Introduction
Metabolic syndrome (MS) is a constellation of
pathologic conditions including hypertension, hyperglycemia,
dyslipidemia and abdominal obesity (1). As one of the core components of MS,
hypertension further leads to the development of atherosclerotic
cardiovascular disease (ASCVD) by inducing myocardial ischemia and
apoptosis. In fact, according to the 1992 Framingham Study,
hypertension accounted for ~25% of cardiac failures (2), with a morbidity rate of ≥50% in
elderly patients (3).
In patients with hyperglycemia, particularly with
type II diabetes mellitus, serum insulin levels may be normal or
abnormally increased compared with the reference range. This is
known as insulin resistance (IR) (4). Hyperglycemia and IR are also
considered core components of MS, serving a central role in the
progression of ASCVD (5).
Isosorbide mononitrate (ISMN) is an organic nitrate
used for the prevention and treatment of ASCVD. ISMN generates
exogenous nitric oxide (NO) to expand coronary arteries and improve
the functions of endothelial cells. The resulting dilation of
coronary vessels improves oxygen supply to the myocardium (6). However, studies have also
demonstrated that NO may induce myocardial apoptosis (7). In one study, Wang et al
(8) reported that ISMN synergized
with aspirin in activating the NO signaling and activated apoptosis
in human colon cancer cells.
To the best of the author's knowledge, a direct
investigation into the association between IR, NO content and
myocardial apoptosis in a background of coexisting hypertension in
a rodent animal model has not yet been conducted. In the present
study, a hypertensive model was established by feeding Wistar and
spontaneously hypertensive rats (SHR) with a high sucrose/fat (HSF)
diet, in conjunction with ISMN. ISMN is extensively prescribed for
patients with ASCVD. The present study also aimed to address the
pathophysiological effects from long-term use of ISMN in
hypertensive patients.
Materials and methods
Hypertensive animal model
The hypertensive animal model was established with
14-week-old male SHR (Beijing Wei Tong Li Hua Experimental Animal
Technology Co., Ltd, China) and 14-week-old male Wistar rats
(Experimental Animal Center of Henan, Henan, China). Rats were
housed in conventional cages (5 rats/cage) with free access to food
and water at a controlled temperature (23±2°C) and humidity (55±5%)
under a 12 h light/dark cycle starting at 6:00 a.m. Body weight and
caloric intake were recorded weekly.
A total of 40 male Wistar rats (body weight,
394.45±10.19 g) were randomly divided into 4 groups (1–4); 40
SHR (body weight, 393.14±11.12 g) were also randomly divided into
four groups (5–8). Group 1 and 5 rats were fed a normal
diet. Group 2 and 6 rats were fed a HSF diet. Group 3 and 7 rats
were fed a normal diet supplemented with ISMN. Group 4 and 8 rats
were fed with the HSF and ISMN. The HSF diet was composed of 79%
normal diet, 10% sucrose, 5% lard, 5% cholesterol, and 1%
lithocholic acid. The experimental protocol followed the guidance
for the Care and Use of Laboratory Animals (US National Institutes
of Health, no. 85–23) (9) and the
guidelines of the Animal Care and Use Committee of Zhengzhou
University. The present study was approved by the Ethics Review
Committee of Second Affiliated Hospital of Zhengzhou
University.
Sampling of arterial blood and
myocardium
A total of 12 weeks post-feeding, the rats were
anesthetized by intraperitoneal injection of chloral hydrate (300
mg/kg, C8383; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Arterial blood (1 ml) was drawn by carotid artery intubation;
arterial blood, instead of peripheral (venous) blood is technically
more convenient and allows a larger blood volume for subsequent
experiments. The blood was centrifuged at 500 × g at room
temperature for 5 min and the plasma was snap frozen in liquid
nitrogen and stored at −20°C until use. Subsequently, the rats were
euthanized by supplementary intraperitoneal injection of sodium
pentobarbital (70 mg/kg, 1507002; Sigma-Aldrich; Merck KGaA)
approved by the guidelines of the Animal Care and Use Committee of
Zhengzhou University. The hearts were surgically dissected and
immersed in ice-cold saline to remove blood. A total of 4 sections
of full-thickness myocardium were taken from the left ventricle. Of
these, one section was used immediately for the terminal
deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling
(TUNEL) assay. The remaining sections were snap frozen in liquid
nitrogen and preserved at −80°C for the NO assay and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis. Snap freezing is the technique of rapid sample freezing
with liquid nitrogen and maintains tissue sample integrity and
delays the actions of proteases and nucleases that inhibit
degradation of RNA or proteins used in molecular assays.
