Introduction
Pediatric stroke is a rare but serious event with
high rates of mortality and morbidity, is among the top 10 causes
of mortality and is as common as brain tumors in children (1,2). The
incidence of childhood stroke is estimated at 2.10–13.02 per
100,000 individuals and intracerebral hemorrhage (ICH) accounts for
almost 50% of all stroke cases (3–6). By
contrast, ICH in adults only comprises ~10–30% of cases (7). Pediatric ICH has a mortality rate of
~25% and leads to significant disability in 42% of those who
survive (8), which results in a
significant burden on families and society.
Small intestinal motility (SIM) disorder presents
primarily with early satiety, bloating, nausea, vomiting and loss
of appetite (9). The motility
disorder may result in the abnormal propulsion of intestinal
content and nutrient malabsorption, leading to impaired intestinal
mucosa and barrier function, deficient immunity, translocation of
intestinal bacteria and endotoxins, and even systemic inflammatory
response syndrome and multiple organ dysfunction syndrome (10,11).
Notably, small intestinal dysmotility is considered to be a common
complication following several brain events, including traumatic
brain injury (TBI) (11–13) and ischemic stroke (14). ICH is a serious brain disease, the
pathophysiological common features of which are in accordance with
the other brain events mentioned above. However, to date, no study
has investigated the complication of SIM following ICH. The present
study hypothesized that ICH may also induce significant small
intestinal dysmotility as occurs in ischemic stroke and TBI. This
is important in relation to the period of growth and development of
anatomy and biomechanics in children, as small intestinal
dysmotility not only affects the prognosis of the disease itself,
but may also affect the future growth, nutritional status and
mental development of the child.
Ghrelin is a 28-amino acid peptide, which is
primarily secreted from the stomach, and is distributed in the
gastrointestinal (GI) tract and the central nervous system
(15,16). Ghrelin has also been found in other
tissues, including the pancreas, lungs, kidney, ovaries, gonads,
adrenal glands, myocardium, adipose tissue and placenta of humans
and rodents (17–20). In various pathological and
physiological conditions, ghrelin is secreted into the circulatory
system. Ghrelin has several physiological functions, including
stimulating food intake (21),
modulating systemic metabolism (16), regulating the secretion of gastric
acid (22), affecting sleep
(23), inducing cell proliferation
(24) and reducing the
thermogenesis of brown adipose tissue (25). There has been increasing interest
regarding its potent effects on gastric emptying and GI motility. A
number of studies have shown that the peptide is vital in
protecting against GI dysmotility in various diseases, including
multiple system atrophy (26),
systemic sclerosis (27), stress
(28), shock (29), diabetes (30) and brain events (11–14).
However, the protective role and underlying mechanisms of ghrelin
in ICH-induced small intestinal dysmotility remain to be
elucidated, which has limited systemic treatment paradigms for
pediatric ICH.
In the present study, the primary aim was to
determine the association between serum levels of ghrelin and SIM
disorder post-ICH in mice. The second objective was to evaluate the
potential effect of exogenous ghrelin administration on ICH-induced
SIM disorder. It was shown that ghrelin administration enhanced SIM
by downregulating the expression of inducible nitric oxide synthase
(iNOS)/nitric oxide (NO) via the cholinergic pathway following
pediatric ICH in mice.
Materials and methods
Animals
Male C57BL/6 mice (4–6 weeks old) were purchased
from the Experimental Animal Center of the Chinese Academy of
Sciences (Shanghai, China). All animal protocols were approved by
the Laboratory Animal Ethics Committee of the First People's
Hospital of Nantong (Nantong, China; no. SYYLS2016009). Animal
experiments were performed in accordance with the rules of the US
National Institutes of Health Guidelines (31). All mice were housed at a constant
temperature (18–22°C) and humidity in a 12 h light/dark cycle under
specific pathogen-free conditions. Sterilized food and water were
provided ad libitum. All efforts were made to minimize
suffering and to reduce the number of mice included.
ICH model
The animal model was induced by injecting autologous
blood in two stages, in a stereotactic manner, as described
previously with minor modifications (32). The mice were anesthetized with a
single intraperitoneal injection of ketamine (100 mg/kg) and
xylazine (10 mg/kg) (Sigma, Merck Millipore, Darmstadt, Germany).
The mice were placed in a stereotactic frame (RWD Life Science,
Shenzhen, China) and subjected to ICH via autologous blood
infusion. A 1 mm burr hole was drilled 2.3 mm lateral to the
midline and 0.2 mm anterior to the bregma. The autologous blood (25
µl) was collected using a Hamilton syringe from a tail cut. A
needle was advanced 3.0 mm into the right striatum. The autologous
blood (25 µl) was then injected using a microinfusion pump (World
Precision Instruments, Sarasota, FL, USA) via a double injection
technique. An initial volume of 5 µl was injected at a rate of 1.5
µl/min. Following a 10 min injection-free interval, the remaining
autologous blood (20 µl) was delivered at the same rate. To
minimize blood backflow, the needle was left in place for another
10 min. Following withdrawal of the needle, the scalp was sutured.
Sham control rats had the needle inserted only. Following surgery,
the animals were returned back into their cage.
Drugs and chemicals
Ghrelin (Tocris Bioscience, Ellisville, MI, USA) was
dissolved in physiological saline and injected intraperitoneally at
a dose of 50, 100 or 200 µg/kg prior to charcoal meal induction.
Atropine (1 mg/kg), phentolamine (30 mg/kg), propranolol (20
mg/kg), all from The Seventh Pharmaceutical Co, Ltd. (Wuxi, China),
L-NAME (50 mg/kg) and L-arginine (300 mg/kg), from Sigma-Aldrich;
Merck Millipore, were freshly prepared in 0.9% saline on the day of
the experiment and pretreated intraperitoneally together with
ghrelin.
