Introduction
Senile dementia is a progressive neurodegenerative
syndrome characterized by cognitive function disorders and decline
caused by degeneration and apoptosis of nerve cells in the brain
and nervous system (1). Clinical
manifestations of senile dementia include memory impairment,
aphasia, damage of visual spatial skill, executive dysfunction and
personality and behavioral dysfunctions (2,3).
Neurodegenerative disorders in patients with senile dementia
frequently result in an increased mortality rate in the elderly
population (4,5). Systematic reviews and meta-analyses
have investigated the association between statins and senile
dementia. Statin drugs reduce the plasma concentration of
cholesterol and have been reported to reduce the risk of senile
dementia (6–8).
Previous reviews have discussed the effectiveness of
statins in the prevention of dementia, but there is insufficient
evidence to recommend statins for the treatment of Alzheimer's
disease, a condition that leads to the development of numerous
forms of dementia (9,10). Pathogenesis of senile dementia
mainly targets the hippocampal area associated with memory and
cognition, and leads to cognitive function disorders and
impairments of cognition including, memory, language and attention
(11,12). Elevated rates of apoptosis and
oxidative stress in hippocampal cells are frequently observed in
patients with senile dementia (13).
Apoptosis of hippocampal cells is a symptom of
senile dementia (14,15). Petit et al (16) reported that the prevention of basal
and induced neuronal apoptosis abrogates mutations associated with
Parkinson disease. Previous reports also indicated that apoptosis
of hippocampal cells contributes to aggravation of senile dementia
and leads to cognitive impairments, memory deterioration, formation
of amyloid plaques, loss of neurons and synapses, and aggregation
neurofibrillary tangles (17,18).
These reports suggest that inhibition of apoptosis of hippocampal
cells is beneficial for the suppression of cognitive
impairments.
A previous study indicated that memory dysfunction
and failure of energy metabolism induced by oxidative stress are
associated with the development of neurodegenerative disorders
(19). Biomarkers of oxidative
stress in patients with vascular dementia have been observed in
clinical patients with senile dementia (20). Furthermore, activation of the
amyloidogenic pathway can cause oxidative stress, which leads to
mitochondrial accumulation and apoptosis (21). Furthermore, protecting execution of
hippocampal cells against apoptosis caused by oxidative stress in
senile dementia disease was also clearly elaborated in previous
study (22).
In the present study, the anti-apoptotic effects of
simvastatin and potential molecular mechanism underlying the
prevention of pathological processes of senile dementia in a rat
model were investigated. A candidate signaling pathway triggered by
simvastatin and leading to the improvement of cognitive impairment
was evaluated in a rat model of senile dementia. The results of the
present study indicated that simvastatin treatment significantly
reduced amyloid plaques, loss of neurons and synapses and
neurofibrillary tangles induced by oxidative stress, and apoptosis
of hippocampal cells, through the extracellular signal-regulated
kinase (ERK)/AKT serine/threonine kinase (AKT) signaling pathway in
a rat model of senile dementia.
Materials and methods
Animal study
A total of 60 male Sprague Dawley rats (8 weeks old,
240-320 g) were purchased from the Chinese Academy of Sciences
Institute of Biophysics (Beijing, China). All rats were
intraperitoneally injected with scopolamine (1 mg/kg;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) to establish a model
of senile dementia (23). The
injection was repeated five times once every three days and rats
were divided into two groups (30 rats each). Rats with senile
dementia were treated with a single daily intravenous 10 mg/kg/day
simvastatin injection for 60 days or with an equivalent amount of
PBS. All rats were housed at 24–26°C with a 12 h light/dark cycle
and fed ad libitum.
Ethical approval
The present study complied with the Guide for the
Care and Use of Laboratory Animals of China. All experimental
surgeries and animal operations were performed in accordance with
the Ethics of Animal Experiments Defense Research. During all
surgical operations and euthanasia, efforts were made to minimize
suffering. The present study was approved by the Ethics Committee
of the Traditional Chinese Medicine Hospital of Weifang (Weifang,
China).
Cell culture
Hippocampal cells were isolated from the CA1 region
of rats with senile dementia as previously described (24). Hippocampal cells were cultured in
RPMI-1640 medium (Sigma-Aldrich; Merck KGaA) and the culture medium
was supplemented with 10% fetal bovine serum (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 µg/ml penicillin
and streptomycin (Sigma-Aldrich; Merck KGaA) and 10% glutamine
(Invitrogen; Thermo Fisher Scientific, Inc.).
ATF-6 overexpression
Hippocampal cells (1×105) were cultured
in a 6-well plate until 85% confluence; the media was then removed
from the culture plate followed by 3 washes with PBS. Hippocampal
cells were transfected by plentivirus-ATF-6 (ATFOP) using
Lipofectamine 2000 (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocols. After 48 h transfection, cells were used
for further analysis.
Transfection of small interference RNA
(Si-RNA)
Hippocampal cells (4×105 cells/well) were
seeded in a 6-well plate for 24 h at 37°C. The medium was removed
and Opti-MEM (Invitrogen) added for 24 h at 37°C. The siRNA
sequences corresponding to the gene were designed and synthesized
by Shanghai GenePharma Co., Ltd. (Shanghai, China). The siRNA
sequences against ATF-6 (Si-ATF-6): 5′-GAAGGUAGUUGAAUGGUGCAUACAA-3′
or siRNA-vector (Control): 5′-CUCGUCUCAUUGATGACAGTT-3′. After 48 h
transfection, cells were used for further analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from hippocampal cells using
an RNAeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA). Expression
levels of superoxide dismutase (SOD), glutathione (GSH), catalase
(CAT) and inducible nitric oxide synthase (iNOS) in hippocampal
cells were measured by an RT-qPCR kit (AB4104C; Invitrogen; Thermo
Fisher Scientific, Inc.) with β-actin as an endogenous control
(25). The PCR cycling conditions
were performed at 95°C for 30 sec and 45 cycles of 95°C for 5 sec,
56.5°C for 10 sec and 72°C for 10 sec. All primers (Table I) were synthesized by Invitrogen
(Thermo Fisher Scientific, Inc.). Relative mRNA expression changes
were calculated by the 2−ΔΔCq method (26). The results are expressed as a fold
change compared with the β-actin control.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Target gene | Forward primer | Reverse primer |
|---|
| SOD |
5′-TCTGGATGGGTGTGGCTTGCTCT-3′ |
5′-GCATGCTCCCAAACATCGATC-3′ |
| GSH |
5′-GAAAGCCCAGTCTTCATTGC-3′ |
5′-TTGGAACCGTGCTAGTCTCA-3′ |
| iNOS |
5′-GACGAGACGGATAGGCAGAG-3′ |
5′-CACATGCAAGGAAGGGAACT-3′ |
| CAT |
5′-CGTGCTGAATGAGGAACAGA-3′ |
5′-AGTCAGGGTGGACCTCAGTG-3′ |
| Foxp2 |
5′-AGCAACCAGCTCTTCAGGTTCC-3′ |
5′-ACGTTGTATTTGTCTGAGTACCG-3′ |
| SHIP |
5′-CCTCAAGATGCACATCCGAAG-3′ |
5′-AAAGTTTTCAATGACCAAGC-3′ |
| cREB |
5′-GATACTCAGGCAGAGATGATCTACCC −3′ |
5′-AGACCAGGCACCAGACCAAAGA-3′ |
| β-actin |
5′-GTGGGCGCCCAGGCACCA-3′ |
5′-CTCCTTAATGTCACGCACGATTT-3′ |
Western blotting
Hippocampal cells (1×106) were
homogenized in lysate buffer containing protease-inhibitor and were
centrifuged at 8,000 × g at 4°C for 10 min. The supernatant was
used for analyzing protein expression. Protein concentration was
measured by a BCA protein assay kit (Thermo Scientific Fisher
Scientific, Inc.). Protein samples (20 µg) were separated on 12%
SDS-PAGE and transferred onto PVDF membranes (EMD Millipore,
Billerica, MA, USA). Following blocking with 5% bovine serum
albumin at 37°C for 1 h, the following primary antibodies were used
in immunoblotting assays: Anti Bcl2-associated agonist of cell
death (Bad; cat. no. ab32445; 1:1,000); apoptosis regulator BAX
(Bax; cat. no. ab32503; 1:1,000); P53 (cat. no. ab1101; 1:1,000);
Bcl-2 apoptosis regulator (Bcl-2; cat. no. ab32124; 1:1,000); SOD
(cat. no. ab13533; 1:1,000); GSH (cat. no. ab94733; 1:1,000); iNOS
(cat. no. ab15323; 1:500); CAT (cat. no. ab78292; 1:500); forkhead
box protein P2 (Foxp2; cat. no. ab16046; 1:1,000); SHIP (cat. no.
