Introduction
Osteoarthritis (OA) is a multifactorial disease that
is characterized by biochemical and morphological alterations
resulting from unbalanced cartilage anabolism and catabolism, and
is a primary cause of pain and disability in the elderly population
(1,2). Although the understanding of the
exact mechanism of OA pathogenesis remains incomplete, the
inflammatory response is considered to be implicated in the
development and progression of OA (3). Interleukin (IL)-1β, which controls
the degradation of articular cartilage matrix, is among the
mediators involved in the disturbance of metabolic processes in OA
pathophysiology (4). The catabolic
effects of IL-1β are primarily mediated through the activation of
various signaling pathways, including mitogen-activated protein
kinase (MAPK) and nuclear factor (NF)-κB (4). Previous studies have demonstrated
that aggrecanase-2 (ADAMTS5) is a major factor involved in OA
(5), and inhibition of ADAMTS5 has
been reported to prevent cartilage degradation in an animal OA
model (6). Thus, ADAMTS5
regulation may be regarded as a rational strategy for therapeutic
intervention in OA (7).
MicroRNAs (miRNAs/miRs) are a class of regulative
non-coding RNA with a length of 18–23 nucleotides that function in
the post-transcriptional regulation of gene expression. miRNAs
serve critical roles in multiple biological processes in human
diseases (1). Previous studies
have reported differential miRNAs expression patterns among OA,
normal plasma (8), synovial fluid
(9) and cartilage (10). A previous study demonstrated that
miRNAs participated in the ‘tug-of-war’ between tissue homeostasis
and OA pathogenesis (11). Further
studies have demonstrated that dysregulation of miR-27a was
involved in cardiovascular risk (12), neuronal autophagy in brain injury
(13) and prostate cancer
progression (14). In addition,
treatment with anti-miR-27a significantly increased the expression
of matrix metallopeptidase (MMP)-13 in human osteoarthritic
chondrocytes (15). Certain miRNAs
have been confirmed to serve an important role in OA pathogenesis
and disease progression through the regulation of ADAMTS5
expression (16,17). However, whether miR-27a-3p
regulates the expression of ADAMTS5 in primary normal chondrocytes
and its role during the progression of OA remains to be
determined.
The present study focused on the role of miR-27a-3p
in OA development and the associated underlying mechanisms.
Materials and methods
Human cartilage collection
The consent and study plan was approved by the
Ethics Committee on Human Experimentation at the First Affiliated
Hospital of Sun Yat-sen University (Guangzhou, China; IRB: 2011011)
and complied with the Declaration of Helsinki 2000. All
participants provided written informed consent. Femoral condyles or
tibial plateaus were selected as specimens. Human OA cartilage was
obtained from knee joints at the time of total knee replacement
operation (n=5, aged 59–72 years). OA was diagnosed according to
the American College of Rheumatology (18). Normal cartilage samples were taken
from patients (n=5, aged 11–28 years), who suffered from various
types of bone/soft tissue cancer affecting the thigh/ilium and had
amputation of the thigh. Patient information is presented in
Table I.
 | Table I.Characteristics of patients included
in the present study. |
Table I.
Characteristics of patients included
in the present study.
| No. | Sex | Age | Diagnosis | Operation | Material
position | Classification |
|---|
| 1 | M | 16 | Right thigh
osteosarcoma | Amputation | Right Knee | Normal
cartilage |
| 2 | M | 23 | Left iliac Ewing
sarcoma | Amputation | Left Knee | Normal
cartilage |
| 3 | F | 22 | Malignant soft
tissue tumor of the left thigh | Amputation | Left Knee | Normal
cartilage |
| 4 | M | 28 | Right thigh
osteosarcoma | Amputation | Right Knee | Normal
cartilage |
| 5 | F | 11 | Left thigh Ewing
sarcoma | Amputation | Left Knee | Normal
cartilage |
| 6 | M | 59 | Osteoarthritis in
both knees | TKA | Right Knee | OA cartilage |
| 7 | F | 65 | Left knee
osteoarthritis | TKA | Left Knee | OA cartilage |
| 8 | F | 65 | Osteoarthritis in
both knees | TKA | Right Knee | OA cartilage |
| 9 | M | 72 | Left knee
osteoarthritis | TKA | Left Knee | OA cartilage |
| 10 | F | 65 | Osteoarthritis in
both knees | TKA | Left Knee | OA cartilage |
Primary chondrocyte isolation and cell
culture
Articular cartilage tissues obtained from normal
articular cartilage were broken up into small pieces (<1
mm3) and digested sequentially in pronase (Roche
Diagnostics, Basel, Switzerland) at 37°C for 90 min and collagenase
P (Roche Diagnostics) for 12 h at 37°C on a stirring plate.
Isolated primary human articular chondrocytes were seeded into
flasks containing Dulbecco's Modified Eagle's medium/F12 (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
5% fetal bovine serum, 1% penicillin and 1% streptomycin at 37°C
and 5% CO2. The medium was replaced every three days.
First-passage chondrocytes were used for subsequent
experiments.
