Introduction
Breast cancer is the most prevalent cancer among
females worldwide, with an incidence ranging from 0.02% in middle
Africa to 1% in North America. Although the overall survival rate
for patients has improved significantly in the past 30 years due to
earlier diagnosis and better treatments, nowadays breast cancer
still represents the second leading cause of cancer-associated
mortality in women, mainly due to its invasion/metastasis to other
organs including lungs and livers (1,2).
However, mechanisms responsible for the migration, invasion and
metastasis of breast cancer cells remain poorly understood.
Chemokines belongs to a super-family of small,
cytokine-like proteins that induce cytoskeletal rearrangement,
adhesion to endothelial and directional migration by interacting
with G-protein-coupled receptors (GPCRs) (3,4).
Among the 46 different human chemokines and 18 GPCRs identified so
far, CXCL12 and its receptor CXCR4 play an important role in
promoting the invasion and metastasis of breast cancers (4–6). It
has been shown that breast cancer cells express aberrant high
levels of CXCR4, and thereby metastasize preferentially to
CXCL12-rich tissues such as lungs and bone marrows (4,6).
Accordingly, reducing the expression of CXCR4 or blocking
CXCR4-CXCL12 interactions reduces the experimental invasion and
metastasis of breast cancer cells (7–9).
Thus, identifying molecules regulating the expression of CXCR4 on
cancer cells may represent a potential target for the treatment of
patients with breast cancer.
JWA, also known as ADP ribosylation factor like
GTPase 6 interacting protein 5 (ARL6IP5), was initially cloned from
human tracheal bronchial epithelial cells as an all-trans retinoic
acid responsive and cytoskeleton-associated gene, and has been
shown to be involved in cell oxidative stress, differentiation and
apoptosis (10–15). In addition, as a
microtubule-associated protein, JWA regulates the proliferation and
migration of cancer cells via MAPK cascades, inhibits the invasion
and metastasis of melanoma cells via integrin signaling, and
suppresses the tumor angiogenesis via ILK signaling or
Sp1-activated MMP-2 expressions (16–19).
Therefore, the downregulated expression of JWA predicts poor
prognosis in human melanoma, esophageal squamous cell carcinoma,
hepatocellular carcinoma and gastric cancers (18–21).
Nonetheless, the functions of JWA in breast cancers remain largely
unknown.
In the present study, we found that the expression
of JWA is significantly reduced in primary human breast tumors than
the paired adjacent normal tissues. Despite the ineffectiveness in
cellular proliferations, increasing the expression of JWA in breast
cancer cell line MDA-MB-231 (with low endogenous JWA) greatly
reduces the expression of CXCR4 and the in vitro cellular
migration/invasion abilities, while downregulating its expressions
in MDA-MB-468 cells (with high endogenous JWA) has the opposite
effect. Moreover, preventing the down-/upregulation of CXCR4
induced by increased/decreased JWA expressions in breast carcinoma
cells almost completely reverses the disturbed cellular invasions
to control levels. These data indicate that JWA suppresses the
migration/invasion of breast carcinoma cells via downregulating the
expression of CXCR4. Our findings further strengthen the importance
of JWA in tumor invasion and metastasis, and suggest that JWA may
represent a potential anti-metastatic target for breast cancer
patients.
Materials and methods
Breast cancer specimens
The tumor specimens and paired normal breast tissue
specimens were obtained from patients undergoing breast surgery.
None of the patients had received radiotherapy or chemotherapy
prior to the surgery. Written informed consent was provided by each
patient recruited and the present study was approved by the local
human Ethics Committee of The Affiliated Changzhou No. 2 People's
Hospital of Nanjing Medical University (Changzhou, Jiangsu,
China).
Cell lines and culture
Breast carcinoma cells MDA-MB-231 and MDA-MB-468
were purchased from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). All the cells were cultured
in DMEM medium supplemented with 10% of fetal bovine serum (FBS),
100 U/ml of penicillin and 100 µg/ml of streptomycin (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified
incubator with 5% CO2.
Plasmids and transfection
The control Flag-vector and Flag-JWA plasmids were
kindly provided by Professor Gang Li (University of British
Columbia, Canada) as described previously (13). HA-tagged CXCR4 vector was obtained
by subcloning the cDNA into the pCMV-HA-dsRed2 expression plasmid
(GV316; Genechem, Shanghai, China). SiRNA specific for JWA
(5′-CGAGCTATTTCCTTATCTC-3′) was synthesized by Riobio (Guangzhou,
China) as previously published (14). To specifically knockdown the
expression of CXCR4, we subcloned the CXCR4-specific sequence
(5′-TGCCTTACTACATTGGGAT-3′) into the pCMV-U6-GFP shRNA vector
(GV248; Genechem). Cells were (co-) transfected with siRNA or
plasmids with Lipofectamine 2000 following the protocols provided
by the manufacturer (Invitrogen; Thermo Fisher, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was isolated from cells with TRIzol (Takara Bio,
Dalian, China) and reversely transcribed into cDNA using an oligo
(dT) primer subsequently (Promega Corp., Madison, WI, USA). RT-qPCR
was performed with SYBR Premix Ex Taq (Takara Bio) using an ABI
7900HT detection system (Thermo Fisher Scientific Inc.). Gene
expression levels were normalized to the endogenous GAPDH in each
sample.