ab59338; 1:1,000); cAMP-response element binding protein (cREB;
cat. no. ab33613; 1:1,000); activating transcription factor-6
(ATF-6; cat. no. ab122897; 1:1,000); ERK (cat. no. ab54230;
1:1,000); AKT (cat. no. ab8805; 1:1,000) and β-actin (cat. no.
ab8226; 1:2,000; all from Abcam, Cambridge, UK) for 12 h at 4°C.
Horseradish peroxidase-conjugated antibody (cat. no. HAF019;
1:5,000; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used as
a secondary antibody for 2 h at 37°C and detected using a western
blotting Luminol reagent (cat. no. 12015218001; Sigma-Aldrich;
Merck KGaA) for enhanced chemiluminescence. The density of the
bands was analyzed by Quantity one software version 4.62 (Bio-Rad
Laboratories, Inc.).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
The TUNEL assay was used for the analysis of
apoptosis of hippocampal neuron cells in experimental rats
following simvastatin treatment (10 mg/kg/day; Sigma-Aldrich; Merck
KGaA) or an equivalent dose of PBS. Procedures were performed as
previously described (27).
Briefly, cells (1×104) were cultured in a 6-well plate
for at 37°C for 12 h. Then cells were fixed with 4%
paraformaldehyde followed by permeabilization with 0.1% Triton
X-100. Subsequently, apoptosis of cells were stained with TUNEL
reaction mixture (Sigma-Aldrich; Merck KGaA) at 37°C for 2 h. Cells
were washed 3 times in TBST. TUNEL assays were conducted using a
TUNEL fluorescence FITC kit (Roche, Indianapolis, IN, USA)
according to the manufacturer's instructions. Hippocampal neuronal
cell images in 6 fields were captured using a Zeiss LSM 510
confocal microscope (Zeiss AG, Oberkochen, Germany) at a wavelength
of 488 nm.
Cognitive tests
Cognitive competence of rats was determined by open
field activity levels in a black Plexiglas box (60×60×25 cm) to
analyze therapeutic effects of simvastatin. Rats were placed in the
open black box for 10 min and their behavior was monitored and
evaluated using an auto-tracking system (SmarTrack GPS Tracker;
SmarTrack, Coalville, UK). The Morris water maze test was performed
prior to and following the treatment with simvastatin to measure
changes in the cognitive competence. The Morris water maze
experiment was performed in a circular stainless-steel tank (155 cm
diameter, 60 cm depth) filled with water to a depth of 40 cm
(27.0±1.0°C) that was made opaque by the addition of skim milk.
Rats learned to find a hidden circular platform (10 cm diameter,
1.5 cm below the surface of the water) in a fixed area in one
quadrant of the tank. A modified Rankin scoring (mRS) system
(28) was used for assessing the
therapeutic effects. Good outcomes and futile outcomes were defined
as mRS scores ≤2 and 5–6, respectively. Rats tend to favor the
closed arm when they feel strong anxiety (29).
Immunological staining
The effects of the simvastatin treatment on neuronal
loss, amyloid plaques and neurofibrillary tangles were evaluated
using immunohistochemical (IHC) staining for
neuroprotection-associated proteins in hippocampi from experimental
rats. Staining was performed on cerebral neurons of hippocampi of
randomly selected animals from simvastatin- or PBS-treated groups.
IHC procedures were previously reported in detail (30). Brains were frozen and coronal
sections were cut in a cryostat following perfusion, fixation using
95% alcohol for 15 min at 37°C and cryoprotection. Free-floating
sections (4 µm) were rinsed and placed in a solution containing
anti-p75 nerve growth factor (NGF) receptor antibody (cat. no.
ab8874; 1:500; Abcam) or anti-homocysteine (Hcy) antibody (cat. no.
ab15154; 1:500; Abcam). Following rinsing, sections were incubated
in the presence of a biotinylated horse anti-rabbit antibody (cat.
no. a0545; 1:500; Chemicon; Merck KGaA) for NGF or Hcy staining for
2 h at 37°C. Sections were washed and observed using fluorescent
video microscopy (BZ-9000; Keyence Corporation, Osaka, Japan).
Thionin staining
The hippocampal area from experimental rats was
post-fixed in Böhm-Sprenger fixative (methanol, formalin, and
acetic acid, in a 16:3:1 volume ratio) for 1 h at 37°C, hydrolyzed
for 1 h in 5N hydrochloric acid, immersed in the thionin
(Sigma-Aldrich; Merck KGaA) staining solution for 1 h, and rinsed
thrice in a bisulphite rinse solution [0.5% sodium bisulphite (w/v)
in 0.05N hydrochloric acid], each separated by water rinses. Images
was observed by light microscope (Olympus BX51; Olympus
Corporation, Tokyo, Japan).
