinylidene fluoride membrane. The
membrane was incubated with primary antibody for 2 h at room
temperature and then incubated with secondary antibody for 1 h at
room temperature. The bands were visualized chemiluminescently
using an ECL kit (Beyotime Institute of Biotechnology, Shanghai,
China). Semi-quantification was verified using ImageJ version 1.41
software (imagej.nih.gov/ij/), with results
representative of three independent experiments.
Cell proliferation assay
A Cell Counting Kit-8 (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used to measure the
effects of siRNA transfection on the proliferation of A431 cells,
according to the manufacturer's protocol. The Cell Counting Kit-8
assay was performed in 96-well plates 24, 48 and 72 h following
siRNA transfection. Cells were plated at a density of 5×104
cells/well into 96-well plates. The detection reagent (10 µl WST-8)
was added to each well and incubated for 1 h at 37°C. Viable cell
numbers were estimated by measurement of the optical density at 450
nm, with results representative of six independent experiments.
Cell apoptosis assay
Cell apoptosis was detected using Annexin
V-fluorescein isothiocyanate/propidium iodide (FITC/PI) double
labeling with an Alexa Fluor 488 Annexin V/Dead Cell Apoptosis kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Cells were plated at 2×105 cells/well into
6-well plates. Annexin V-FITC/PI double labeling was performed in
6-well plates 24, 48 and 72 h following siRNA transfection. Annexin
V-FITC (5 µl) was added to each well and incubated for 15 min at
4°C, following which 10 µl of PI was added and incubated for 5 min
at 4°C. The stained cells were analyzed using BD FACSVerse flow
cytometry (BD Biosciences, San Diego, CA, USA), with results
representative of three independent experiments.
Statistical analysis
Data were statistically analyzed using the
Statistical Package for Social Sciences, version 12.0 (SPSS, Inc.,
Chicago, IL, USA). The results are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a significant
difference in all analyses. For the results of the
immunohistochemical staining, the statistical significance between
two groups was determined using a Mann-Whitney U test. For other
results, the statistical significance between two groups was
determined using a paired-samples t-test. The statistical
significance between multiple groups was determined using one-way
analysis of variance in conjunction with a Newman Keuls post-hoc
test.
Results
Expression of AQP3 is increased in
tissue samples of AK, BD and SCC
The present study examined the expression of AQP3 in
tissue samples of normal skin, AK, BD and SCC using
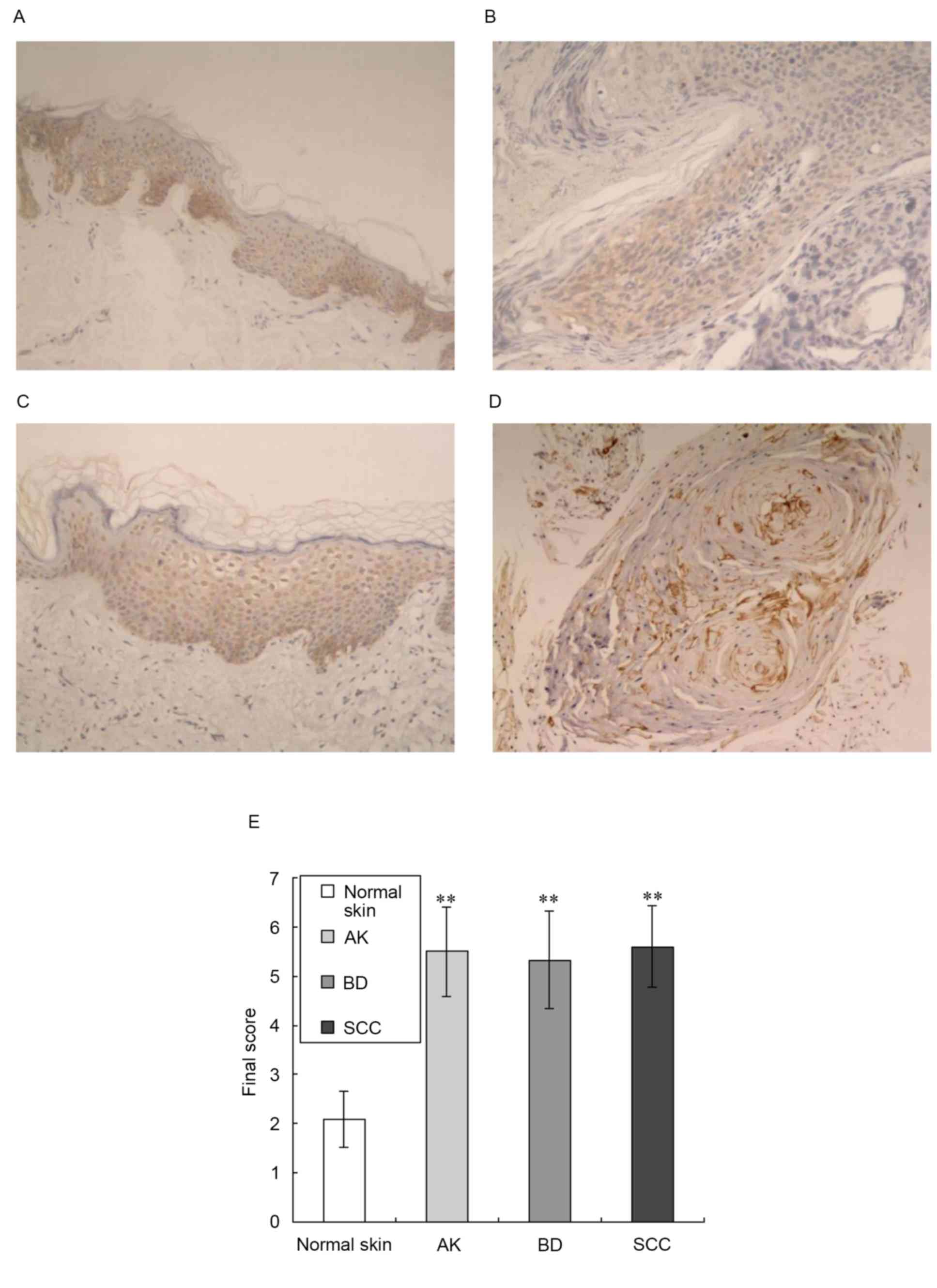
immunohistochemistry, as shown in Fig.