Three experiments were performed to determine the
association between serum levels of ghrelin and SIM, the effect of
the exogenous ghrelin administration on SIM disorder, and its
underlying mechanisms post-ICH. In the first experiment, the
animals were randomly divided into six groups (n=6/group): Sham
group and ICH groups at 3, 6, 12, 24, and 72 h. In the second
experiment, the animals were randomly divided into seven groups
(n=9/group): Sham; ICH; ICH + ghrelin (50 µg/kg); ICH + ghrelin
(100 µg/kg); ICH + ghrelin (200 µg/kg); ICH+ghrelin + L-NAME; and
ICH + ghrelin +L-arginine. In the third experiment, the animals
were divided into four groups (n=6/group): ICH + ghrelin; ICH +
ghrelin + atropine; ICH + ghrelin + phentolamine; and ICH + ghrelin
+ propranolol.
Neurobehavioral deficits
Neurobehavioral deficits in the mice were examined
by investigators in a blinded-manner. The Rotarod test was
performed to evaluate motor coordination. Prior to ICH induction,
the mice were trained three times per day for 3 days; each trial
lasted for 5 min with a 2 h interval between each trial at a slow
speed of 4 rpm. The speed was gradually increased to 40 rpm when
the animals were habituated. Following ICH induction, the animals
were tested three times per day on days 1, 3, 5 and 7, and the
average latency to falling was recorded.
The forelimb placement test was performed to
evaluate the reflexive motor ability of the contralateral forelimb.
The investigator stimulated the respective vibrissae to elicit
excitation once per trial for 10 trials. The percentages of
successful left paw placing responses were determined for the
impaired forelimb.
For the corner turn test, the animals were allowed
to advance into a 30° corner and turn either left or right to exit.
The test was repeated 10–15 times, and the percentage of turning
direction was calculated as follows: Number of left turns/total
turns ×100.
Histopathology
Segments of ileum were harvested from the mice in
each group (3 h, 12 h and 3 days in the sham surgery and post-ICH
groups) and fixed in 10% neutral formalin for 24 h. The samples
were embedded in paraffin and cut into 4 µm sections, which were
then mounted on glass slides. The sections were stained with
hematoxylin and eosin (H&E). The histopathological changes in
the samples were observed in a blinded-manner using an inverted
microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Enzyme-linked immunosorbent assay
(ELISA)
The serum levels of ghrelin and NO were quantified
at each time point using an ELISA kit (R&D systems,
Minneapolis, MN, USA) according to the manufacturer's protocol. The
absorbance at 450 nm was recorded and a standard curve was
established to calculate the protein concentration.
SIM
The mice were fasted for 24 h prior to the
experiment. A 0.1 ml volume of charcoal meal (5% activated charcoal
suspended in 10% aqueous gum arabic) was injected into the stomach
of each mouse via a gastric tube. After 30 min, the mice were
sacrificed at each time point. Following laparotomy, the entire
small intestine was harvested. The distance traveled by the
charcoal of from the pylorus to the furthest point was recorded.
The ratio of small intestine impelling force was measured using the
following formula: Ratio of intestinal impelling force (%)=distance
traveled by the charcoal/total length of the small intestine
×100.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from the tissues of the small intestine
was extracted using TRIzol reagent (Invitrogen, Watltham, MA, USA)
according to the manufacturer's protocol. Spectrophotometric
analysis (OD260/280) was used to determine the quantity
of RNA. Reverse transcription of the RNA into cDNA was performed
using a Prime Script RT reagent kit (Takara, Otsu, Japan). Primers
(Table I), which were synthesized
commercially by Sangon Biotech Co. Ltd. (Shanghai, China), were
designed to amplify the target genes. RT-qPCR was performed
according to the manufacturer's protocol using an ABI 7500 PCR
instrument with SYBR Green (Takara Bio, Inc.). Briefly, the
reactions were performed using 1 µl cDNA, 0.4 µl PCR forward
primer, and 0.4 µl reverse primer in a 10 µl total reaction volume.
Cycling condition were as follows: An initial predenaturation step
at 95°C for 30 sec, followed by 40 cycles of denaturation at 95°C
for 5 sec and annealing at 60°C for 30 sec (33). Data were determined using the
2−∆∆Cq (34) method
with SDS software version 2.4.1 (Applied Biosystems, Thermo Fisher
Scientific, Inc.) and β-actin was quantified as an endogenous
control.
 | Table I.Polymerase chain reaction primer
sequences. |
Table I.
Polymerase chain reaction primer
sequences.
| Target gene | Sense primer
(5′-3′) | Antisense primer
(5′-3′) |
|---|
| iNOS |
CCTCCTCCACCCTACCAAGT |
CACCCAAAGTGCTTCAGTCA |
| nNOS |
GCTTCAGGAATATGAGGAATGG |
TGATGGAATAGTAGCGAGGTTGT |
| eNOS |
TGTCTGCGGCGATGTCACT |
CATGCCGCCCTCTGTTG |
| β-actin |
GTGACGTTGACATCCGTAAAGA |
GCCGGACTCATCGTACTCC |
Western blot analysis
Total protein from the small intestine tissues was
extracted with a mixture of RIPA buffer (EMD Millipore, Bedford,
MA, USA). Following centrifugation at 12,000 × g for 10 min at 4°C,
the supernatant was collected. Protein concentrations were measured
using a bicinchoninic acid assay (Thermo Fisher Scientific, Inc.).
The samples were diluted in protein loading buffer (Sunshine
Biotechnology, Nanjing, China) and boiled for 5 min at 95°C.
