Introduction
Drug-eluting stents (DESs) have become the preferred
choice to decrease stent thrombosis rates after stent implantation
(1). Rapamycin (RPM), also known
as sirolimus, is a macrolide compound that is usually used as an
anti-proliferative and immunosuppressive drug to prevent rejection
of transplanted organs. Along with the well-known
CYPHER™ sirolimus-eluting stents, RPM-eluting stents
have been widely used to inhibit vascular smooth muscle cell
proliferation and therefore decrease the restenosis rates of the
implanted stents (2,3). Although RPM has a remarkable
anti-thrombotic action when eluted from the stent, it also inhibits
endothelial cell proliferation, which impairs re-endothelialization
after DES implantation (4,5). Therefore, the attenuation of
RPM-induced injury of vascular endothelial cells is of great
importance to the development of new RPM-eluting stents.
Long non-coding RNAs (lncRNAs) have emerged as novel
regulators of gene expression and are able to regulate several
cellular processes, such as proliferation, migration, invasion and
chemoresistance (6–13). Additionally, lncRNAs are also
involved in cardiovascular development and pathophysiology
(14). Michalik et al
(15), provided the first evidence
for the regulation of vascular endothelial cell functions, such as
migration and sprouting, by lncRNA metastasis-associated lung
adenocarcinoma transcript 1 (MALAT1). Besides, smooth muscle and
endothelial cell-enriched migration/differentiation-associated
lncRNA (lncRNA SENCR), also known as FLI1-AS1 or lncRNA9, has been
confirmed to have the capacity to stabilize the differentiated
state and maintain contractile phenotype of smooth muscle cells
(16). LncRNA SENCR, highly
expressed in endothelial cells, smooth muscle cells and aortic
tissue and a recent study demonstrated that a high level of lncRNA
SENCR promoted the sprouting of cultured endothelial cells, as well
as the expression of proangiogenic genes, suggesting enhancement of
endothelial cell function (17).
However, the mechanisms of vascular endothelial cell functions
regulation by lncRNAs especially under RPM existing remain largely
unknown.
Based on these findings, we speculated that there
may be an association between RPM and lncRNAs in the inhibition of
proliferation and migration of vascular endothelial cells. To
validate this hypothesis, in the present study, we selected two
relevant lncRNAs MALAT1 and SENCR, especially lncRNA SENCR, and
chose the stable endothelial cell line HUVEC to investigate the
underlying mechanisms post RPM treatment. The results suggested the
potential application of lncRNA SENCR as endothelial cell function
predictors and provided a promising candidate for intervention of
the side effect of RPM in patients who received RPM-eluting
stents.
Materials and methods
Cell culture and RPM treatment
Human umbilical vein endothelial cells (HUVECs;
ATCC® PCS-100-013) were bought from ACTT (Manassas, VA,
USA) and were cultured in RPMI 1640 medium (HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in 5%
CO2. HUVECs in the logarithmic growth phase were
digested with trypsin and then cell suspensions of
1–5×104 cells/ml were made. A total of 100 µl of the
above cell suspensions was seeded per well in 96-well plates in
triplicate. RPM (Selleck Chemicals, Houston, TX, USA) solutions at
serials of concentrations (0, 1, 10 and 100 nM) were added to the
cells. After incubation for another 48 h, a Cell Counting Kit-8
(CCK8) assay was used to determine the appropriate RPM
concentration for subsequent experiments.
The appropriate intervention time for RPM treatment
was then determined. HUVECs were treated with the selected RPM
concentration determined by the above CCK8 experiment and were
cultured for 0, 24, 48 and 72 h. The control group treated with an
equal volume of pbs instead at each time point was normally
cultured and treated with no RPM. The CCK8 assay was then
performed.
CCK8 assay
A CCK8 assay was performed according to the
supplier's instructions (Beyotime Institute of Biotechnology,
Haimen, China). Specifically, 10 µl CCK-8 and 90 µl serum-free
culture medium were added to each well and cultured at 37°C and 5%
CO2 for 1 h. The absorbance at 450 nm wavelength was
measured with a plate reader (DNM-9602, Beijing, China) and the
value of each well was recorded.
Expression of endogenous lncRNAs
MALAT1 and SENCR
The mRNA from each group of cells was extracted and
reverse transcribed into cDNA using reverse transcription kit
(Fermentas; Thermo Fisher Scientific, Inc.). The resultant cDNA was
used as a template for quantitative polymerase chain reaction
(qPCR) detection. The primers for lncRNAs MALAT1 and SENCR are
listed in Table I. β-actin was
used as the reference gene. qPCR procedure followed the standard
procedure of the SYBR-Green PCR kit (Thermo Fisher Scientific,
Inc.): 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec
and 60°C for 45 sec, then 95°C for 15 sec, 60°C for 1 min, 95°C for
15 sec and 60°C for 15 sec. The results were analyzed with
real-quantitative PCR & the 2−ΔΔCt method.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| MALAT1 |
CTTCCCTAGGGGATTTCAGG |
GCCCACAGGAACAAGTCCTA |
| SENCR |
CAGCCAGAAAGGACTCCAACTCC |
GGAGGCAGCTGGTGCTGAAAG |
| β-actin |
CATCGTCCACCGCAAATGCTTC |
AACCGACTGCTGTCACCTTCAC |
Construction and transfection of the
lncRNA SENCR overexpression plasmid
The plasmid pcDNA 3.1+ (1.52 µg/µl; Invitrogen;
Thermo Fisher Scientific, Inc.) was used to construct the
overexpression vector for lncRNA SENCR. Specifically, EcoR1 and
Not1 restriction enzyme sites were introduced to clone SENCR which
was synthesized in vitro and this created a EcoR1-SENCR-Not1
fragment, then the full-length SENCR sequence was ligated to pcDNA
3.1+ vector with DNA ligase. Finally, the recombinant plasmid was
verified by sequencing analysis. The transfection was performed
according to the recommended protocol of Lipofectamine™
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) as follows: i)
HUVEC cells in the logarithmic growth phase were cultured at
5×105 cells per well in a 6-well culture plate and
incubated at 37°C and 5% CO2 for 24 h; ii) the culture
medium was changed to serum-free opti-MEM medium (Gibco; Thermo
Fisher Scientific, Inc.) 2 h before transfection; iii) the
transfection reagent Lipofectamine™ 2000 was diluted in
opti-MEM at 1:20, and the mixture was kept at room temperature for
5 min; iv) Lipofectamine 2000 and lncRNA SENCR-overexpression
plasmid (2.5 µg/well, empty plasmid vector with equivalent
concentration was used as control in the transfection experiment)
were mixed gently and kept at room temperature for 20 min; v) the
mixture was added to the wells with 200 µl per well and the plate
was cultured in the incubator at 37°C and 5% CO2 for 6
h; vi) the mixture was aspirated and replaced with a complete
medium and vii) the plate was cultured overnight at 37°C and 5%
CO2.