Statistical analysis
All data are expressed as the mean of triplicate
experiments ± standard deviation and analyzed using Student's t
test or one-way analysis of variance followed by a Tukey honest
significant difference post-hoc test. All data were analyzed using
SPSS software (version 19.0; IBM Corp., Armonk, NY, USA), GraphPad
Prism (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA) and
Microsoft Excel (Microsoft Corporation, Redmond, WA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
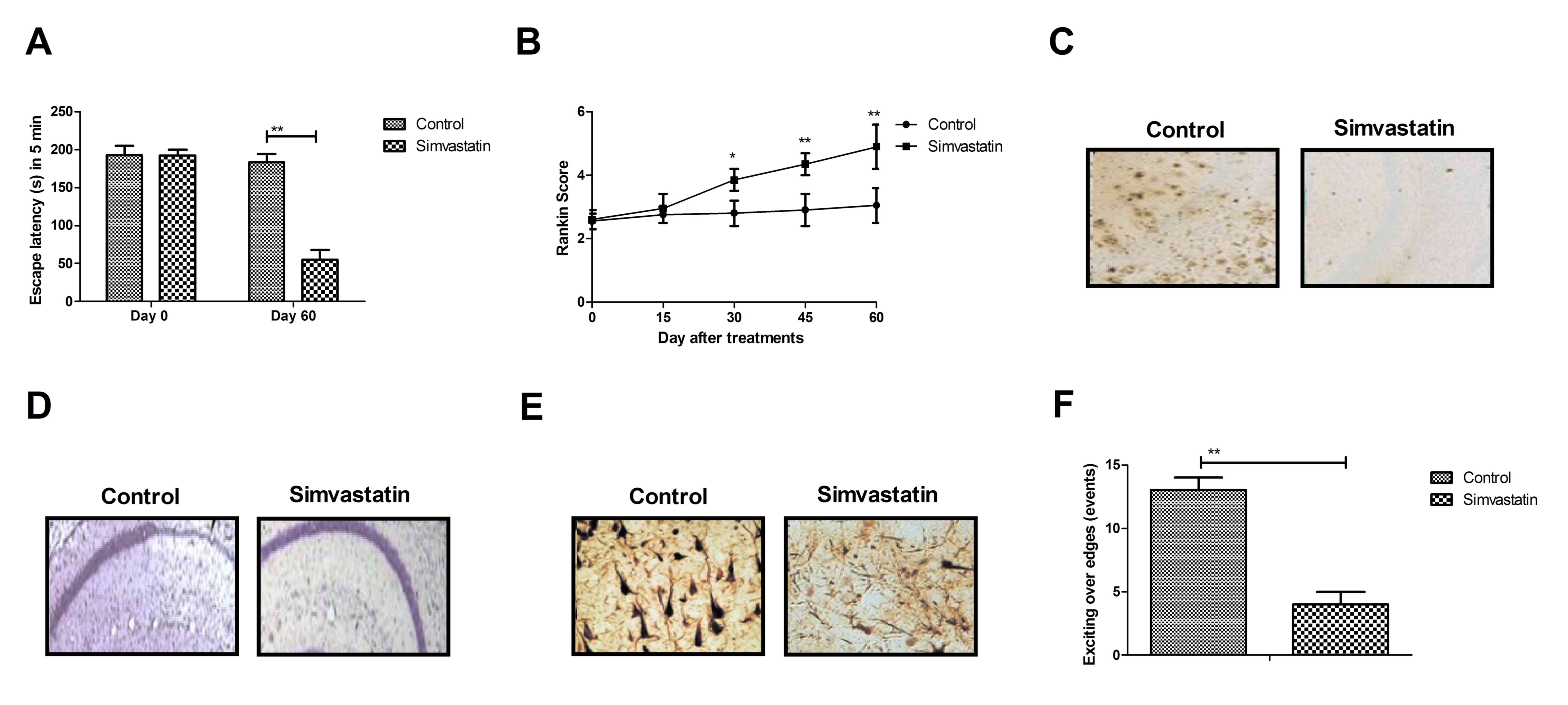
Simvastatin demonstrates beneficial
effects on cognitive competence of hippocampal network in rats with
senile dementia
In vivo efficacy of simvastatin on the
treatment of senile dementia in rats was analyzed using cognitive
experiments. As presented in Fig.
1A, simvastatin treatment improved cognitive impairments
determined by the escape latency during a 5-min study period. The
memory competence, evaluated by the Rankin preclinical score of
cognitive competences, was ameliorated by simvastatin treatment
(Fig. 1B). Histological analysis
of hippocampus demonstrated that amyloid plaques and loss of
neurons were decreased in simvastatin-treated rats with senile
dementia compared with the control (Fig. 1C and D). The results of the present
study also demonstrated that the abundance of neurofibrillary
tangles and anxiety was improved by simvastatin treatment in rats
with senile dementia determined by Morris water maze (Fig. 1E and F). These results suggest that
simvastatin treatment induces beneficial effects on cognitive
competence of the hippocampal network.
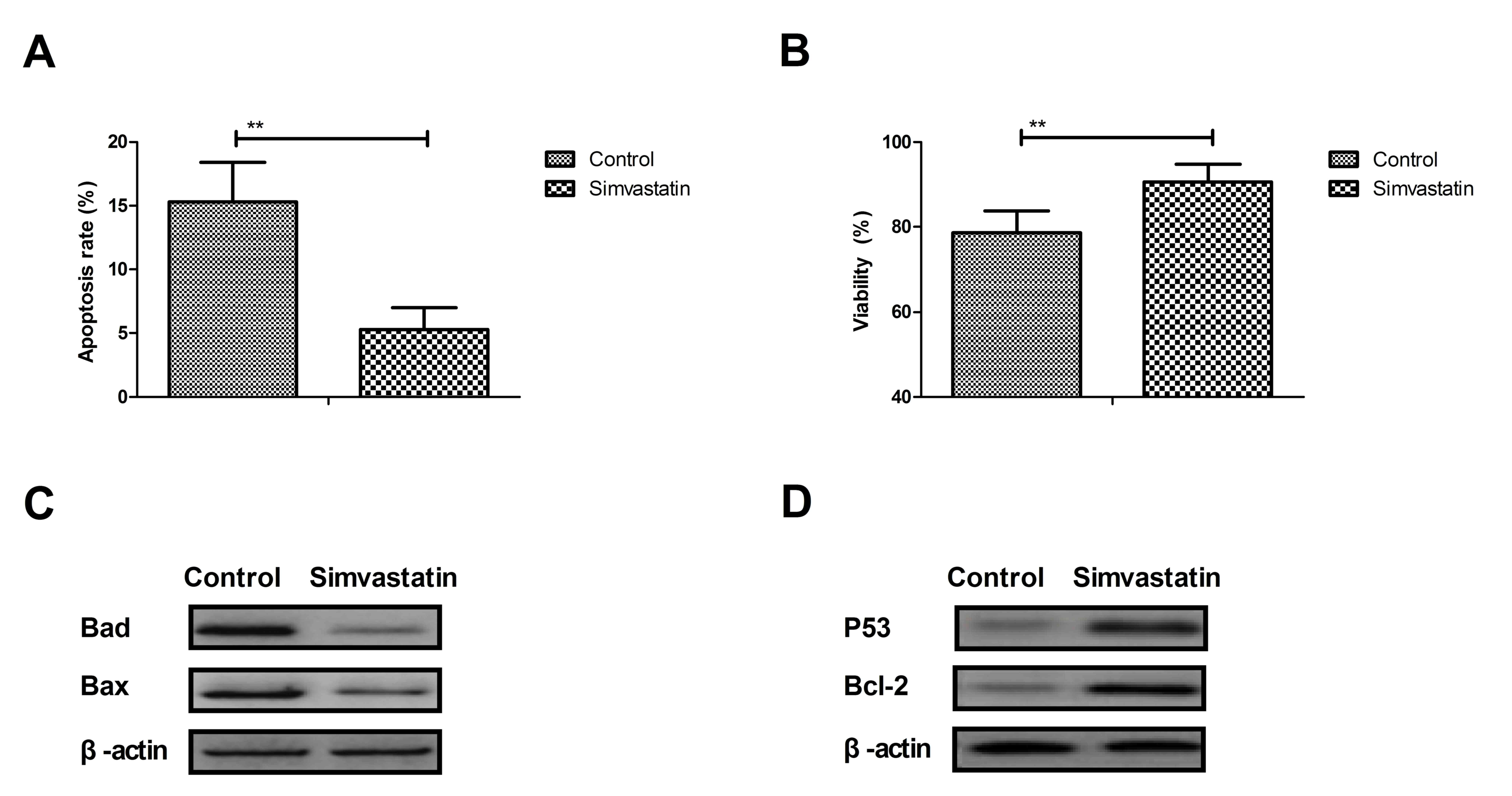
Simvastatin inhibits apoptosis of
hippocampal cells in the rat model of senile dementia
Apoptosis of hippocampal cells is associated with
senile dementia. The efficacy of simvastatin in the prevention of
apoptosis of hippocampal cells in the rat model of senile dementia
was investigated. As determined by the TUNEL assay and presented in
Fig. 2A, simvastatin (10
mg/kg/day) treatment inhibited apoptosis of hippocampal cells. The
results of the present study demonstrated that simvastatin improved
the viability of hippocampal cells compared with the control group
(Fig. 2B). Western blotting
demonstrated that Bad and Bax were downregulated, while P53 and
Bcl-2 were upregulated in hippocampal cells from the CA1 region in
experimental rats compared with the control group (Fig. 2C and D). These results indicate
that simvastatin potentially inhibits apoptosis of hippocampal
cells in the rat model of senile dementia through the regulation of
apoptosis-associated protein expression.