Subsequently, 30 µg total protein was separated on a 10% sodium
dodecyl sulfate-polyacrylamide gel by electrophoresis. The proteins
were then electrotransferred onto a 0.45 µm PVDF membrane (EMD
Millipore). The membranes were blocked for 1 h in 5% skim milk and
probed with primary antibodies from Abcam (Cambridge, UK) at the
following dilutions: iNOS (1:50 dilution, catalog no. ab3523), nNOS
(1:2,000 dilution, catalog no. ab76067), eNOS (1:1,000 dilution,
catalog no. ab95254), and β-actin (1:10,000 dilution, catalog no.
ab3280) at 4°C overnight, respectively. The blots were then
incubated with goat anti-rabbit (catalog no. HA1001-100) or goat
anti-mouse (catalog no. HA1006) HRP-conjugated secondary antibody
(1:5,000 dilution; HuaAn Biotechnology, Hangzhou, Zhejiang, China)
for 1 h at room temperature. The immunoreactive bands were detected
using ECL chemiluminescence reagent (Pierce, Rockford, IL, USA) and
quantified using Quantity One software version 4.6.2 (Bio-Rad,
Hercules, CA, USA).
Statistical analysis. The statistical analysis was
performed using SPSS 16.0 (SPSS, Chicago, IL, USA). Differences
between groups were analyzed using one-way analysis of variance
followed by Student-Newman-Keuls test. Correlation was assessed
using Pearson's correlation coefficient. Quantitative data are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Changes in neurological behavior
To evaluate establishment of the ICH model induced
by intracerebral infusion of autologous blood, neurological
behavior was observed and recorded. No neurobehavioral deficits
were observed in the sham group, whereas the mice subjected to
intracerebral blood infusion had significant neuroethological
abnormalities within 7 days following surgery. As shown in Fig. 1A, a significant reduction in
retention time on the Rotarod was detected on days 1–7 post-ICH.
Following ICH, the retention times on the Rotarod decreased by
~50.92, 57.03, 49.82 and 47.59% on days 1, 3, 5 and 7, respectively
(all P<0.01). In the forelimb-placing test, the blood-infused
mice presented with marked forelimb placing deficits, compared with
the sham mice, at each time point (Fig. 1B). Following ICH, the
forelimb-placing score was decreased by ~73.61, 78.49, 75.00 and
69.56% on days 1, 3, 5 and 7, respectively (all P<0.01).
Compared with the sham controls, the corner turn deficits were
significantly higher on days 1, 3, 5, and 7 post-ICH (Fig. 1C). The corner turn score was
increased by ~60.60, 64.45, 54.30 and 48.68% on days 1, 3, 5 and 7
(all P<0.01) post-ICH. Together, these results indicated that
the ICH model using the intracerebral autologous blood infusion
were established successfully, and the experimental ICH model was
suitable for use in the subsequent experiments aimed for
determining the role of ghrelin on SIM disorder post-ICH in
vivo.
Changes in intestinal mucosa
tissues
To determine the intestinal mucosal injury post-ICH,
sections of ileum from sham group and ICH groups at 3, 12 and 72 h
were stained using H&E staining. Visually, the sections showed
approximately intact mucosa in the sham group (Fig. 2A). By contrast, mucosal damage
occurred as early as 3 h post-ICH, shown as mild lifting of
epithelial cells from the top of villi, and shortened and thickened
villi (Fig. 2B). In addition,
severe lifting of epithelial cells, epithelial disorganization,
fusion of adjacent villi, inflammatory cell infiltration, and naked
lamina propria were observed at 12 h (Fig. 2C) and the changes were maximal at
72 h (Fig. 2D).
Correlations between serum levels of
ghrelin and SIM
To determine the changes in serum levels of ghrelin
following ICH, ELISA was performed. As shown in Fig. 3A, the serum levels of ghrelin were
significantly elevated between 3 and 72 h post-ICH (6.60±0.81,
6.99±0.77, 8.63±0.92, 8.00±0.74 and 6.68±0.72 ng/ml post-ICH, vs.
5.48±0.77 ng/ml in the sham group, respectively; P<0.05). The
serum level of ghrelin increased as early as 3 h, peaked at 12 h,
and remained at a significantly higher level until 72 h.
To examine the changes in SIM post-ICH, charcoal
meal staining was performed at each time point. Compared with the
sham controls, SIM was decreased between 3 and 72 h post-ICH
(56.82±2.51, 42.80±3.93, 34.61±4.10, 38.64±2.01 and 45.63±3.64%,
vs. 75.83±2.46% in the sham group, respectively; Fig. 3B; P<0.01). Consistently, small
intestine dysmotility peaked at 12 h post-ICH. Based on these
results, exogenous ghrelin was administered for 12 h following ICH
for the subsequent experiments aimed at determining the effect of
ghrelin on SIM.
The correlations between serum levels of ghrelin and
SIM were analyzed using Pearson's correlation coefficients. There
was a significant negative correlation between the serum level of
ghrelin and SIM (r=−0.783; P<0.01; Fig. 3C).