Computational prediction of miR-27a-3p
target gene
The miRNA target prediction software miRanda
(http://www.microrna.org) and the TargetScan
Database (http://www.targetscan.org/) were
employed to predict miR-27a-3p binding sites in human 3′UTRs as
described in a previous study (19), and only those identified by two
software were selected for further study.
Treatment with IL-1β and
transfection
When the cells reached 80% confluence,
1×106 cells were seeded into 6-well plates for
recombinant human IL-1β (PeproTech, Inc., Rocky Hill, NJ, USA)
treatment and stimulated with 0, 1, 5 or 10 ng/ml IL-1β for 0, 3,
4, 5, 8 or 24 h (0–5 h for RNA extraction and 0–24 h for protein
extraction). For miR-27a-3p overexpression experiments,
2×104 primary human chondrocytes were transfected with
50 nM miR-27a-3p mimic (5′-UUCACAGUGGCUAAGUUCCGC-3′; Guangzhou
RiboBio, Co., Ltd., Guangzhou, China) using
Lipofectamine® 2000 (Gibco; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. miR-Control (cat.
no. miR01101; Guangzhou RiboBio, Co., Ltd.) with 50 nM was used as
a control. After 24 h transfection, the cells were treated with
IL-1β (5 ng/ml) at 37°C for 5 and 24 h to collect the mRNA and
protein, respectively. For the involvement of NF-κB and MAPK
signaling pathways investigation, 1×106 primary human
chondrocytes were treated with pathway-specific inhibitors. The
NF-κB inhibitor, SN50 (5 µM) and various MAPK inhibitors, including
SB203580 (1 µM), PD98059 (10 µM) and SP600125 (10 µM) (p38, MEK-1/2
and c-Jun N-terminal kinase inhibitors, respectively), were used to
treat cells for 2 h prior to the exposure of IL-1β, which was
followed by treatment with IL-1β (5 ng/ml; 5 h for RNA extraction
and 24 h for protein extraction) at 37°C. The cells were then
harvested for subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cartilage tissues and
chondrocytes using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was generated using the PrimeScript® miRNA
cDNA Synthesis kit (Takara Biotechnology, Inc.). To analyze
miR-27a-3p expression, qPCR was performed using SYBR Premix Ex Taq
II (Takara Bio, Inc., Otsu, Japan) on a CFX96 Real-Time PCR
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
miR-27a-3p expression was normalized to U6 expression (20). For GAPDH and ADAMTS5, total RNA was
reverse transcribed using the miRNeasy Mini kit (Qiagen Sciences,
Inc., Gaithersburg, MD, USA) and qPCR was performed using the SYBR
green system (Takara Bio, Inc.) (21). GAPDH was used as a reference.
Relative expression levels were quantified by the 2−ΔΔCq
method (22). Each experiment was
repeated three times and each assay was performed in triplicate.
All primers were designed by Sangon Biotech Co., Ltd., (Shanghai,
China). Gene-specific primer pairs were as follows: ADAMTS5
forward, 5′-AATGCACTTCAGCCACCATCA-3′ and reverse,
5′-TCGTAGGTCTGTCCTGGGAGTTC-3′; GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′.
The primer sequence for RT-PCR miR-27a-3p forward,
5′-TTCACAGTGGCTAAGTTCCGC-3′, miRNA-specific primer was provided by
Mir-X miRNA qRT-PCR SYBR kits; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′.
Western blot analysis
Western blot experiments were performed according to
standard methods. Total protein was isolated from primary human
chondrocytes or cartilage tissues. Cells were placed on ice
immediately following treatments washed with ice-cold PBS and
harvested in Mammalian Protein Extraction Reagent buffer (Pierce;
Thermo Fisher Scientific, Inc.). All of the wash buffers and
Mammalian Protein Extraction Reagent buffer included a 1X protease
inhibitor mixture (Roche Diagnostics), NaF (5 mM) and
Na3VO4 (200 µM). The concentration of the
protein was detected by the bicinchoninic acid method.
Subsequently, 50 mg total cell proteins from each sample was
resolved by 10% SDS-PAGE and transferred by electroblotting to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% nonfat dry milk in 0.1%
TBS-Tween-20 (TBST) at 37°C for 1 h and subsequently incubated
overnight at 4°C in 5% nonfat dry milk in TBST with a primary
antibody against ADAMTS5 (1:1,000 dilution; cat. no. ab135656;
Abcam, Cambridge, UK). A primary antibody against GAPDH (1:2,000
dilution, cat. no. 5174S; Cell Signaling Technology, Inc.) was also
used. After washing, the membranes were incubated with anti-rabbit
antibody (1:3,000; cat. no. 111-035-003; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) conjugated to horseradish
peroxidase at 37°C for 1 h and proteins were detected using
Enhanced Chemiluminescence Plus reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and an ImageQuant LAS 4000 Mini detection system
(GE Healthcare Life Sciences, Little Chalfont, UK). Densitometric
analysis was performed using ImageJ software version 1.39 (National
Institutes of Health, Bethesda, MD, USA).
