Introduction
Lung cancer represents the leading reason of
worldwide cancer deaths, and non-small cell lung cancer (NSCLC) is
the major subtype of lung cancer and accounts for ~80% of all
patients with lung cancer (1). The
5-year survival rate of lung cancer remains as low as ~15%, long
term survival rate of NSCLC patients remains poor, indicating the
incomplete comprehension of the underlying pathogenesis of NSCLC
(2).
Hypoxia is closely related to various
pathophysiological processes and has been found to be a
characteristic feature in lung cancer. In many cancers,
overexpression of HIF-1α caused by intratumoral hypoxia genetically
alters some crucial oncogenes and tumor suppressor genes (3). HIF-1α has been shown to be involved
in the stubborn resistance to radiotherapy and some forms of
chemotherapy (4). Overexpression
of HIF-1α has been reported at both protein and mRNA level in NSCLC
patients with poor prognosis (5–7).
Previous studies revealed that miRNAs had been demonstrated to be
involved in the molecular response to hypoxia and regulate the
expression of HIF-1α protein (8).
For example, miRNA-519c restrained the hypoxia-induced
proliferation of NSCLC cells through inhibiting HIF1α via the
direct binding to the 3′untranslated region (3′-UTR) of the HIF-1α
transcripts (8). Evidence is also
accumulating that miRNA-199a is involved in regulation HIF-1α
(9).
Accumulating evidences of ectopic lncRNA expression
in many cancer types in response to hypoxic condition imply
profound and potentially that lncRNAs involves in hypoxia (10). PVT1 is a strongly conserved lncRNA
between mouse and human and amplification of PVT1 is one of the
most common events in lung cancer (11–13).
PVT1 could act as ceRNA for miRNAs (14), for instance, a net binding sequence
towards the miR-200 family in PVT1 is revealed; through interacting
with miRNAs, PVT1 regulates the expression of hundreds of mRNAs
(14).
Here, we detect the expression of PVT1 and
miR-199a-5p in response to hypoxia, emphasize the relationship
between the dysfunctional expressions of PVT1, miR-199a-5p of NSCLC
cells in response to hypoxia and highlight the specific mechanistic
roles of PVT1 and miR-199a-5p in NSCLC under hypoxia.
Materials and methods
Tissue samples and clinical data
collection
The human NSCLC cell lines A549 and SPCA-1 cell
lines were obtained from ATCC and cultured with DMEM supplemented
with 10% FBS and maintained at 37°C in 5% CO2. For cell hypoxia,
cells were incubated in 2% O2 at 37°C.
Tumor tissue samples, normal adjacent tissue samples
and clinical data were collected from 60 patients with NSCLC
enrolled at the Weifang People's Hospital from September 2011 to
September 2013. Written informed consent which conformed to the
principles outlined in the Declaration of Helsinki was given by all
patients. Specimen collection was approved by the Research and
Ethical Committee of Weifang People's Hospital.
Cell culture and transient
transfection
NSCLC cell lines A549 and SPCA-1 were transfected
with siRNA targeting for miR-199a-5p and PVT1 using Lipofectamine
2000 (Invitrogen, Grand Island, USA) following the manufacturer's
instructions. siRNAs targeting for PVT1 and normal control were
purchased from Cosmo Bio (Tokyo, Japan). The siRNA target sequence
for PVT1 is si-PVT1 sense, 5′-gcuuggaggcugaggaguutt-3′ and
antisense, 5′-aacuccucagccuccaagctt-3′. The siRNA target sequences
for miR-199a-5p are sense, 5′-ggagatcctgctccgtcgccc-3′ and
antisense, 5′-aacuccucagccuccaagctt-3′. The synthetic miR-199a-5p
mimic, 5′-acaguagucugcacauugguua-3′, miR-199a-5p mock,
5′-uuguacuacacaaaaguacug-3′. The transfected cells were harvested
at 48 h after transfection.