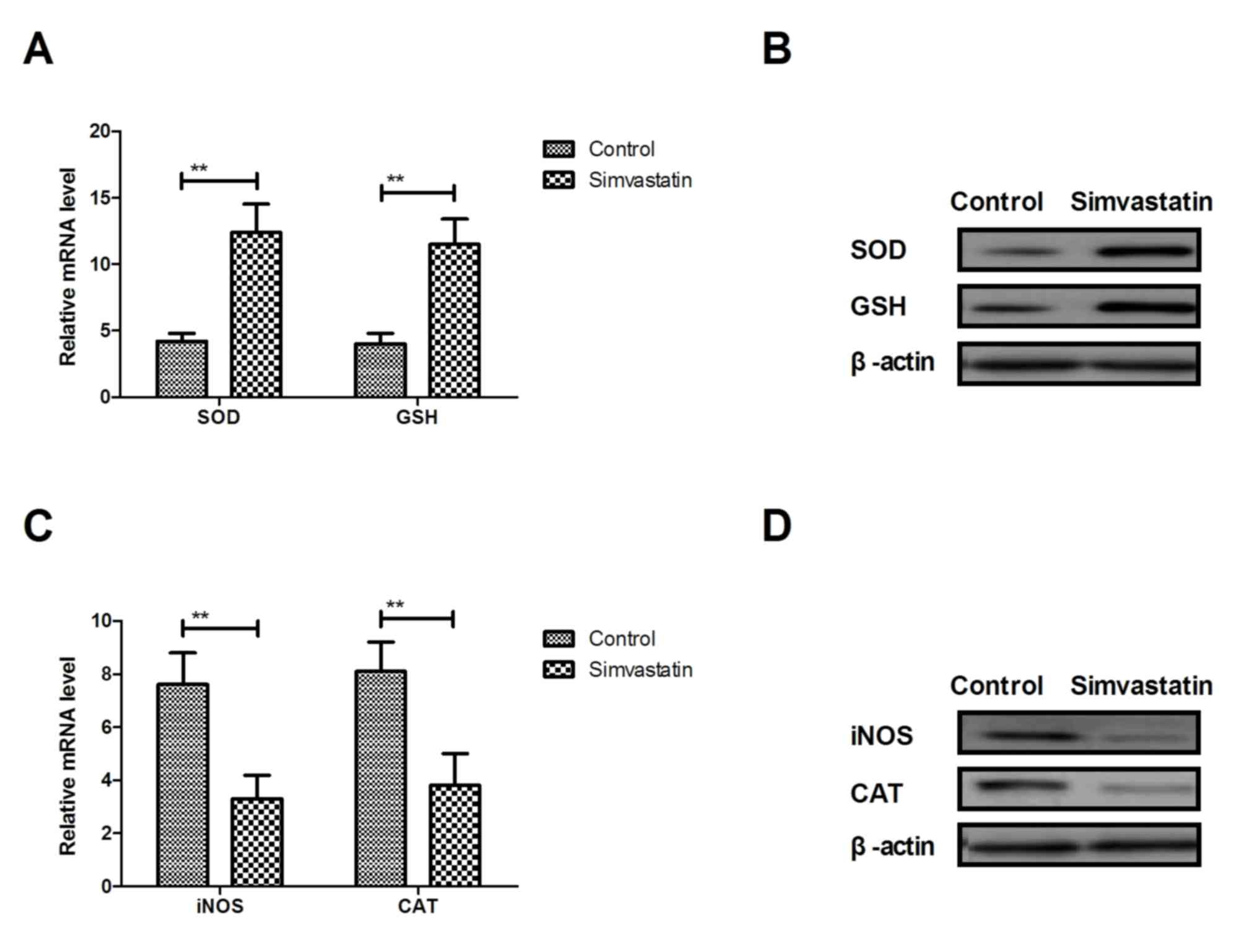
Simvastatin ameliorates oxidative
stress of hippocampal cells in the rat model of senile
dementia
Oxidative stress serves an essential role in the
programmed hippocampal cell death. As presented in Fig. 3A and B, gene and protein expression
levels of SOD and GSH were upregulated by simvastatin in
hippocampal cells in the rat model of senile dementia. However,
gene and protein expression levels of iNOS and CAT were
downregulated in hippocampal cells in the rat model of senile
dementia (Fig. 3C and D). These
results indicate that simvastatin treatment ameliorates oxidative
stress of hippocampal cells in the rat model of senile
dementia.