TUNEL assay
The TUNEL assay was performed on cardiomyocytes
seeded on chamber slides as previously described (10,11).
In brief, 2 days following isolation, the primary rat
cardiomyocytes were incubated with 1% pericardial fluid for 48 h at
37°C. The cells were fixed in 10% neutral buffered formalin for 10
min at room temperature. The TUNEL assay was performed on fixed
cardiomyocytes with an in-situ Apoptosis Detection kit,
according to the manufacturer's protocols (MK500; Takara Bio Inc.,
Otsu, Japan). Individual nuclei were observed and images were
captured at ×400 with a standard Olympus bright field microscopy
(Olympus Corporation, Tokyo, Japan) for quantitative analysis.
NO assay
NO contents in rat myocardium were determined using
the nitric acid deoxidize enzyme method with a commercial assay kit
according to the manufacturer's protocol (A012; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China).
RT-PCR
A total of 100 mg frozen rat myocardium was
homogenized in 1 ml TRIzol® (15596018; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Total RNA was extracted with
chloroform, precipitated with isopropyl alcohol and ethanol wash,
according to the manufacturer's protocol. Total RNA was dissolved
in nuclease-free water with the concentration determined by UV
spectroscopy at a wavelength of 260 nm. RT was conducted according
to the manufacturer's protocols using a high capacity cDNA reverse
transcription kit (4368814; Thermo Fisher Scientific, Inc.). The
semi-quantitative PCR reactions were carried out on an Eppendorf
thermal cycler with a Taq PCR kit (New England BioLabs,
Inc., Ipswich, MA, USA) and PCR primers listed as below: B-cell
lymphoma 2 (Bcl-2)-associated X protein (Bax) forward,
5′GGGTGGTTGCCCTTTTCTAC3′ and reverse, 5′GGTGAGTGAGGCAGTGAGGA3′;
BCL-2 forward, 5′CTGTGGTCCACCTGACCCTC3′ and reverse,
5′GGCATCCCAGCCTCCGTTAT3′; GAP DH forward, 5′TCAACGGCACAGTCAAGG3′
and reverse, 5′GGGTAGGAACACGGAAGG3′. The PCR reaction included the
following thermocycling conditions: Initial denaturation at 95°C
for 5 min, 35 cycles of denaturation at 95°C for 30 sec, annealing
at an oligo-specific temperature (Bax, 52°C; BCL-2, 58°C; GAPDH,
55°C) for 30 sec, and extension at 72°C for 30 sec. The PCR
products were analysed by 1% agarose gel electrophoresis,
visualized using ethidium bromide and quantified using densitometry
with ImageJ software bundled with 64-bit Java (1.6.0_24; National
Institutes of Health, Bethesda, MD, USA). NAPDH was used as the
internal control.
Western blotting
Total proteins from rat myocardial tissue were
extracted with radioimmunoprecipitation assay lysis buffer
supplemented with protease and phosphatase inhibitors (MSSAFE-5VL;
Sigma-Aldrich, Merck KGaA). Protein concentration was determined
using the standard Bicinchoninic Acid protein assay following the
supplier's protocol (Thermo Fisher Scientific, Inc.). Equal amounts
(typically 30 µg/lane) of proteins were resolved by 4–12% SDS-PAGE
and electrotransferred to nitrocellulose membranes. The membranes
were blocked with 5% non-fat milk in Tris buffer saline containing
0.2% Tween-20 (TBST) at room temperature for 1 h and incubated with
primary antibodies against Bcl-2 (1:1,000), Bax (1:1,000), GAPDH
(1:2,500) at 4°C overnight. The membranes were washed with TBST 3
times then incubated with horseradish peroxidase-conjugated
secondary antibodies (1:2,500) at room temperature for 2 h. All
primary antibodies (sc-20067, sc-56015, sc-516142) were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Secondary
antibodies were acquired from Zymed Laboratories (31460; Thermo
Fisher Scientific, Inc.). The western blot bands were visualized
using enhanced chemiluminescence reagent (cat. no. RPN2232; Beijing
Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) and
quantified using densitometric analysis with ImageJ software
bundled with 64-bit Java (1.6.0_24; National Institutes of
Health).