RT-qPCR
SYBR-Green-based quantitative qRT-PCR was performed
to assess expression of miRNA and lncRNA in tissue samples and lung
cancer cells with the 7500 Real-time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA
was extracted from lung tumor samples or lung cancer cells using
the miRVana miRNA Isolation kit (Ambion, Austin TX, USA). The PCR
primer sequences were as follows: Primers for PVT1 RNA forward,
5′-ccgactcttcctggtgaagc-3′ and reverse, 5′-gtatggtcagctcaagccca-3′;
primers for miR-199a-5p, stem-loop primer
5′-ctcaactggtgtcgtggagtcggcaattcagttgagacaggtag-3′, forward,
5′-acactccagctgggcccagt-3′ and reverse, 5′-tggtgtcgtggagtcg-3′; for
18S rRNA forward, 5′-gtaacccgttgaaccccatt-3′ and reverse,
5′-ccatccaatcggtagtagcg-3′; HIF-1α forward,
5′-gtctcgagatgcagccagat-3′ and reverse, 5′-tcaccagcatcca
gaagtttc-3′; β-actin forward, 5′-ctacaatgagctgcgtgtgg-3′ and
reverse, 5′-aaggaaggctggaagagtgc-3′.
Cell proliferation assay
Cell proliferation was analyzed by the cell count
assay. Lung cancer cells were plated in 96-well plates at a density
of 1×103 cells/well. Cell Counting kit-8 (Solarbio,
Beijing, China) was used to detect cell viability according to the
manufacturer's protocol. The reaction was measured at 570 nm with
enzyme immunoassay analyzer (Bio-Rad, Berkeley, CA, USA). All
experiments were performed in triplicate.
Western blot analysis
Cells lysates were prepared and Bio-Rad Protein
Assay was performed to detect the protein concentration. Cell
lysates were separated by SDS-PAGE gels, and then separated
proteins were transferred into polyvinylidene fluoride (PVDF)
membrane. The PVDF membrane was blocked with 5% non-fat milk and
then incubated with the primary antibodies dilution against HIF-1α
overnight at 4°C. The blot was developed with horseradish
peroxidase-linked secondary antibodies at a dilution ratio of
1:1,000 at room temperature for 1 h. The immunoreactive bands were
visualized using ECL system. Quantification of band intensities was
performed with Gel-pro Analyzer software (Media Cybernetics,. Inc.,
Rockville, MD, USA).
RNA immunoprecipitation
pcDNA3.1-MS2, pcDNA3.1-MS2-PVT1 or
pcDNA3.1-MS2-PVT1-Mut and pMS2-GFP (Addgene, Inc., Cambridge, MA,
USA) were co-transfected into A549 cells with in a 10 cm dish.
After 48 h, formaldehyde was added at a final concentration of 1%
at room temperature for 10 min and terminated by 200 mM glycine.
The cells were washed with PBS and then lysed using lysis buffer.
After 15 min centrifugation at 15,000 rpm, the supernatant was
collected and mixed with GFP antibody to perform RIP experiments
with the Magna RIP™ RNA-Binding Protein Immunoprecipitation kit
(Millipore, Bedford, MA, USA).
Immunohistochemical assay
Immunohistochemistry for HIF-1α was performed on
NSCLC tissues slides using a primary antibody against HIF-1α
(Maxim, Fuzhou, China) and a horseradish peroxidase-conjugated
secondary antibody (Maxim). The color reaction was performed with
3,3-diaminobenzidine. The staining intensities were evaluated in
each sample using integrated optical density (IOD) and graded on a
scale of 0 to 9 by two pathologists. The staining index (SI) score
>mIOD defined tumors with high expression, and an SI score ≤mIOD
regarded as low expression.