Western blot analysis
Western blots were performed as previously described
(12). Briefly, cells were lysed
in keratin extraction buffer (1% Triton-X 100, 0.02 mM Tris, 0.6 M
KCl, and 1 mM PMSF, pH 7.0) and protein concentrations were
determined by bicinchoninic acid (BCA) assays (Beyotime, Nantong,
China). Proteins were separated in SDS-PAGE 12.5% gels and blotted
onto PVDF membrane (Millipore). After incubation for 1 h in
blocking buffer (Tris-buffered saline with 5% nonfat milk), the
membrane was incubated with primary antibodies overnight at 4°C,
followed by a further incubation with HRP-coupled secondary
antibodies at room temperature for 2 h. Signals were visualized
with an enhanced chemiluminescent kit (GE Healthcare, Chicago, IL,
USA). The following antibodies were used: Mouse monoclonal anti-JWA
(contract produced by AbMax, Beijing, China) and anti-GAPDH (6C5;
Beyotime); rabbit polyclonal anti-Flag (Beyotime); rabbit
monoclonal anti-CXCR4 (UMB2, Abcam); Rabbit monoclonal anti-AKT
(C67E7) and anti-pAKT (D25E6; Cell Signaling Technology, Inc.,
Danvers, MA, USA); and HRP-coupled polyclonal goat anti-mouse or
rabbit IgG (Beyotime).
Transwell invasion assay
The 24-well Transwell chambers with a pore size of 8
mm (Corning, Tewksbury, MA, USA) were pre-coated with 50 ml 100
mg/ml fibronectin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
105 cells in 100 ml serum-free medium were seeded into
the upper chamber while 600 ml medium with 10% serum was added into
the lower chamber. After incubation at 37°C for 12 h, cells in the
upper chamber were carefully removed with a cotton swab and cells
that had traversed to the reverse side of the membrane were fixed
in methanol, stained with Giemsa, and imaged with a microscopy
(IX70; Olympus Tokyo, Japan). Experiments were performed in
triplicates, and five random fields of each well were recorded to
count the cell numbers. In some experiments, cells were pretreated
with a CXCR4 specific antagonist AMD3100 (octahydrochloride
hydrate; Sigma-Aldrich; Merck KGaA) at 100 nM for 2 h before
seeding.
Wound healing migration assays
Cells were seeded into 24-well plates and cultured
till 80–90% confluence. Cells were washed once with PBS, and then a
scratch was gently introduced onto the cell monolayer with 200 ml
pipette tips. After washing with PBS twice, cells were grown in
serum-free medium, and migrating cells were imaged with a
microscopy (IX70; Olympus) at 36 or 48 h. Experiments were
performed in duplicates, and three random fields of each well were
recorded. The rate of wound closure was calculated on the basis of
the average distance between the two wound edges.
Proliferation assay
Cells were plated at a density of 5,000 cells/well
in triplicates in 96-well plates. Cellular viabilities were
determined by Cell Counting kit-8 (CCK-8; Beyotime) at indicated
times according to the manufacturer's instructions. Proliferation
index was calculated as the ratio of OD value at the indicated
time/OD value of the input cells.
Statistical analysis
The differences among different groups were
determined by the parametric unpaired Student's t-test, and values
at P<0.05 were considered significant.
Results
JWA negatively regulates the
invasion/migration abilities of breast cancer cells
We first analyzed the expression levels of JWA in
two breast cell lines, MDA-MB-231 and MDA-MB-468, with differential
migratory abilities. Our data clearly showed that the endogenous
protein level of JWA is significantly lower in MDA-MB-231 than that
in MDA-MB-468 cells (Fig. 1A). As
the endogenous levels of JWA seem to inversely correlate with
cellular invasion/migration abilities (22), we subsequently
downregulated/upregulated the protein levels of JWA in
MDA-MB-468/MDA-MB-231 cells and determined its impact on cellular
invasion/migration capabilities in vitro. Ectopically high
expression of JWA in MDA-MB-231 cells remarkably repressed their
invasion abilities in a Transwell assay (Fig. 1B, D and E). By contrast, knockdown
of endogenous JWA by siRNA significantly increased the number of
MDA-MB-468 cells that had traversed the membrane (Fig. 1C, F and G). Similar results were
obtained in would healing migration assays (Fig. 2A and B). Notably, this observed
effect of JWA on cellular migration/invasion cannot be attributed
to the disturbed cellular proliferations as manipulation of JWA
levels has no impact on the proliferations of MDA-MB-231 or
MDA-MB-468 cells (Fig. 2C).
Moreover, side-by-side comparisons between the primary tumor and
paired adjacent normal tissues revealed significantly lower levels
of JWA in tumor tissues in all six breast cancer patients (Fig. 3A). Together, these data indicate
that JWA negatively regulates the invasion/migration capabilities
of breast carcinoma cells.