IR index
The homeostatic model assessment of higher insulin
resistance (HOMA-IR) was used to quantify the IR as follows:
Fasting blood glucose (mmol/l) × fasting insulin (mIU/l)/22.5. The
fasting glucose was determined by our hospital chemistry laboratory
using the oxidase test. The insulin was similarly determined using
the 2-site electrochemiluminescent insulin immunoassay.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Comparison between two groups was analyzed with
two-sample t-test. Comparison of data in more than two groups was
performed with one-way analysis of variance, followed by Fisher's
least significant difference comparison-t-test. Comparison and
Pearson's correlation analysis were performed using SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
HSF and ISMN result in increased
HOMA-IR and NO content in myocardial tissue
In Wistar and SHR rats, 12 weeks of HSF feeding
resulted in significantly increased HOMA-IR compared with the
normal diet (Fig. 1A and B).
Alterations largely consistent with the elevated IR in the two rat
strains were also observed for the blood glucose (data not shown).
HSF feeding had no notable impact on the NO production in
myocardial tissue (Fig. 1C and D).
Supplementing ISMN in the two rat species fed with HSF induced an
increase in HOMA-IR levels. In addition, ISMN significantly
increased the NO content in myocardial tissue (Fig. 1C and D). A combination of HSF and
ISMN was associated with more pronounced increases in the HOMA-IR
in Wistar and SHR rats, indicating the possible synergistic effects
of HSF and ISMN on HOMA-IR. Although not significant by statistical
analysis, HSF also appeared to visually augment the activity of
ISMN in producing NO in myocardial tissue.
 | Figure 1.HSF and ISMN result in HOMA-IR and NO
content increase in the rat myocardial tissue. Following 12 weeks
of feeding with HSF, ISMN or a combination, resulted in increased
HOMA-IR in the arterial blood of (A) Wistar rats and (B) SHR, with
possible synergistic effects between HSF and ISMN. HSF alone
exhibited no notable impact on NO production in (C) Wistar rats or
(D) SHR; however, HSF increased the myocardial NO production in the
two strains of rats. ISMN alone, or in combination with HSF
resulted in pronounced increases in NO content in Wistar and SHR
rats compared with the NS. Data were expressed as mean ± standard
deviation (n=10). **P<0.01, ***P<0.001. HOMA-IR, higher
insulin resistance; HSF, high sucrose/fat diet; ISMN, isosorbide
mononitrate; NO, nitric oxide; NS, normal diet; SHR, spontaneously
hypertensive rats. |
HSF and ISMN activate myocardial
apoptosis
Subsequently, myocardial apoptosis was analyzed by
quantifying the mRNA transcription and protein expression of key
apoptotic components. RT-qPCR with total RNA extracted from two rat
strains revealed that HSF and ISMN feeding resulted in reduced
transcription of the anti-apoptotic gene Bcl-2 (Fig. 2A and B), and increased the
transcription of the pro-apoptotic gene Bax (Fig. 2C and D). The combination of HSF and
ISMN appeared to have synergistic effects. At the protein level,
similar alterations were observed. HSF and ISMN feeding suppressed
the protein expression of Bcl-2 and activated Bax, with a notable
synergism between HSF and ISMN (Fig.
3).