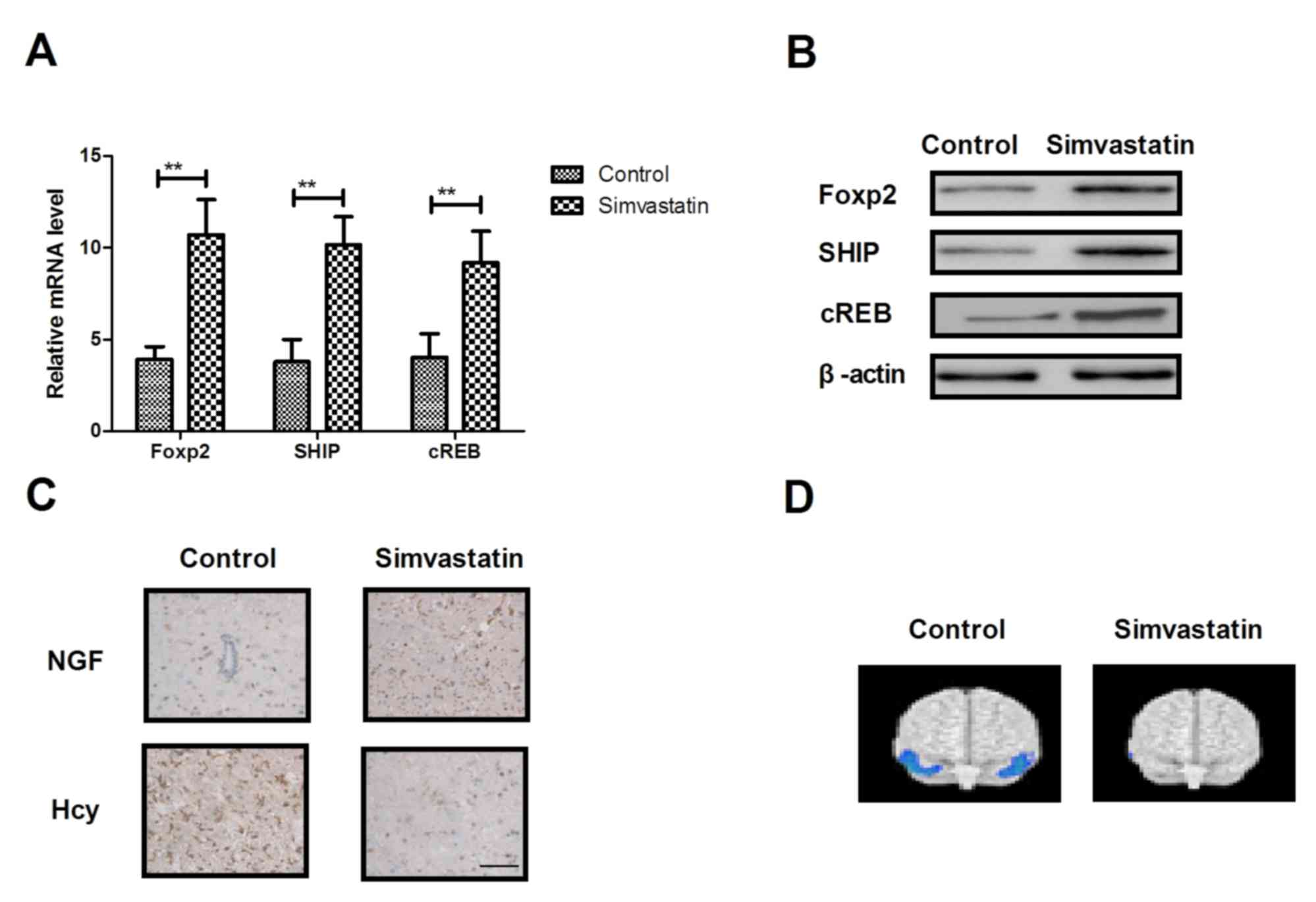
Simvastatin improves neuroprotective
protein expression in hippocampal cells in the rat model of senile
dementia
The neuroprotective effect is an important aspect of
anti-dementia drugs. Therefore, the present study analyzed changes
in the expression of neuroprotective proteins in hippocampal cells
in the rat model of senile dementia following treatment with
simvastatin. As presented in Fig. 4A
and B, simvastatin treatment increased gene and protein
expression levels of Foxp2, SHIP and cREB in hippocampal cells.
Immunohistochemistry demonstrated that simvastatin increased the
expression of NGF and down-regulated Hcy expression levels in
neurons (Fig. 4C). It was also
observed that thionin staining of hippocampal area in
simvastatin-treated rats demonstrated a marked difference in the
dispersion of the pyramidal cell layer (Fig. 4D). These results indicate that
simvastatin treatment can improve neuroprotective protein
expression in hippocampal cells in the rat model of senile
dementia.
Simvastatin regulates apoptosis of
hippocampal cells through the ATF-6-mediated ERK/AKT signaling
pathway
In order to analyze the potential mechanism
underlying the activity of simvastatin in the progression of senile
dementia, the ERK/AKT signaling pathway in hippocampal cells was
investigated. As presented in Fig.
5A, expression levels of ATF-6, ERK and AKT were downregulated
by simvastatin treatment in hippocampal cells compared with the
control group. In vitro assays demonstrated that ATF-6
overexpression increased apoptosis in simvastatin-induced
hippocampal cells (Fig. 5B).
Expression and phosphorylation levels of ERK and AKT were also
increased by ATF-6 overexpression in hippocampal cells (Fig. 5C). However, ATF-6 knockdown
enhanced apoptosis of hippocampal cells and downregulation of
ERK1/2 and AKT expression levels in hippocampal cells (Fig. 5D and E). ATF-6 overexpression
blocked simvastatin-regulated oxidative stress in hippocampal cells
(Fig. 5F). These results suggest
that simvastatin regulates apoptosis of hippocampal cells through
ATF-6-mediated ERK/AKT signaling pathway.