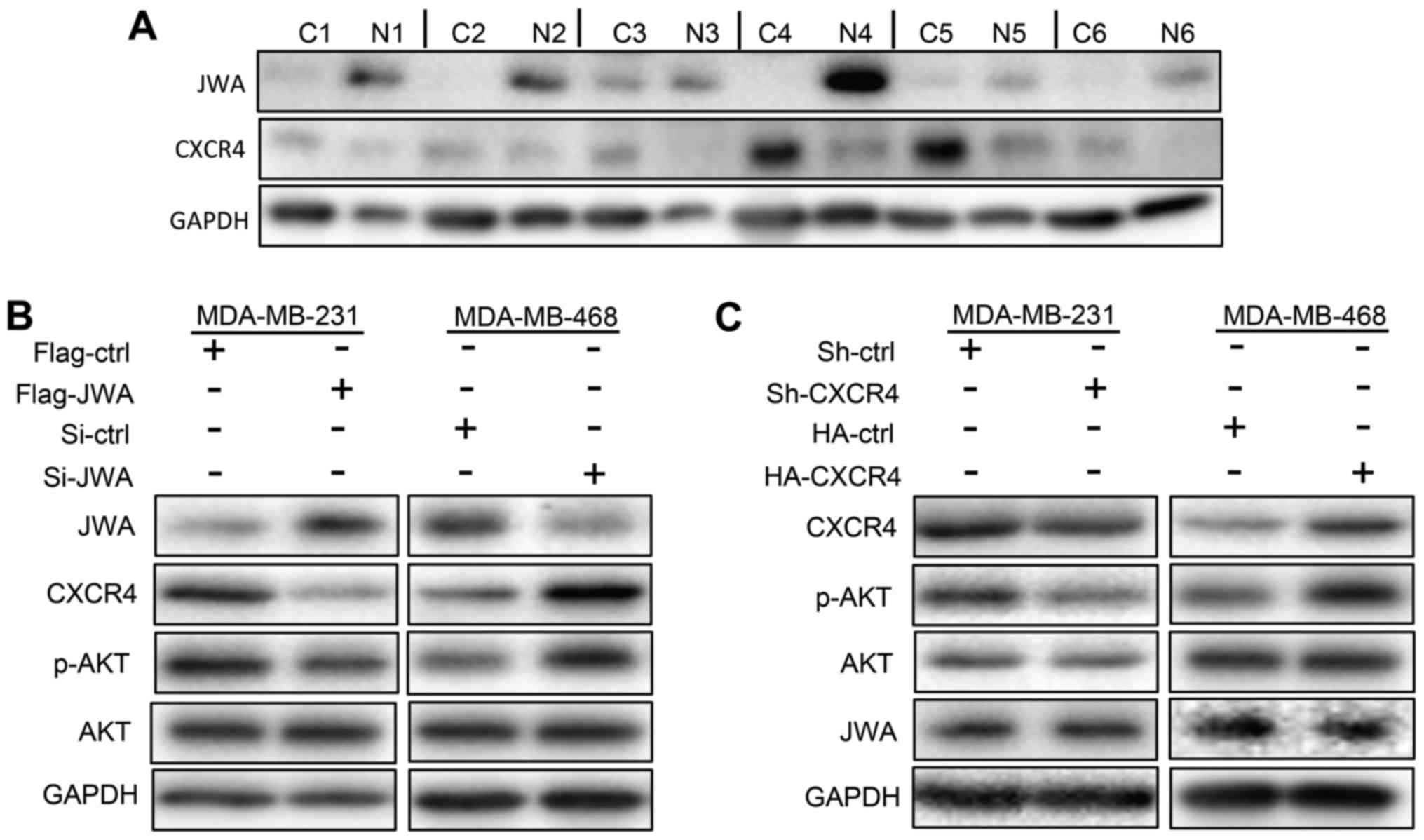 | Figure 3.JWA suppresses the expression of CXCR4
and the activation of AKT in breast cancer cells. (A) Expression of
JWA and CXCR4 in paired cancer (indicated by C) and adjacent normal
(indicated by N) tissues taken from 6 breast cancer patients. (B)
Overexpression and downregulation of JWA in MDA-MB-231 (left panel)
and MDA-MB-468 (right panel) cells, respectively, resulted in
decreased and increased expressions of CXCR4 and p-AKT,
respectively. (C) Manipulations of CXCR4 levels in MDA-MB-231 and
MDA-MB-468 cells do not affect the expression of JWA. MDA-MB-231
cells were transfected with a control (Sh-ctrl) or CXCR4-targeting
vector to knockdown endogenous CXCR4 expressions. By contrast,
MDA-MB-468 cells were transfected with a control (HA-ctrl) or
CXCR4-expression (HA-CXCR4) plasmid to overexpress CXCR4. Levels of
each protein were detected by western blotting 48 h
post-transfection. CXCR4, C-X-C motif chemokine receptor type 4;
ctrl, control; Si, small interfering RNA; Sh, small hairpin RNA;
p-, phosphorylated; AKT, protein kinase B. |
JWA downregulates the expression of
CXCR4 in breast carcinoma cells
In line with previous reports (4), lower levels of CXCR4 were observed in
breast tumors than the paired normal tissues (Fig. 3A). Given that CXCR4 promotes the
in vitro migratory as well as the in vivo metastatic
abilities of breast cancer cells through the PI3K/AKT signaling
pathway (4,6,23,24),
the inversed correlation of JWA and CXCR4 levels in breast cancer
tissues prompted us to investigate whether JWA regulates the
expression of CXCR4 and/or phosphorylated AKT (p-AKT). As shown in
Fig. 3B, overexpression of JWA in
MDA-MB-231 cells represses the expression of CXCR4 and p-AKT, while
knockdown of JWA in MDA-MB-468 cells has the opposite effect
(Fig. 3B). We next
down-/upregulated the expression of CXCR4 in MDA-MB-231/468 cells
with shRNA/overexpression plasmids, respectively, to determine
whether it affects JWA levels as well. As expected, levels of p-AKT
were positively correlated with CXCR4 in both cell lines. However,
comparable expressions of JWA were observed in control and
MDA-MB-468/231 cells with up-/downregulated CXCR4 (Fig. 3C). We thus concluded that JWA
represses the expression of CXCR4 and AKT signaling pathway in
human breast cancer cells.