Statistical analysis
All data were presented as the mean ± SD, Student's
t-test was performed for comparisons. All P-values were obtained
with the SPSS 18.0 software package (SPSS, Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Downregulation of miR-199a-5p and
upregulation of PVT1 in lung cancer cells in response to
hypoxia
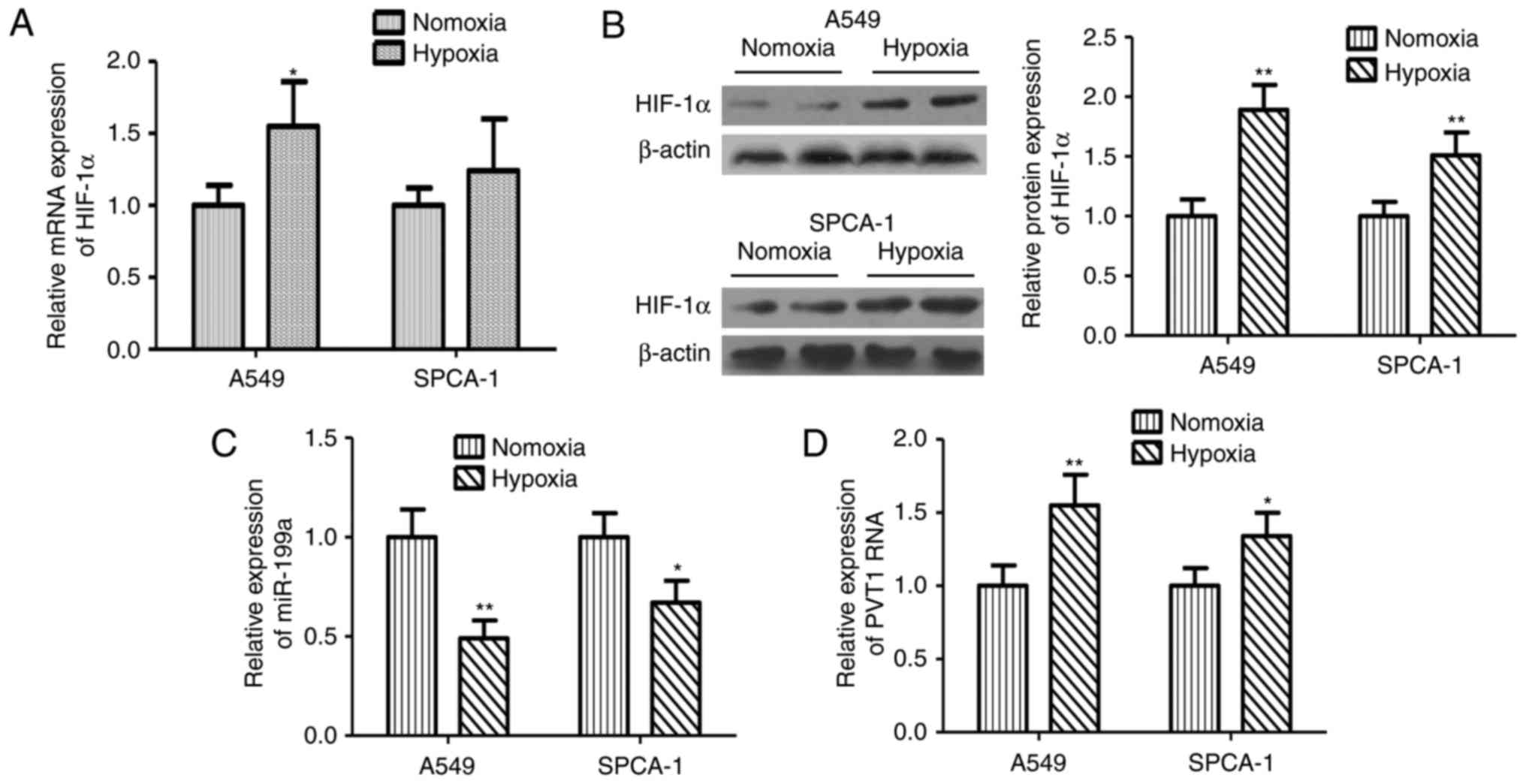
The effects of hypoxia on the mRNA and protein
expression levels of HIF-1α were investigated. As expected, hypoxia
resulted in the increases of HIF-1α mRNA and protein (Fig. 1A, B). To investigate the role of
miR-199a-5p and PVT1 in NLCSC cells subjected to hypoxic
conditions, the expression of miR-199a-5p and PVT1 in both SPCA-1
and A549 cell lines in response to hypoxia were initially detected.
Expression of miR-199a-5p was decreased in the both cell lines
under hypoxic conditions; on the contrary, expression of PVT1 was
unregulated in both SPCA-1 and A549 cell lines. Compared with
normoxic conditions, the expression of miR-199a-5p under hypoxic
was 48 and 32% lower in SPCA-1 and A549 cell lines, respectively
(Fig. 1C). Furthermore, in both
SPCA-1 and A549 cell lines, the PVT1 expression level under hypoxia
was 55 and 34% higher compared with the expression under normoxic
conditions (Fig. 1D).
