Introduction
Cross-talk between the sympathetic nervous system
and immune system is necessary for health and well-being (1,2).
Communication with immune cells occurs directly by neurotransmitter
release from sympathetic nerves that bind to receptors expressed on
immune cells (3). Norepinephrine
(NE) is a member of the catecholamines family and is widely located
in the central nervous system as well as peripheral tissues
(4,5). NE modulates immune responses by
binding to adrenergic receptors (ARs) expressed on immune cells
(6).
Natural killer (NK) cells are large granular
lymphocytes, which serve a role in the innate immune response
(7). NK cells kill tumor cells and
virally infected cells by direct cell-mediated cytotoxicity in a
non-major histocompatibility complex restricted manner (8). Certain studies have demonstrated that
NE has a regulatory effect on NK cell activation (9–11).
However, the underlying mechanisms are not clear. NK92-MI cells
established from patients with NK malignancies, serve an important
role in the study of NK cell biology (12). In the present study, NK92-MI cells
were used to investigate the mechanism by which NE regulates NK
cell function.
NE exerts its effects by binding to α- and β-ARs
(13). β2-AR is associated with G
proteins via a stimulatory G protein subunit, which positively
regulates the adenylate cyclase-cyclic adenosine monophosphate
(cAMP) signal transduction pathway and cAMP response
element-binding protein (CREB) activation. CREB (14,15),
a transcription factor that has previously been revealed to be
activated by protein kinase A (PKA), has important roles in
regulating the activity of NK cells (16,17).
However, whether the β2-AR/cAMP/PKA/phosphorylated (p)-CREB
signaling pathway is involved in regulation of NK cells remains to
be determined.
The results of the present study suggested that NE
may inhibit cytotoxicity and expression levels of perforin,
granzyme B and interferon (IFN)-γ in NK92-MI cells, predominantly
via the β2-adrenergic receptor/cAMP/PKA/p-CREB signal pathway. The
present study may further the present understanding regarding the
role of neuropeptides in the regulation of NK cell-associated
innate immunity.
Materials and methods
Cell culture
NK92-MI cells were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA) and maintained in a
modification of Eagle's minimum essential medium (α-MEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 0.01 mM 2-mercaptoethanol, 0.02 mM folic acid, 2
mM L-glutamine, 12.5% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) 0.2 mM inositol and 12.5%
horse serum (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C
in a humidified 5% CO2 incubator. K562 cells were also
obtained from ATCC and cultured in RPMI-1640 (Hyclone; GE
Healthcare Life Sciences) medium with 10% FBS at 37°C in a
humidified 5% CO2 incubator.
Cytotoxicity assay
NK92-MI cell cytotoxicity was determined using a
colorimetric, non-radioactive, assay that quantitatively measures
the release of lactate dehydrogenase (LDH) following cell lysis.
NK92-MI (effector) cells were incubated with a series of NE
(Sigma-Aldrich; Merck KGaA) concentrations 10−12,
10−10, 10−9, 10−8 and
10−7 M at 37°C for 48 h and then co-cultured with K562
(target) cells at an effector-to-target (E:T) ratio of 4:1 at 37°C
for 4 h. Samples were centrifuged at 1,500 × g at 4°C for 5 min.
The supernatants were collected and LDH release in the supernatants
was evaluated using a colorimetric reaction (absorbance at 490 nm).
The spontaneous and maximum LDH release was measured by adding 100
µl α-MEM or 1% NP-40 (Sigma-Aldrich, Merck KGaA) to the effector
cells or target cells. NE demonstrated no direct cytotoxic effect
on K562 cells or NK92-MI cells alone (data not shown). The
percentage specific lysis was calculated as follows:
Specific lysis (%)=[optical density
(OD)experimental group-ODtarget cell natural
release control]/(OD target cell maximum release
control-OD target cell natural release control)
×100%.
To evaluate the possible function of the AR α, β1
and β2 in mediating NE-induced effects, NK92-MI cells were
pre-incubated with specific α receptor antagonist phentol-amine
(PH), β1 receptor antagonist CGP20712A (CGP) or β2 receptor
antagonist ICI118551 (ICI; all were provided by Sigma-Aldrich;
Merck KGaA) at 37°C for 30 min prior to stimulation by NE. The
concentration of antagonists were 0.1, 0.3 and 1.0 µM. The
above-described procedures were used to detect NK92-MI cell
cytotoxic activity against K562 cells.