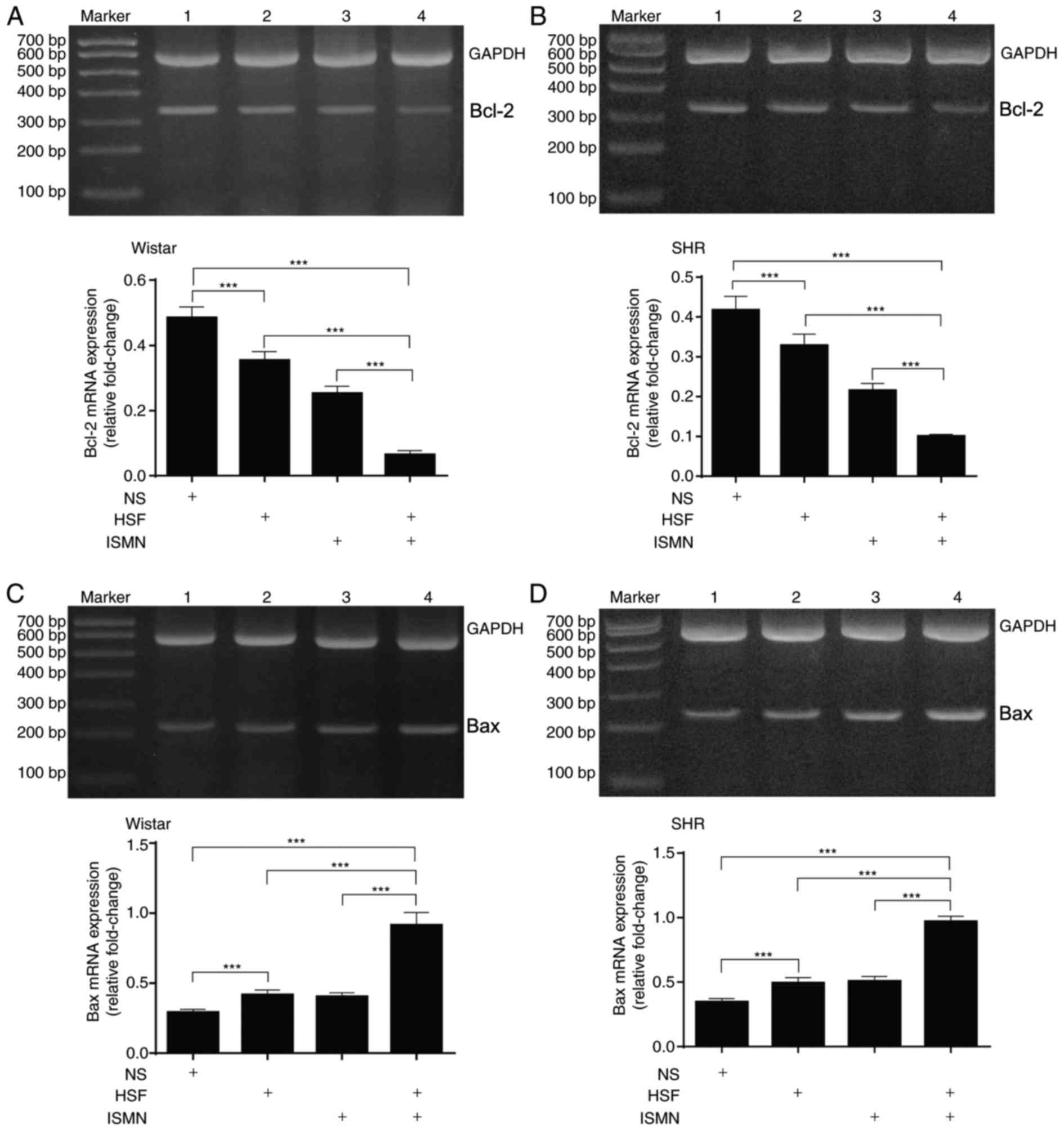 | Figure 2.HSF and ISMN activate the
mitochondrial apoptotic pathway in rat myocardium by affecting the
transcription of Bcl-2 and Bax. Following 12 weeks of feeding with
HSF and ISMN, transcription levels of the anti-apoptotic gene BCL-2
reduced within (A) Wistar rats and (B) SHR. Representative images
of agarose gel electrophoresis of the quantitative polymerase chain
reaction products are also presented. Transcription of the
pro-apoptotic gene Bax increased in (C) Wistar rats and (D) SHR.
The combination of HSF and ISMN appeared to have synergistic
effects. GADPH served as the internal control. Lane 1, normal diet;
lane 2, HSF; lane 3, ISMN; lane 4, combination. Bar graph data were
expressed as mean ± standard deviation (n=10). ***P<0.001.