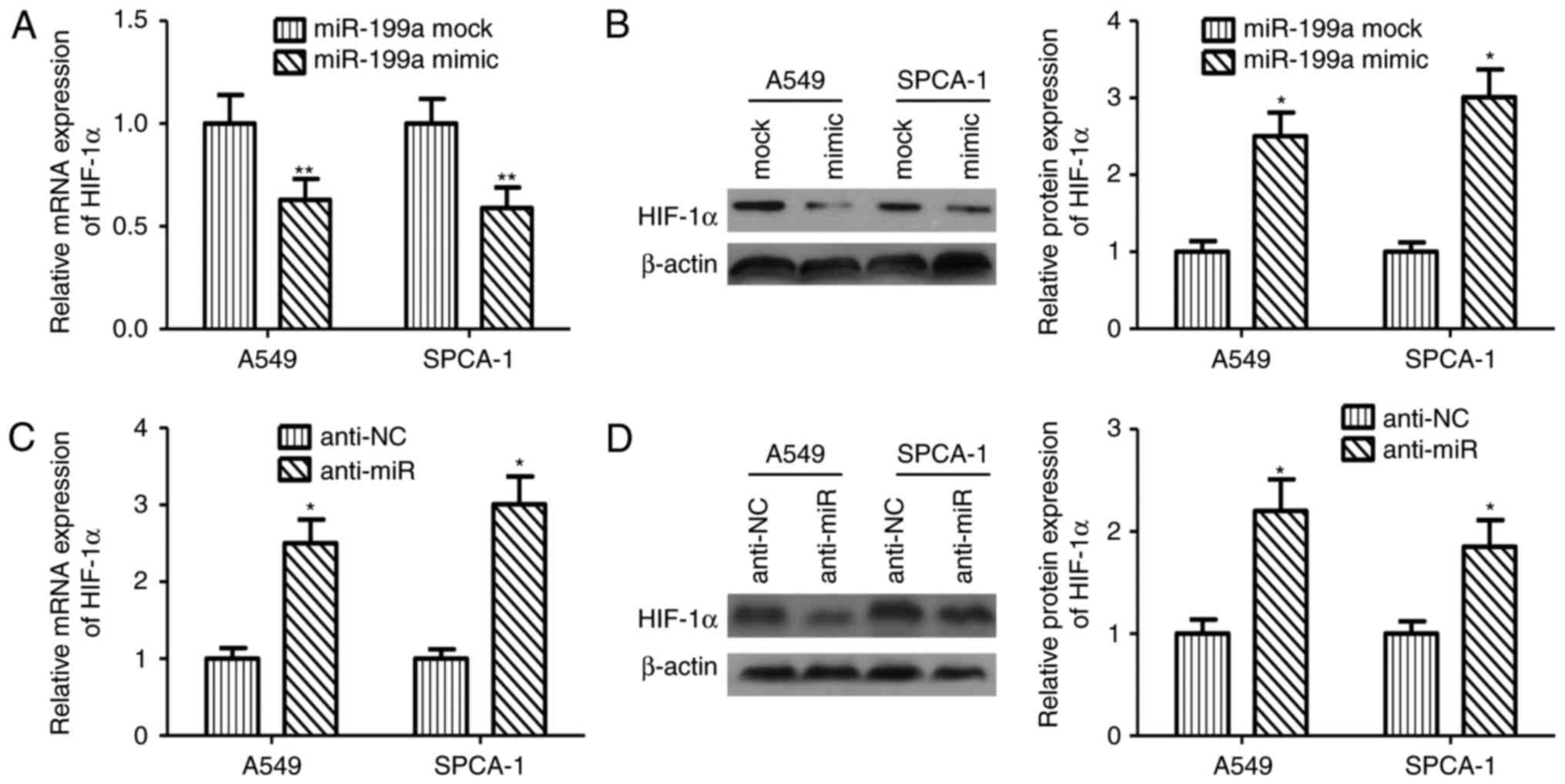
miR-199a-5p regulates HIF-1α and cell
response to hypoxia in NSCLC
Activation of HIF-1α under hypoxia has been regarded
as a important event for sustained tumor growth, migration and
metastasis by regulating angiogenesis and promoting the expression
of tumor-associated genes in various tumor cells. To examine the
direct effects of miR-199a-5p on expression of HIF-1α in NSCLC
cell, overexpression of miR-199a-5p was performed in both breast
cancer cell lines. Ectopic expression miR-199a-5p in A549 and
SPCA-1 cells decreased in mRNA and protein levels of HIF-1α
significantly (Fig. 2A, B). On the
contrary, silencing of miR-199a-5p augmented the mRNA and protein
levels of HIF-1α (Fig. 2C, D).