The present study also aimed to evaluate the role of
cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA)
signaling pathway in inhibitory effect on cytotoxicity of NK92-MI
cell by NE. NK92-MI cells were pre-incubated with PKA inhibitor
(Rp-8-Br-cAMP) 10 or 50 µM at 37°C for 30 min prior to stimulation
by NE. The above-described procedures were used to detect NK92-MI
cell cytotoxic activity against K562 cells.
Western blotting
NK92-MI cells exposed to NE (10−8 M) at
37°C for 24 h were harvested and washed with ice-cold PBS. Cells
were resuspended in SDS-protein lysis buffer [0.1% SDS, 50 mM
Tris-HCl (pH 8.0), 150 mM NaCl, 1% NP-40 and 1%
phenylmethylsulfonyl fluoride], solubilized for 30 min on ice and
then centrifuged at 4°C at 12,000 × g for 5 min. Protein
concentrations were measured using Bradford reagent (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). For western blotting,
equivalent amounts (40 µg) total protein per sample were separated
by SDS-PAGE on a 12.5% gel. Proteins in the gel were transferred
onto a polyvinylidene fluoride membrane by semi-dry blotting. The
membrane was blocked with 5% bovine serum albumin (BSA;
Sigma-Aldrich; Merck KGaA) overnight at 4°C and then incubated with
primary antibodies at room temperature for 2.5 h against the
following proteins: Perforin (cat. no. ab97305; 1:1,000; Abcam,
Cambridge, UK), granzyme B (cat. no. ab53097; 1:1,000; Abcam) and
β-actin (cat. no. ab8277; 1:2,000; Abcam). Following washing, the
membrane was incubated with a horseradish peroxidase
(HRP)-conjugated anti-rabbit IgG secondary antibody (cat. no.
ab107866; 1:10,000; Abcam) at room temperature for 1 h. The bands
were then visualized using Pierce ECL Western Blotting Substrate
(Thermo Fisher Scientific Inc.) at 0.5–10 min post-washing. The
protein expression was analyzed by Image-Pro plus software 6.0
(Media Cybernetics, Inc., Rockville, MD, USA), which was
represented as the density ratio vs. β-actin.
To investigate the possible function of the β2 AR in
mediating NE-induced effects, NK92-MI cells were pre-incubated with
ICI for at 37°C 30 min prior to stimulation by NE. The
concentrations of antagonists used were 0.1, 0.3 and 1.0 µM. The
above-described procedures were used to detect the expression of
CREB and p-CREB using the following primary antibodies: Anti-CREB
(cat. no. ab31387; 1:1,000; Abcam) and anti-p-CREB (cat. no.
ab10564; 1:1,000; Abcam). Following this, the membrane was
incubated with HRP-conjugated anti-rabbit IgG, (cat no. ab107866;
1:10,000; Abcam).
Measurement of interferon (IFN)-γ
production
IFN-γ levels in culture supernatants were measured
using an IFN-γ ELISA kit (Cusabio Biotech, Co., Ltd., Wuhan, China;
cat no. CSB-E04577h) following manufacturer's protocol.
Measurement of intracellular cAMP
levels
Intracellular cAMP levels were measured using cAMP
Enzyme immunoassay kit according to the manufacturer's protocol
(Sigma-Aldrich; Merck KGaA; cat no. CA200).