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; HSF,
high sucrose/fat diet; ISMN, isosorbide mononitrate; NS, normal
diet; SHR, spontaneously hypertensive rats. |
 | Figure 3.HSF and ISMN induce the mitochondrial
apoptotic pathway by altering the protein expression levels of
Bcl-2 and Bax. Following 12 weeks of feeding with HSF and ISMN,
Bcl-2 expression was suppressed and the expression of Bax was
activated. (A) Representative image and quantitative analysis of
(B) Bcl-2 and (C) Bax protein expression levels as revealed by
western blotting in Wistar rats. (D) Representative image and
quantitative analysis of (E) Bcl-2 and (F) Bax protein expression
levels as revealed by western blotting in SHR. There was a notable
synergism between HSF and ISMN. Representative western blotting
data were presented in (A and D). GADPH served as the loading
control. Lane 1, normal diet; lane 2, HSF; lane 3, ISMN; lane 4,
combination. Bar graph data were expressed as mean ± standard
deviation (n=10). ***P<0.001. Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; HSF, high sucrose/fat diet; ISMN,
isosorbide mononitrate; NS, normal diet; SHR, spontaneously
hypertensive rats. |
Finally, myocardial apoptosis was analyzed via a
TUNEL assay, which detected DNA fragmentation, a characteristic
hallmark of apoptosis. The immunohistochemical staining of the
myocardium revealed enhanced apoptosis within rats fed with the HSF
or ISMN diet (Fig. 4). The Wistar
and SHR rats fed with the combinational diet of HSF and ISMN
demonstrated more intense staining in the TUNEL assay.
NO production in rat myocardium is
positively associated with HOMA-IR
To understand the association between NO content and
HOMA-IR, Pearson's correlation test was performed. In group 2
Wistar hypertensive rats fed with HSF, a positive association
between the NO content and HOMA-IR was observed (Fig. 5).
Discussion
NO serves important roles in diverse physiological
and pathological processes, including vasodilatation, oxidative
stress and inflammation (6). NO
produced predominantly by the vascular endothelium, leads to direct
relaxation of the vascular smooth muscles (12). The dilation of veins promotes
peripheral pooling of blood and reduces venous return to the
right-side of the heart, thereby lessening preload (12). Arteriolar relaxation, however,
reduces systemic vascular resistance and systolic arterial pressure
(12). NO also directly dilates
the coronary arteries thereby increasing the blood flow to
myometrium. ISMN, an exogenous NO inducer, is widely prescribed for
the prophylactic treatment of angina pectoria and early management
of myocardial infarction (13).
In the present study, SHR and Wistar rats served as
the experimental model. SHR rats are widely used as an animal model
of essential hypertension and cardiovascular disease. They are
derived from the parental strain Wistar rats. Generally, rats are
favored over mice for a more assimilated physiology to humans,
making them better suited for the study of pathological conditions,
including cardiovascular disease. Rats are also technically more
advantageous, with their larger size allowing advanced surgical
procedures and larger volume of body fluids and tissue for adequate
experimental readouts. The hypertensive rat model of the present
study revealed that the basal myocardial NO content in SHR rats was
increased compared with in the Wistar rats. The elevated NO level
in SHR rats may be attributed to the increased protein-bound
dinitrosyl nonheme iron complexes, which release NO to the
peripheral circulation to combat the hypertensive state (14). This protective mechanism may be
compensatory to maintaining the systemic blood pressure at low
levels (12). In addition,
increased activity of NO synthase (NOS) III and augmented
expression of NOS II have been reported in the cardiac and aortic
endothelia (15). These two
enzymes may regulate the vasoreactivity in the SHR rats.
The data of the present study demonstrated that
feeding with HSF or ISMN increased the proapoptotic protein Bax and
suppressed the anti-apoptotic Bcl-2. This was accompanied by the
induction of myocardial apoptosis, as demonstrated by the TUNEL
assay. Excessive production of NO by HSF or ISMN feeding has been
reported to mediate the apoptotic cell death of myocardium via the
cyclic guanosine monophosphate pathway, and generate peroxynitrite
to damage myocardium by reacting with the superoxide anion
(16,17).
In eukaryotes, there are two primary apoptotic
pathways: The extrinsic or death receptor pathway and the intrinsic
or mitochondrial pathway. The Bcl-2 family of proteins regulate
apoptosis by controlling the mitochondrial permeability. The
anti-apoptotic proteins, Bcl-2 and Bcl-xL, reside in the outer
mitochondrial wall and inhibit cytochrome c release.
Conversely, the cytoplasmic pro-apoptotic protein Bax translocates
to mitochondria following apoptotic signaling, promoting the
release of cytochrome c. Upon release, cytochrome c
associates with apoptosis protease activating factor 1 to form the
apoptosome, that recruits and activates procaspase-9. This is
followed by activation of executioner caspases including caspase-3
or −7 and terminal events proteolysis and DNA fragmentation. It has
been reported that NO promotes apoptosis by activating the
mitochondria-dependent apoptotic cell death, which suggests the
involvement of tumor suppressor p53 as a target during cell death
execution (18).