Dual luciferase assays
DNA sequences of the ADAMTS5 3′untranslated region
(3′UTR) were amplified by PCR (98°C 10 sec; 55°C 5 sec or 15 sec;
72°C 1 min/kb; 30 cycles), and used the Prime STAR HS DNA
Polymerase (Takara Bio, Inc.). The amplified DNA sequences were
cloned into the pMIR-REPORT Luciferase (Obio Technology Corp.,
Ltd., Shanghai, China) and the restriction enzymes used were Mlu
and Hind III (NEB), and co-transfected using Lipofectamine 2000
(Thermo Fisher Scientific, Inc., with the control or hsa-miR-27a-3p
into 293 cells (purchased from American Type Culture Collection,
Manassas, VA, USA). The DNA sequence of ‘ADAMTS5 3′UTR’ was
amplified on the genomic DNA. The primers that were used for
amplification are H4597-4-1 and H4597-4-2, the sequence for
H4597-4-1 is 5′-ATAGGCCGGCATAGACGCGTCAACTTAACTGGCTAGTACATTG-3′, and
H4597-4-2 is
5′-AAAGATCCTTTATTAAGCTTTACTTTAACCTAGTTTACAATTTATATTTATTATG-3′. The
sequencing primers for ADAMTS5 3′UTR Vector are Luc-C-F, M13F and
H4597-F1, with Luc-C-F: GAGGAGTTGTGTTTGTGGAC, M13F:
TGTAAAACGACGGCCAGT, and H4597-F1: GTGAGGAAAACTGTGATTTGTAGG.
For the dual luciferase assay, 1.2×104
293 cells in a 96-well plate were transfected with 50 nM miR-27a-3p
or miR-NC (Guangzhou RiboBio Co., Ltd.). The cells were then
co-transfected with 2 mg/ml of vector with the wild-type or mutant
3′UTR of ADAMTS5 gene. Cell lysates were harvested 48 h after
transfection and luciferase activity was determined using the
Dual-Luciferase® Reporter Assay System (Promega
Corporation, Madison, WI, USA). Firefly luciferase values were
normalized to the Renilla signal, and the ratio of the
Firefly/Renilla values was determined. All experiments were
performed in triplicate.
Statistical analysis
Continuous data are presented as the mean ± standard
deviation. One-way analysis of variance or Student's t-tests were
used to identify significant differences among or between groups.
P<0.05 was considered to indicate a statistically significant
difference. Data were analyzed using SPSS statistical software
version 19.0 (IBM Corp., Armonk, NY, USA). All experiments were
performed at least three times.
Results
Expression levels of miR-27a-3p and
ADAMTS5 in normal and OA cartilage
To investigate the potential effect of miR-27a-3p in
the progression of OA, the present study determined the expression
of miR-27a-3p in normal and OA articular cartilage. The results
demonstrated that miR-27a-3p expression was significantly decreased
in OA cartilage compared with normal cartilage (Fig. 1A), while the mRNA and protein
expression levels of ADAMTS5 were significantly upregulated in OA
cartilage compared with normal cartilage (Fig. 1B-D).
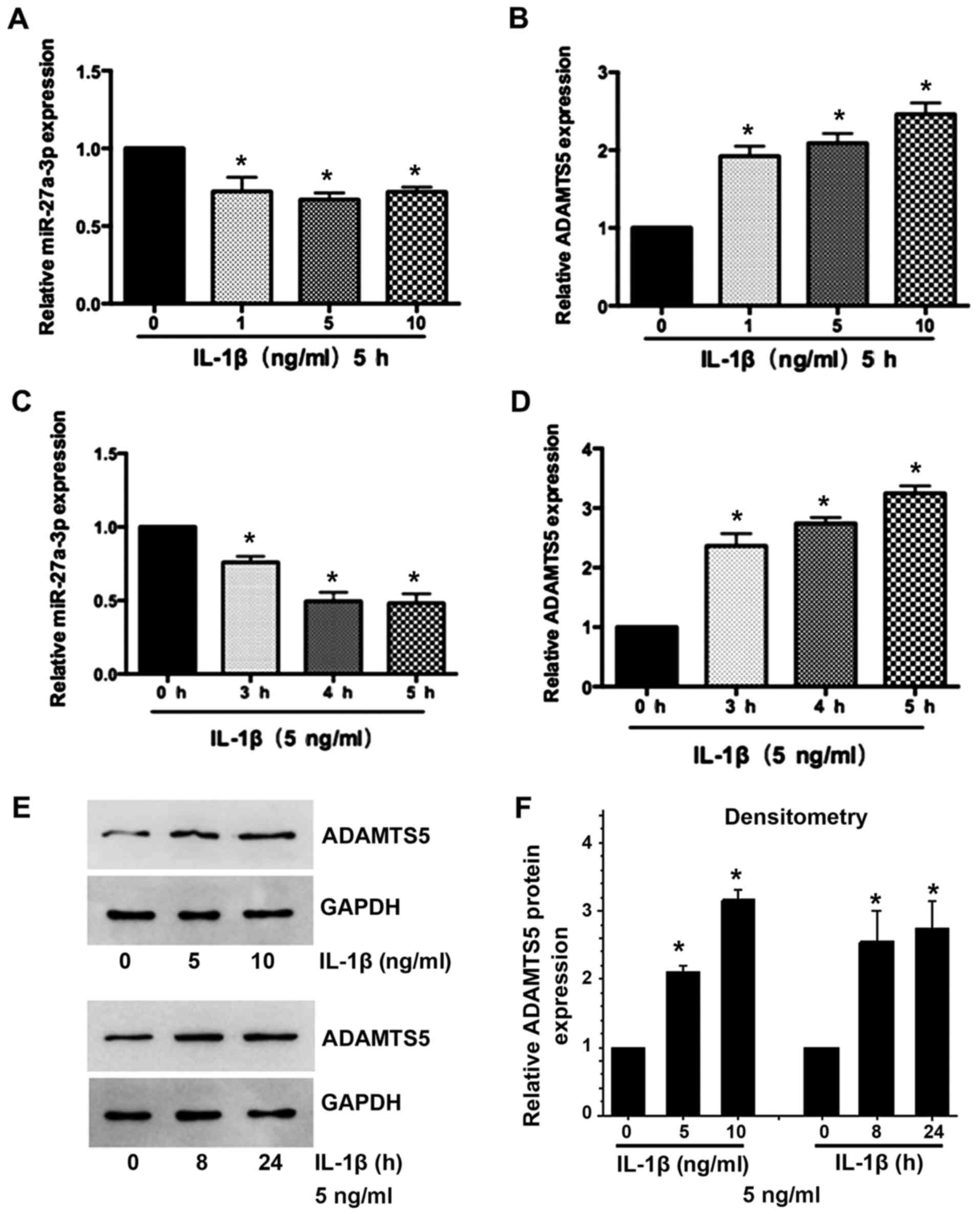
IL-1β suppresses miR-27a-3p expression
and induces ADAMTS5 expression in primary human chondrocytes
Subsequently, the present study determined the
expression of miR-27a-3p and ADAMTS5 in IL-1β-stimulated human