PVT1 reverses the growth inhibition of
miR-199a-5p
To examine the negative effects of miR-199a-5p on
proliferation of NLCSC, miR-199a-5p mimic was transfected into A549
and SPCA-1 cells. Compared with the control group, ectopic
expression of miR-199a-5p inhibited the viability of both A549 and
SPCA-1 cells in both normal and hypoxic conditions (Fig. 3A, B). In contrast, silencing of
miR-199a-5p by RNAi promoted the proliferation capacity of A549 and
SPCA-1cells (Fig. 3C, D).
To further explore whether PVT1 expression
correlated with miR-199a-5p expression and subsequent progression,
scramble or PVT1 targeted siRNA were transfected into A549 and
SPCA-1cells. qPCR was performed to detect the expression of PVT1.
As shown in Fig. 3E, qPCR assays
indicated that expression of PVT1 was significantly reduced.
miR-199a-5p expression was significantly upregulated (Fig. 3F). Correspondingly the mRNA and
protein expression of HIF-1α in A549 and SPCA-1 cells decreased
significantly (Fig. 3G, H). Next,
MTT assay revealed that knockdown of PVT1 significantly suppressed
proliferation of A549 and SPCA-1 cells significantly compared with
the control cells (Fig. 3I,
J).
PVT1 is inversely correlated with
miR-199a-5p in NSCLC tissues
HIF-1α protein mainly expressed in NSCLC nuclei or
cytoplasm, according to the SI score, NLCSC tissues were organized
into HIF-1α high and low expression group (Fig. 4A). To demonstrated the function
role of PVT1 in NSCLC, the expressions of PVT1 and miR-199a-5p in
60 pairs NSCLC tissues were detected by qPCR. These results
indicated that PVT1 was upregulated in HIF-1α high group compared
with HIF-1α low group (Fig. 4B).
Similarly, qPCR was performed to detect the expression of
miR-199a-5p in NSCLC tissues. HIF-1α low group showed highly
expression of miR-199a-5p, in contrast, the corresponding HIF-1α
high group showed lower expression of miR-199a-5p (Fig. 4C). These results revealed that PVT1
expression was negatively correlated with miR-199a-5p expression in
NSCLC tissues (Fig. 4D).
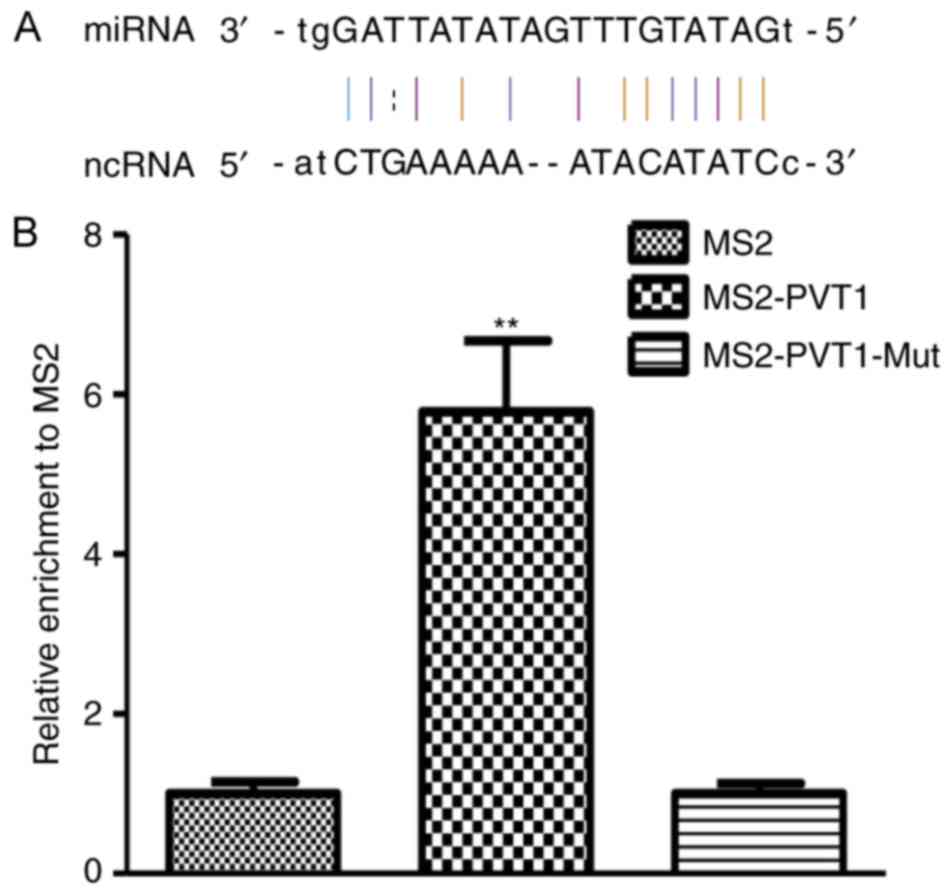
PVT1 acts as a molecular sponge for
miR-199a-5p
To examine whether PVT1 functioned as sponges
binding with specific miRNAs, interactions between lncRNA-miRNA
were predicted by miRcode (http://www.mircode.org/) and starBase v2.0 (http://starbase.sysu.edu.cn/). As shown in Fig. 5A, PVT1 contains a predicted
miR-199a-5p targeting site. To analyze the direct combination
between miR-199a-5p and PVT1, RNA immunoprecipitation was performed
to pull down endogenous miRNAs associated with PVT1, results of
qPCR demonstrated that the PVT1 RIP in A549 cells was significantly
enriched for miRbase 21 compared with IgG, the empty vector and