Proliferation, migration and
angiogenesis of HUVECs
In all the proliferation, migration and angiogenesis
experiments, cells were firstly treated by control medium, lncRNA
SENCR overexpression (lncRNA SENCR OE), RPM (100 nM) or RPM plus
lncRNA SENCR OE (RPM+lncRNA SENCR OE) for 24 h. For the detection
of proliferation, CCK8 assay was used and the procedure was the
same as described above. For the analysis of cell migration,
scratch test and transwell test were performed. The wound was
measured at 0, 8 and 12 h in the scratch test. In the transwell
test, the above treated cells were collected and reseeded into the
up chamber of the transwell insert (24-well plate; Corning
Incorporated, Corning, NY, USA) at the density of 2×105
cells per well with 200 µl serum free basic medium. 500 µl complete
endothelial cell growth medium was placed in the lower chamber. The
cells were incubated for 12 h at 37°C. Cells migrated to the lower
surface of the filter were fixed with 70% methanol and stained with
0.5% crystal violet solution and then were imaged (magnification,
×100). For the quantification of the migrated cells, cell numbers
were counted in 4 randomly selected fields (magnification, ×100).
For angiogenesis detection, Matrigel was dissolved overnight at 4°C
before being used. A total of 50 µl per well was added and
incubated at 37°C for 1 h. The above treated cells were collected
and reseeded into the Matrigel-coated wells at a density of
104 cells per well. After 4 and 8 h of incubation at
37°C, 4 fields of view (magnification, ×200) were selected per well
to analyze the tube length and the number of branches of the blood
vessels.
Cell cycle analysis of HUVECs
After 24 h treatment with RPM, the cell cycle stage
was detected in the HUVECs by flow cytometry. The cells were
collected and stained by PI (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) staining. Specifically: i) the cells were washed by PBS
twice and the cell concentration was set at 1×106/ml;
ii) the cell suspension was fixed at 70% ethanol at −20°C for 24 h
and washed with PBS before staining; iii) 100 µg/ml RNase A was
added and the mixture was incubated at 37°C for 30 min; iv) 50
µg/ml PI solution was added and the mixture was incubated at 4°C
for 30 min in the dark; v) the sample was run on machine and the
recording excitation wavelength was 488 nm and vi) cell cycle
analysis was performed with FLOWJO software (v7.6.2).
mRNA expression of vascular
endothelial growth factor (VEGFA), vascular cell adhesion protein-1
(VCAM-1) and p21
Total mRNA was extracted and then reverse
transcribed into cDNA. The resultant cDNA was used as the template
for qPCR detection. The qPCR primers for VEGFA, VCAM-1 and
p21 are listed in Table
II. β-actin was used as the reference gene. The qPCR procedure
followed the standard thermocycling protocol: 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 45 sec, then
95°C for 15 sec; 60°C for 1 min, 95°C for 15 sec and 60°C for 15
sec. The results were analyzed with the real-quantitative PCR &
the 2−ΔΔCq method (18).
 | Table II.Quantitative polymerase chain reaction
primer sequences. |
Table II.
Quantitative polymerase chain reaction
primer sequences.
| Primer | Sequence (5′-3′) |
|---|
| hVEGFA_F |
TCACCAAGGCCAGCACATAG |
| hVEGFA_R |
TCGTTTTTGCCCCTTTCCCT |
| hP21_F |
TCCTCATCCCGTGTTCTCCT |
| hP21_R |
ACAAGTGGGGAGGAGGAAGT |
| hVCAM1_F |
AGATTGGTGACTCCGTCTCA |
| hVCAM1_R |
TCATTGTCAGCGTAGATGTGG |
| hβ-actin_F |
CATCGTCCACCGCAAATGCTTC |
| hβ-actin_R |
AACCGACTGCTGTCACCTTCAC |
Protein expression of the RPM-related
signaling pathway in HUVECs
HUVECs were cultured in 6-well plates for 24 h, then
the total protein was extracted. Western blotting was performed to
detect the protein expression levels of phosphorylated (p-ERK1/2,
ERK1/2, p-mTOR and mTOR, with GAPDH as the reference gene. The
procedure of western blotting followed the standard protocol. In
brief, about 106 cells were taken and lysed and the
total protein was obtained; a 10% SDS-PAGE gel was prepared; 30 µg
total protein was loaded in each lane and gel electrophoresis was
performed at 60 V for 30 min and then at 90 V for 1 h; the protein
bands on the gel were transferred to a polyvinylidene difluoride
(PVDF) membrane under a steady current of 200 mA for 120 min; the
PVDF membrane was blocked with 5% skimmed milk powder for 2 h, then
the membrane was incubated with appropriate antibodies for p-ERK1/2
(Affinity, AF1014), ERK1/2 (Affinity, AF0155), p-mTOR (Affinity,
AF3308), mTOR (Affinity, AF6308) with a dilution ratio of 1:1,000
and GAPDH (ab37168; Abcam) as the reference with a dilution ratio
of 1:2,000. For chemiluminescence detection, an ECL western
blotting kit (Thermo Fisher Scientific, Inc.) was used to react
with the interest proteins. Tanon Automatic Chemiluminescence Image
Analysis System (5200, China) was used to scan the light bands 5
min after the reaction and the matching software accompanying the
machine was used for analysis.