chondrocytes. Decreased miR-27a-3p (Fig. 2A and B) and increased ADAMTS5 mRNA
and protein (Fig. 2C-F) expression
was observed in a time- and dose-dependent manner in chondrocytes
treated with IL-1β. These results indicated that there may be a
negative association between miR-27a-3p and the expression of
ADAMTS5 in the development of OA.
miR-27a-3p inhibits ADAMTS5 expression
in human chondrocytes
RT-qPCR was performed to confirm the successful
overexpression of miR-27a-3p in human chondrocytes (Fig. 3A). RT-qPCR and western blot
analysis demonstrated that overexpression of miR-27a-3p suppressed
the mRNA and protein expression of ADAMTS5 in human chondrocytes,
compared with miR-Control-transfected cells (Fig. 3B-D). Furthermore, miR-27a-3p
overexpression downregulated IL-1β-induced increases in the mRNA
and protein expression of ADAMTS5 in human chondrocytes (Fig. 3B-D). These results indicated that
the expression of ADAMTS5 may be downregulated by miR-27a-3p in
human chondrocytes.
miR-27a-3p targets the 3′UTR of
ADAMTS5 mRNA
To further clarify the molecular mechanisms that
underlie the regulation of ADAMTS5 expression by miR-27a-3p, the
sequence in the 3′UTR of ADAMTS5 gene was analyzed. Bioinformatics
prediction such as miRanda (http://www.microrna.org) and the TargetScan Database
(http://www.targetscan.org/) demonstrated
that ADAMTS5 may be a potential miR-27a-3p target gene (Fig. 4A). 293 cells were co-transfected
with miR-27a-3p mimics or miR-Control and wild-type or mutant
ADAMTS5 plasmids. A double-luciferase reporter gene system
demonstrated that the miR-27a-3p mimics had no significant effect
on the luciferase activity of the mutant ADAMTS5 plasmid, but
significantly decreased the luciferase activity of the wild-type
reporter plasmid compared with those co-transfected with
miR-Control (P<0.05; Fig. 4B).
These results indicated that ADAMTS5 may be a direct target of
miR-27a-3p.
IL-1β regulates miR-27a-3p expression
via NF-κB and MAPK signaling pathways in chondrocytes
A previous study confirmed that IL-1β induced the
activation of NF-κB and MAPK in human chondrocytes (23), and IL-1β has also been reported to
modulate the transcription of MMPs and tissue inhibitor of
metallopeptidases via NF-κB and MAPK (24). To determine whether the NF-κB and
MAPK signaling pathways may regulate IL-1β-dependent expression of
miR-27a-3p and ADAMTS5, primary human chondrocytes were treated
with pathway-specific inhibitors. Compared with IL-1β treatment
alone, the expression of miR-27a-3p was enhanced by pretreatment
with NF-κB and MAPK inhibitors (Fig.
5A) and ADAMTS5 expression was suppressed at both the mRNA and
protein levels (Fig. 5B-D). These
results indicated that the effect of IL-1β on miR-27a-3p and
ADAMTS5 expression in human chondrocytes may be associated with the
activation of the NF-κB and MAPK signaling pathways.