1. This analysis revealed low expression levels of AQP3 in
normal epidermal keratinocytes and prominent expression in the
middle and lower epidermis (Fig.
1A). In the tissue samples of AK and BD, AQP3 was moderately
expressed in the epidermis (Fig. 1B
and C). In the tissue samples of SCC, AQP3 was moderately
expressed in the horn pearls and carcinoma cells nests (Fig. 1D). This analysis also showed
prominent expression of AQP3 in the plasma membrane of
keratinocytes, consistent with the fact that AQP3 is an integral
membrane protein (6). AQP3 was
negatively expressed in the dermal tunica intima of the normal
skin, AK, BD and SCC samples.
Compared with normal skin, the expression levels of
AQP3 in the tissue samples of AK, BD and SCC were significantly
increased (z-values of 4.175, 4.369 and 4.818 respectively;
P<0.01), as shown in Fig.
1E.
Expression of PLD2 is increased in
tissue samples of AK, BD and SCC
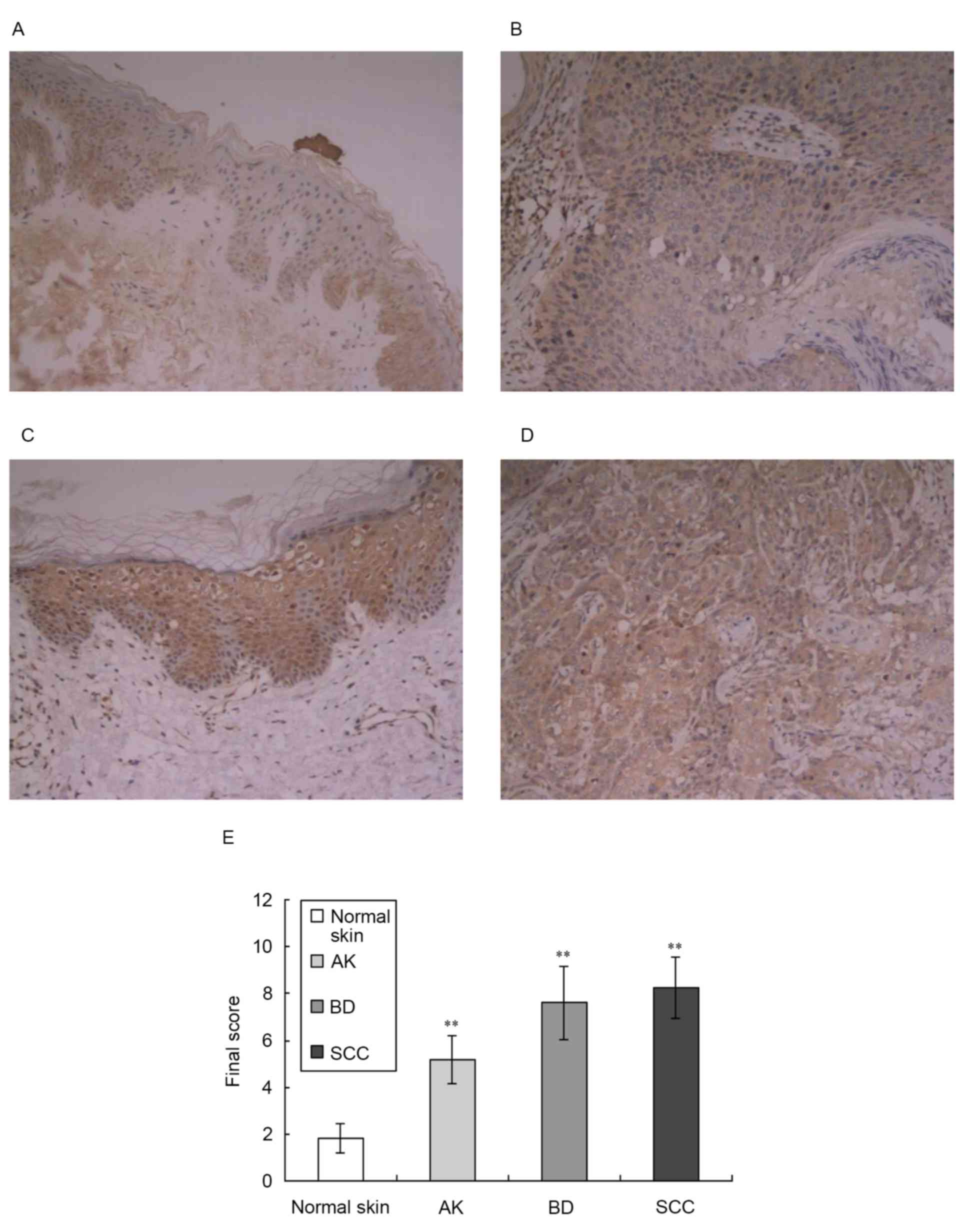
The present study also examined the expression
levels of PLD2 in tissue samples of normal skin, AK, BD and SCC
using immunohistochemistry, as shown in Fig. 2. This analysis revealed a low level
of expression of PLD2 in normal epidermal keratinocytes and
prominent expression in the middle and lower epidermis (Fig. 2A). In the tissue samples of AK,
PLD2 was moderately expressed in the epidermis (Fig. 2B). In the tissue samples of BD and
SCC, PLD2 was expressed at high levels (Fig. 2C and D). The majority of previous
investigations have reported that PLD2 localizes to the plasma
membrane (18), however, it has
also been reported to have a cytosolic distribution and localize to
the Golgi apparatus (7). The
results of the present study showed that PLD2 localized to the
cytoplasm and plasma membrane of keratinocytes.