PVT1 with mutations in miR-199a-5p targeting sites (Fig. 5B).
Discussion
Hypoxia-inducible factor 1 (HIF1) is upregulated in
many types of cancers subjected to intratumoral hypoxic condition.
HIF-1α subunit, an endogenous hypoxia marker, has been extensively
studied (3,15). Upregulation of HIF-1α is associated
with tumor growth and survival rate of NSCLC (5–7).
Previous studies have shown that the suppression of HIF-1α activity
notably restrained tumor proliferation in animal models (16,17).
Screening of chemical HIF-1α inhibitors is ongoing for HIF-1α
targeted cancer therapy, however the specificity for HIF-1α is the
main problem (18).
Previous researches suggested that miR-199a-5p could
regulate HIF-1α expression in cardiac myocytes (19). Targeted regulation of HIF-1α
expression by miRNAs remains unclear in NSCLC, searching for
specific miRNA targeting HIF-1α might assist to generate
selectively novel approaches for cancer therapy.
We speculated that miR-199a-5p might also
participate in the regulation of HIF-1α expression and the process
of cell proliferation in hypoxia-induced NSCLC cells. To reveal the
functional interaction between HIF-1α and miR-199a-5p, silencing of
HIF-1α by siRNA was applied to A549 and SPCA-1 cells which
expressed miR-199a-5p. Consistent with previous findings (9), downregulation of miR-199a-5p leads to
increased expression of HIF-1α and promote cell proliferation. On
the contrary, ectopic expression of miR-199a-5p decreased the
expression of HIF-1α and blocked the proliferation ability of A549
and SPCA-1 cells under hypoxic conditions. These results hinted
that miR-199a-5p negatively regulated proliferation induced by
hypoxia via targeting HIF-1α.
Since the identification of lncRNA in malignancy
tumors, a growing number of researches on the functional roles of
lncRNAs have been performed in various cancers. The abnormal
expression of lncRNAs is participated in the regulating tumor
progression and biological behaviors in lung cancer via
interactions with miRNAs or mRNAs (20,21).
PVT1 has been shown to involve in colorectal cancer (22), ovarian and breast cancers (23). Previous studies have demonstrated
that unrestrained activation of HIF in pVHL-defective renal cancer
enhances expression of MYC and PVT1 via long distance interactions
with a HIF-binding intergenic enhancer (24). In this study, we confirmed that
PVT1 was overexpressed in the hypoxic lung cancer cells. Our
results showed that PVT1 knockdown could significantly suppress
lung cancer cell proliferation in vitro. Nevertheless, the
potential role and molecular mechanism of PVT1 in lung cancer in
response to hypoxia remains to be clarified.