Statistical analysis
The difference among groups was analyzed with SPSS
statistical software (20.0; IBM, Corps., Armonk, NY, USA) using
one-way analysis of variance with a post-hoc Fisher's least
significant difference test. All values were expressed as mean ±
standard deviation. All experiments were performed in triplicate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
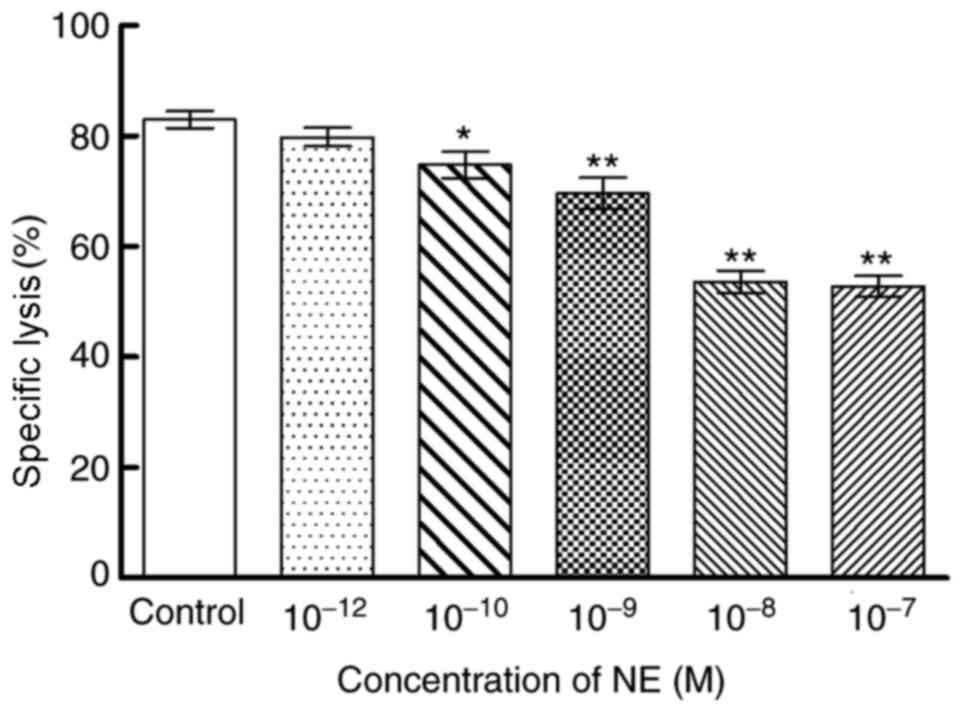
Effects of NE on cytotoxicity of
NK92-MI cells
An LDH-release cytotoxicity assay was used to
determine the cytotoxicity of NK92-MI cells against K562 cells
(Fig. 1). Compared with the
control group, NE (10−10-10−7 M)
significantly inhibited NK92-MI cells cytotoxicity in a
dose-dependent manner (P<0.05 and P<0.01).
Effects of AR antagonists on
inhibition of NK92-MI cell cytotoxicity by NE
AR antagonists were adopted to assess the roles of
ARs in mediating the inhibition of NK92-MI cell cytotoxicity by NE
(Fig. 2). Results demonstrated
that, compared with the NK92-MI cells stimulated by NE alone, α, β1
and β2 AR antagonists PH (1.0 µM), CGP (0.3 and 1.0 µM) and ICI
(0.1, 0.3 and 1.0 µM) could all partly block the inhibitory effect
on cytotoxicity of NK92-MI cells by NE (at optimal concentration
10−8 M; P<0.05). Among them, the effect of β2 AR
antagonists ICI was the most marked, the cytotoxicity of NK92-MI
cells stimulated by NE+ICI (1.0 µM) was significantly increased
compared with both PH and CGP (0.1, 0.3 and 1.0 µM; P<0.05).
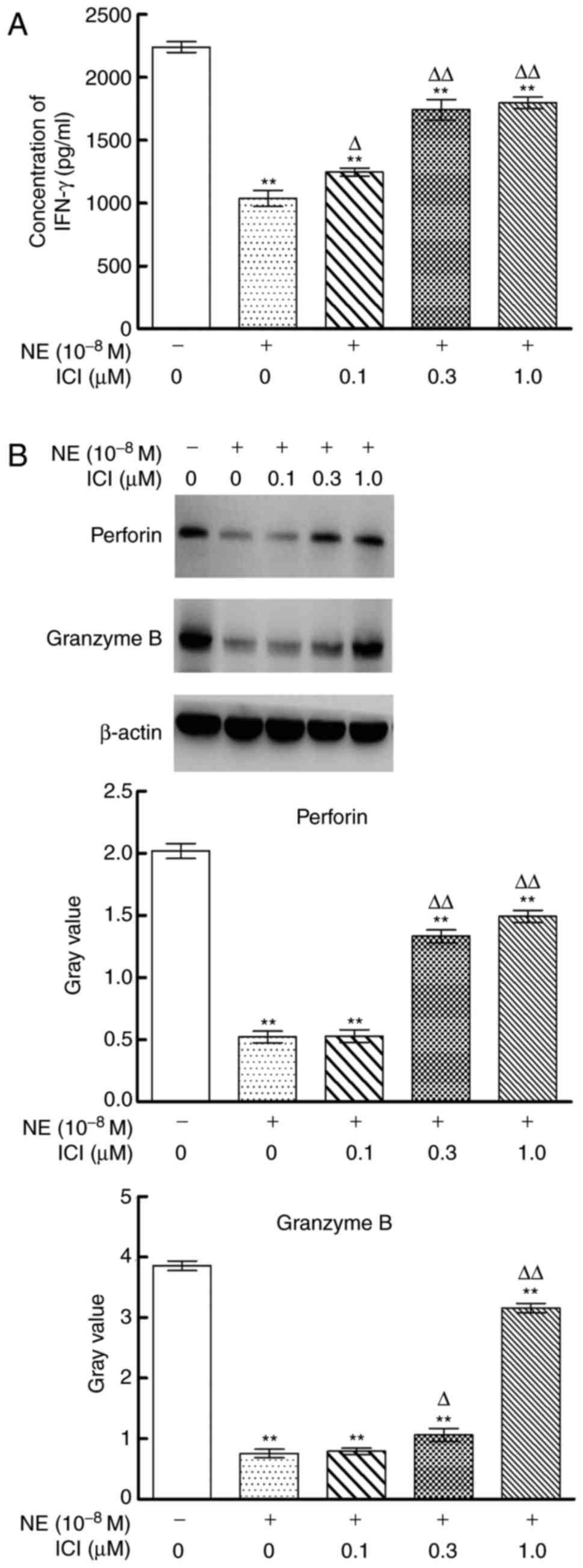
Effects of β2 AR antagonist on
expression of perforin, granzyme B and IFN-γ by NK92-MI cells
treated by NE
The effects of ICI on the expression of perforin,
granzyme B and IFN-γ of NK92-MI cells treated by NE were assessed
(Fig. 3). Compared with NE alone
treated NK92-MI cells, blockade of β2 AR by ICI (0.1, 0.3 and 1.0
µM) in NE treated NK92-MI cells resulted in significantly increased
expression of IFN-γ (P<0.05), ICI (0.3 and 1.0 µM) could
upregulate the expression of both perforin and granzyme B in NE
treated NK92-MI cells (P<0.05).
Role of cAMP-PKA signal pathway in the
inhibitory effect on the cytotoxicity of NK92-MI cell by NE
NK92-MI cells were pre-incubated with ICI for 30 min
prior to stimulation by NE, expression of CREB, p-CREB and
intracellular cAMP concentration were detected (Fig. 4A and B). NE could significantly
increase the expression of p-CREB and intracellular cAMP
concentration (P<0.01). Compared with NE alone treated NK92-MI
cells, blockade of β2 AR by ICI (0.3 and 1.0 µM) in NE treated