Compared with normal skin, the expression levels of
PLD2 in the tissue samples of AK, BD and SCC were significantly
increased (z-value: 4.09, 4.31 and 4.74, respectively; P<0.01),
as shown in Fig. 2E.
Selection and identification of
siRNA
Based on the cDNA sequences of AQP3 and PLD2 in
GenBank, three sequences of AQP3 siRNA and three sequences of PLD2
siRNA were designed. The sequences used are shown in Table II. The A431 cells were transfected
with siRNA using Lipofectamine™ 2000. The inhibitory effect of each
siRNA sequence on the mRNA expression of the target gene was
detected using RT-qPCR analysis, as shown in Fig. 3. In the three AQP3 siRNA sequences,
AQP3-homo-363 had the most marked inhibitory effect on the mRNA
expression of AQP3, however, it also inhibited the mRNA expression
of PLD2. Therefore, AQP3-homo-612 was selected for use in the
following experiments. In the three PLD2 siRNA sequences,
PLD2-homo-602 had the highest inhibitory effect on the mRNA
expression of PLD2, therefore, PLD2-homo-602 was selected for use
in the following experiments.
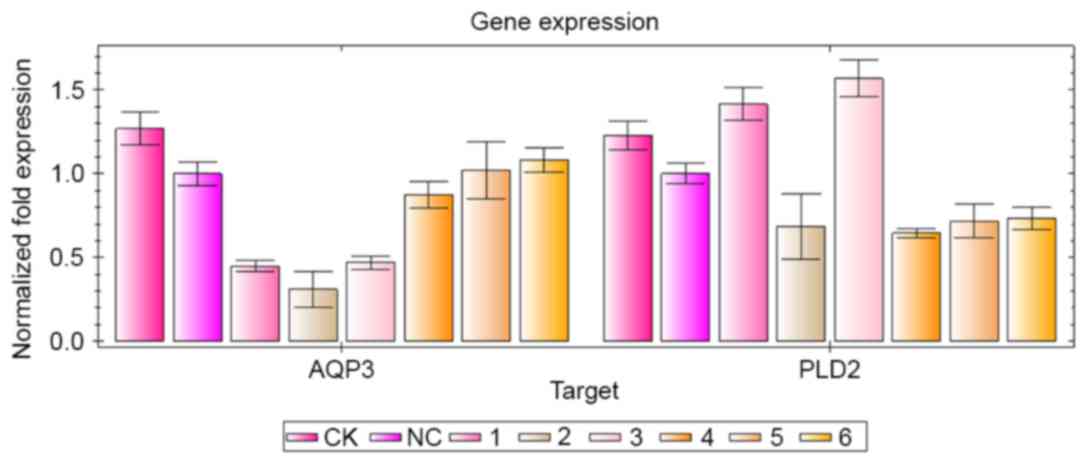 | Figure 3.Inhibitory effects of AQP3 and PLD2
siRNA sequences on the target gene. siRNA transfection and reverse
transcription-quantitative polymerase chain reaction analyses were
performed. The groups were as follows: CK group, normal cultured
A431 cells; NC group, A431 cells were transfected with NC siRNA; 1,
AQP3-homo-612 siRNA group; 2, AQP3-homo-363 siRNA group; 3,
AQP3-homo-360 group; 4, PLD2-homo-602 group; 5, PLD2-homo-1352
group; 6, PLD2-homo-5338 group. siRNA, small interfering RNA; AQP3,
aquaporin 3; PLD2, phospholipase D2; CK, control check; NC,
negative control. |
The inhibitory effect of AQP3-homo-612 siRNA on the
protein expression of AQP3, and the inhibitory effect of
PLD2-homo-602 siRNA on the protein expression of PLD2 were detected
using western blot analysis, as shown in Fig. 4A. The protein level of AQP3 was
significantly downregulated following siRNA transfection, compared
with negative control siRNA transfection (t=60.884;
P<0.001; Fig. 4B). The protein
level of PLD2 was also significantly downregulated following siRNA
transfection, compared with negative control siRNA transfection
(t=12.419; P=0.006) (Fig.
4C). No significant difference was found among the CK group,
transfection regent group and negative control group (P>0.05).
These results indicated that the repression of protein levels of
AQP3 and PLD2 occurred post-transcriptionally.