Statistical analysis
Data were analyzed with SPSS v19 statistical
software (SPSS, Inc., Chicago, IL, USA). All data are presented in
the form of mean ± standard deviation. Comparisons among >2
groups were performed with one-way analysis of variance followed by
the least significant difference post hoc method, and comparisons
between two groups were performed with Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of RPM treatment on the
proliferation of HUVECs
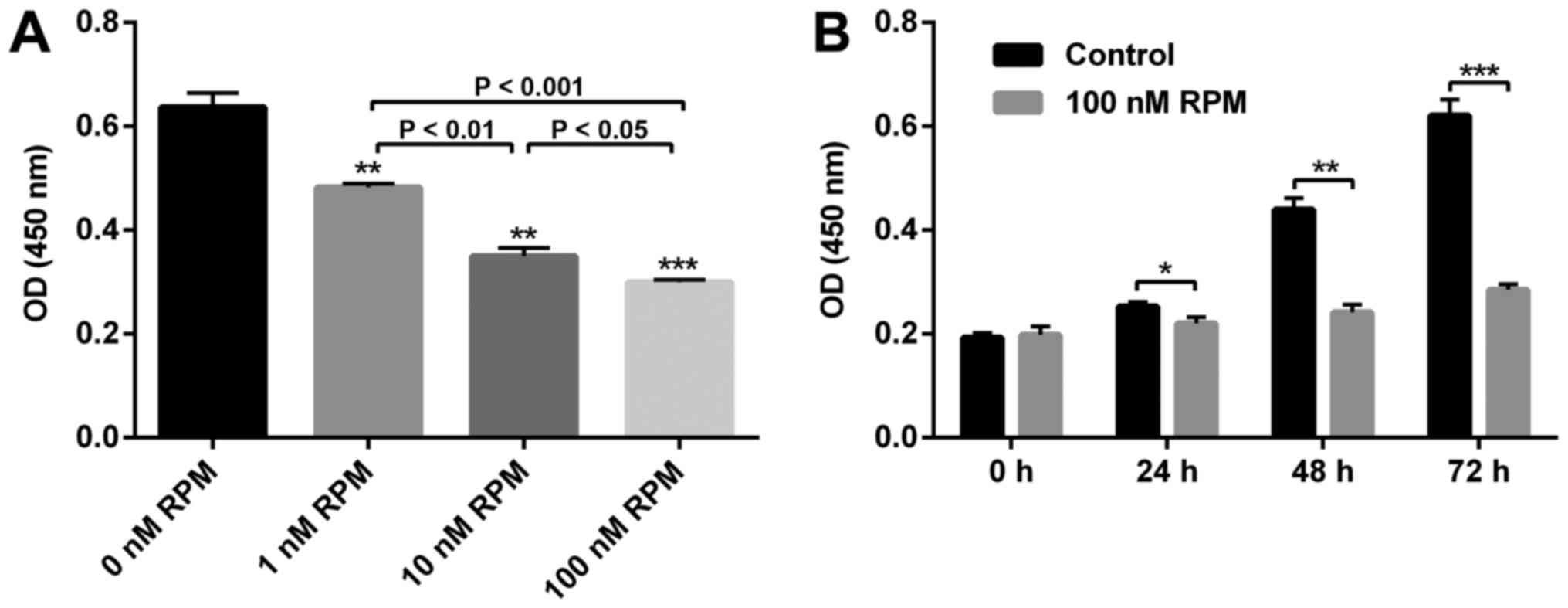
To confirm the effect of RPM on the proliferation of
vascular endothelial cells, a CCK8 assay was performed on the
HUVECs. As shown in Fig. 1,
compared with the control group (0 nM RPM treatment), the
proliferative capacity of HUVECs was significantly inhibited in a
concentration-dependent manner. Specifically, the rate of
inhibition was about 50% when the RPM concentration was 100 nM.
Since the suppression was the most effective at this concentration,
we selected 100 nM RPM to be taken forward in the present study. An
obviously inhibited HUVECs proliferation after RPM treatment (100
nM) at all the time points could be observed (Fig. 1B). Therefore, to study the
relationship between lncRNA SENCR and RPM treatment on HUVEC
behavior, treatment with 100 nM RPM for 24 h was used in the
follow-up experiments.
RPM treatment downregulates the
endogenous expression of lncRNAs MALAT1 and SENCR in HUVECs
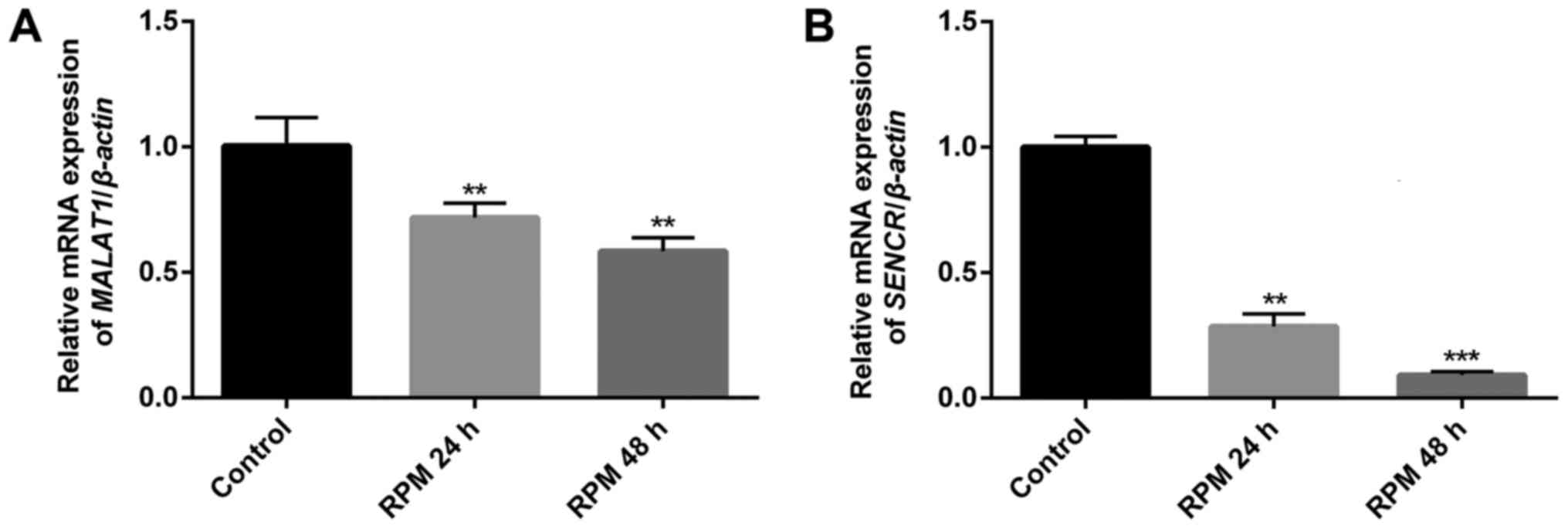
To study the relationship between RPM treatment and
the endogenous expression of the two vascular endothelial cell
function-related lncRNAs, MALAT1 and SENCR, in HUVECs, qPCR
analysis was performed. Fig. 2
showed that both lncRNAs MALAT1 (P<0.01) and SENCR (P<0.01 in
24 h and P<0.001 in 48 h) genes were significantly downregulated
in HUVECs after exposure to 100 nM RPM. It was noted that lncRNA
SENCR showed a more remarkable downregulation than lncRNA MALAT1
after RPM treatment in 48 h. Therefore, lncRNA SENCR was selected
to study the effect of lncRNA on the cellular functions of vascular
endothelial cells after RPM treatment.