In 2011, competing endogenous RNA (ceRNA) hypothesis
was presented, which consolidated the transcripts and constituted a
regulatory RNA network (25).
ceRNAs could worked as sponges for a set of miRNAs and functionally
prevent these targeted transcripts of mRNA from being degraded by
miRNA (26,27). Recently, PVT1 has been proposed to
function as ceRNA by competitively binding miR-199a-5p using
miRTarBase (25,28). Therefore we hold that PVT1 also has
roles as miRNAs sponges to modulate the functions of
miR-199a-5p.
In this study, we demonstrated that PVT1 could work
as a molecular sponge for miR-199a-5p, upregulated expression of
its endogenous targets HIF-1α, and inhibited its function. We
revealed that PVT1 inhibited the function of miR-199a-5p through
competitively binding miR-199a-5p and blocked growth and migration
of lung cancer cells. The effects of PVT1 on cell proliferation and
expression of HIF-1α were abrogated by the mutation of miR-199a-5p
binding sites. The inhibitory effects of depletion of PVT1 on
proliferation and migration are overcome by inhibition of
miR-199a-5p. These findings suggest that inhibition ability on
HIF-1α of PVT1 is dependent upon its binding to miR-199a-5p.
In conclusion, we first report that PVT1 promotes
expression of HIF-1α, a critical endogenous hypoxia marker, in
NSCLC. With this finding, we demonstrated that PVT1 modulated
HIF-1α by competing for miR-199a-5p as a ceRNA to regulate cell
proliferation. Our findings established a novel connection among
PVT1, miR-199a-5p, and HIF-1α in cell response to hypoxia. Above
data indicate that PVT1 might work as vital target for hypoxia
therapy.
Acknowledgements
The present study was supported by grants from The
Natural Science Foundation of Shandong Province (2015ZRB14132).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller YE: Pathogenesis of lung cancer:
100 year report. Am J Respir Cell Mol Biol. 33:216–223. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adams JM, Difazio LT, Rolandelli RH, Luján
JJ, Haskó G, Csóka B, Selmeczy Z and Németh ZH: HIF-1: A key
mediator in hypoxia. Acta Physiol Hung. 96:19–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takasaki C, Kobayashi M, Ishibashi H,
Akashi T and Okubo K: Expression of hypoxia-inducible factor-1α
affects tumor proliferation and antiapoptosis in surgically
resected lung cancer. Mol Clin Oncol. 5:295–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang SL, Ren QG, Wen L and Hu JL:
Clinicopathological and prognostic significance of
hypoxia-inducible factor-1 alpha in lung cancer: A systematic
review with meta-analysis. J Huazhong Univ Sci Technolog Med Sci.
36:321–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren W, Mi D, Yang K, Cao N, Tian J, Li Z
and Ma B: The expression of hypoxia-inducible factor-1α and its
clinical significance in lung cancer: A systematic review and
meta-analysis. Swiss Med Wkly. 143:w138552013.PubMed/NCBI
|
|
8
|
Cha ST, Chen PS, Johansson G, Chu CY, Wang
MY, Jeng YM, Yu SL, Chen JS, Chang KJ, Jee SH, et al: MicroRNA-519c
suppresses hypoxia-inducible factor-1alpha expression and tumor
angiogenesis. Cancer Res. 70:2675–2685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding G, Huang G, Liu HD, Liang HX, Ni YF,
Ding ZH, Ni GY and Hua HW: MiR-199a suppresses the hypoxia-induced
proliferation of non-small cell lung cancer cells through targeting
HIF1α. Mol Cell Biochem. 384:173–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei X, Wang C, Ma C, Sun W, Li H and Cai
Z: Long noncoding RNA ANRIL is activated by hypoxia-inducible
factor-1α and promotes osteosarcoma cell invasion and suppresses
cell apoptosis upon hypoxia. Cancer Cell Int. 16:732016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang C, Liu S, Wang H, Zhang Z, Yang Q
and Gao F: LncRNA PVT1 overexpression is a poor prognostic
biomarker and regulates migration and invasion in small cell lung
cancer. Am J Transl Res. 8:5025–5034. 2016.PubMed/NCBI
|
|
12
|
Wan L, Sun M, Liu GJ, Wei CC, Zhang EB,
Kong R, Xu TP, Huang MD and Wang ZX: Long noncoding RNA PVT1
promotes non-small cell lung cancer cell proliferation through
epigenetically regulating LATS2 expression. Mol Cancer Ther.