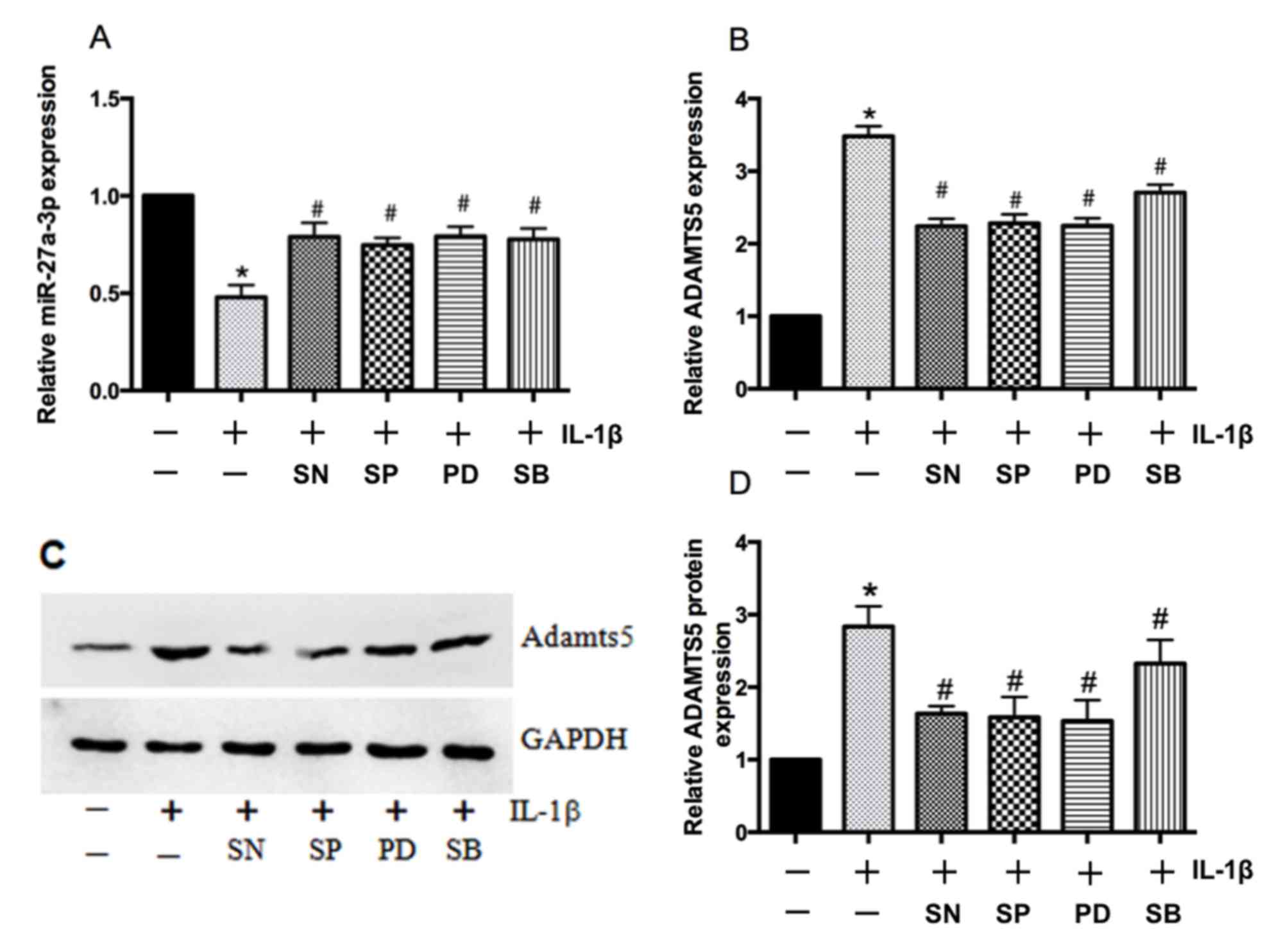 | Figure 5.Mitogen-activated protein kinases and
NF-κB regulate IL-1β-mediated effects on miR-27a-3p and ADAMTS5
expression. Reverse transcription-quantitative polymerase chain
reaction analysis of (A) miR-27a-3p and (B) ADAMTS5 mRNA expression
in primary human chondrocytes following IL-1β treatment for 5 h
with or without a p38 inhibitor (SB203580), ERK inhibitor
(PD98059), JNK inhibitor (SP60025) or NF-κB inhibitor (SN50). (C)
Representative western blot bands for ADAMTS5 protein expression in
primary human chondrocytes treated with IL-1β for 24 h with or
without p38, ERK, JNK and NF-κB inhibitors. (D) Densitometric
analysis of western blotting results demonstrated that all of the
inhibitors significantly reduced IL-1β-induced increases in the
protein expression of ADAMTS5. Data are presented as the mean ±
standard deviation of three independent experiments. *P<0.05 vs.
non-stimulated controls and #P<0.05 vs.