NK92-MI cells resulted in a significant reduction of the expression
of p-CREB and intracellular cAMP concentration (P<0.05).
NK92-MI cells were pre-incubated with PKA inhibitor
(Rp-8-Br-cAMP) for 30 min prior to stimulation by NE, cytotoxicity
of NK92-MI cells was detected (Fig.
4C). Compared with NE alone treated NK92-MI cells, inhibiting
the activity of PKA by Rp-8-Br-cAMP (10 and 50 µM) in NE treated
NK92-MI cells resulted in significantly increased cytotoxicity
(P<0.01).
Discussion
In mammals under stress, an increased level of
stress-associated hormone can be induced by the activation of the
hypothalamic-pituitary-adrenal and the sympathetic-adrenal
medullary axes, such as corticotropin releasing hormone,
adrenocorticotropic hormone and NE (18,19).
Activation by stress of the sympathetic nervous system results in
the release of catecholamines from the adrenal medulla and
sympathetic nerve terminals (20,21).
Catecholamines consist of several types of substances including NE,
dopamine, histamine, serotonin and epinephrine. NE is one of the
primary catecholamines of the sympathetic nervous system released
during a stress response and serves an important role in modulating
immune function (22).
In the present study, the effects of NE on NK92-MI
cells and associated mechanisms in vitro were investigated.
The major findings from the present study are that NE reduced
NK92-MI cell cytotoxicity, downregulated the expression of
perforin, granzyme B and IFN-γ in a dose-dependent manner. Granzyme
B, an essential cytotoxic effector, is the main NK cell granule
serine protease, and is produced by activated NK cells and
initially stored in cytoplasmic granules with other cytolytic
proteins including perforin (23).
Perforin not only induces necrotic death but also apoptosis
independently of granzyme B and facilitates entry of granzyme B
into the target cells (24). NK
cells are a major source of IFN-γ (25). NE exerts its effects through
binding to α- and β-ARs (13).
Blockade of NK α-, β1- and β2-ARs could suppress the inhibitory
cytotoxicity and expression of perforin, granzyme B and IFN-γ of NK
cells by NE. The results indicated that NK ARs (α, β1 and β2) are
all involved in the reduction of NK cells cytotoxicity and
perforin, granzyme B, IFN-γ expression by NE. Among ARs (α, β1 and
β2) antagonists, the effect of β2 AR antagonist (ICI) was the most
marked. The results of the present study indicated that β2 AR
served a more dominant role in this process than the two
others.