Validation of the overexpression of
lncRNA SENCR
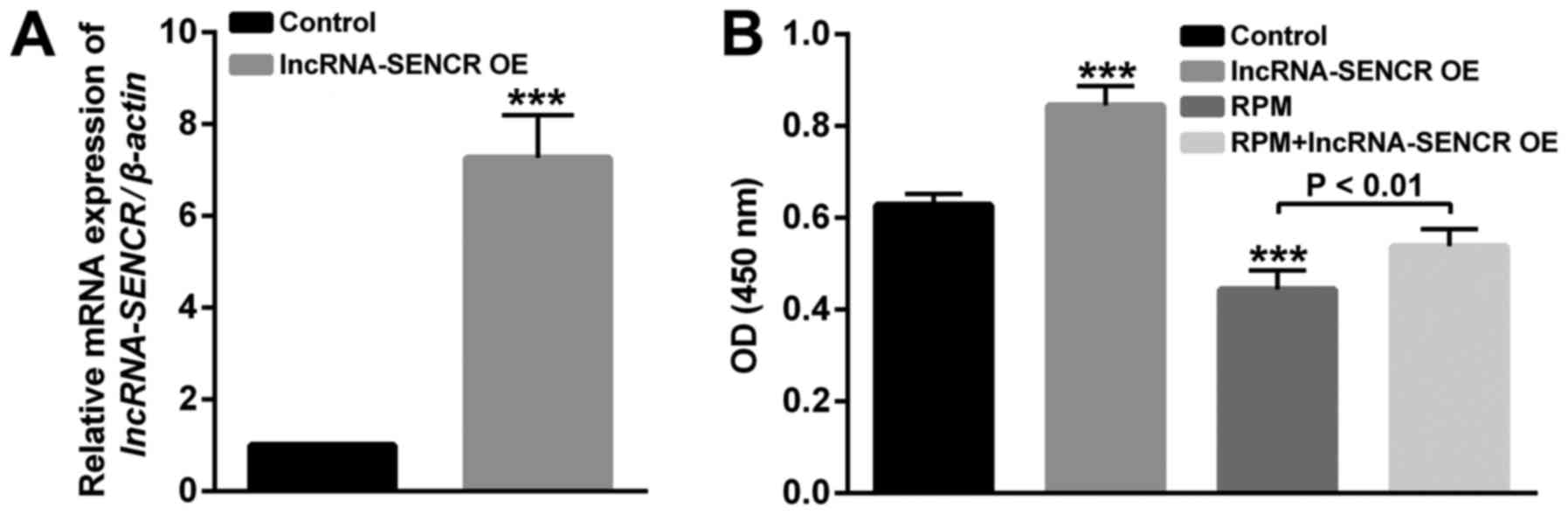
After the construction and transfection of the
lncRNA SENCR-overexpression plasmid, we verified the overexpression
of lncRNA SENCR in HUVECs. As shown in Fig. 3A, compared with the control group,
the relative expression of the SENCR gene in transfected HUVECs was
significantly increased, indicating the successful overexpression
of lncRNA SENCR in HUVECs (P<0.001).
To study the effects of RPM treatment and
overexpression of lncRNA SENCR on the functions of vascular
endothelial cells, proliferation, migration and angiogenesis
analysis of HUVECs was performed in detail. Firstly, the
proliferation of HUVECs in Fig. 3B
showed that compared with the control group, the lncRNA SENCR
overexpression group enhanced and RPM treatment (100 nM for 24 h)
decreased proliferation of HUVECs. However, RPM treatment
associated with SENCR overexpression (RPM+lncRNA-SENCR OE) showed
increased proliferation compared with RPM treatment alone. These
results indicate that RPM could inhibit the proliferation of HUVEC
cells and overexpression of lncRNA SENCR could alleviate the
inhibitory effect of RPM on the proliferation of HUVECs.
Effects of RPM treatment and
overexpression of lncRNA SENCR on the functions of HUVECs
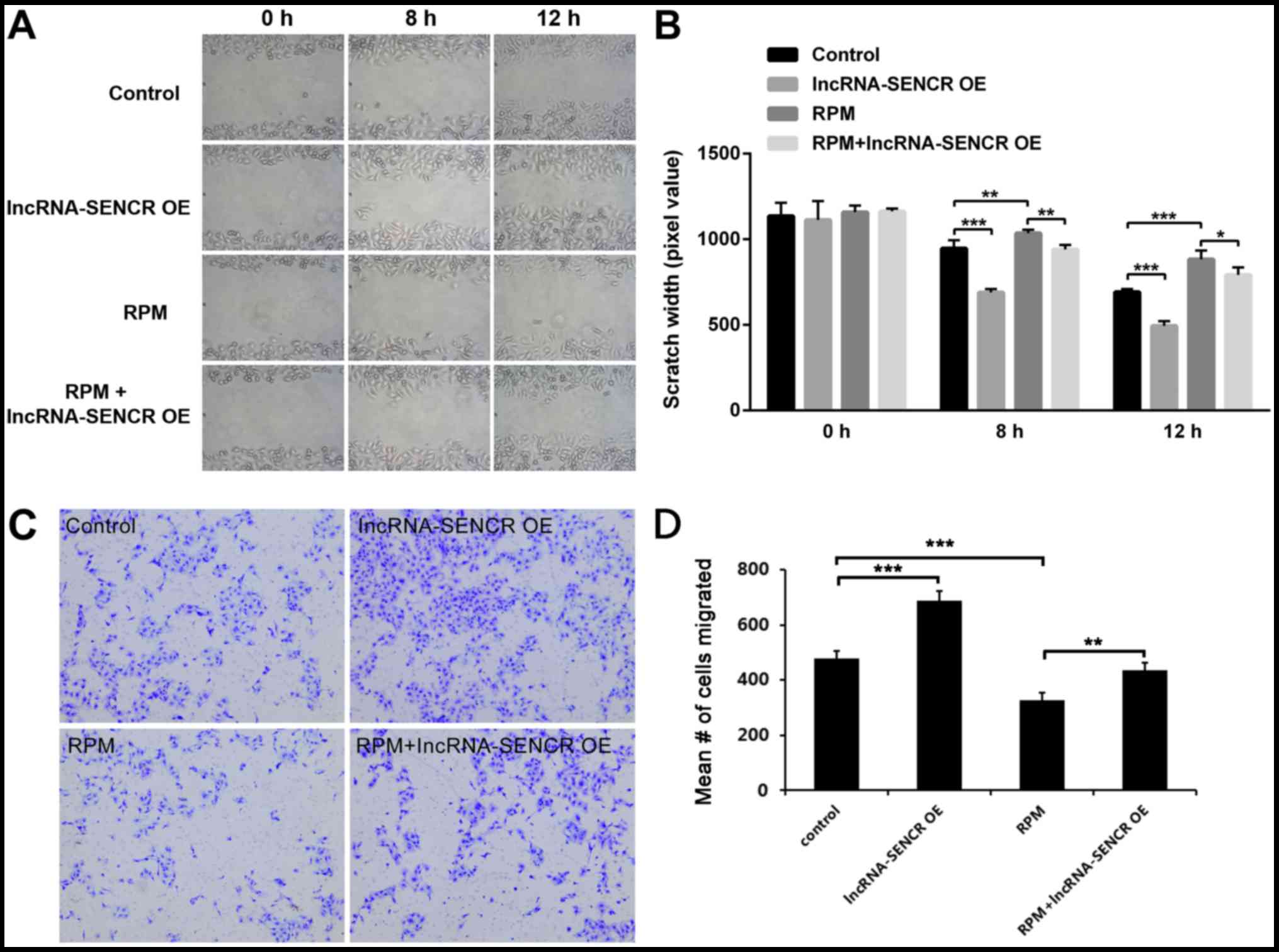
We then examined the migration profile of HUVECs.