IL-1β-stimulated controls. NF-κB, nuclear factor-κB; IL-1β,
interleukin-1β; miR, microRNA; ADAMTS5, aggrecanase-2; ERK,
extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; SB, SB203580; PD, PD98059; SP, SP60025; SN, SN50. |
Discussion
Disruptions in extracellular matrix (ECM)
homeostasis are key events in the pathogenesis of OA (25) and ADAMTS5 has a key role in ECM
homeostasis due to its capacity to degrade a wide range of ECM
components (26). The current
study demonstrated that the expression of miR-27a-3p was
significantly lower in OA cartilage compared with normal cartilage,
while the overexpression of miR-27a-3p in human chondrocytes
inhibited ADAMTS5 expression. In addition, the expression levels of
ADAMTS5 and miR-27a-3p were demonstrated to be mediated by IL-1β,
which may be associated with the activation of the NF-κB and MAPK
signaling pathways. Mutation of the miR-27a-3p binding site in the
3′-UTR of ADAMTS5 mRNA abolished miR-27a-3p-mediated repression of
reporter activity. Overall, the results of the present study
indicated that miR-27a-3p may serve a role in regulating the
expression of ADAMTS5 in OA.
Aggrecan depletion in arthritic cartilage has been
considered to be the primary pathological feature of OA (27). During OA development, ADAMTS5
activity is regulated at the transcriptional level and via
post-translational modifications and the proprotein may be
processed by various convertases or proteases, including furin,
proprotein convertase 7 and syndecan 4 (28,29).
Previous studies have investigated the pathogenesis of OA using
ADAMTS4/5 knockout mice, the results of which revealed that
inhibition of ADAMTS5, but not ADAMTS4, relieved aggrecan
degradation and cartilage destruction, which indicated that ADAMTS5
served a role in OA cartilage aggrecan degradation in mice
(5,6). Consistent with previously published
data (30), the present study
demonstrated that ADAMTS5 was upregulated in human OA cartilage
compared with normal cartilage.
The catabolic and anabolic effects of miRNAs on OA
cartilage has received attention (31). miRNAs have also been reported to be
involved in the pathogenesis of ADAMTS5-associated OA.
miRNA-140-knock-out mice developed OA-like features, which were
attributed to the elevated expression of ADAMTS5 (29). In addition, IL-1β suppressed the
expression of miRNA-140 in chondrocytes in vitro (32) and Miyaki et al (29) reported that miR-140 downregulated
ADAMTS5 expression in IL-1β-induced OA chondrocytes. Furthermore,
Hu et al (33) reported
that miR-302, nuclear receptor subfamily 2 group F member 2 and
octamer-binding transcription factor 4 may be involved in a novel
mechanism for understanding and inducing pluripotency in somatic
cells, and that NR2F2 may be directly targeted by miR-302. Liang
et al (34) demonstrated
that miR-302 was downregulated in irradiated breast cancer cells
and is a potential sensitizer to radiotherapy. However, the role of
miR-27a-3p in OA and whether miR-27a-3p regulates ADAMTS5 remains
unknown. In the present study, miR-27a-3p was downregulated in OA
cartilage compared with normal cartilage. The overexpression of
miR-27a-3p inhibited the expression of ADAMTS5 in human
chondrocytes. These observations, along with the results of the
luciferase reporter assay, indicated that miR-27a-3p may interact
with the 3′UTR of ADAMTS5 mRNA and downregulate its expression at
the post-transcriptional level. These results indicated that
miR-27a-3p may serve important roles in the pathogenesis and
development of OA.
Cartilage matrix degradation may be stimulated and
enhanced by cytokines, such as IL-1β or tumor necrosis factor-α,
which promote aggrecan degradation through the regulation of MMPs
and aggrecanases (35). IL-1β may
stimulate ADAMTS5 expression, which leads to cartilage
extracellular matrix degradation. A previous study demonstrated a
critical role of miR-30a in regulating IL-1β-meditated OA
pathogenesis and provided novel insight into the mechanisms of
cytokine modulation of ADAMTS5 expression (30). In the present study, IL-1β
treatment repressed miR-27a-3p expression in chondrocytes. It was
also demonstrated that IL-1β-induced suppression of miR-27a-3p was
reversed by NF-κB and MAPK inhibitors. A similar induction of
ADAMTS5 in OA by NF-κB and MAPK activation was previously reported
(36). These results indicate that
miR-27a-3p may serve an important role in the degeneration of
cartilage and may be regulated by NF-κB and MAPK catabolic pathways
in chondrocytes.
The current study has several limitations. Only 5
unpaired samples were used, and studies with a higher number of
samples with similar age should be performed to confirm these
results. Although it is difficult to collect more normal cartilage
in a short time because of the limited number of patients
undergoing the right type of surgery to obtain these tissues and
who are willing to enroll in the study, therefore greater numbers
will be collected to enhance validity in the future. Another
limitation is that the current study only focused on the regulation
of ADAMTS5 by miR-27a-3p; the regulation of aggrecan degradation by
miR-27a-3p will be investigated in future studies. In addition, the
present study primarily consisted of in vitro experiments,
and in vivo experiments should be performed in future
studies.