β2 AR, a G-protein linked receptor that classically
leads to intracellular accumulation of cAMP and activation of PKA
upon stimulation (14,15). The second messenger cAMP regulates
a number of cellular processes (26,27).
With the exception of certain ion channels, all known effects of
cAMP are mediated via the activation of PKA (28). It has demonstrated that increased
cAMP levels could inhibit the killing effect of NK-cell on tumor
cells (16,29). Blockade of β2 AR by ICI in NE
treated NK92-MI cells resulted in a reduction of the expression of
p-CREB and intracellular cAMP concentration. Inhibition of the
activity of PKA by Rp-8-Br-cAMP in NE treated NK92-MI cells
resulted in increased cytotoxicity. Results demonstrated that NE
inhibited NK92-MI cells through the β2-AR/cAMP/PKA/p-CREB signaling
pathway.
In conclusion, the results of the present study
suggest that NE can inhibit cytotoxicity and expression of
perforin, granzyme B and IFN-γ of NK92-MI cell mainly through the
β2-AR/cAMP/PKA/p-CREB signaling pathway. This novel study should
aid understanding of the role of neuropeptides in the regulation of
NK cell-associated innate immunity.
Acknowledgements
The authors would like to thank all members of the
Center of Experiment and Technology, China Medical University
(Shenyang, China) for their support and assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZS and ZL conceived and designed the experiments;
ZS, DH, SL and WF performed the experiments; JW analyzed the data;
and ZS wrote the manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed in
accordance with the guidelines provided by the Ethical committee of
Experimental Animal Care at China Medical University (Shenyang,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NK
|
natural killer
|
|
NE
|
norepinephrine
|
|
AR
|
adrenergic receptor
|
References
|
1
|
Bellinger DL and Lorton D: Autonomic
regulation of cellular immune function. Auton Neurosci. 182:15–41.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lorton D and Bellinger DL: Molecular
mechanisms underlying β-adrenergic receptor-mediated cross-talk
between sympathetic neurons and immune cells. Int J Mol Sci.
16:5635–5665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winerdal M, Winerdal ME, Wang YQ, Fredholm
BB, Winqvist O and Aden U: Adenosine A1 receptors contribute to
immune regulation after neonatal hypoxic ischemic brain injury.
Purinergic Signal. 12:89–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsuda K, Tsuda S and Nishio I: Role of
alpha2-adrenergic receptors and cyclic adenosine
monophosphate-dependent protein kinase in the regulation of
norepinephrine release in the central nervous system of
spontaneously hypertensive rats. J Cardiovasc Pharmacol. 42 Suppl
1:S81–S85. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilson CB, Ebenezer PJ, McLaughlin LD and
Francis J: Predator exposure/psychosocial stress animal model of
post-traumatic stress disorder modulates neurotransmitters in the
rat hippocampus and prefrontal cortex. PLoS One. 9:e891042014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kohm AP and Sanders VM: Norepinephrine: A
messenger from the brain to the immune system. Immunol Today.
21:539–542. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu C, Li Z, Sheng W, Fu R, Li L, Zhang T,
Wu Y, Xing L, Song J, Wang H and Shao Z: Abnormalities of
quantities and functions of natural killer cells in severe aplastic
anemia. Immunol Invest. 43:491–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakamoto N, Ishikawa T, Kokura S, Okayama
T, Oka K, Ideno M, Sakai F, Kato A, Tanabe M, Enoki T, et al: Phase
I clinical trial of autologous NK cell therapy using novel
expansion method in patients with advanced digestive cancer. J
Transl Med. 13:2772015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puerto M, Guayerbas N, Alvarez P and De la
Fuente M: Modulation of neuropeptide Y and norepinephrine on
several leucocyte functions in adult, old and very old mice. J
Neuroimmunol. 165:33–40. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takamoto T, Hori Y, Koga Y, Toshima H,
Hara A and Yokoyama MM: Norepinephrine inhibits human natural
killer cell activity in vitro. Int J Neurosci. 58:127–131. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gan X, Zhang L, Solomon GF and Bonavida B:
Mechanism of norepinephrine-mediated inhibition of human NK
cytotoxic functions: Inhibition of cytokine secretion, target
binding, and programming for cytotoxicity. Brain Behav Immun.