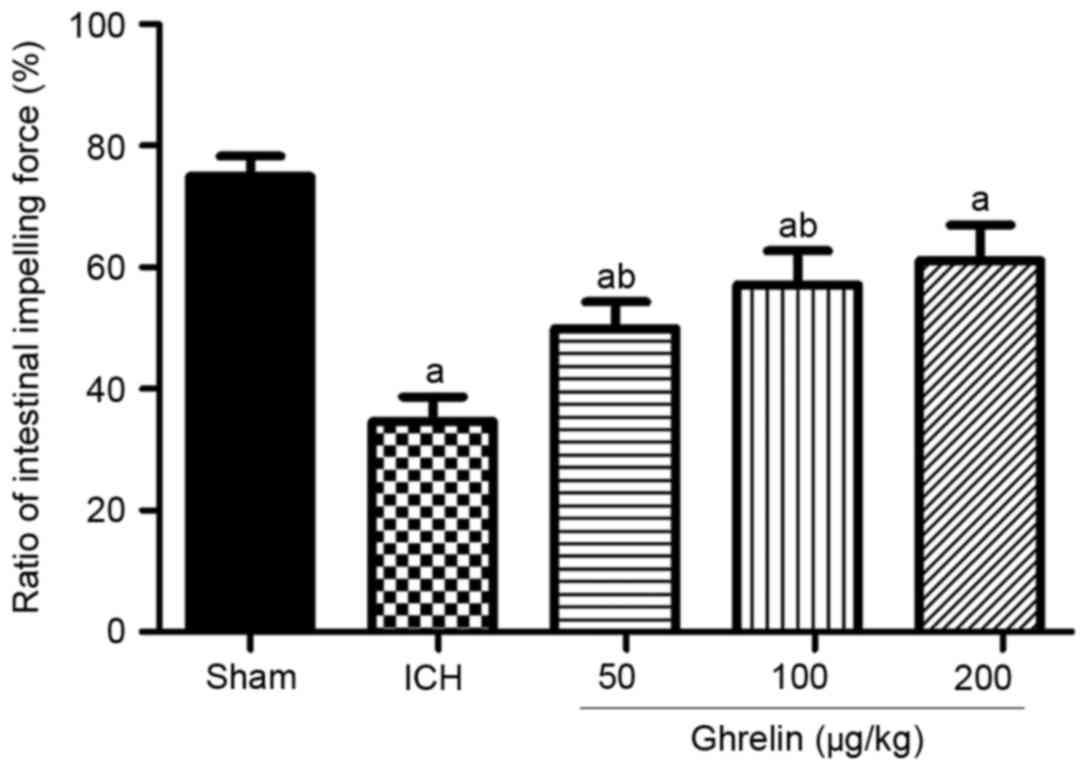
Effect of ghrelin on SIM
To investigate the effect of ghrelin on SIM in
vivo, the ratio of small intestinal impelling force was
measured at 12 h post-ICH. As shown in Fig. 4, SIM was significantly increased in
a dose-dependent manner by a single injection of ghrelin of 50, 100
and 200 µg/kg (49.95±3.27, 57.11±5.61 and 61.11±5.84%, vs.
34.61±4.10% in the vehicle, respectively) following the induction
of ICH (P<0.01). However, no significant difference was found
between 200 µg/kg ghrelin treatment and 100 µg/kg ghrelin treatment
(P>0.05). Therefore, a dose of 100 µg/kg ghrelin was used for
administration in the subsequent experiments to determine its
underlying mechanisms.
Effect of ghrelin on the iNOS/NO
pathway
To determine the NO levels following ghrelin
administration, ELISA was performed. Compared with the sham group,
the vehicle-treated group exhibited higher NO levels at 12 h
post-ICH (52.19±5.25, vs. 99.59±6.84 µmol/l; P<0.01). However,
ghrelin administration markedly reduced the ICH-induced levels of
NO (78.79±6.51, vs. 99.59±6.84 µmol/l; P<0.01; Fig. 5A).
 | Figure 5.Effect of ghrelin on the expression
of NOS/NO. (A) Serum levels of NO were determined at 12 h in the
sham, ICH, and ICH + ghrelin groups. (B) Reverse
transcription-quantitative polymerase chain reaction and (C)
western blot analyses were performed to detect the mRNA and protein
expression levels of the three isoforms of NOS (iNOS, nNOS and
eNOS) in the sham group and ICH groups + /-ghrelin at 12 h,
respectively. Values are presented as the mean ± standard deviation
(n=6). aP<0.01, vs. sham group;
bP<0.05, vs. former group. Data are representative of
three independent experiments. ICH, intracerebral hemorrhage; NO,
nitric oxide; iNOS, inducible nitric oxide synthase; nNOS, neuronal
nitric oxide synthase; eNOS, endothelial nitric oxide synthase. |
To further determine whether the ghrelin-induced
reduction of NO was NOS-dependent or not, the expression levels of
iNOS, nNOS and eNOS were examined using RT-qPCR and western blot
analyses. At the mRNA level, the expression levels of iNOS, nNOS
and eNOS were significantly increased in the vehicle-treated ICH
group, compared with those in the sham group (2.62±0.26, vs.
0.41±0.17; 1.06±0.13, vs. 0.21±0.10; and 2.28±0.25, vs. 0.72±0.13,
respectively; P<0.01; Fig. 5B).
Ghrelin (100 mg/kg) significantly reduced the mRNA expression of
iNOS, compared with the ICH group (1.71±0.37, vs. 2.62±0.26;
P<0.01); however, it did not significantly alter the mRNA
expression of nNOS (0.92±0.17, vs. 1.06±0.13; P>0.05) or eNOS
(2.67±0.36, vs. 2.28±0.25; P>0.05). At the protein level, a
similar effect was observed (Fig.
5C). Compared with the sham group, ICH induced a significant
increase in the protein expression levels of iNOS, nNOS and eNOS
(1.90±0.21, vs. 1.00±0.17; 1.50±0.16, vs. 1.00±0.16; and 1.83±0.21,
vs. 1.00±0.15, respectively; P<0.01). Similarly, compared with
the ICH group, the protein expression of iNOS (1.41±0.22, vs.
1.90±0.21; P<0.01), but not of nNOS (1.34±0.16, vs. 1.50±0.16;
P>0.05) or eNOS (1.92±0.16, vs. 1.83±0.21; P>0.05), was
downregulated by ghrelin administration.