In conclusion, this study demonstrated that
miR-27a-3p was downregulated in human OA and was suppressed by
IL-1β, acting as a crucial regulator of ADAMTS5, in OA
chondrocytes. The data may provide insight into the roles of
miR-27a-3p in OA pathogenesis as a therapeutic target for OA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Guangdong
Natural Science Foundation (grant no. 2016A030313259), the Startup
Foundation for Doctors of the Guangdong Natural Science Foundation
(grant no. 2015A030310451), the National Natural Science Foundation
of China (grant no. 81401840) and Sun Yat-sen University Starting
Funds for Young Teachers (grant no. 16ykpy31).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PH and HW conceived and designed the study. XL, YK,
ZL and HXW performed the experiments. HW, ZL and HXW wrote the
paper. YX, ML and DX analyzed the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee on
Human Experimentation at the First Affiliated Hospital of Sun
Yat-Sen University (Guangzhou, China; IRB: 2011011). Written
informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OA
|
osteoarthritis
|
|
ADAMTS5
|
aggrecanase-2
|
|
ECM
|
extracellular matrix
|
|
MAPK
|
mitogen-activated protein kinase
|
|
miRNA
|
microRNA
|
References
|
1
|
Jin R, Shen M, Yu L, Wang X and Lin X:
Adipose-derived stem cells suppress inflammation induced by IL-1β
through down-regulation of P2X7R mediated by miR-373 in
chondrocytes of osteoarthritis. Mol Cells. 40:222–229.
2017.PubMed/NCBI
|
|
2
|
Blalock D, Miller A, Tilley M and Wang J:
Joint instability and osteoarthritis. Clin Med Insights Arthritis
Musculoskelet Disord. 8:15–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis!).
Osteoarthritis Cartilage. 21:16–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stanton H, Rogerson FM, East CJ, Golub SB,
Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, et
al: ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and
in vitro. Nature. 434:648–652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glasson SS, Askew R, Sheppard B, Carito B,
Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, et al:
Deletion of active ADAMTS5 prevents cartilage degradation in a
murine model of osteoarthritis. Nature. 434:644–648. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deng H, O'Keefe H, Davie CP, Lind KE,
Acharya RA, Franklin GJ, Larkin J, Matico R, Neeb M, Thompson MM,
et al: Discovery of highly potent and selective small molecule
ADAMTS-5 inhibitors that inhibit human cartilage degradation via
encoded library technology (ELT). J Med Chem. 55:7061–7079. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cuadra Borgonio VM, González-Huerta NC,
Romero-Córdoba S, Hidalgo-Miranda A and Miranda-Duarte A: Altered
expression of circulating microRNA in plasma of patients with
primary osteoarthritis and in silico analysis of their pathways.
PLoS One. 9:e976902014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu JF, Zhang SJ, Zhao C, Qiu BS, Gu HF,
Hong JF, Cao L, Chen Y, Xia B, Bi Q and Wang YP: Altered microRNA
expression profile in synovial fluid from patients with knee
osteoarthritis with treatment of hyaluronic acid. Mol Diagn Ther.
19:299–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Díaz-Prado S, Cicione C, Muiños-López E,
Hermida-Gómez T, Oreiro N, Fernández-López C and Blanco FJ:
Characterization of microRNA expression profiles in normal and
osteoarthritic human chondrocytes. BMC Musculoskelet Disord.
13:1442012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldring MB and Marcu KB: Epigenomic and
microRNA-mediated regulation in cartilage development, homeostasis,
and osteoarthritis. Trends Mol Med. 18:109–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arroyo B A, Salloum-Asfar S, Pérez-Sánchez
C, Teruel-Montoya R, Navarro S, García-Barberá N, Luengo-Gil G,
Roldán V, Hansen JB, López-Pedrera C, et al: Regulation of TFPIα
expression by miR-27a/b-3p in human endothelial cells under normal
conditions and in response to androgens. Sci Rep. 7:435002017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun L, Zhao M, Wang Y, Liu A, Lv M, Li Y,
Yang X and Wu Z: Neuroprotective effects of miR-27a against
traumatic brain injury via suppressing FoxO3a-mediated neuronal
autophagy. Biochem Biophys Res Commun. 482:1141–1147. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wan X, Huang W, Yang S, Zhang Y, Zhang P,
Kong Z, Li T, Wu H, Jing F and Li Y: Androgen-induced miR-27A acted
as a tumor suppressor by targeting MAP2K4 and mediated prostate
cancer progression. Int J Biochem Cell Biol. 79:249–260. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tardif G, Hum D, Pelletier JP, Duval N and
Martel-Pelletier J: Regulation of the IGFBP-5 and MMP-13 genes by
the microRNAs miR-140 and miR-27a in human osteoarthritic
chondrocytes. BMC Musculoskelet Disord. 10:1482009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Djuranovic S, Nahvi A and Green R:
miRNA-mediated gene silencing by translational repression followed
by mRNA deadenylation and decay. Science. 336:237–240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin X, Wang JQ and Yan SY: Reduced miR26a
and miR26b expression contributes to the pathogenesis of
osteoarthritis via the promotion of p65 translocation. Mol Med Rep.