JWA suppresses the invasion abilities
of breast cancer cells lines via downregulating the expression of
CXCR4
Data presented in Figs.
1–3 implied that JWA may
inhibit the invasion of breast cancer cells by regulating the
expression of CXCR4. We thus tried to normalize the disturbed
expressions of CXCR4 in MDA-MB-231/468 cells caused by the
overexpression/knockdown of JWA before measuring cellular invasion
abilities. As shown in Fig. 4A and
B, despite the high expression of JWA, MDA-MB-231 cells
cotransfected both with JWA and CXCR4 expression plasmids displayed
comparable levels of CXCR4 and p-AKT as control cells. Importantly,
upregulation of CXCR4 almost completely abrogated the inhibitory
effect of overexpressed JWA on cellular invasion abilities in
MDA-MB-231 cells (Fig. 4A).
Likewise, preventing the upregulation of CXCR4 and p-AKT by
transfecting MDA-MB-468 cells with a CXCR4-targeting shRNA vector
reversed the enhanced cellular invasions conferred by downregulated
JWA (Fig. 4C and D). Moreover,
blocking CXCR4 signaling by treating JWA-downregulated MDA-MB-468
cells with AMD3100 (a CXCR4 specific antagonist) greatly reduced
their transmigrations across the membrane in Transwell assays
(Fig. 5). Therefore, cellular
invasion capabilities are closely correlated to the expression
levels of CXCR4/p-AKT, but not JWA, in both cell lines.
 | Figure 4.JWA inhibited the invasion abilities
of breast cancer cells via CXCR4. (A) Preventing the downregulation
of CXCR4 induced by the overexpressed JWA reversed the reduced
cellular invasion ability of MDA-MB-231 cells. (B) Cells were
co-transfected with a JWA-expressing vector (Flag-JWA) in the
absence (indicated by M) or presence of a CXCR4-expressing
(HA-CXCR4), or a control (HA-ctrl) construct by Lipofectamine
2000™. (C) Downregulation of CXCR4 inhibited the augmented cellular
invasion of MDA-MB-468 cells induced by the decreased levels of
endogenous JWA. (D) MDA-MB-468 cells were co-transfected with a
JWA-targeting siRNA (Si-JWA) in the absence (indicated by M) or
presence of a CXCR4-targeting (Sh-CXCR4), or control (Sh-ctrl)
shRNA-containing plasmids using Lipofectamine 2000™. Cellular
invasive capabilities were determined in Transwell assays (A and C)
and levels of each protein were analyzed by western blotting (B and
D) 48 h post-transfection. Cells that had traversed to the reverse
side of the membrane were fixed, stained and imaged. Experiments
were performed in triplicate, and 5 random fields in each well were
recorded to count the cell numbers. The results were expressed as
the mean number ± standard error mean of three independent