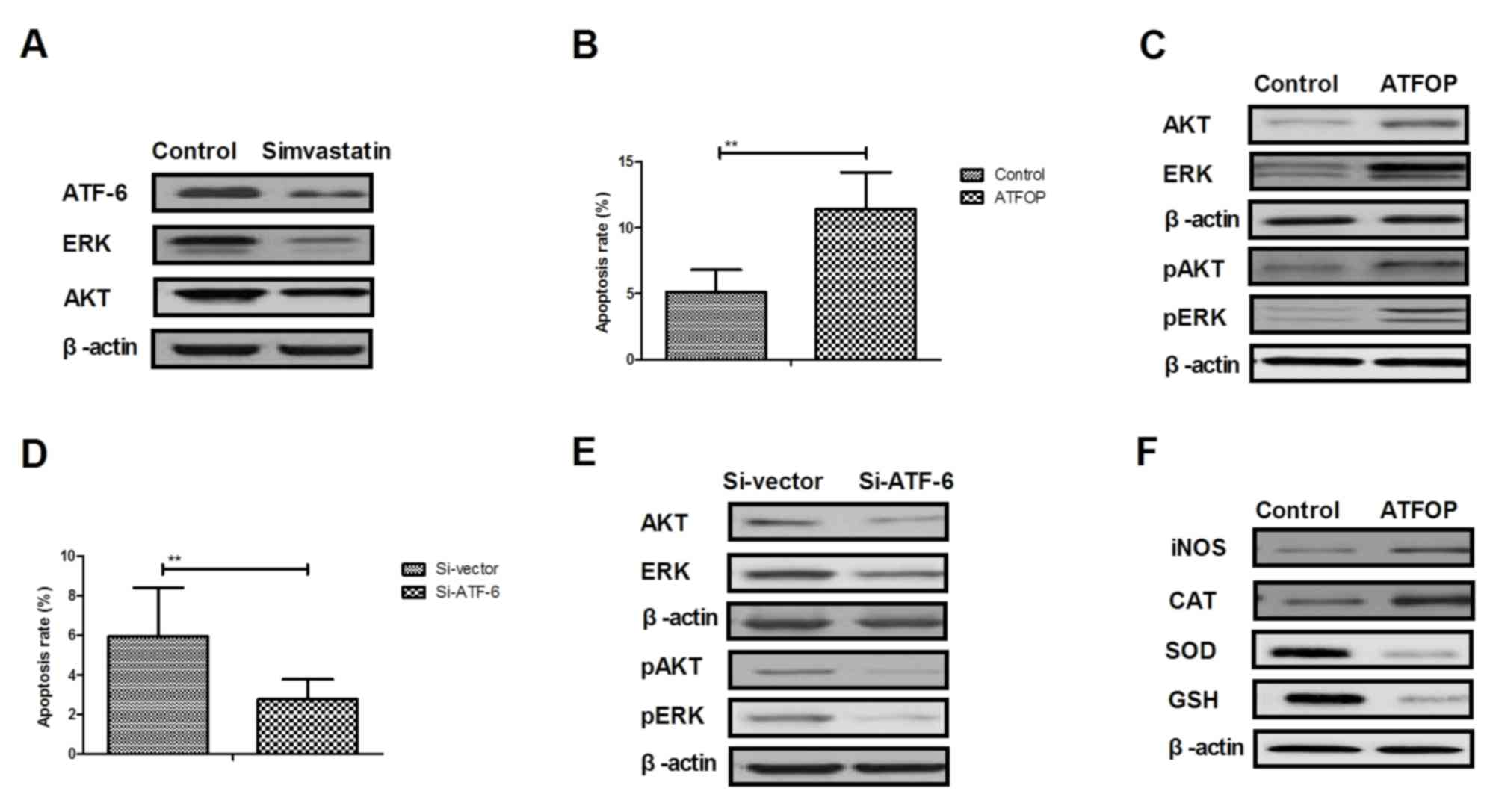 | Figure 5.Simvastatin regulates apoptosis of
hippocampal cells through ATF-6-mediated ERK/AKT signaling pathway.
(A) Western blotting of expression levels of ATF-6, ERK and AKT in
hippocampal cells. (B) ATF-6 overexpression abolishes
simvastatin-inhibited apoptosis of hippocampal cells. (C)
Expression and phosphorylation levels of pERK and pAKT were
reverses by ATF-6 overexpression in hippocampal cells. (D) ATF-6
knockdown enhances simvastatin-inhibited apoptosis of hippocampal
cells. (E) Si-ATF-6 downregulates of ERK1/2 and AKT expression
levels in hippocampal cells. (F) ATF-6 overexpression blocks
simvastatin-regulated oxidative stress in hippocampal cells. ERK,
extracellular signal-regulated kinase; AKT, AKT serine/threonine
kinase; ATF-6, activating transcription factor-6; ATFOP, ATF-6
overexpression; Si, small interfering RNA; p, phosphorylated; ROS,
reactive oxygen species; CAT, catalase; SOD, superoxide dismutase;
GSH, glutathione. |
Discussion
The majority of patients with senile dementia suffer
from vascular dementia, which decreases the quality of their life
(31). Senile dementia damages the
nervous system and impairs the cognitive ability in the elderly
(32). Hippocampal cell apoptosis
is highly correlated with hypoxia, ischemia and injury evidenced by
changes in the infarct volume during chronic cerebral hypoperfusion
(33). It has been demonstrated
that simvastatin has a crucial role in apoptosis of hippocampal
cells, memory recovery, cognitive rehabilitation and neuronal
survival (34). In addition,
oxidative damage of brain regions may induce apoptosis of
hippocampal cells, which could lead to cognitive dysfunction in
patients with senile dementia (35). Furthermore, upregulation of the
activity-dependent neuroprotective protein in the Tau mutation is
regarded a novel marker for the onset of frontotemporal dementia
(36). In the present study, the
benefits of simvastatin for the treatment of senile dementia were
further investigated and a potential mechanism of
simvastatin-mediated anti-apoptosis in hippocampal cells was
analyzed in a rat model of senile dementia.
Simvastatin is a statin that reduces
hypercholesterolemia. Simvastatin has neuroprotective potential
against 6-hydroxydopamine-induced Parkinson's disease-like symptoms
(37). Neuronal marker recovery
following simvastatin treatment of dementia in the rat brain was
investigated in vivo by magnetic resonance (38). El-Dessouki et al (39) revealed that the neuroprotective
effect of simvastatin is mediated via regulation of
neurotransmitters and activity of acetylcholinesterase in
L-methionine-induced vascular dementia. In addition, a previous
study proposed a novel mechanism of simvastatin-mediated
neuroprotection in neurodegenerative diseases through the
modulation of seladin-1-associated metabolic regulation (40). A previous molecular analysis
demonstrated that the neuroprotective effect of simvastatin is
derived from the improvement of endoplasmic reticulum stress
response in a rat model of global forebrain ischemia/reperfusion
(41). In the present study,
simvastatin improved cognitive impairments, memory competence,
reduced the number of amyloid plaques and diminished the loss of
neurons and synapses, neurofibrillary tangles and oxidative damage
in experimental rats.
Apoptosis of hippocampal cells aggravates the degree
of dementia and damages the cranial nerve system in patients with
senile dementia (17). In the
present study, simvastatin treatment suppressed apoptosis of
hippocampal cells in CA1 regions and contributed to the improvement
of cognitive competence. Previous research indicated that
simvastatin exerts beneficial outcomes on patients with senile
dementia due to the anti-neoplastic and anti-apoptotic effects in a
number of cell types (42). In
addition, attenuation of apoptosis, inflammation and oxidative
stress in the blood and brain tissues is beneficial for the
treatment of scopolamine-induced dementia in rats (23). A previous study suggested that
ATF-6 is associated with apoptosis of dopaminergic neurons and
accumulates in the core of Lewy bodies in Parkinson's disease
(43). The results of the present
study suggest that simvastatin treatment inhibits ATF-6-mediated
ERK/AKT signaling pathway in hippocampal cells in CA1 regions in
the rat model of senile dementia.
Expression of ERK is upregulated in hippocampal
neurons of mice with vascular dementia (44). Hu et al (45) demonstrated that autophagy and
AKT/cREB signaling are involved in the neuroprotective effects of
nimodipine in a rat model of vascular dementia. Yao et al
(46) demonstrated that ATF-6
mediates oxidized low-density lipoprotein-induced cholesterol
accumulation and apoptosis in macrophages by upregulating DNA
damage-inducible transcript 3 protein expression. In the present
study, simvastatin treatment downregulated ATF-6, ERK and AKT
expression in CA1 hippocampal cells in the rat model of senile
dementia, which is consistent with a previous report (47). Endogenous expression of ATF-6
abolished the protective effects of simvastatin-inhibited neuronal
damage in hippocampal cells in the CA1 region through the
modulation of the activity of the ERK/AKT signal pathway.