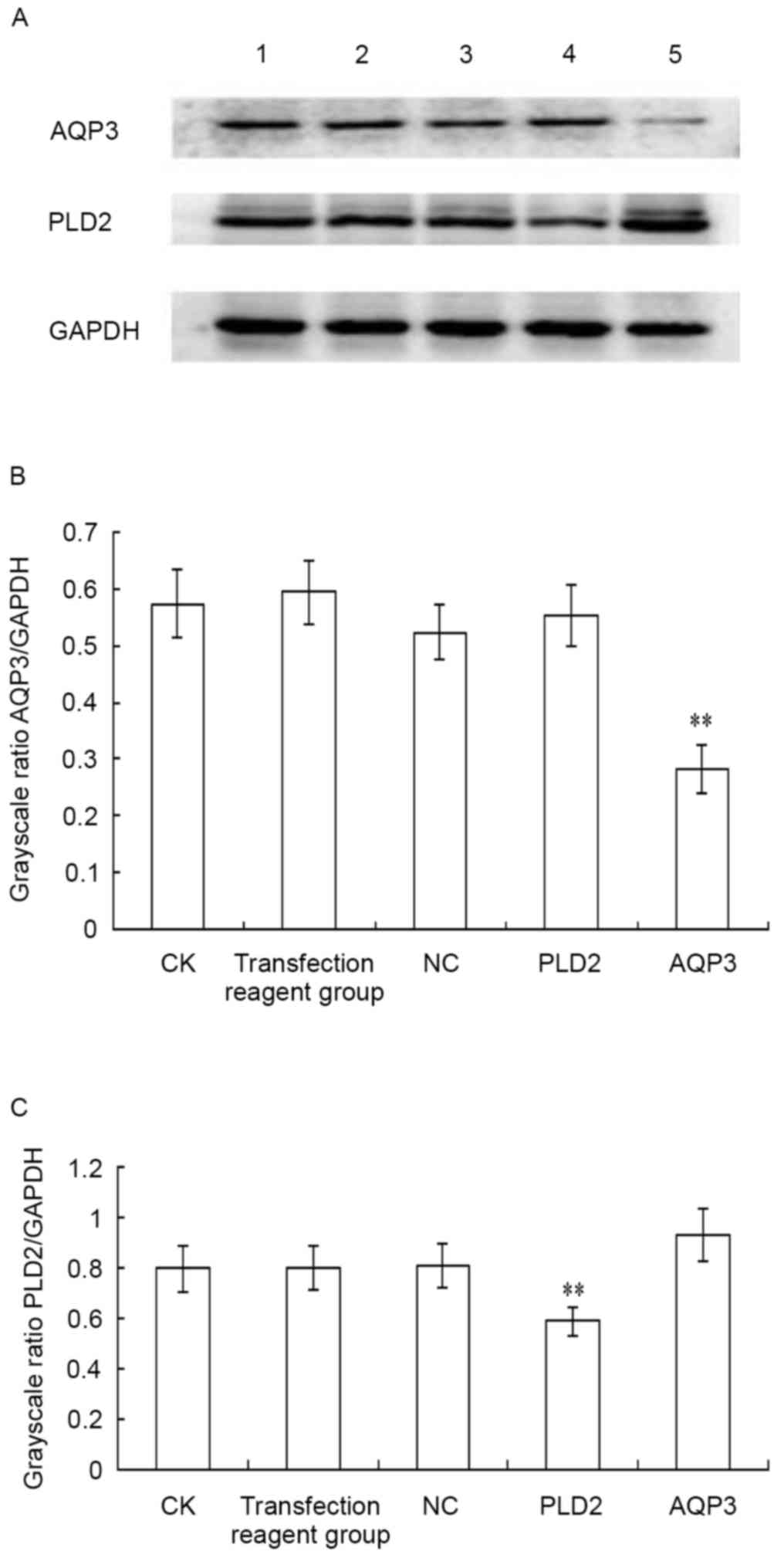 | Figure 4.Inhibitory effect of siRNA
transfection on the protein expression of AQP3 and PLD2. siRNA
transfection and western blot analysis were performed. (A) Results
of the western blot analysis; results are representative of three
independent experiments. Quantification of the protein expression
of (B) AQP3 and (C) PLD2. The statistical significance between two
groups was determined using a paired-samples t-test. **P<0.01,
compared with the NC group. Lane 1, CK group; lane 2, transfection
reagent group (transfection reagent added to the culture medium);
lane 3, NC group; lane 4, PLD2-siRNA group; lane 5, AQP3-siRNA
group. siRNA, small interfering RNA; AQP3, aquaporin 3; PLD2,
phospholipase D2; CK, control check; NC, negative control. |
AQP3 siRNA and PLD2 siRNA suppress the
proliferation of A431 cells
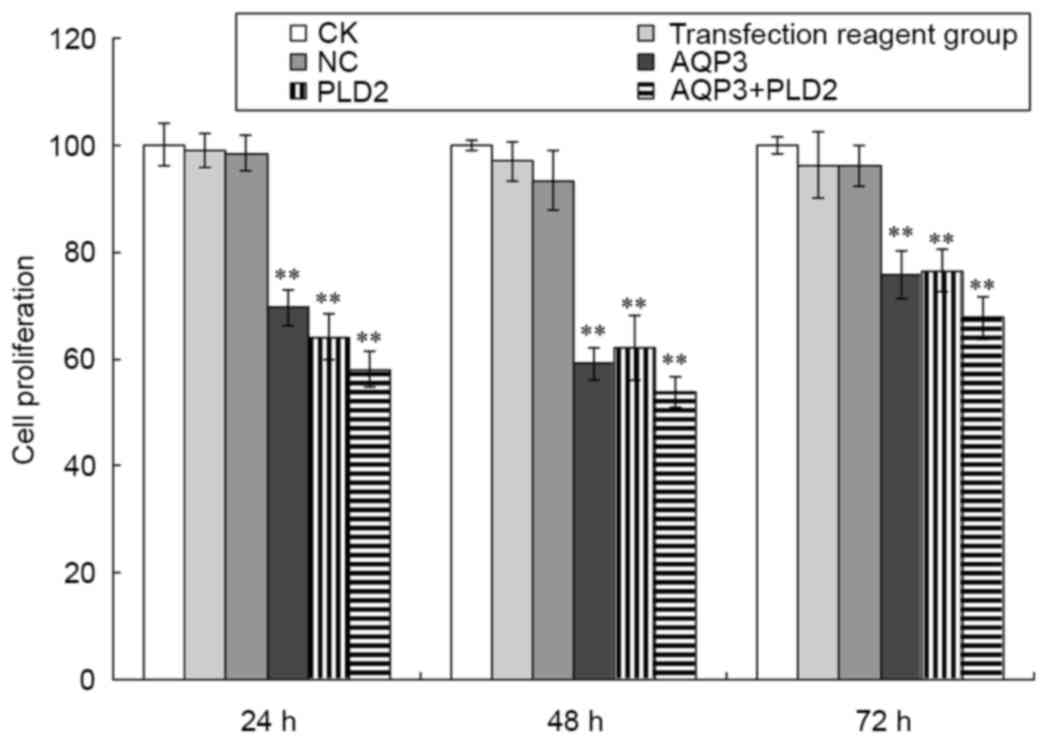
The present study also determined the proliferation
of A431 cells following AQP3 siRNA and PLD2 siRNA transfection. As
shown in Fig. 5, compared with the
negative control group, the proliferation of A431 cells following
AQP3 siRNA transfection was significantly inhibited at 24, 48 and
72 h (t=10.997, 24.103 and 9.54, respectively; P<0.01). Compared
with the negative control group, the proliferation of A431 cells
following PLD2 siRNA transfection was also significantly inhibited