16:227–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diandong H, Kefeng S, Weixin F and Zaifu
L: Proteomic analysis of NK92-MI cells activated by neuropeptide
substance P. Neuropeptides. 47:157–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maletic V, Eramo A, Gwin K, Offord SJ and
Duffy RA: The role of norepinephrine and its α-Adrenergic receptors
in the pathophysiology and treatment of major depressive disorder
and schizophrenia: A systematic review. Front Psychiatry. 8:422017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guereschi MG, Araujo LP, Maricato JT,
Takenaka MC, Nascimento VM, Vivanco BC, Reis VO, Keller AC, Brum PC
and Basso AS: Beta2-adrenergic receptor signaling in CD4+ Foxp3+
regulatory T cells enhances their suppressive function in a
PKA-dependent manner. Eur J Immunol. 43:1001–1012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang D, Ma QY, Hu HT and Zhang M:
β2-adrenergic antagonists suppress pancreatic cancer cell invasion
by inhibiting CREB, NFkB and AP-1. Cancer Biol Ther. 10:19–29.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao W, Huang Y, Liu Z, Cao BB, Peng YP
and Qiu YH: Dopamine receptors modulate cytotoxicity of natural
killer cells via cAMP-PKA-CREB signaling pathway. PLoS One.
8:e658602013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martinet L, Jean C, Dietrich G, Fournié JJ
and Poupot R: PGE2 inhibits natural killer and gamma delta T cell
cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA
type I-dependent signaling. Biochem Pharmacol. 80:838–845. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stuempfle KJ, Nindl BC and Kamimori GH:
Stress hormone responses to an ultraendurance race in the cold.
Wilderness Environ Med. 21:22–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eshkevari L, Permaul E and Mulroney SE:
Acupuncture blocks cold stress-induced increases in the
hypothalamus-pituitary-adrenal axis in the rat. J Endocrinol.
217:95–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verbrugghe E, Boyen F, Gaastra W, Bekhuis
L, Leyman B, Van Parys A, Haesebrouck F and Pasmans F: The complex
interplay between stress and bacterial infections in animals. Vet
Microbiol. 155:115–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng GH, Liu J, Zhang J, Wang Y, Peng XC,
Wei YQ and Jiang Y: Exogenous norepinephrine attenuates the
efficacy of sunitinib in a mouse cancer model. J Exp Clin Cancer
Res. 33:212014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slota C, Shi A, Chen G, Bevans M and Weng
NP: Norepinephrine preferentially modulates memory CD8 T cell
function inducing inflammatory cytokine production and reducing
proliferation in response to activation. Brain Behav Immun.
46:168–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spaeny-Dekking EH, Hanna WL, Wolbink AM,
Wever PC, Kummer JA, Swaak AJ, Middeldorp JM, Huisman HG, Froelich
CJ and Hack CE: Extracellular granzymes A and B in humans:
Detection of native species during CTL responses in vitro and in
vivo. J Immunol. 160:3610–3616. 1998.PubMed/NCBI
|
|
24
|
Fu WX, Qin B, Zhou AP, Yu QY, Huang QJ and
Liang ZF: Regulation of NK92-MI cell cytotoxicity by substance P.
Scand J Immunol. 74:107–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barakat FM, McDonald V, Di Santo JP and
Korbel DS: Roles for NK cells and an NK cell-independent source of
intestinal gamma interferon for innate immunity to Cryptosporidium
parvum infection. Infect. Immun. 77:5044–5049. 2009.
|
|
26
|
Chen H, Wild C, Zhou X, Ye N, Cheng X and
Zhou J: Recent advances in the discovery of small molecules
targeting exchange proteins directly activated by cAMP (EPAC). J
Med Chem. 57:3651–3665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Skalhegg BS and Tasken K: Specificity in
the cAMP/PKA signaling pathway. Differential expression,
regulation, and subcellular localization of subunits of PKA. Front
Biosci. 5:D678–D693. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Torgersen KM, Vaage JT, Levy FO, Hansson
V, Rolstad B and Tasken K: Selective activation of cAMP-dependent
protein kinase type I inhibits rat natural killer cell
cytotoxicity. J Biol Chem. 272:5495–5500. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martinet L, Jean C, Dietrich G, Fournié JJ
and Poupot R: PGE2 inhibits natural killer and gamma delta T cell
cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA
type I-dependent signaling. Biochem Pharmacol. 80:838–845. 2010.
View Article : Google Scholar : PubMed/NCBI
|