In conclusion, the present study indicates that
simvastatin may be an efficient agent for the treatment of senile
dementia. Simvastatin treatment protects hippocampal neurons
against apoptosis that further repairs the nervous system in the
brain. The results of the present study indicate that simvastatin
treatment suppresses apoptosis of hippocampal cells in the CA1
region through ATF-6-mediated ERK/AKT signaling pathway. These
results suggest that simvastatin is a promising agent for the
treatment of senile dementia.
References
|
1
|
Zeng L, Zou Y, Kong L, Wang N, Wang Q,
Wang L, Cao Y, Wang K, Chen Y, Mi S, et al: Can Chinese Herbal
medicine adjunctive therapy improve outcomes of senile vascular
dementia? Systematic review with meta-analysis of clinical trials.
Phytother Res. 29:1843–1857. 2015. View
Article : Google Scholar
|
|
2
|
Song MH, Hamada H and Mimura M:
Semiological differences between late-life schizophrenia and senile
dementia. Keio J Med. 63:34–38. 2014. View Article : Google Scholar
|
|
3
|
Kawahara M, Mizuno D, Koyama H, Konoha K,
Ohkawara S and Sadakane Y: Disruption of zinc homeostasis and the
pathogenesis of senile dementia. Metallomics. 6:209–219. 2014.
View Article : Google Scholar
|
|
4
|
Disse M, Reich H, Lee PK and Schram SS: A
Review of the association between parkinson disease and malignant
melanoma. Dermatol Surg. 42:141–146. 2016. View Article : Google Scholar
|
|
5
|
Rizzo G, Copetti M, Arcuti S, Martino D,
Fontana A and Logroscino G: Accuracy of clinical diagnosis of
Parkinson disease: A systematic review and meta-analysis.
Neurology. 86:566–576. 2016. View Article : Google Scholar
|
|
6
|
Vos E and Nehrlich HH: Use of statins and
incidence of dementia and cognitive impairment without dementia in
a cohort study. Neurology. 73:406–407. 2009. View Article : Google Scholar
|
|
7
|
Wilson PW and Vega GL: Counterpoint:
Lipoproteins and dementia: Is there compelling evidence to treat
Alzheimer's patients with statins? J Clin Lipidol. 2:394–396. 2008.
View Article : Google Scholar
|
|
8
|
Horsdal HT, Olesen AV, Gasse C, Sørensen
HT, Green RC and Johnsen SP: Use of statins and risk of
hospitalization with dementia: A Danish population-based
case-control study. Alzheimer Dis Assoc Disord. 23:18–22. 2009.
View Article : Google Scholar :
|
|
9
|
McGuinness B, O'Hare J, Craig D, Bullock
R, Malouf R and Passmore P: Statins for the treatment of dementia.
Cochrane Database Syst Rev: CD007514. 2010. View Article : Google Scholar
|
|
10
|
McGuinness B, Craig D, Bullock R and
Passmore P: Statins for the prevention of dementia. Cochrane
Database Syst Rev: CD003160. 2009. View Article : Google Scholar
|
|
11
|
Pettigrew C, Soldan A, Zhu Y, Wang MC,
Brown T, Miller M and Albert M: BIOCARD Research Team: Cognitive
reserve and cortical thickness in preclinical Alzheimer's disease.
Brain Imaging Behav. 11:357–367. 2017. View Article : Google Scholar
|
|
12
|
Fritz NE, Kegelmeyer DA, Kloos AD, Linder
S, Park A, Kataki M, Adeli A, Agrawal P, Scharre DW and Kostyk SK:
Motor performance differentiates individuals with Lewy body
dementia, Parkinson's and Alzheimer's disease. Gait Posture.
50:1–7. 2016. View Article : Google Scholar
|
|
13
|
Morimoto S, Kuzuhara S and Kokubo Y:
Increased oxidative stress in patients with amyotrophic lateral
sclerosis/Parkinsonism-dementia complex in the Kii peninsula,
Japan. Mov Disord. 24:123–126. 2009. View Article : Google Scholar
|
|
14
|
Jahng GH, Oh J, Lee DW, Kim HG, Rhee HY,
Shin W, Paik JW, Lee KM, Park S, Choe BY and Ryu CW: Glutamine and
glutamate complex, as measured by functional magnetic resonance
spectroscopy, Alters during Face-name association task in patients
with mild cognitive impairment and Alzheimer's disease. J
Alzheimers Dis. 53:7452016. View Article : Google Scholar
|
|
15
|
Nesteruk T, Nesteruk M, Styczynska M,
Barcikowska-Kotowicz M and Walecki J: Radiological evaluation of
strategic structures in patients with mild cognitive impairment and
Early Alzheimer's disease. Pol J Radiol. 81:288–294. 2016.
View Article : Google Scholar :
|
|
16
|
Petit A, Kawarai T, Paitel E, Sanjo N, Maj
M, Scheid M, Chen F, Gu Y, Hasegawa H, Salehi-Rad S, et al:
Wild-type PINK1 prevents basal and induced neuronal apoptosis, a
protective effect abrogated by Parkinson disease-related mutations.
J Biol Chem. 280:34025–34032. 2005. View Article : Google Scholar
|
|
17
|
Sun ZK, Ma XR, Jia YJ, Liu YR, Zhang JW
and Zhang BA: Effects of resveratrol on apoptosis in a rat model of
vascular dementia. Exp Ther Med. 7:843–848. 2014. View Article : Google Scholar :
|
|
18
|
Kaul M and Lipton SA: Signaling pathways
to neuronal damage and apoptosis in human immunodeficiency virus
type 1-associated dementia: Chemokine receptors, excitotoxicity,
and beyond. J Neurovirol. 10 Suppl 1:S97–S101. 2004. View Article : Google Scholar
|
|
19
|
Huang JL, Fu ST, Jiang YY, Cao YB, Guo ML,
Wang Y and Xu Z: Protective effects of Nicotiflorin on reducing
memory dysfunction, energy metabolism failure and oxidative stress
in multi-infarct dementia model rats. Pharmacol Biochem Behav.
86:741–748. 2007. View Article : Google Scholar
|
|
20
|
Shi GX, Liu CZ, Wang LP, Guan LP and Li
SQ: Biomarkers of oxidative stress in vascular dementia patients.
Can J Neurol Sci. 39:65–68. 2012. View Article : Google Scholar
|
|
21
|
Hernández-Zimbron LF and Rivas-Arancibia
S: Oxidative stress caused by ozone exposure induces β-amyloid 1–42
overproduction and mitochondrial accumulation by activating the
amyloidogenic pathway. Neuroscience. 304:340–348. 2015. View Article : Google Scholar
|
|
22
|
Bayir H, Kapralov AA, Jiang J, Huang Z,
Tyurina YY, Tyurin VA, Zhao Q, Belikova NA, Vlasova II, Maeda A, et
al: Peroxidase mechanism of lipid-dependent cross-linking of
synuclein with cytochrome C: Protection against apoptosis versus
delayed oxidative stress in Parkinson disease. J Biol Chem.
284:15951–15969. 2009. View Article : Google Scholar :
|
|
23
|
Demirci K, Nazıroğlu M, Övey İS and
Balaban H: Selenium attenuates apoptosis, inflammation and
oxidative stress in the blood and brain of aged rats with
scopolamine-induced dementia. Metab Brain Dis. 32:321–329. 2017.