at 24, 48 and 72 h (t=8.726, 30.468 and 7.017, respectively;
P<0.01). Compared with the negative control group, the
proliferation of A431 cells following AQP3 siRNA and PLD2 siRNA
transfection was also significantly inhibited at 24, 48 and 72 h
(t=12.496, 34.732 and 11.459, respectively; P<0.01). No
significant differences were found among the CK group, transfection
reagent group and negative control group (P>0.05).
AQP3 siRNA and PLD2 siRNA promote the
apoptosis of A431 cells
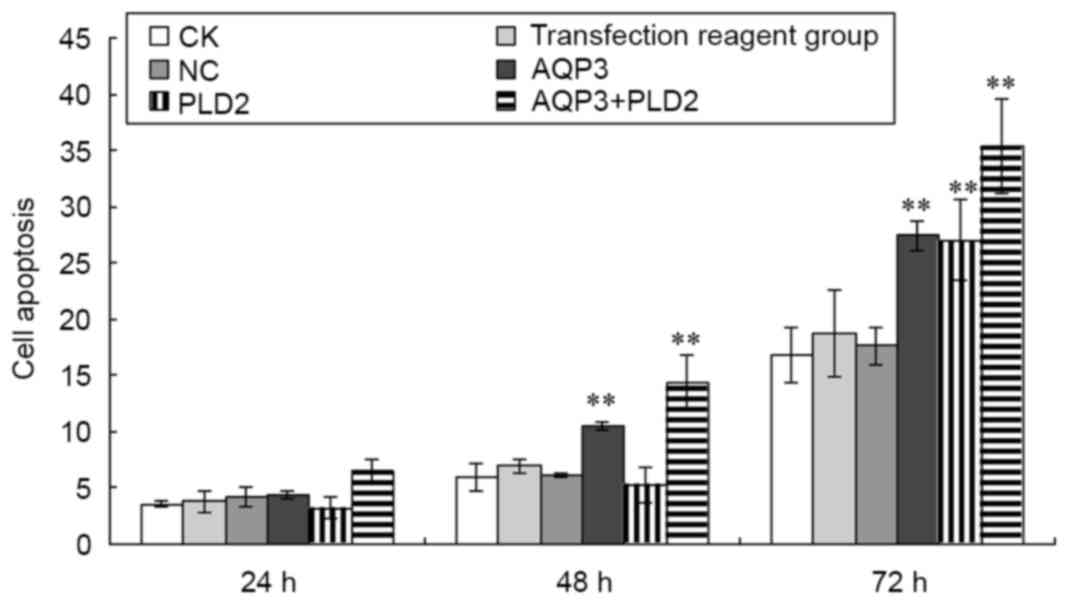
The apoptosis of A431 cells following transfection
with AQP3 siRNA and PLD2 siRNA were also determined. As shown in
Fig. 6, compared with the negative
control group, the apoptosis of A431 cells following AQP3 siRNA
transfection was significantly increased at 48 and 72 h (t=11.359
and 20.912, respectively; P<0.01). Compared with the negative
control group, the apoptosis of A431 cells following PLD2 siRNA
transfection was significantly increased at 72 h (t=19.891;
P<0.01). Compared with the negative control group, the apoptosis
of A431 cells following AQP3 siRNA and PLD2 siRNA transfection was
significantly increased at 48 and 72 h (t=15.559 and 27.692,
respectively; P<0.01). No significant differences were found
among the CK group, transfection regent group and negative control
group (P>0.05).