experiments. *P<0.05, **P<0.01 and ***P<0.001, as
indicated. CXCR4, C-X-C motif chemokine receptor type 4; ctrl,
control; Si, small interfering RNA; Sh, small hairpin RNA; p-,
phosphorylated; AKT, protein kinase B. |
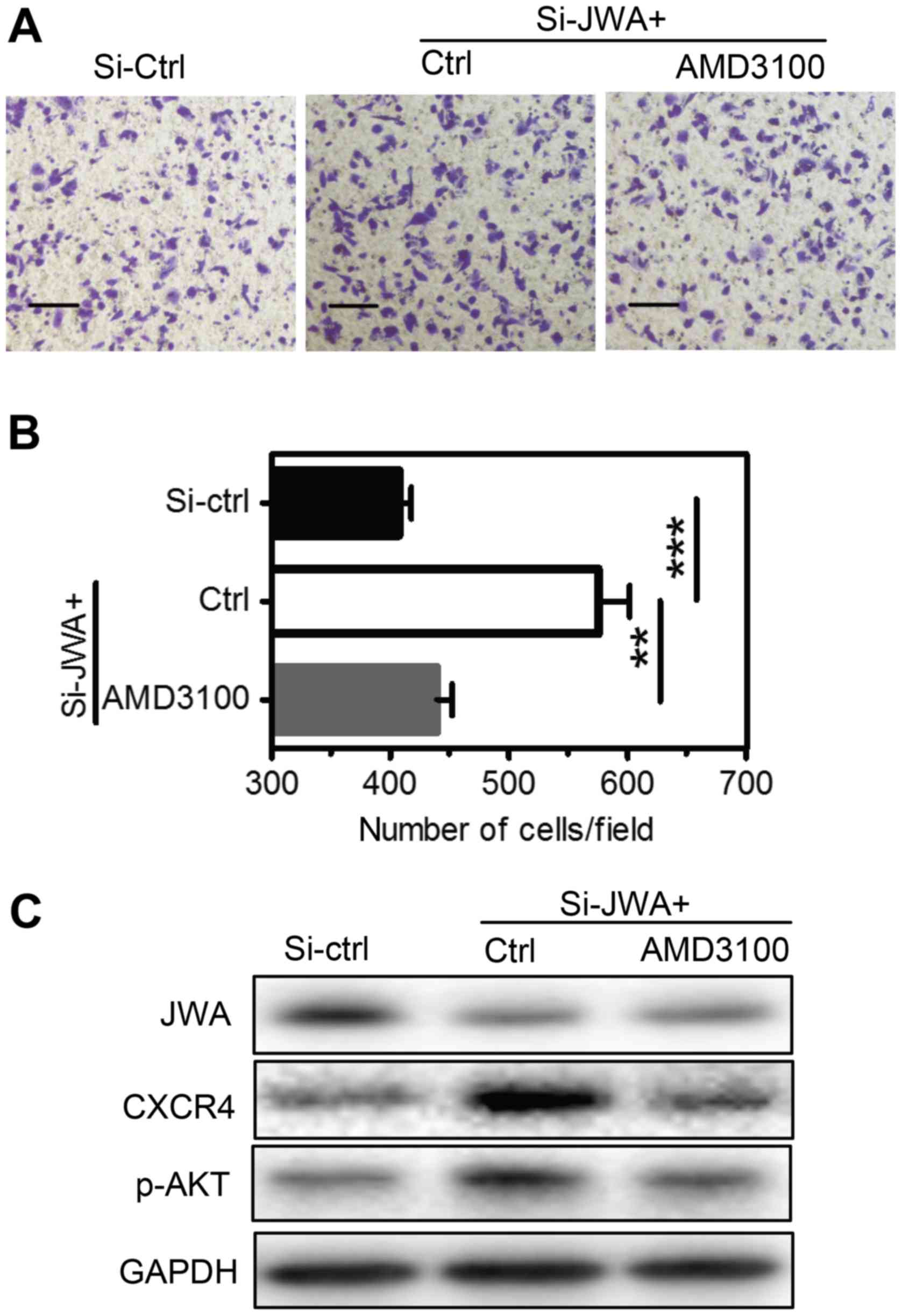 | Figure 5.JWA inhibits the invasive abilities
of breast cancer cells via the CXCR4-mediated signaling pathway.
(A) MDA-MB-468 cells were transfected with a control (Si-ctrl) or
JWA-targeting siRNA (Si-JWA) with Lipofectamine 2000™. Following 48
h, cells were treated with a vehicle control (Ctrl) or AMD3100 (a
CXCR4 specific antagonist) at 100 nM for 2 h. Cellular invasion
abilities were then determined by (B) Transwell assays and protein
levels were measured by (C) western blot analysis. Cells that had
traversed to the reverse side of the membrane were fixed, stained
and imaged (magnification, ×400; scale bars=100 µm). Experiments
were performed in triplicate, and 5 random fields of each well were
recorded to count the cell numbers. The results were expressed as
the mean number ± standard error mean of three independent
experiments. **P<0.01 and ***P<0.001, as indicated. CXCR4,
C-X-C motif chemokine receptor type 4; ctrl, control; Si, small
interfering RNA; p-, phosphorylated; AKT, protein kinase B. |
Collectively, these data indicate that JWA
negatively regulates the invasion/migration abilities of breast
cancer cells by inhibiting the expression of CXCR4.
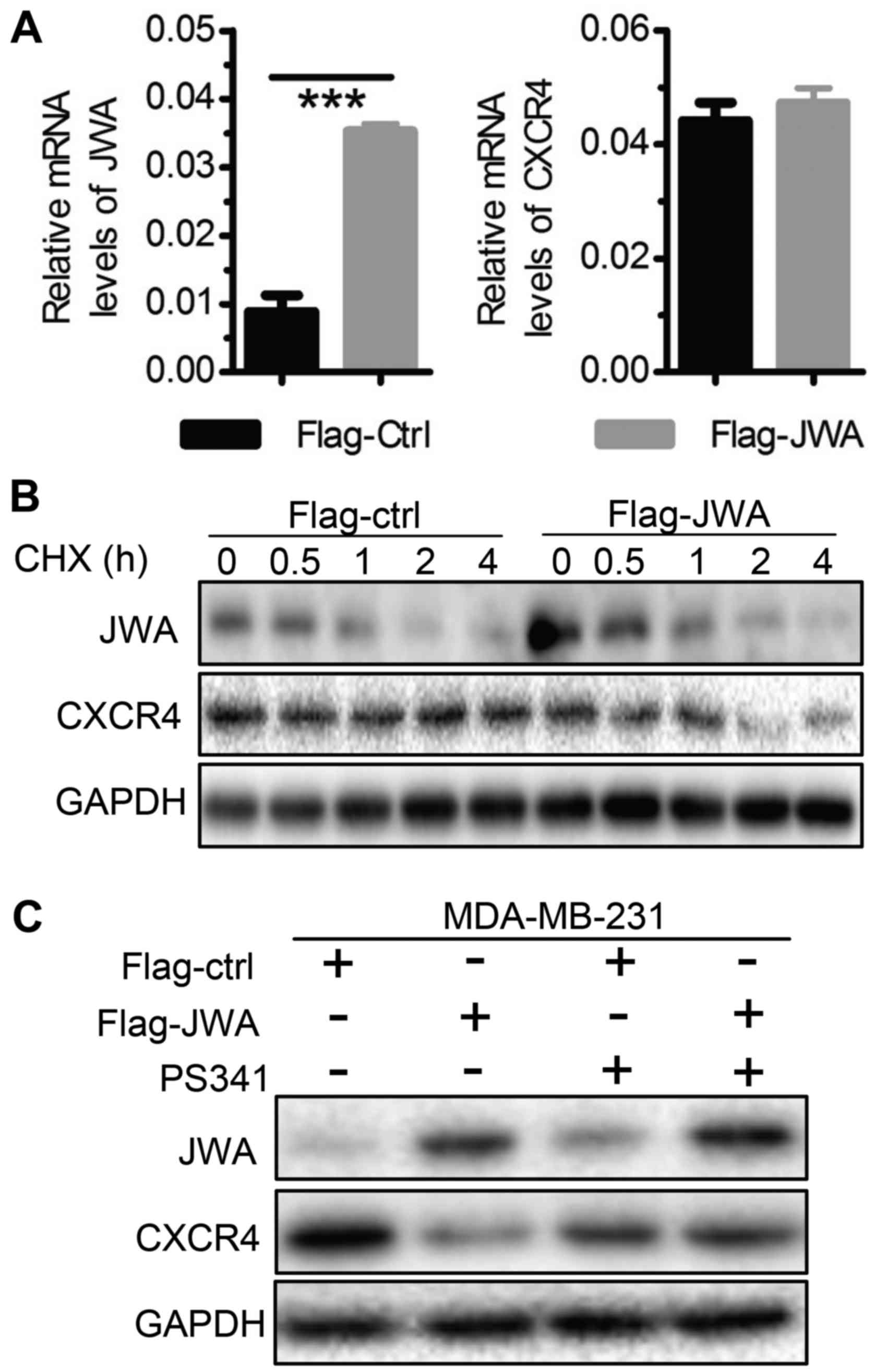
JWA inhibits the expression of CXCR4
by promoting protein degradations
To explore how JWA regulates the expression of CXCR4
in breast cancer cells, we first measured the mRNA levels of CXCR4
in MDA-MB-231/468 cells upon JWA up- or downregulation,
respectively. Interestingly, in contrast to the greatly reduced
CXCR4 protein levels, comparable mRNA levels of CXCR4 were detected
in MDA-MB-231 cells with overexpressed JWA (Figs. 4B and 6A). Similar results were obtained in
MDA-MB-468 cells after knockdown of JWA by siRNA (data not shown).
These data clearly demonstrated that JWA has no effect on the
transcription of CXCR4 in breast cancer cells. Given that JWA is
capable of suppressing protein expressions via
ubiquitin-proteasome-dependent mechanism (14,18),
we subsequently treated control or JWA-overexpressing MDA-MB-231
cells with Cycloheximide (CHX, a protein synthesis inhibitor) or
Bortezomib (PS341, a proteasome inhibitor). We found that
overexpressed JWA gradually but significantly reduced the protein
levels of CXCR4 in cells after Cycloheximide treatment (Fig. 6B), which was largely reversed by