View Article : Google Scholar
|
|
24
|
Cao Y, Guo N, Lv Y, Shi H and Chen X:
Isolation, primary culture and characterization of mouse glomerular
mesangial cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 29:1315–1318.
2013.(In Chinese).
|
|
25
|
Xiao S, Wang J and Xiao N: MicroRNAs as
noninvasive biomarkers in bladder cancer detection: A diagnostic
meta-analysis based on qRT-PCR data. Int J Biol Markers.
31:e276–e285. 2016. View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Naganuma Y, Ichii O, Otsuka S, Hashimoto Y
and Kon Y: Analysis of TdT-mediated dUTP nick end labeling
(TUNEL)-positive cells associated with cardiac myogenesis in mouse
embryo. J Vet Med Sci. 75:283–290. 2013. View Article : Google Scholar
|
|
28
|
Han M, Choi JW, Rim NJ, Kim SY, Suh HI,
Lee KS, Hong JM and Lee JS: Cerebral infarct volume measurements to
improve patient selection for endovascular treatment. Medicine.
95:e47022016. View Article : Google Scholar :
|
|
29
|
Tanaka Y, Akiyoshi J, Kawahara Y, Ishitobi
Y, Hatano K, Hoaki N, Mori A, Goto S, Tsuru J, Matsushita H, et al:
Infrared radiation has potential antidepressant and anxiolytic
effects in animal model of depression and anxiety. Brain Stimul.
4:71–76. 2011. View Article : Google Scholar
|
|
30
|
Dirani M, Nasreddine W, Abdulla F and
Beydoun A: Seizure control and improvement of neurological
dysfunction in Lafora disease with perampanel. Epilepsy Behav Case
Rep. 2:164–166. 2014. View Article : Google Scholar :
|
|
31
|
Venketasubramanian N, Sahadevan S, Kua EH,
Chen CP and Ng TP: Interethnic differences in dementia
epidemiology: Global and Asia-Pacific perspectives. Dement Geriatr
Cogn Disord. 30:492–498. 2010. View Article : Google Scholar
|
|
32
|
Battistin L and Cagnin A: Vascular
cognitive disorder. A biological and clinical overview. Neurochem
Res. 35:1933–1938. 2010. View Article : Google Scholar
|
|
33
|
Yang HY, Liu Y, Xie JC, Liu NN and Tian X:
Effects of repetitive transcranial magnetic stimulation on synaptic
plasticity and apoptosis in vascular dementia rats. Behav Brain
Res. 281:149–155. 2015. View Article : Google Scholar
|
|
34
|
Wolozin B, Wang SW, Li NC, Lee A, Lee TA
and Kazis LE: Simvastatin is associated with a reduced incidence of
dementia and Parkinson's disease. BMC Med. 5:202007. View Article : Google Scholar :
|
|
35
|
Luca M, Luca A and Calandra C: The role of
oxidative damage in the pathogenesis and progression of Alzheimer's
disease and vascular dementia. Oxid Med Cell Longev.
2015:5046782015. View Article : Google Scholar :
|
|
36
|
Schirer Y, Malishkevich A, Ophir Y, Lewis
J, Giladi E and Gozes I: Novel marker for the onset of
frontotemporal dementia: Early increase in activity-dependent
neuroprotective protein (ADNP) in the face of Tau mutation. PLoS
One. 9:e873832014. View Article : Google Scholar :
|
|
37
|
Kumar A, Sharma N, Gupta A, Kalonia H and
Mishra J: Neuroprotective potential of atorvastatin and simvastatin
(HMG-CoA reductase inhibitors) against 6-hydroxydopamine (6-OHDA)
induced Parkinson-like symptoms. Brain Res. 1471:13–22. 2012.
View Article : Google Scholar
|
|
38
|
Tušková R, Lipták B, Szomolányi P, Vančová
O, Uličná O, Sumbalová Z, Kucharská J, Dubovický M, Trattnig S,
Liptaj T and Kašparová S: Neuronal marker recovery after
Simvastatin treatment in dementia in the rat brain: In vivo
magnetic resonance study. Behav Brain Res. 284:257–264. 2015.
View Article : Google Scholar
|
|
39
|
El-Dessouki AM, Galal MA, Awad AS and Zaki
HF: Neuroprotective effects of simvastatin and cilostazol in
L-Methionine-Induced vascular dementia in rats. Mol Neurobiol.
54:5074–5084. 2017. View Article : Google Scholar
|
|
40
|
Ramos MC, Sierra S, Ramirez C, Velasco J
and Burgos JS: Simvastatin modulates the Alzheimer's
disease-related gene seladin-1. J Alzheimers Dis. 28:297–301.
2012.
|
|
41
|
Urban P, Pavliková M, Sivonová M, Kaplán
P, Tatarková Z, Kaminska B and Lehotský J: Molecular analysis of
endoplasmic reticulum stress response after global forebrain
ischemia/reperfusion in rats: Effect of neuroprotectant
simvastatin. Cell Mol Neurobiol. 29:181–192. 2009. View Article : Google Scholar
|
|
42
|
Bartolomé F, Muñoz U, Esteras N, Alquezar
C, Collado A, Bermejo-Pareja F and Martín-Requero A: Simvastatin
overcomes the resistance to serum withdrawal-induced apoptosis of
lymphocytes from Alzheimer's disease patients. Cell Mol Life Sci.
67:4257–4268. 2010. View Article : Google Scholar
|
|
43
|
Vitte J, Traver S, De Paula Maués A,
Lesage S, Rovelli G, Corti O, Duyckaerts C and Brice A:
Leucine-rich repeat kinase 2 is associated with the endoplasmic
reticulum in dopaminergic neurons and accumulates in the core of
Lewy bodies in Parkinson disease. J Neuropathol Exp Neurol.
69:959–972. 2010. View Article : Google Scholar
|
|
44
|
Hu YZ, Lu PY and Ling L: The expression of
ERK in the hippocampal neurons of mice with vascular dementia.
Zhongguo Ying Yong Sheng Li Xue Za Zhi. 25(466–467): 5202009.(In
Chinese).
|
|
45
|
Hu M, Liu Z, Lv P, Wang H, Zhu Y, Qi Q and
Xu J: Autophagy and Akt/CREB signalling play an important role in
the neuroprotective effect of nimodipine in a rat model of vascular
dementia. Behav Brain Res. 325:79–86. 2017. View Article : Google Scholar
|
|
46
|
Yao S, Zong C, Zhang Y, Sang H, Yang M,
Jiao P, Fang Y, Yang N, Song G and Qin S: Activating transcription
factor 6 mediates oxidized LDL-induced cholesterol accumulation and
apoptosis in macrophages by up-regulating CHOP expression. J
Atheroscler Thromb. 20:94–107. 2013. View Article : Google Scholar
|
|
47
|
Chen PY, Sun JS, Tsuang YH, Chen MH, Weng
PW and Lin FH: Simvastatin promotes osteoblast viability and
differentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutr Res.
30:191–199. 2010. View Article : Google Scholar
|