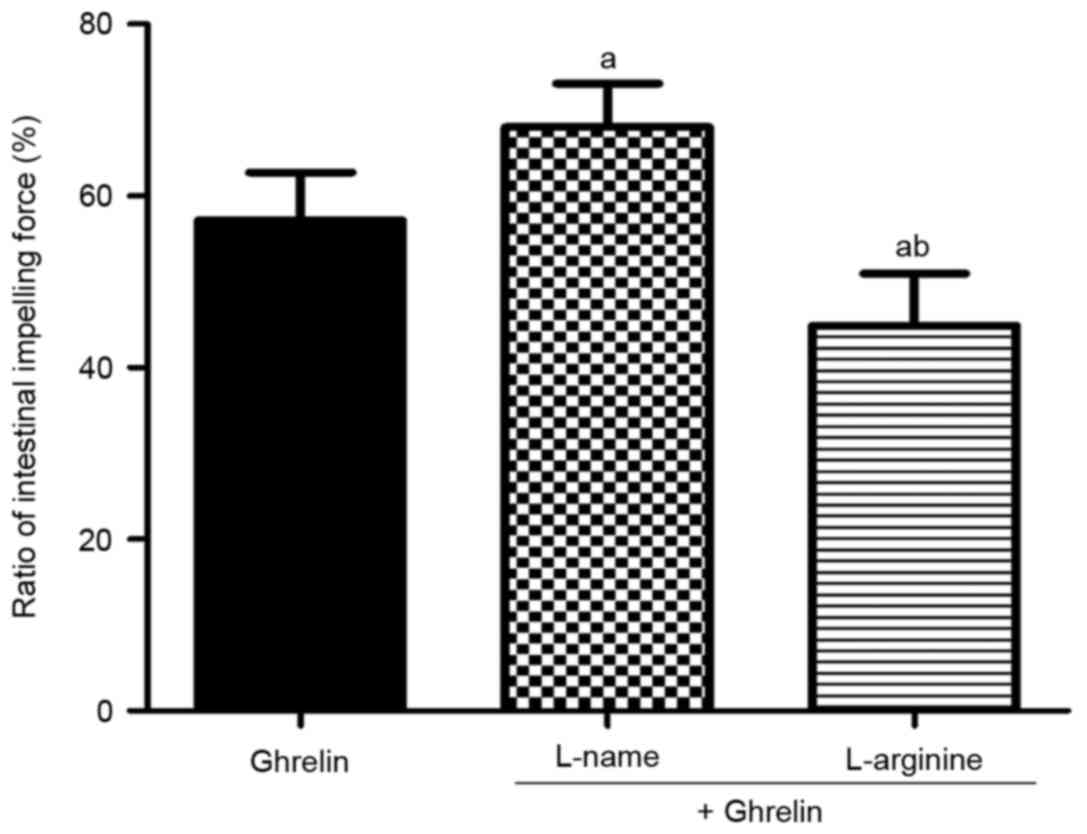
To further confirm the protective effect of ghrelin
on SIM via the iNOS/NO pathway, L-NAME and L-argnine were used. As
shown in Fig. 6, co-treatment with
L-NAME, a nonselective NOS inhibitor, markedly enhanced SIM,
compared with treatment with ghrelin alone at 12 h post-ICH
(67.99±5.09, vs. 57.11±5.61%; P<0.01). By contrast, co-treatment
with L-argnine, a precursor of NOS, weakened SIM in comparison with
ghrelin treatment alone at 12 h post-ICH (44.86±6.11, vs.
57.11±5.61%; P<0.01).
Effect of ghrelin on SIM via the
cholinergic excitatory pathway
To further determine whether the ghrelin-mediated
reduction of iNOS/NO was cholinergic pathway-dependent or not,
M-cholinergic and adrenergic receptor antagonists were
administered. As shown in Fig. 7,
atropine, an antagonist of M-cholinergic receptor, significantly
reversed the effect of ghrelin on SIM (40.61±4.9, vs. 57.11±5.61%;
P<0.01). By contrast, no significant inhibitory effects were
observed on the excitatory effect of ghrelin by the adrenergic
receptor antagonists phentolamine or propranolol (60.61±4.95, vs.
57.11±5.61 and 57.28±5.03, vs. 57.11±5.61%, respectively;
P>0.05), compared with the control group.
Discussion
SIM disorder is a common complication following
pediatric ICH. However, it has not been investigated extensively
and the pathophysiological changes have not been discussed or
evaluated extensively in the past. Of note, small intestinal
dysmotility may not only affect the prognosis of pediatric ICH
itself, but it may affect the child's future mental and physical
development. In the present study, the primary findings were as
follows: i) Neurological dysfunction was observed in mice
administered with an intracerebral infusion of autologous blood;
ii) temporal profiles of intestinal mucosal damage and SIM disorder
were induced by ICH in mice; iii) evaluation of the time-course
showed that the serum levels of ghrelin increased markedly between
3 h and 3 days, peaked at 12 h, and showed a significant negative
correlation with SIM; iv) administration of ghrelin effectively
attenuated ICH-induced small intestinal dysmotility in a
dose-dependent manner; v) ghrelin administration appeared to
ameliorate ICH-induced small intestinal dysmotility by reducing
iNOS/NO via the cholinergic excitatory pathway. To the best of our
knowledge, the present study is the first in vivo
investigation to show the association between ghrelin and SIM
following experimental ICH, further clarifying the effect of
ghrelin on SIM disorder.
It is important to note that intestinal mucosal
damage may progress to ulcer formation and upper GI bleeding
according to severity. Clinically, the frequency of upper GI
bleeding following ICH is almost 30% (35,36),
which leads to long-term hospitalization and higher mortality
rates. In the present study, severe damage of the intestinal mucosa
occurred rapidly as early as 3 h following ICH and persisted for 3
days. These findings agree with the results of other brain event
models (11,14,37),
including TBI and ischemic stroke. This suggests that ICH induces
intestinal mucosal damage, which may develop into upper GI bleeding
without reasonable intervention. In addition, previous evidence has
revealed that the mucosal damage leads to GI dysmotility, and vice
versa (11,14).