It remains uncertain whether IR, as a core component
of the metabolic syndrome, directly leads to myocardial apoptosis.
The present study reported that myocardial apoptosis was
significantly augmented in the hypertensive rats exposed to HSF or
ISMN. This was accompanied by a greater degree of IR; thus, it may
be assumed that there was a direct association between IR and
myocardial apoptosis. The underlying mechanism may be too complex
for the scope of the present study; the causal association was not
examined. However, a number of earlier studies have suggested that
certain pathological processes, including oxidative stress,
inflammation, endothelial dysfunction and metabolic imbalance
resulting from, IR may directly damage myocardial cells, resulting
in myocardial apoptosis and infarction (19–22).
In particular, IR may induce systemic inflammatory factors,
including C-reactive protein, tumor necrosis factor-α and
interleukin-6, which initiate and aggravate atherosclerosis,
leading to myocardial ischemia and apoptosis. Furthermore, IR has
been reported to directly contribute to the pathogenesis of
hypertension and the subsequent myocardial apoptosis. A possible
theory of this causal association is the secondary hyperinsulinemia
of IR, which enhances the ability of kidneys to reabsorb sodium and
water, resulting in hypertension. The correlation analysis of the
present study demonstrated a positive correlation between NO
content within the myocardium of rats fed with HSF and the degree
of HOMA-IR. This may be explained by the secondary hyperinsulinemia
in IR, which induces endogenous NO secretion. A few studies have
also suggested that IR was associated with an elevation in skeletal
muscle inducible NOS (iNOS) (23–25),
which may produce large amounts of NO. NO may alter the
S-nitrosation of proteins involved in insulin signal transduction.
S-nitrosation of insulin receptor β-subunit and protein kinase B
may impair kinase activities, whereas S-nitrosation of insulin
receptor substrate 1 reduces the tissue expression.
The present study may be limited by the capability
of a rat model to efficiently represent a human disease.
Furthermore, dynamic alterations in blood pressure were not
monitored due to technical challenges. The conventional tail-cuff
method requires special technical expertise and is disregarded by
certain experts in the cardiovascular field due to the artefactual
results from the physical restraint of animals and human influence
(26). The gold-standard blood
pressure measurement; however, requires the use of implanted
telemetry. This technology is expensive requiring an elaborate
technical setup. Various clinical studies have suggested that with
a long-term application of ISMN, endothelial functions of patients
may be compromised (27). The
short duration (12 weeks) of the present study may not recapitulate
the long-term and chronic alterations within the myocardium.
In conclusion, the findings of the present study
suggested that HSF- and ISMN-feeding in Wistar and SHR rats may
simultaneously induce IR and increase NO content in the myocardium.
This process was accompanied by the activation of the mitochondrial
death cascade and apoptosis in the myocardium.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
TL, BB and HW designed the experiments. TL, DJ and
FM carried out all the experiments. TL and MS analyzed the data and
wrote the manuscript. CT provided advice and guidance regarding
analysis of data.
Ethics approval and consent to
participate
The experimental protocol followed the guidance for
the Care and Use of Laboratory Animals (US National Institutes of
Health, no. 85–23) and the guidelines of the Animal Care and Use
Committee of Zhengzhou University. The present study was approved
by the Ethics Review Committee of Second Affiliated Hospital of
Zhengzhou University (Zhengzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alberti KG, Zimmet P and Shaw J: IDF
Epidemiology Task Force Consensus Group: The metabolic syndrome-a
new worldwide definition. Lancet. 366:1059–1062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kannel WB and Cobb J: Left ventricular
hypertrophy and mortality-results from the framingham study.
Cardiology. 81:291–298. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chae CU, Pfeffer MA, Glynn RJ, Mitchell
GF, Taylor JO and Hennekens CH: Increased pulse pressure and risk
of heart failure in the elderly. JAMA. 281:634–639. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reaven GM: Role of insulin resistance in
human disease (syndrome X): An expanded definition. Annu Rev Med.