Discussion
Hara-Chikuma and Verkman demonstrated AQP3
immunoreactivity in human SCC (8).
It was suggested that the protein expression of AQP3 is correlated
with proliferation in SCC as the channel is co-localized with
keratin 14, a marker of basal keratinocytes (8). Verkman et al (19) also indicated that the levels of
AQP3 were increased in SCC and correlated with the
hyperproliferation observed in the disease (19). However, Voss et al (13) examined AQP3 immunoreactivity in SCC
and found that AQP3 immunoreactivity was ‘patchy’, with certain
regions of the lesion staining intensely for AQP3 and others
showing minimal or no staining. The regions showing reduced levels
of AQP3 exhibited positivity for Ki67, a marker of proliferating
cells, whereas regions of the tumor with high levels of AQP3 were
negative for Ki67 immunoreactivity (13). This suggested that proliferation is
correlated with the downregulation of AQP3 in SCC (13). In the present study, the levels of
AQP3 were determined in AK, BD and SCC tissues using
immunohistochemistry, and compared with the normal epidermis. It
was found that the expression of AQP3 was low in the normal
epidermis, whereas AQP3 was moderately expressed in AK and BD, and
moderately expressed in the horn pearls and carcinoma cells nests
of SCC. Compared with normal skin, the expression levels of AQP3 in
AK, BD and SCC tissues were significantly increased. AK is an
intra-epidermal keratinocyte-derived precancerous lesion in humans.
BD is a variant of SCC in the skin and the mucocutaneous junction
(20). The findings of the present
study suggested that increased levels of AQP3 were correlated with
proliferation in SCC, and with tumor progression from a
precancerous lesion to a cancerous lesion in situ, and then
to an invasive lesion.
The role of PLD in cancer and tumorigenesis has been
investigated in detail in the last decade. The activity of PLD has
been shown to be significantly elevated in several types of tumor
(11), suggesting the possibility
that PLD may be involved in tumorigenesis. The overexpression of
PLD1 and PLD2 has been found to induce upregulation of the activity
of matrix metalloprotease-9, induce undifferentiated sarcoma when
transplanted into nude mice and increase the fraction of cells in
the S phase. These results suggest that overexpression of PLD
isozymes may be important in neoplastic transformation (21). A431 cells express PLD1 and PLD2,
however, the regulatory mechanism through which PLD1 and PLD2 are
activated is different. Hydrogen peroxide induces the tyrosine
phosphorylation of PLD1 and PLD2, whereas epidermal growth factor
only causes the tyrosine phosphorylation of PLD2 (22). The involvement of PLD2 in cell
signaling continues to expand geometrically. It involves gene
transcription, and mitogenic and cell migration effects as observed
in normal growth, tumor development and inflammation (23). The present study also determined
the levels of PLD2 in AK, BD and SCC using immunohistochemistry. It
was found that the expression of PLD2 was low in the normal
epidermis, whereas PLD2 was moderately expressed in AK and
expressed at high levels in BD and SCC. Compared with normal skin,
the expression levels of PLD2 in the tissue samples of AK, BD and
SCC were significantly increased. These findings suggested that
increased levels of PLD2 may be correlated with tumor progression
and development in SCC.
On investigating gene function, the specific
knockdown of target genes without affecting other genes is
critically important. RNA interference mediated by siRNA and short
hairpin RNA is a specific gene-silencing technology. Based on the
AQP3 and PLD2 cDNA sequences in GenBank, three specific sequences
of AQP3 siRNA and three specific sequences of PLD2 siRNA were
constructed in the present study for transfection into the human
A431 SCC cell line. Different siRNAs had different inhibitory
effects on the mRNA expression of the target gene. The positional
effects and a variance in the secondary structure of the nucleotide
sequence at different sites are involved in the different
inhibitory effects of the siRNA targeting the same gene (24,25).
The ability to evade apoptosis is a hallmark of cancer cells,
resulting in tumor growth, metastasis, and resistance to
chemotherapy and radiotherapy. Using in vitro experiments,
the present study confirmed the anticancer effect of AQP3 siRNA and
PLD2 siRNA on SCC. The results showed that AQP3 siRNA and PLD2
siRNA affected the proliferation and apoptosis of SCC. Transfection
with AQP3 siRNA and PLD2 siRNA significantly inhibited
proliferation and promoted apoptosis of SCC cells. Several
investigations have shown that silencing PLD has a negative effect
on the migration, adhesion and invasion of cancer cells. The
concomitant downregulation of siRNA mediated by aberrant AQP3/PLD2
signaling may be important for the treatment of SCC.
The suggestion that AQP3 and PLD2 are coupled is
based on immunocytochemical co-localization and their ability to
coimmunoprecipitate (12). The
association of AQP3 and PLD2 in caveolin-rich membrane microdomains
of keratinocytes and their functional association to generate the
lipid signaling molecule PG provides a novel mechanism by which
AQP3 regulates epidermal function. AQP3-mediated glycerol transport
in skin is involved in a complex regulation of cell proliferation
and differentiation, which are central features of epidermal
homeostasis and regeneration. It is possible that AQP3 mediates
different effects depending on whether or not it associated with
PLD2 (26). In addition,
manipulation of the AQP3/PLD2 signaling module appears to inhibit
keratinocyte proliferation and trigger early differentiation
(27). The mechanism by which the
AQP3/PLD2 signaling module exerts its effects on the pathogenesis
of SCC remain to be fully elucidated, however, substantial data
supporting an involvement of this signaling module in tumor
progression and development indicate that further investigation is
warranted.