As is already known, the brain-gut axis and
brain-gut peptides are important in regulating GI motility. Primary
and secondary brain injuries lead to changes in the synthesis and
secretion of brain-gut peptides, which are involved in altering
small intestine motility. To date, multiple brain-gut peptides have
been identified (38–42), including somatostatin, gastri),
cholecystokinin, vasoactive intestinal peptide, motilin, substance
P and ghrelin. Ghrelin is an endogenous ligand of the growth
hormone secretagogue receptor, and has been shown to be important
in several diseases (26–30). However, the dynamic changes in
serum levels of ghrelin during the early stages of ICH, and its
time-dependent correlation with SIM, have not been reported
previously. In the present study, the levels of ghrelin increased
in a time-dependent manner in the serum of ICH mice. By contrast,
SIM showed ab opposite trend between 3 h and 3 days post-ICH. At 12
h, the changes in serum levels of ghrelin and SIM peaked following
ICH. The present study also demonstrated a negative correlation
between the serum level of ghrelin and SIM. These data are in
agreement with the findings of another previous study, which
demonstrated the association in an ischemic stroke model (14). Therefore, the serum level of
ghrelin may serve as a potential hallmark for predicting
ICH-induced SIM disorder in clinical application. However, the
cause-and-effect association between serum levels of ghrelin and
SIM disorder remains to be elucidated. In another study, Xu et
al (14) offered a potential
explanation for this association in a middle cerebral artery
occlusion model. Their results showed that the brain event caused
neurohumoral regulation disorders, leading to gastrointestinal
motility disorder. The concomitant effect caused the decrease in
SIM, whereas the increase in the serum level of ghrelin was
compensatory. Therefore, the negative correlation between the serum
level of ghrelin and intestinal motility can be explained as a
compensatory reaction. The adaptive upregulation of serum levels of
ghrelin may lead to ICH-induced intestinal hypomotility to restore
GI kinetic energy. The decrease in intestinal kinetics demonstrates
that the compensation cannot sustain the physiological small
intestinal function. To determine the effect of ghrelin on
ICH-induced SIM disorder in the present study, exogenous ghrelin
was administrated. Notably, exogenous ghrelin administration
significantly attenuated small intestine hypomotility in a
dose-dependent manner following ICH. Taken together, these results
indicated that the induction of endogenous ghrelin serves as an
adaptive self-defense mechanism, although it is insufficient to
completely attenuate the small intestinal hypomotility caused by
ICH. Ghrelin offers a potential novel approach to alleviate SIM
disorder following ICH.
NOS neurons are well-known inhibitory cells to GI
motility, which are distributed extensively in the submucosal and
myenteric nerve networks (29,43).
NOS has three isoforms (iNOS, nNOS and eNOS) and can synthesize NO,
which inhibits GI motility. In the present study, three lines of
evidence demonstrated the effect of ghrelin on SIM disorder via the
iNOS/NO inhibitory pathway. Firstly, experiments revealed that the
ICH-induced serum expression of NO was significantly inhibited by
ghrelin administration, which was consistent with the findings of a
lipopolysaccharide-induced GI motility disturbance model (29). However, the results from a series
of rodent models (29,44,45)
showed that ghrelin administration downregulated the expression of
NOS/NO. The present study found that ghrelin administration
markedly reduced the level of NO by downregulating the expression
of iNOS post-ICH, rather than that of nNOS or eNOS, which was
determined using RT-qPCR and western blot analysis. These results
are in accordance with those of Chen et al (29); ghrelin had no significant effect on
the expression of nNOS/NO or eNOS/NO, however, it had a beneficial
effect on SIM via inhibiting the expression of iNOS/NO. The present
study further confirmed that ghrelin reversed ICH-induced small
intestinal hypomotility via the iNOS-dependent pathway by
administration with L-NAME or L-argnine. Taken together, these
findings demonstrated that ghrelin modulated SIM post-ICH through
suppressing the expression of iNOS/NO.
Several studies have demonstrated that NOS/NO
decreases GI motility through the autonomic nervous system
(46,47). Ghrelin has also been found to be
critical in the regulation of gastric emptying via the cholinergic
excitatory pathway (48,49). However, whether ghrelin reduces the
expression of iNOS/NO via the cholinergic pathway following ICH has
not been elucidated previously. In the present study, atropine,
phentolamine and propranolol were administered to inhibit the
cholinergic, α-adrenergic, and β-adrenergic pathway, respectively.
The results suggested that the prokinetic effect of ghrelin was
eradicated by atropine, but not by phentolamine or propranolol.
These data are in agreement with those previously reported
(50). Together, these results
suggested that ghrelin attenuated SIM disorder by reducing the
expression of NOS/NO via the cholinergic excitatory pathway, but
not the α- or β-adrenergic excitatory pathways.
In conclusion, the present study is the first, to
the best of our knowledge, to investigate the changes in serum
levels of ghrelin and SIM following ICH over time. The results
provided quantitative evidence suggesting that the administration
of ghrelin reversed ICH-induced small intestinal dysmotility by
downregulating the expression of iNOS-NO via the cholinergic
pathway. Ultimately, based on an improved understanding of ghrelin
in ICH-induced intestinal hypomotility, the use of ghrelin as a
potential marker for predicting post-ICH kinetic disorders and
pharmacological agents may be an effective intervention in clinical
application.
Acknowledgements
This study was supported by the Science and
Technology Project Fund (grant no. WQ2014002) from the Health
Bureau of Nantong Municipality.
References
|
1
|
Ganesan V, Hogan A, Shack N, Gordon A,
Isaacs E and Kirkham FJ: Outcome after ischaemic stroke in
childhood. Dev Med Child Neurol. 42:455–461. 2010. View Article : Google Scholar
|
|
2
|
Heideman RL, Packer RJ, Albright LA,
Freeman CR and Rorke LB: Tumors of the central nervous
systemPrinciples and Practice of Pediatric Oncology. Pizzo PA and
Poplack DG: Lippincott Williams & Wilkins; Philadelphia: pp.