blocking the function of proteasomes (Fig. 6C). These data indicate that JWA
promotes the proteasome degradation of CXCR4 and thereby negatively
regulates its expression in breast cancer cells.
Discussion
In the present study, we investigated the expression
and function of JWA in breast cancer cells as well as the
mechanisms behind. Our data demonstrated a significantly reduced
expression of JWA in primary breast cancers than paired adjacent
normal tissues. Moreover, downregulating JWA increases while
overexpressing JWA inhibits the in vitro cellular migration
and invasion abilities of two breast cancer cell lines via
modulating the proteasome degradation of CXCR4. Our study thus not
only confirms the well-accepted negative roles of JWA in cancer
invasion and metastasis, but also extends these studies by
uncovering that JWA exerts this function via a previously
unidentified mechanism, regulating the surface expression of
chemokine receptor CXCR4 (16–21).
JWA thus may represent a potential target for the treatment of
breast cancer patients.
Numerous studies have indicated that JWA is a tumor
suppressor gene as its downregulation promotes the survival,
migration, invasion, metastasis and angiogenesis of a variety of
human cancer cells (14,16–18,20,21,25–27).
Regarding breast cancers, we have previously shown that JWA
enhances As2O3-induced apoptosis of MCF-7
cells (12,13). Moreover, Chen et al
(28) recently demonstrated that
human primary breast cancer tissues express significantly lower
levels of JWA, and its knockdown promotes the proliferation,
migration and invasion of MDA-MB-231 cells in vitro. By
using two different breast cancer cell lines, we confirmed Chen's
findings except the negative role of JWA in cellular proliferations
(Figs. 1–4). This discrepancy may be attributed to
different approaches used as Chen et al (28) downregulated while we upregulated
JWA in MDA-MB-231 cells expressing low levels of endogenous JWA
(Figs. 1 and 2). It is possible that cellular survival
and proliferations will only be affected when the expression level
of JWA, a microtubule- and cytoskeleton-associated protein, is
below a certain threshold. In this scenario, comparable
proliferations between control and JWA-downregulated MDA-MB-468
cells could be explained by that the level of JWA in shRNA-treated
cells was still above this threshold due to the high endogenous
expressions of JWA (Figs. 1 and
2). Nevertheless, these data
indicate that low expressions of JWA represent a bad prognostic
maker for human breast cancers, in addition to previously reported
melanoma, esophageal squamous, hepatocellular and gastric cancers
(18–21).