15:551–558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altman R, Asch E, Bloch D, Bole G,
Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg
M, et al: Development of criteria for the classification and
reporting of osteoarthritis. Classification of osteoarthritis of
the knee. Diagnostic and Therapeutic Criteria Committee of the
American Rheumatism Association. Arthritis Rheum. 29:1039–1049.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao WJ, Zhang HF and Su JY:
Downregulation of microRNA-195 promotes angiogenesis induced by
cerebral infarction via targeting VEGFA. Mol Med Rep. 16:5434–5440.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wa Q, Liu Y, Huang S, He P, Zuo J, Li X,
Li Z, Dong L, Peng J, Wu S, et al: miRNA-140 inhibits C3H10T1/2
mesenchymal stem cell proliferation by targeting CXCL12 during
transforming growth factor-β3-induced chondrogenic differentiation.
Mol Med Rep. 16:1389–1394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Tian Y, Wang J, Phillips KL, Binch
AL, Dunn S, Cross A, Chiverton N, Zheng Z, Shapiro IM, et al:
Inflammatory cytokines induce NOTCH signaling in nucleus pulposus
cells: Implications in intervertebral disc degeneration. J Biol
Chem. 288:16761–16774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang J, Cui W, Song F, Zhai C, Hu H, Zuo Q
and Fan W: Effects of mesenchymal stem cells on
interleukin-1β-treated chondrocytes and cartilage in a rat
osteoarthritic model. Mol Med Rep. 12:1753–1760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie J, Fu N, Cai LY, Gong T, Li G, Peng Q
and Cai XX: The effects of interleukin-1β in modulating
osteoclast-conditioned medium's influence on gelatinases in
chondrocytes through mitogen-activated protein kinases. Int J Oral
Sci. 7:220–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Si HB, Zeng Y, Liu SY, Zhou ZK, Chen YN,
Cheng JQ, Lu YR and Shen B: Intra-articular injection of
microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression
by modulating extracellular matrix (ECM) homeostasis in rats.
Osteoarthritis Cartilage. 25:1698–1707. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoshi H, Akagi R, Yamaguchi S, Muramatsu
Y, Akatsu Y, Yamamoto Y, Sasaki T, Takahashi K and Sasho T: Effect
of inhibiting MMP13 and ADAMTS5 by intra-articular injection of
small interfering RNA in a surgically induced osteoarthritis model
of mice. Cell Tissue Res. 368:379–387. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bondeson J, Wainwright S, Hughes C and
Caterson B: The regulation of the ADAMTS4 and ADAMTS5 aggrecanases
in osteoarthritis: A review. Clin Exp Rheumatol. 26:139–145.
2008.PubMed/NCBI
|
|
28
|
Echtermeyer F, Bertrand J, Dreier R,
Meinecke I, Neugebauer K, Fuerst M, Lee YJ, Song YW, Herzog C,
Theilmeier G and Pap T: Syndecan-4 regulates ADAMTS-5 activation
and cartilage breakdown in osteoarthritis. Nat Med. 15:1072–1076.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyaki S, Sato T, Inoue A, Otsuki S, Ito
Y, Yokoyama S, Kato Y, Takemoto F, Nakasa T, Yamashita S, et al:
MicroRNA-140 plays dual roles in both cartilage development and
homeostasis. Genes Dev. 24:1173–1185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji Q, Xu X, Zhang Q, Kang L, Xu Y, Zhang
K, Li L, Liang Y, Hong T, Ye Q and Wang Y: The
IL-1β/AP-1/miR-30a/ADAMTS-5 axis regulates cartilage matrix
degradation in human osteoarthritis. J Mol Med (Berl). 94:771–785.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang B, Kang X, Xing Y, Dou C, Kang F, Li
J, Quan Y and Dong S: Effect of microRNA-145 on IL-1β-induced
cartilage degradation in human chondrocytes. FEBS Lett.
588:2344–2352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyaki S, Nakasa T, Otsuki S, Grogan SP,
Higashiyama R, Inoue A, Kato Y, Sato T, Lotz MK and Asahara H:
MicroRNA-140 is expressed in differentiated human articular
chondrocytes and modulates interleukin-1 responses. Arthritis
Rheum. 60:2723–2730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu S, Wilson KD, Ghosh Z, Han L, Wang Y,
Lan F, Ransohoff KJ, Burridge P and Wu JC: MicroRNA-302 increases
reprogramming efficiency via repression of NR2F2. Stem Cells.
31:259–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang Z, Ahn J, Guo D, Votaw JR and Shim
H: MicroRNA-302 replacement therapy sensitizes breast cancer cells
to ionizing radiation. Pharm Res. 30:1008–1016. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mort JS and Billington CJ: Articular
cartilage and changes in arthritis: Matrix degradation. Arthritis
Res. 3:337–341. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kobayashi H, Hirata M, Saito T, Itoh S,
Chung UI and Kawaguchi H: Transcriptional induction of ADAMTS5
protein by nuclear factor-κB (NF-κB) family member RelA/p65 in
chondrocytes during osteoarthritis development. J Biol Chem.
288:28620–28629. 2013. View Article : Google Scholar : PubMed/NCBI
|