633–697. 1997
|
|
3
|
Fullerton HJ, Wu YW, Zhao S and Johnston
SC: Risk of stroke in children: Ethnic and gender disparities.
Neurology. 61:189–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giroud M, Lemesle M, Gouyon JB, Nivelon
JL, Milan C and Dumas R: Cerebrovascular disease in children under
16 years of age in the city of Dijon, France: A study of incidence
and clinical features from 1985 to 1993. J Clin Epidemiol.
48:1343–1348. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lynch JK, Hirtz DG, DeVeber G and Nelson
KB: Report of the National institute of neurological disorders and
stroke workshop on perinatal and childhood stroke. Pediatrics.
109:116–123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung B and Wong V: Pediatric stroke among
Hong Kong Chinese subjects. Pediatrics. 114:206–212. 2004.
View Article : Google Scholar
|
|
7
|
Balami JS and Buchan AM: Complications of
intracerebral haemorrhage. Lancet Neurol. 11:101–118. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lynch JK and Han CJ: Pediatric stroke:
What do we know and what do we need to know? Semin Neurol.
25:410–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tack J and Lee KJ: Pathophysiology and
treatment of functional dyspepsia. J Clin Gastroenterol.
39:211–216. 2005. View Article : Google Scholar
|
|
10
|
Yuan DD, Chi XJ, Jin Y, Li X, Ge M, Gao
WL, Guan JQ, Zhang AL and He ZQ: Intestinal injury following liver
transplantation was mediated by TLR4/NF-κB activation-induced cell
apoptosis. Mol Med Rep. 13:1525–1532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang YB, Liu J and Yang ZX: Effects of
intestinal mucosal blood flow and motility on intestinal mucosa.
World J Gastroenterol. 17:657–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keshavarzi Z, Khaksari M and Shahrokhi N:
The effects of cyclooxygenase inhibitors on the gastric emptying
and small intestine transit in the male rats following traumatic
brain injuery. Iran J Basic Med Sci. 17:406–410. 2014.PubMed/NCBI
|
|
13
|
Olsen AB, Hetz RA, Xue H, Aroom KR,
Bhattarai D, Johnson E, Bedi S, Cox CS Jr and Uray K: Effects of
traumatic brain injury on intestinal contractility.
Neurogastroenterol Motil. 25:e593–e463. 2013. View Article : Google Scholar
|
|
14
|
Xu X, Zhu Y and Chuai J: Changes in serum
ghrelin and small intestinal motility in rats with ischemic stroke.
Anat Rec (Hoboken). 295:307–312. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong Y, Xu L, Guo F, Pang M, Shi Z, Gao S
and Sun X: Effects of ghrelin on gastric distension sensitive
neurons and gastric motility in the lateral septum and arcuate
nucleus regulation. J Gastroenterol. 49:219–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cowley MA, Smith RG, Diano S, Tschöp M,
Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M,
Heiman ML, et al: The distribution and mechanism of action of
ghrelin in the CNS demonstrates a novel hypothalamic circuit
regulating energy homeostasis. Neuron. 37:649–661. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schellekens H, Dinan TG and Cryan JF: Lean
mean fat reducing ‘ghrelin’ machine: Hypothalamic ghrelin and
ghrelin receptors as therapeutic targets in obesity.
Neuropharmacology. 58:2–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Date Y, Kojima M, Hosoda H, Sawaguchi A,
Mondal MS, Suganuma T, Matsukura S, Kangawa K and Nakazato M:
Ghrelin, a novel growth hormone-releasing acylated peptide, is
synthesized in a distinct endocrine cell type in the
gastrointestinal tracts of rats and humans. Endocrinology.
141:4255–4261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang G, Lee HM, Englander E and Greeley GH
Jr: Ghrelin-not just another stomach hormone. Regul Pept.
105:75–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gnanapavan S, Kola B, Bustin SA, Morris
DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB
and Korbonits M: The tissue distribution of the mRNA of ghrelin and
subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol
Metab. 87:29882002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakazato M, Murakami N, Date Y, Kojima M,
Matsuo H, Kangawa K and Matsukura S: A role for ghrelin in the
central regulation of feeding. Nature. 409:194–198. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peeters TL: Ghrelin and the gut. Endocr
Dev. 25:41–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Broussard JL, Kilkus JM, Delebecque F,
Abraham V, Day A, Whitmore HR and Tasali E: Elevated ghrelin
predicts food intake during experimental sleep restriction. Obesity
(Silver Spring). 24:132–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lien GS, Lin CH, Yang YL, Wu MS and Chen
BC: Ghrelin induces colon cancer cell proliferation through the
GHS-R, Ras, PI3K, Akt, and mTOR signaling pathways. Eur J
Pharmacol. 776:124–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin L, Saha PK, Ma X, Henshaw IO, Shao L,
Chang BH, Buras ED, Tong Q, Chan L, McGuinness OP and Sun Y:
Ablation of ghrelin receptor reduces adiposity and improves insulin
sensitivity during aging by regulating fat metabolism in white and
brown adipose tissues. Aging Cell. 10:996–1010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ozawa T, Tokunaga J, Arakawa M, Ishikawa
A, Takeuchi R, Mezaki N, Miura T, Sakai N, Hokari M, Takeshima A,
et al: Abnormal ghrelin secretion contributes to gastrointestinal
symptoms in multiple system atrophy patients. J Neurol.