15:1082–1094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui D, Yu CH, Liu M, Xia QQ, Zhang YF and
Jiang WL: Long non-coding RNA PVT1 as a novel biomarker for
diagnosis and prognosis of non-small cell lung cancer. Tumour Biol.
37:4127–4134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Conte F, Fiscon G, Chiara M, Colombo T,
Farina L and Paci P: Role of the long non-coding RNA PVT1 in the
dysregulation of the ceRNA-ceRNA network in human breast cancer.
PLoS One. 12:e01716612017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harris AL: Hypoxia-a key regulatory factor
in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guerin E, Raffelsberger W, Pencreach E,
Maier A, Neuville A, Schneider A, Bachellier P, Rohr S, Petitprez
A, Poch O, et al: In vivo topoisomerase I inhibition attenuates the
expression of hypoxia-inducible factor 1α target genes and
decreases tumor angiogenesis. Mol Med. 18:83–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Lin X, Shoemaker AR, Albert DH,
Fesik SW and Shen Y: Hypoxia-inducible factor-1 inhibition in
combination with temozolomide treatment exhibits robust antitumor
efficacy in vivo. Clin Cancer Res. 12:4747–4754. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang CM and Yu J: Hypoxia-inducible
factor-1 as a therapeutic target in cancer. J Gastroenterol
Hepatol. 28:401–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rane S, He M, Sayed D, Vashistha H,
Malhotra A, Sadoshima J, Vatner DE, Vatner SF and Abdellatif M:
Downregulation of miR-199a derepresses hypoxia-inducible
factor-1alpha and Sirtuin 1 and recapitulates hypoxia
preconditioning in cardiac myocytes. Circ Res. 104:879–886. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui M, Xiao Z, Wang Y, Zheng M, Song T,
Cai X, Sun B, Ye L and Zhang X: Long noncoding RNA HULC modulates
abnormal lipid metabolism in hepatoma cells through an
miR-9-mediated RXRA signaling pathway. Cancer Res. 75:846–857.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Quagliata L, Matter MS, Piscuoglio S,
Arabi L, Ruiz C, Procino A, Kovac M, Moretti F, Makowska Z,
Boldanova T, et al: Long noncoding RNA HOTTIP/HOXA13 expression is
associated with disease progression and predicts outcome in
hepatocellular carcinoma patients. Hepatology. 59:911–923. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi Y, Sawada G, Kurashige J, Uchi
R, Matsumura T, Ueo H, Takano Y, Eguchi H, Sudo T, Sugimachi K, et
al: Amplification of PVT-1 is involved in poor prognosis via
apoptosis inhibition in colorectal cancers. Br J Cancer.
110:164–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guan Y, Kuo WL, Stilwell JL, Takano H,
Lapuk AV, Fridlyand J, Mao JH, Yu M, Miller MA, Santos JL, et al:
Amplification of PVT1 contributes to the pathophysiology of ovarian
and breast cancer. Clin Cancer Res. 13:5745–5755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grampp S, Platt JL, Lauer V, Salama R,
Kranz F, Neumann VK, Wach S, Stöhr C, Hartmann A, Eckardt KU, et
al: Genetic variation at the 8q24.21 renal cancer susceptibility
locus affects HIF binding to a MYC enhancer. Nat Commun.
7:131832016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer
A, Llobet-Navas D, Rajbhandari P, Bansal M, Guarnieri P, Silva J
and Califano A: An extensive microRNA-mediated network of RNA-RNA
interactions regulates established oncogenic pathways in
glioblastoma. Cell. 147:370–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karreth FA, Tay Y, Perna D, Ala U, Tan SM,
Rust AG, DeNicola G, Webster KA, Weiss D, Perez-Mancera PA, et al:
In vivo identification of tumor-suppressive PTEN ceRNAs in an
oncogenic BRAF-induced mouse model of melanoma. Cell. 147:382–395.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsu SD, Tseng YT, Shrestha S, Lin YL,
Khaleel A, Chou CH, Chu CF, Huang HY, Lin CM, Ho SY, et al:
miRTarBase update 2014: An information resource for experimentally
validated miRNA-target interactions. Nucleic Acids Res.
42:(Database Issue). D78–D85. 2014. View Article : Google Scholar : PubMed/NCBI
|