The results of the scratch assay showed that lncRNA SENCR
overexpression obviously decreased the scratch width of HUVECs, at
either 8 or 12 h (P<0.001 vs. control; Fig. 4A and B) and RPM treatment
significantly inhibited the cell migration, which got effectively
relieved by high level of lncRNA SENCR (P<0.01 vs. RPM at 8 h,
P<0.05 vs. RPM at 12 h; Fig. 4A and
B). However, interestingly, the RPM+lncRNA-SENCR OE group
demonstrated a smaller scratch width than the RPM group. Transwell
assay provided a similar data (Fig. 4C
and D) and it revealed that lncRNA SENCR upregulation partly
reversed the inhibitory effect on cell migration in presence of
RPM. These results suggest that RPM inhibits the migration of
HUVECs and overexpression of the lncRNA SENCR could alleviate the
inhibitory effect of RPM on the migration of HUVECs.
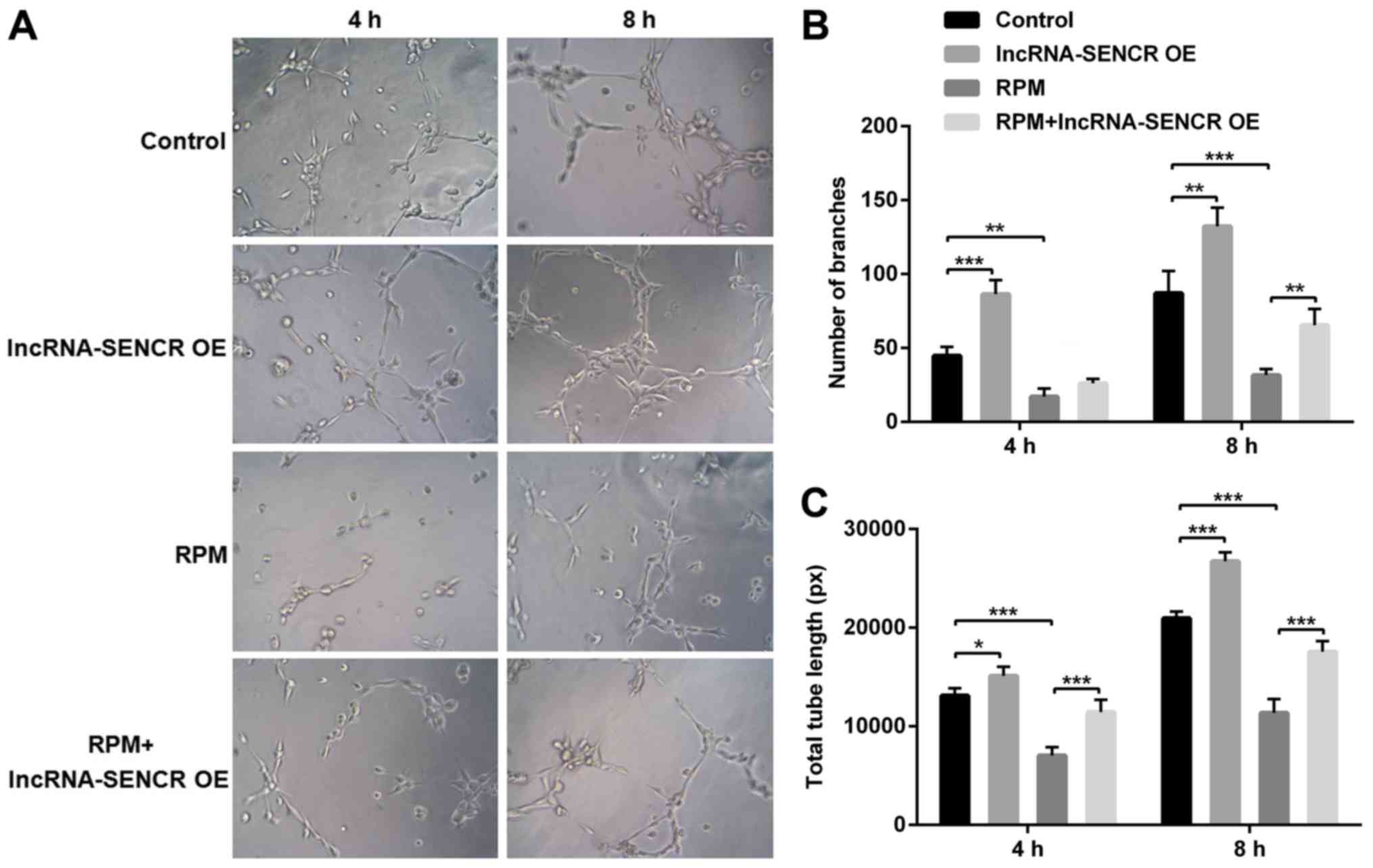
The angiogenesis detection was performed using the
Matrigel method. The results in Fig.
5 are after cultivation for 4 and 8 h. Compared with the
control group, the number and length of branches were significantly
increased in the lncRNA-SENCR OE group and significantly reduced in
the RPM treatment group at 8 h (P<0.01). However, consistent
with the aforementioned cellular function results, the
RPM+lncRNA-SENCR OE group showed an increased cell branch number
and increased vessel length compared with the RPM treatment group
alone, indicating that the overexpression of lncRNA SENCR relieves
the inhibitory effect of RPM treatment on angiogenesis in
HUVECs.