AQP3 and PLD2 are abnormally expressed and/or
localized in SCC. AQP3 transports glycerol to PLD2 for the
synthesis of lipid second messengers, which potentially mediate the
effects of the AQP3/PLD2 signaling module in regulating the
proliferation and apoptosis of SCC. PLD exhibits cross talk with a
variety of cancer regulators and also provides survival signals.
There are multiple mechanisms by which PLD-mediated survival
signals are generated in cancer cells. PLD suppresses
phosphoprotein 2A, reduces its association with E4BP and S6K, and
assists in the transformation of cells (28). PLD2 interacts with mammalian target
of rapamycin and activates it, which provides survival signals
(29). PLD stabilizes mutant p53
in a mitogen-activated protein kinase-dependent manner. In turn,
PLD-generated survival signals dependent on mutant p53 (30). PLD also acts as a survival signal
for cancer. PLD regulates hypoxia inducible factor 1α at the
translational level and promotes cancer cell proliferation
(29). Another mechanism by which
PLD promotes cancer growth is by preventing the apoptosis of cancer
cells. PLD2 promotes the survival of cancer cells by preventing
apoptosis (31). PLD2 also
enhances the expression of anti-apoptotic proteins, including
B-cell lymphoma 2 (Bcl-2) and Bcl-extra large (32). To further elucidate the effective
mechanism of AQP3 siRNA and PLD2 siRNA in the proliferation and
apoptosis of SCC, further investigation is necessary.
Taken together, the findings of the present study
suggested that increased levels of AQP3 and PLD2 may be correlated
with tumor progression and development in SCC. The results showed
that AQP3 siRNA and PLD2 siRNA affected the proliferation and
apoptosis of SCC. Transfection with AQP3 siRNA and PLD2 siRNA
significantly inhibited the proliferation and promoted the
apoptosis of SCC cells. The results also showed that AQP3 siRNA and
PLD2 siRNA significantly downregulated the mRNA and protein levels
of AQP3 and PLD2 in A431 cells, and that they inhibited
proliferation and promoted apoptosis in vitro. The AQP3/PLD2
signaling module may be involved in, or serve as surrogate markers
for the pathogenesis of SCC, and the concomitant downregulation of
siRNA mediated by aberrant AQP3/PLD2 signaling may be important for
the treatment of SCC. These findings provide novel insights for the
development of gene therapy technology to treat patients with SCC
in the future.
Acknowledgements
The authors would like to thank the staff of the
Department of Pathology, Hangzhou Hospital of Traditional Chinese
Medicine (Hangzhou, China), for their assistance with skin tissue
sample collection and immunohistochemical analysis. This study was
funded by the Natural Science Foundation of Zhejiang Province
(grant no. LY12H11010).
Glossary
Abbreviations
Abbreviations:
|
AQP3
|
aquaporin 3
|
|
PLD2
|
phospholipase D2
|
|
SCC
|
squamous cell carcinoma
|
|
AK
|
actinic keratosis
|
|
BD
|
Bowen's disease
|
|
siRNA
|
small interfering RNA
|
|
FITC/PI
|
annexin V-fluorescein
isothiocyanate/propidium iodide
|
References
|
1
|
Mobasheri A and Marples D: Expression of
the AQP-1 water channel in normal human tissues: A semiquantitative
study using tissue microarray technology. Am J Physiol Cell
Physiol. 286:C529–C537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boury-Jamot M, Sougrat R, Tailhardat M, Le
Varlet B, Bonté F, Dumas M and Verbavatz JM: Expression and
function of aquaporins in human skin: Is aquaporin-3 just a
glycerol transporter? Biochim Biophys Acta. 1758:1034–1042. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song Y, Sonawane N and Verkman AS:
Localization of aquaporin-5 in sweat glands and functional analysis
using knockout mice. J Physiol. 541:561–568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hara-Chikuma M, Sohara E, Rai T, Ikawa M,
Okabe M, Sasaki S, Uchida S and Verkman AS: Progressive adipocyte
hypertrophy in aquaporin-7-deficient mice: Adipocyte glycerol
permeability as a novel regulator of fat accumulation. J Biol Chem.
280:15493–15496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boury-Jamot M, Daraspe J, Bonté F, Perrier
E, Schnebert S, Dumas M and Verbavatz JM: Skin aquaporins: Function
in hydration, wound healing, and skin epidermis homeostasis. Handb
Exp Pharmacol. 190:205–217. 2009. View Article : Google Scholar
|
|
6
|
Sougrat R, Morand M, Gondran C, Barré P,
Gobin R, Bonté F, Dumas M and Verbavatz JM: Functional expression
of AQP3 in human skin epidermis and reconstructed epidermis. J
Invest Dermatol. 118:678–685. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugiyama Y, Ota Y, Hara M and Inoue S:
Osmotic stress up-regulates aquaporin-3 gene expression in cultured
human keratinocytes. Biochim Biophys Acta. 1522:82–88. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hara-Chikuma M and Verkman AS: Prevention
of skin tumorigenesis and impairment of epidermal cell
proliferation by epidermal cell proliferation by targeted
aquaporin-3 gene disruption. Mol Cell Biol. 28:326–332. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liscovitch M, Czarny M, Fiucci G and Tang
X: Phospholipase D: Molecular and cell biology of a novel gene
family. Biochem J. 3:401–415. 2000. View Article : Google Scholar
|
|
10
|
Liscovitch M, Chalifa V, Pertile P, Chen
CS and Cantley LC: Novel function of phosphatidylinositol
4,5-bisphosphate as a cofactor for brain membrane phospholipase D.