260:2073–2077. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ariyasu H, Iwakura H, Yukawa N, Murayama
T, Yokode M, Tada H, Yoshimura K, Teramukai S, Ito T, Shimizu A, et
al: Clinical effects of ghrelin on gastrointestinal involvement in
patients with systemic sclerosis. Endocr J. 61:735–742. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ochi M, Tominaga K, Tanaka F, Tanigawa T,
Shiba M, Watanabe T, Fujiwara Y, Oshitani N, Higuchi K and Arakawa
T: Effect of chronic stress on gastric emptying and plasma ghrelin
levels in rats. Life Sci. 82:862–868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen YT, Tsai SH, Sheu SY and Tsai LH:
Ghrelin improves LPS-induced gastrointestinal motility
disturbances: Roles of NO and prostaglandin E2. Shock. 33:205–212.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malin SK, Samat A, Wolski K, Abood B,
Pothier CE, Bhatt DL, Nissen S, Brethauer SA, Schauer PR, Kirwan JP
and Kashyap SR: Improved acylated ghrelin suppression at 2 years in
obese patients with type 2 diabetes: Effects of bariatricsurgery
vs. standard medical therapy. Int J Obes. 38:364–370. 2014.
View Article : Google Scholar
|
|
31
|
Garber J, Barbee RW, Bielitzki J, et al:
Guide for the care and use of laboratory animals. 8th edition.
Washington DC: National Academies Press; pp. 1–246. 2011,
PubMed/NCBI
|
|
32
|
Rynkowski MA, Kim GH, Komotar RJ, Otten
ML, Ducruet AF, Zacharia BE, Kellner CP, Hahn DK, Merkow MB,
Garrett MC, et al: A mouse model of intracerebral hemorrhage using
autologous blood infusion. Nat Protoc. 3:122–128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu YQ, Tang G, Wang Y, Chen X, Gu X,
Zhang Z, Wang Y and Yang GY: Metformin attenuates blood-brain
barrier disruption in mice following middle cerebral artery
occlusion. J Neuroinflammation. 11:1772014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Misra UK, Kalita J, Pandey S and Mandal
SK: Predictors of gastrointestinal bleeding in acute intracerebral
haemorrhage. J Neurol Sci. 208:25–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang TC, Li JG, Shi HM, Yu DM, Shan K, Li
LX, Dong XY and Ren TH: Gastrointestinal bleeding after
intracerebral hemorrhage: A retrospective review of 808 cases. Am J
Med Sci. 346:279–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun B, Hu C, Fang H, Zhu L, Gao N and Zhu
J: The effects of lactobacillus acidophilus on the intestinal
smooth muscle contraction through PKC/MLCK/MLC signaling pathway in
TBI mouse model. PLoS One. 10:e01282142015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Raybould HE and Taché Y: Cholecystokinin
inhibits gastric motility and emptying via a capsaicin-sensitive
vagal pathway in rats. Am J Physiol. 255:242–246. 1988.
|
|
39
|
Liu J, Li ZS, Wan XJ and Wang W:
Expression and function of apoptosis-related genes Bcl-2/Bax and
Fas/Fas L in the course of stress ulcer. Zhonghua Yi Xue Za Zhi.
83:504–509. 2003.(In Chinese). PubMed/NCBI
|
|
40
|
Schmidt WE, Creutzfeldt W, Schleser A,
Choudhury AR, Nustede R, Höcker M, Nitsche R, Sostmann H, Rovati LC
and Fölsch UR: Role of CCK in regulation of pancreaticobiliary
functions and GI motility in humans: Effects of loxiglumide. Am J
Physiol. 260:G197–G206. 1991.PubMed/NCBI
|
|
41
|
Nguyen NQ, Fraser RJ, Chapman MJ, Bryant
LK, Holloway RH, Vozzo R, Wishart J, Feinle-Bisset C and Horowitz
M: Feed intolerance in critical illness is associated with
increased basal and nutrient-stimulated plasma cholecystokinin
concentrations. Crit Care Med. 35:82–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chapman MJ, Nguyen NQ and Deane AM:
Gastrointestinal dysmotility: Evidence and clinical management.
Curr Opin Clin Nutr Metab Care. 16:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bult H, Boeckxstaens GE, Pelckmans PA,
Jordaens FH, Van Maercke YM and Herman AG: Nitric oxide as an
inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature.
345:346–347. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Winter BY, Bredenoord AJ, De Man JG,
Moreels TG, Herman AG and Pelckmans PA: Effect of inhibition of
inducible nitric oxide synthase and guanylyl cyclase on
endotoxin-induced delay in gastric emptying and intestinal transit
in mice. Shock. 18:125–131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
De Winter BY, De Man JG, Seerden TC,
Depoortere I, Herman AG, Peeters TL and Pelckmans PA: Effect of
ghrelin and growth hormone-releasing peptide 6 on septic ileus in
mice. Neurogastroenterol Motil. 16:439–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Dong L, Cheng Y and Zhao P:
Effects of ghrelin on feeding regulation and interdigestive
migrating complex in rats. Scand J Gastroenterol. 42:447–453. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shuto Y, Shibasaki T, Wada K, Parhar I,
Kamegai J, Sugihara H, Oikawa S and Wakabayashi I: Generation of
polyclonal antiserum against the growth hormone secretagogue
receptor (GHS-R): Evidence that the GHS-R exists in the
hypothalamus, pituitary and stomach of rats. Life Sci. 68:991–996.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Greenwood-Van Meerveld B, Krieqsman M and
Nelson R: Ghrelin as a target for gastrointestinal motility
disorders. Peptides. 32:2352–2356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ogiso K, Asakawa A, Amitani H and Inui A:
Ghrelin: A gut hormonal basis of motility regulation and functional
dyspepsia. J Gastroenterol Hepatol. 26 Suppl 3:S67–S72. 2011.
View Article : Google Scholar
|
|
50
|
Tümer C, Oflazoğlu HD, Obay BD, Kelle M
and Taşdemir E: Effect of ghrelin on gastric myoelectric activity
and gastric emptying in rats. Regul Pept. 146:26–32. 2008.
View Article : Google Scholar : PubMed/NCBI
|