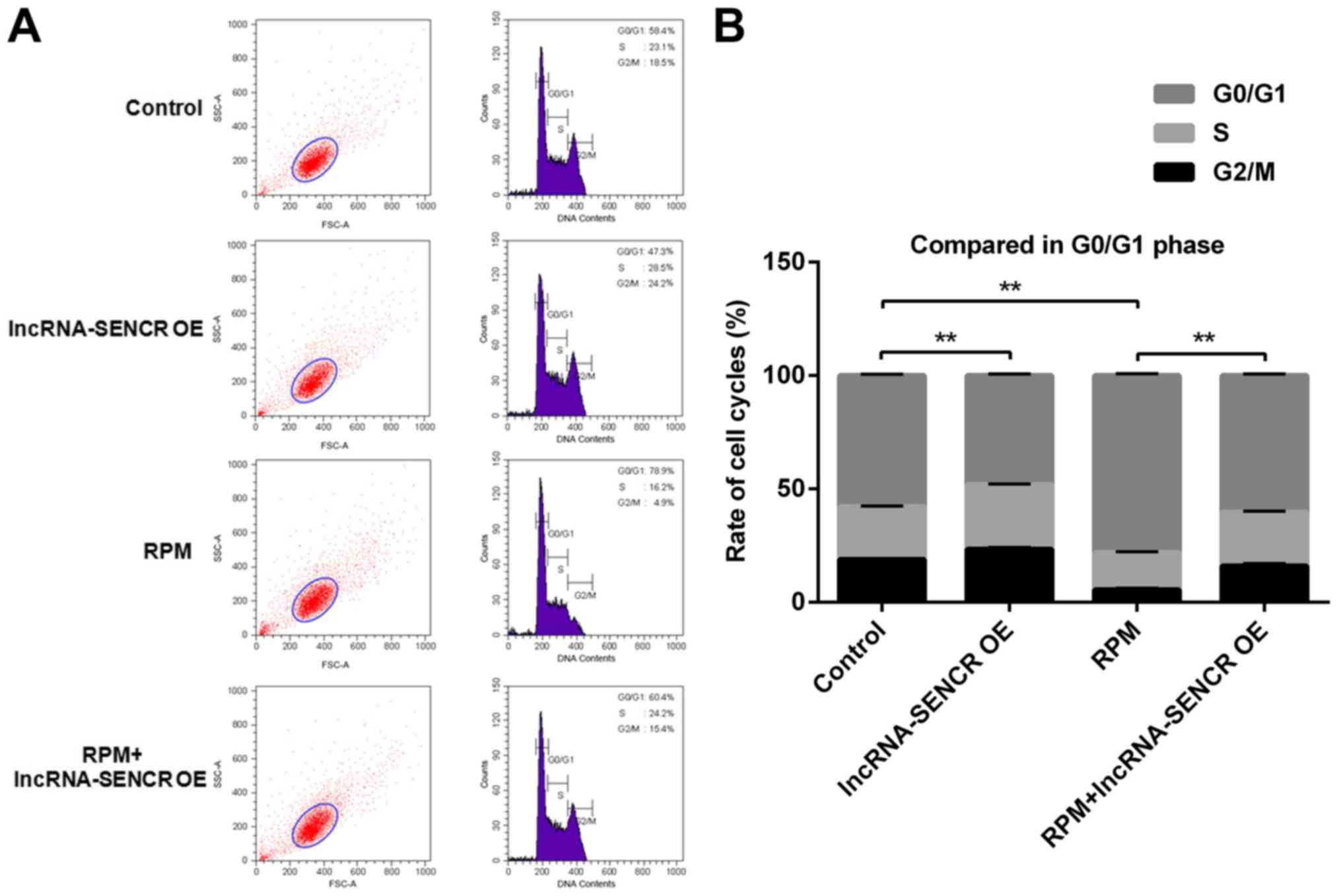
Effects of RPM treatment and
overexpression of lncRNA SENCR on the cell cycle of HUVECs
The effect of RPM treatment and overexpression of
lncRNA SENCR on cell cycle progression was detected by flow
cytometry. The flow cytometry analysis in Fig. 6 showed that the lncRNA-SENCR OE
group had a reduced proportion of G0/G1 phase cells and the RPM
treatment group had an increased proportion of G0/G1 phase cells
compared with the control group. Not unexpectedly and also
consistent with the above results, there was a decreased proportion
of G0/G1 phase cells in the RPM+lncRNA-SENCR OE group. These data
demonstrate that RPM treatment (100 nM for 24 h) blocks the
proliferation of HUVECs in the G0/G1 stage, and overexpression of
lncRNA SENCR could reverse the blocking effect of RPM on HUVEC
proliferation and decreased the percentage of G0/G1 cells from 78.9
to 60.4%.
Effects of RPM treatment and
overexpression of lncRNA SENCR on the expression of VEGFA, p21 and
VCAM-1
In order to further investigate the mechanism that
supports the above results, we performed analysis by qPCR of the
level of related genes in HUVECs after different treatments.
Fig. 7 showed that the
VEGFA and VCAM-1 genes were significantly
downregulated after RPM treatment, however the p21 gene was
remarkably upregulated (P<0.01). Moreover, RPM+lncRNA-SENCR OE
treatment reversed the expression of VEGFA almost to control cells
(P=0.053; Fig. 7A) and
significantly relieved the upregulation of the p21 gene and
downregulation of the VCAM-1 gene caused by RPM treatment
(P<0.05; Fig. 7B and C).
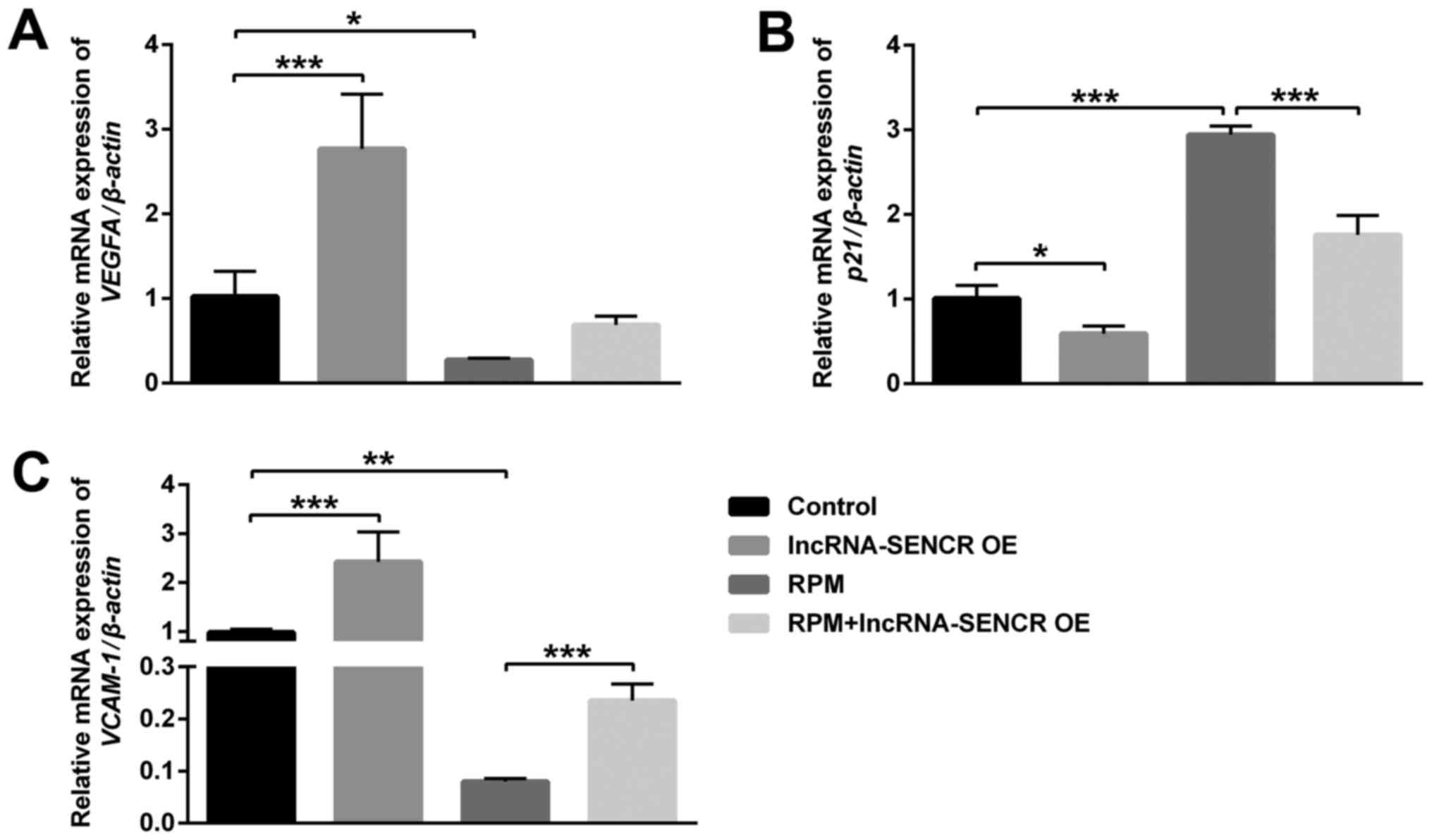 | Figure 7.mRNA expression of VEGFA, p21
and VCAM-1 of HUVECs after treatments with lncRNA-SENCR OE,
RPM and RPM+lncRNA-SENCR OE. (A) VEGFA. (B) p21. (C) VCAM-1. (n=3).