Despite the high efficacy of current mainstream
strategies including surgical resection and adjuvant therapies for
well-confined primary breast tumors, metastatic breast cancer
remains largely incurable and is responsible for 90% of the patient
deaths (6). Given the role of
‘chemokine-receptor’ in metastasis and the most common expression
of CXCR4 in human solid tumors, recent studies have drawn much
attention to its role in tumor metastasis (9). It has been shown that CXCR4 is highly
expressed in human breast cancer tissues and cell lines. Moreover,
the expression levels of CXCR4 are closely correlated with the
lymph node metastasis (4,6,24),
and antagonizing or silencing of CXCR4 blocks the metastasis of
breast cancer cells (7–9). We thus investigated the potential
crosstalks between JWA and CXCR4 by first measuring the expressions
of CXCR4 and JWA in paired breast tumors and adjacent normal
tissues side-by-side. In this western-blot assay, expressions of
JWA (~21 Kd) and CXCR4 (~40 Kd) were detected on the same membrane,
while GAPDH (~36 Kd) was blotted on another membrane/gel loaded
with the same amount of proteins as the protein size for CXCR4 and
GAPDH are too close to be distinguished in one membrane. Moreover,
a negative control (slot without proteins) was not included because
of the limited loading slots. Nonetheless, our data showed that
levels of CXCR4 are inversely-correlated with that of JWA in all
human primary samples (Fig. 3A).
Furthermore, we found that JWA is capable of repressing the
migration and invasion of breast cancer cells through promoting the
protein-degradation of CXCR4 (Figs.
3B–6). Together, these data
indicated that JWA represents a novel negative regulator of CXCR4
in breast cancers, albeit that the detailed mechanisms behind merit
further investigations. Additionally, given the broad expression of
CXCR4 in human solid tumors (9),
it is worth investigating whether findings described here can be
extrapolated to other tumor types.
In conclusion, we have demonstrated that the
expression of JWA is significantly reduced in primary human breast
tumors, and more importantly, that JWA represses the migration and
invasion of breast tumor cell lines via promoting the protein
degradation of CXCR4. These findings are relevant as they suggest
that JWA may represent not only a prognostic maker but also an
anti-metastatic therapeutic target for patients with breast
cancers.
Acknowledgements
The authors would like to thank Dr Jin Xu, Dr Qiang
Wang and Dr Yuling Huang (School of Public Health, Nanjing Medical
University, Jiangsu, China) for their valuable comments, and
expertise in performing experiments and preparing figures.
Funding
The present study was financially supported in part
by the National Natural Science Foundation of China (grant no.
81502294) and the Applied Basic Research Project of Changzhou
(grant no. CZ20160035).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LX and LC conceived and designed the study,
performed the experiments, analyzed the data and drafted the
manuscript. FY and BP were involved in recruiting patients and
collecting patients' samples. XL performed the experiments, and JZ
was involved in the study design and manuscript preparation. YZ and
SW conceived and designed the study, analyzed the data and drafted
the manuscript.
Ethics approval and consent to
participate
Written informed consent was provided by each
patient recruited and the present study was approved by the local
Human Ethics Committee of The Affiliated Changzhou No. 2 People's
Hospital of Nanjing Medical University.
Consent for publication
Written informed consent was provided by each
patient recruited.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Geiger TR and Peeper DS: Metastass
mechanisms. Biochim Biophys Acta. 1796:293–308. 2009.PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cabioglu N, Gong Y, Islam R, Broglio KR,
Sneige N, Sahin A, Gonzalez-Angulo AM, Morandi P, Bucana C,
Hortobagyi GN and Cristofanilli M: Expression of growth factor and
chemokine receptors: New insights in the biology of inflammatory
breast cancer. Ann Oncol. 18:1021–1029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu W, Qian L, Chen X and Ding B:
Prognostic significance of CXCL12, CXCR4, and CXCR7 in patients
with breast cancer. Int J Clin Exp Pathol. 8:13217–13224.
2015.PubMed/NCBI
|
|
5
|
Fernandis AZ, Prasad A, Band H, Klösel R
and Ganju RK: Regulation of CXCR4-mediated chemotaxis and
chemoinvasion of breast cancer cells. Oncogene. 23:157–167. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mukherjee D, Lu H, Yu L, He C, Lahiri SK,
Li T and Zhao J: Kruppel-like factor 8 activates the transcription
of C-X-C cytokine receptor type 4 to promote breast cancer cell
invasion, transendothelial migration and metastasis. Oncotarget.
7:23552–23568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang EH, Singh B, Cristofanilli M,
Gelovani J, Wei C, Vincent L, Cook KR and Lucci A: A CXCR4
antagonist CTCE-9908 inhibits primary tumor growth and metastasis
of breast cancer. J Surg Res. 155:231–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang Z, Yoon Y, Votaw J, Goodman MM,
Williams L and Shim H: Silencing of CXCR4 blocks breast cancer
metastasis. Cancer Res. 65:967–971. 2005.PubMed/NCBI
|
|
9
|
Katkoori VR, Basson MD, Bond VC, Manne U
and Bumpers HL: Nef-M1, a peptide antagonist of CXCR4, inhibits
tumor angiogenesis and epithelialtomesenchymal transition in colon
and breast cancers. Oncotarget. 6:27763–27777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen R, Qiu W, Liu Z, Cao X, Zhu T, Li A,
Wei Q and Zhou J: Identification of JWA as a novel functional gene
responsive to environmental oxidative stress induced by
benzo[a]pyrene and hydrogen peroxide. Free Radic Biol Med.