J Biol Chem. 269:21403–21406. 1994.PubMed/NCBI
|
|
11
|
Gomez-Cambronero J: Phosphatidic acid,
phospholipase D and tumorigenesis. Adv Biol Regul. 54:197–206.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng X and Bollag W Bollinger: Aquaporin
3 colocates with phospholipase d2 in caveolin-rich membrane
microdomains and is downregulated upon keratinocyte
differentiation. J Invest Dermatol. 121:1487–1495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Voss KE, Bollag RJ, Fussell N, By C,
Sheehan DJ and Bollag WB: Abnormal aquaporin-3 protein expression
in hyperproliferative skin disorders. Arch Dermatol Res.
303:591–600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ozdemir E, Kakehi Y, Okuno H and Yoshida
O: Role of matrix metalloproteinase-9 in the basement membrane
destruction of superficialurothelial carcinomas. J Urol.
161:1359–1363. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang XY, Tao CJ, Wu QY and Yuan CD:
Protein extract of ultraviolet-irradiated human skin keratinocytes
promote the expression of mitogen-activated protein kinases,
nuclear factor-κB and interferon regulatory factor-3 in Langerhans
cells via Toll-like receptor 2 and 4. Photodermatol Photoimmunol
Photomed. 29:41–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Bi Z, Chu W and Wan Y: IL-1
receptor antagonist attenuates MAP kinase/AP-1 activation and MMP1
expression in UVA-irradiated human fibroblasts induced by culture
medium from UVB-irradiated human skin keratinocytes. Int J Mol Med.
16:1117–1124. 2005.PubMed/NCBI
|
|
18
|
Du G, Huang P, Liang BT and Frohman MA:
Phospholipase D2 localizes to the plasma membrane and regulates
angiotensin II receptor endocytosis. Mol Biol Cell. 15:1024–1030.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verkman AS: A cautionary note on cosmetics
containing ingredients that increase aquaporin-3 expression. Exp
Dermatol. 17:871–872. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Majores M and Bierhoff E: Actinic
keratosis, Bowen's disease, keratoacanthoma and squamous cell
carcinoma of the skin. Pathologe. 36:16–29. 2015.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Min DS, Kwon TK, Park WS, Chang JS, Park
SK, Ahn BH, Ryoo ZY, Lee YH, Lee YS, Rhie DJ, et al: Neoplastic
transformation and tumorigenesis associated with overexpression of
phospholipase D isozymes in cultured murine fibroblasts.
Carcinogenesis. 22:1641–1647. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Min DS, Ahn BH and Jo YH: Differential
tyrosine phosphorylation of phospholipase D isozymes by hydrogen
peroxide and the epidermal growth factor in A431 epidermoid
carcinoma cells. Mol Cells. 11:369–378. 2001.PubMed/NCBI
|
|
23
|
Gomez-Cambronero J: New concepts in
phospholipase D signaling in inflammation and cancer.
ScientificWorldJournal. 10:1356–1369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holen T, Amarzguioui M, Wiiger MT, Babaie
E and Prydz H: Positional effects of short interfering RNAs
targeting the human coagulation trigger tissue factor. Nucleic
Acids Res. 30:1757–1766. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elbashir SM, Lendeckel W and Tuschl T: RNA
interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev.
15:188–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin H, Zheng X, Zhong X, Shetty AK, Elias
PM and Bollag WB: Aquaporin-3 in keratinocytes and skin: Its role
and interaction with phospholipase D2. Arch Biochem Biophys.
508:138–143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bollag WB, Xie D, Zhong X and Zheng X: A
potential role for the phospholipase D2-aquaporin-3 signaling
module in early keratinocyte differentiation: Production of a novel
phosphatidylglycerol lipid signal. J Invest Dermatol.
127:2823–2831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hui L, Rodrik V, Pielak RM, Knirr S, Zheng
Y and Foster DA: mTOR-dependent suppression of protein phosphatase
2A is critical for phospholipase D survival signals in human breast
cancer cells. J Biol Chem. 280:35829–35835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toschi A, Edelstein J, Rockwell P, Ohh M
and Foster DA: HIF alpha expression in VHL-defficient renal cancer
cells is dependent on phospholipase D. Oncogene. 27:2746–2753.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hui L, Zheng Y, Yan Y, Bargonetti J and
Foster DA: Mutant p53 in MDA-MB-231 breast cancer cells is
stabilized by elevated phospholipase D activity and contributes to
survival signals generated by phospholipase D. Oncogene.
25:7305–7310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho JH, Hong SK, Kim EY, Park SY, Park CH,
Kim JM, Kwon OJ, Kwon SJ, Lee KS and Han JS: Overexpression of
phospholipase D suppresses taxotere-induced cell death in stomach
cancer cells. Biochim Biophys Acta. 1783:912–923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oh KJ, Lee SC, Choi HJ, Oh DY, Kim SC, Min
do S, Kim JM, Lee KS and Han JS: Role of phospholipase D2 in
anti-apoptotic signaling through increased expressions of Bcl-2 and
Bcl-xL. J Cell Biochem. 101:1409–1422. 2007. View Article : Google Scholar : PubMed/NCBI
|