44:121–131. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meshkani R and Adeli K: Hepatic insulin
resistance, metabolic syndrome and cardiovascular disease. Clin
Biochem. 42:1331–1346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Torfgård KE and Ahlner J: Mechanisms of
action of nitrates. Cardiovasc Drugs Ther. 8:701–717. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boyd CS and Cadenas E: Nitric oxide and
cell signaling pathways in mitochondrial-dependent apoptosis. Biol
Chem. 383:411–423. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Diao Y, Liu Y, Gao N, Gao D, Wan
Y, Zhong J and Jin G: Synergistic apoptosis-inducing effect of
aspirin and isosorbide mononitrate on human colon cancer cells. Mol
Med Rep. 12:4750–4758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. National Academy Press;
Washington, DC: pp. 1401996
|
|
10
|
Frustaci A, Kajstura J, Chimenti C,
Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B and Anversa P:
Myocardial cell death in human diabetes. Circ Res. 87:1123–1132.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sam F, Sawyer DB, Chang DL, Eberli FR,
Ngoy S, Jain M, Amin J, Apstein CS and Colucci WS: Progressive left
ventricular remodeling and apoptosis late after myocardial
infarction in mouse heart. Am J Physiol Heart Circ Physiol.
279:H422–H428. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen HI and Hu CT: Endogenous nitric oxide
on arterial hemodynamics: A comparison between normotensive and
hypertensive rats. Am J Physiol. 273:H1816–H1823. 1997.PubMed/NCBI
|
|
13
|
Thadani U and Lipicky RJ: Short and
long-acting oral nitrates for stable angina pectoris. Cardiovasc
Drugs Ther. 8:611–623. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu CC and Yen MH: Higher level of plasma
nitric oxide in spontaneously hypertensive rats. Am J Hypertens.
12:476–482. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Förstermann U and Sessa WC: Nitric oxide
synthases: Regulation and function. Eur Heart J. 33(829–837):
837a–837d. 2012.
|
|
16
|
Hua W, Chen Q, Gong F, Xie C, Zhou S and
Gao L: Cardioprotection of H2S by downregulating iNOS and
upregulating HO-1 expression in mice with CVB3-induced myocarditis.
Life Sci. 93:949–954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li YC, Luo Q, Ge LS, Chen YH, Zhou ND,
Zhang T, Guan XQ and Lin JF: Ivabradine inhibits the production of
proinflammatory cytokines and inducible nitric oxide synthase in
acute coxsackievirus B3-induced myocarditis. Biochem Biophys Res
Commun. 431:450–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brüne B: Nitric oxide: NO apoptosis or
turning it ON? Cell Death Differ. 10:864–869. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dobrin JS and Lebeche D: Diabetic
cardiomyopathy: Signaling defects and therapeutic approaches.
Expert Rev Cardiovasc Ther. 8:373–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boudina S and Abel ED: Diabetic
cardiomyopathy, causes and effects. Rev Endocr Metab Disord.
11:31–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khavandi K, Khavandi A, Asghar O,
Greenstein A, Withers S, Heagerty AM and Malik RA: Diabetic
cardiomyopathy-a distinct disease? Best Pract Res Clin Endocrinol
Metab. 23:347–360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh S, Duggal J, Khosla N and Arora R:
Screening guidelines for coronary heart disease in diabetes:
Current recommendations. J Cardiometab Syndr. 4:107–112. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carvalho-Filho MA, Ueno M, Carvalheira JB,
Velloso LA and Saad MJ: Targeted disruption of iNOS prevents
LPS-induced S-nitrosation of IRbeta/IRS-1 and Akt and insulin
resistance in muscle of mice. Am J Physiol Endocrinol Metab.
291:E476–E482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carvalho-Filho MA, Ueno M, Hirabara SM,
Seabra AB, Carvalheira JB, de Oliveira MG, Velloso LA, Curi R and
Saad MJ: S-nitrosation of the insulin receptor, insulin receptor
substrate 1, and protein kinase B/Akt: A novel mechanism of insulin
resistance. Diabetes. 54:959–967. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perreault M and Marette A: Targeted
disruption of inducible nitric oxide synthase protects against
obesity-linked insulin resistance in muscle. Nat Med. 7:1138–1143.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fritz M and Rinaldi G: Blood pressure
measurement with the tail-cuff method in Wistar and spontaneously
hypertensive rats: Influence of adrenergic- and nitric
oxide-mediated vasomotion. J Pharmacol Toxicol Methods. 58:215–221.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lai J, Wu B, Sun J, Shang Y and Zhu J:
Long-term isosorbide mononitrate treatment impairs endothelial
function in patients with coronary artery disease. Coron Artery
Dis. 24:566–571. 2013. View Article : Google Scholar : PubMed/NCBI
|



