*P<0.05, **P<0.01, ***P<0.001. VEGF, vascular endothelial
growth factor; VCAM-1, vascular cell adhesion protein-1; HUVEC,
human umbilical vein endothelial cell; lnc, long non-coding; SENCR,
smooth muscle and endothelial cell-enriched
migration/differentiation-associated RNA; OE, overexpression; RPM,
rapamycin. |
p21 is a negative regulator of the cell cycle, which
can arrest the cell cycle in the G1 phase and inhibit DNA
replication. VEGFA and VCAM-1 are related genes for vascular
endothelial growth and cell adhesion. These results demonstrate
that RPM treatment could inhibit angiogenesis and cell
proliferation, and the overexpression of the lncRNA SENCR could
alleviate the inhibitory effect of RPM on the angiogenesis and
proliferation of HUVECs.
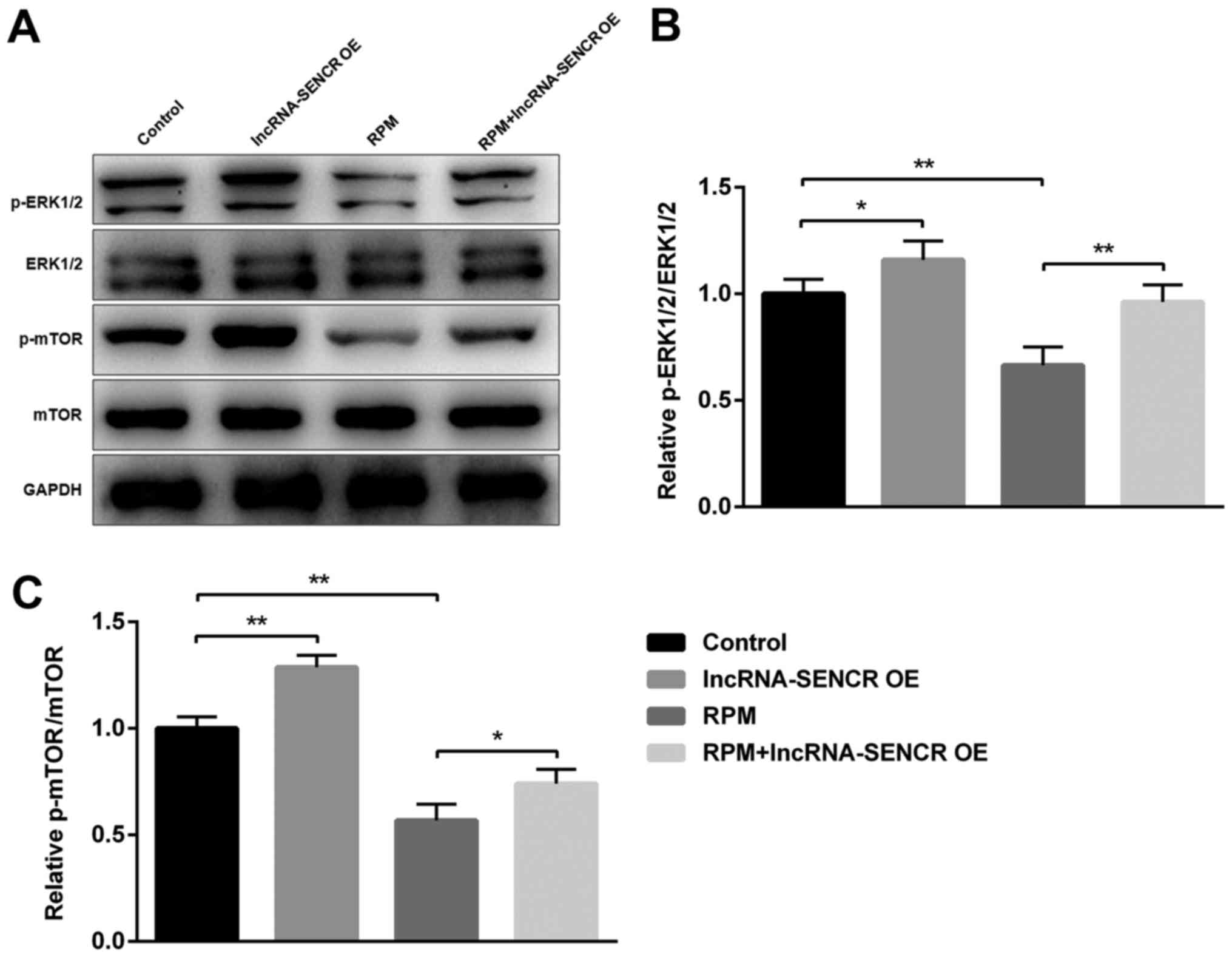
Effects of RPM and lncRNA SENCR on
p-ERK1/2/ERK1/2 and p-mTOR/mTOR
As the ERK1/2 pathway is one of the most influential
pathways associated with cellular responses to various external
stimuli and mTOR has an important role as the mammalian target of
RPM, we next examined the involvement of these proteins during the
RPM treatment and lncRNA SENCR overexpression in HUVECs. Fig. 8A showed the expression of several
proteins involved in the RPM-related signaling pathway in HUVECs,
demonstrating that the phosphorylation of ERK1/2 and mTOR proteins
decreased upon RPM treatment. However RPM+lncRNA-SENCR OE treatment
alleviated the downregulation of p-ERK1/2 and p-mTOR proteins
caused by RPM treatment. Specifically, RPM+lncRNA-SENCR OE
treatment reversed the decrease of the p-ERK1/2/ERK1/2 ratio caused
by RPM treatment to the normal level (P<0.01, vs. RPM group;
Fig. 8B). It was noted that
RPM+lncRNA-SENCR OE treatment also significantly increased the
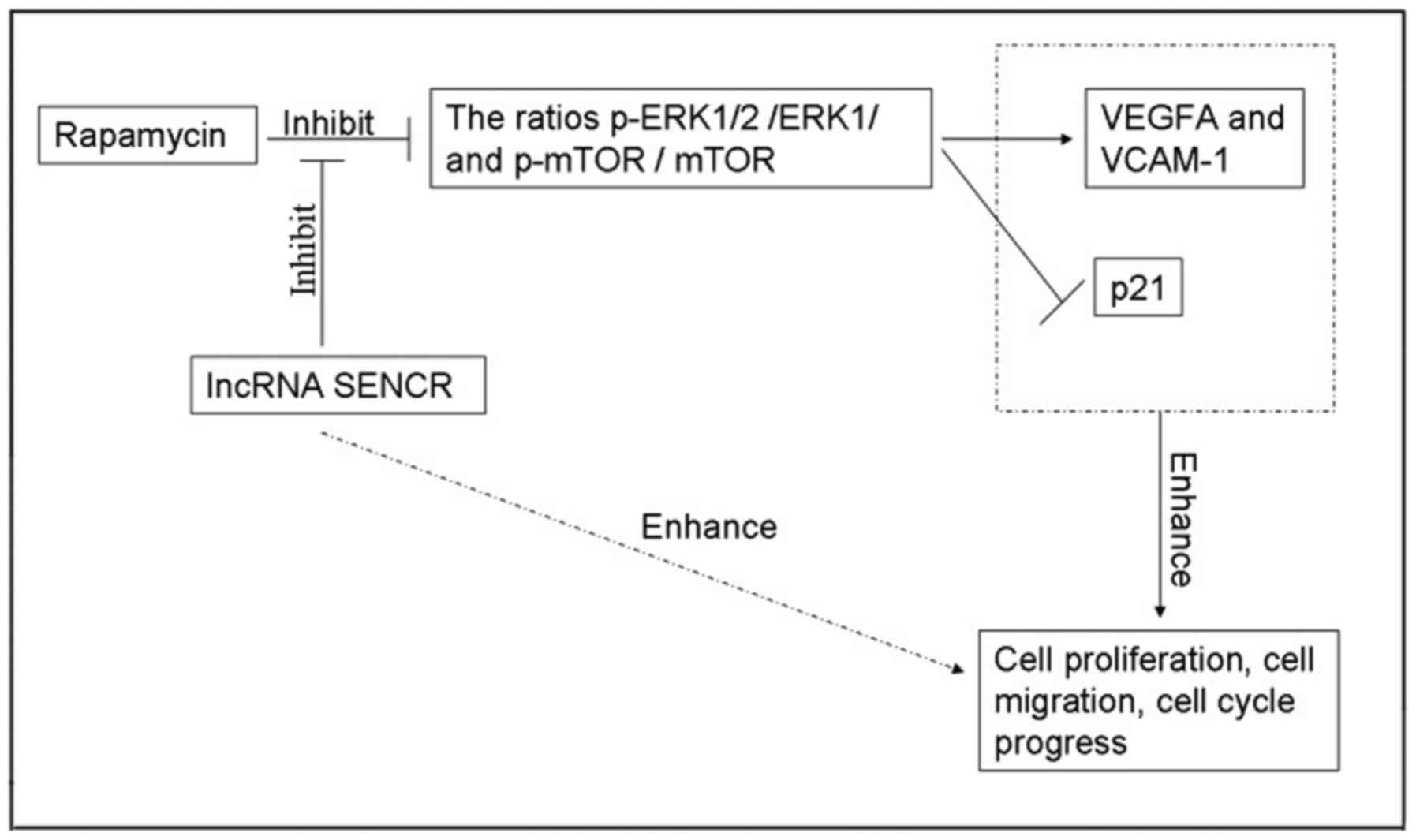
ratio of p-mTOR/mTOR (P<0.05 vs. RPM group; Fig. 8C). These results suggested that, as
shown in Fig. 9, RPM treatment
could inhibit the activation of the ERK1/2 and mTOR pathway, and
overexpression of lncRNA SENCR could relieve the inhibitory effect
of RPM on the ERK1/2 and mTOR pathway.