42:1704–1714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang S, Shen Q, Mao WG, Li AP, Ye J, Liu
QZ, Zou CP and Zhou JW: JWA, a novel signaling molecule, involved
in the induction of differentiation of human myeloid leukemia
cells. Biochem Biophys Res Commun. 341:440–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou J, Ye J, Zhao X, Li A and Zhou J: JWA
is required for arsenic trioxide induced apoptosis in HeLa and
MCF-7 cells via reactive oxygen species and mitochondria linked
signal pathway. Toxicol Appl Pharmacol. 230:33–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen L, Xu W, Li A, Ye J and Zhou J: JWA
enhances As2O3-induced tubulin polymerization
and apoptosis via p38 in HeLa and MCF-7 cells. Apoptosis.
16:1177–1193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu W, Chen Q, Wang Q, Sun Y, Wang S, Li A,
Xu S, Røe OD, Wang M, Zhang R, et al: JWA reverses cisplatin
resistance via the CK2-XRCC1 pathway in human gastric cancer cells.
Cell Death Dis. 5:e15512014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei B, Han Q, Xu L, Zhang X, Zhu J, Wan L,
Jin Y, Qian Z, Wu J, Gao Y, et al: Effects of JWA, XRCC1 and BRCA1
mRNA expression on molecular staging for personalized therapy in
patients with advanced esophageal squamous cell carcinoma. BMC
Cancer. 15:3312015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen H, Bai J, Ye J, Liu Z, Chen R, Mao W,
Li A and Zhou J: JWA as a functional molecule to regulate cancer
cells migration via MAPK cascades and F-actin cytoskeleton. Cell
Signal. 19:1315–1327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bai J, Zhang J, Wu J, Shen L, Zeng J, Ding
J, Wu Y, Gong Z, Li A, Xu S, et al: JWA regulates melanoma
metastasis by integrin alphaVbeta3 signaling. Oncogene.
29:1227–1237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Huang Y, Huang Y, Xia X, Zhang J,
Zhou Y, Tan Y, He S, Qiang F, Li A, et al: JWA suppresses tumor
angiogenesis via Sp1-activated matrix metalloproteinase-2 and its
prognostic significance in human gastric cancer. Carcinogenesis.
35:442–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu J, Tang Y, Farshidpour M, Cheng Y,
Zhang G, Jafarnejad SM, Yip A, Martinka M, Dong Z, Zhou J, et al:
JWA inhibits melanoma angiogenesis by suppressing ILK signaling and
is an independent prognostic biomarker for melanoma.
Carcinogenesis. 34:2778–2788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou J, Ge Z, Tan Y, Jiang G, Feng J, Wang
H and Shi G: Downregulation of JWA expression in human esophageal
squamous cell carcinoma and its clinical significance. Oncol Res.
20:157–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu X, Chen H, Gao Q, Bai J, Wang X, Zhou
J, Qiu S, Xu Y, Shi Y, Wang X, et al: Downregulation of JWA
promotes tumor invasion and predicts poor prognosis in human
hepatocellular carcinoma. Mol Carcinog. 53:325–336. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lim RC, Price JT and Wilce JA:
Context-dependent role of Grb7 in HER2+ve and triple-negative
breast cancer cell lines. Breast Cancer Res Treat. 143:593–603.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peng Y, Zhong Y and Li G: Tubeimoside-1
suppresses breast cancer metastasis through downregulation of CXCR4
chemokine receptor expression. BMB Rep. 49:502–507. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lian X, Jiao Y, Yang Y, Wang Z, Xuan Q,
Liu H, Lu S, Wang Z, Liu Y, Li S, et al: CrkL regulates
SDF-1-induced breast cancer biology through balancing Erk1/2 and
PI3K/Akt pathways. Med Oncol. 32:4112015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin J, Ma T, Jiang X, Ge Z, Ding W, Wu Y,
Jiang G, Feng J, Cui G and Tan Y: JWA regulates human esophageal
squamous cell carcinoma and human esophageal cells through
different mitogen-activated protein kinase signaling pathways. Exp
Ther Med. 7:1767–1771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu J, Tang Y, Cheng Y, Zhang G, Yip A,
Martinka M, Dong Z, Zhou J and Li G: ING4 regulates JWA in
angiogenesis and their prognostic value in melanoma patients. Br J
Cancer. 109:2842–2852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qian J, Zhu W, Wang K, Ma L, Xu J, Xu T,
Røe OD, Li A, Zhou J and Shu Y: JWA loss promotes cell migration
and cytoskeletal rearrangement by affecting HER2 expression and
identifies a high-risk subgroup of HER2-positive gastric carcinoma
patients. Oncotarget. 7:36865–36884. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen X, Feng J, Ge Z, Chen H, Ding W, Zhu
W, Tang X, Chen Y, Tan Y and Ma T: Effects of the JWA gene in the
regulation of human breast cancer cells. Mol Med Rep. 11:3848–3853.
2015. View Article : Google Scholar : PubMed/NCBI
|