Discussion
In this study, we provided evidence for the fact
that the lncRNA SENCR alleviates the effects of RPM inhibition on
HUVECs. The results indicated that the lncRNA SENCR alleviated the
inhibition of cellular proliferation, migration, angiogenesis and
cell cycle progression of HUVECs directly or interfering with the
inhibition progress of ERK1/2 and m-TOR phosphorylation in the
presence of RPM. It is reasonable to conclude that RPM and lncRNA
SENCR interact negatively during the processes of proliferation and
migration of HUVECs.
RPM has been used in first-generation intracoronary
DESs, and its use markedly reduces restenosis following stenting
(1–3). However, RPM not only inhibits smooth
muscle cell proliferation but also reduces endothelial cell
proliferation, preventing re-endothelialization post-DES
implantation (4,5). More recently, second-generation
stents that use biocompatible or biodegradable polymers and more
effective agents appeared and replaced RPM stents in the western
world. However, RPM stents dominate in China and most Chinese
patients select RPM stents because of price and medical insurance.
Therefore, the side effects of RPM on endothelial cells and
corresponding intervention strategies remain worthwhile areas of
study.
Recently, lncRNAs, which is more than 200 base pairs
between protein-coding genes and has their own promoters, are
emerging as novel molecular switches in cellular differentiation,
movement and apoptosis by altering gene expression patterns. A
number of studies have demonstrated that lncRNAs can be targeted to
change cellular physiology and functions of vascular endothelial
cell function and may be a promising therapeutic target for
angiogenesis (19–24). Michalik et al found that
genetic deletion of MALAT1 gene have demonstrated reduced retinal
vascular growth and endothelial growth in vivo (15). One recent study proposed the
circulating lncRNAs including SENCR could be used as biomarkers of
heart function and remodeling in type 2 diabetes (25). However, few reports have focused on
the action of these two lncRNAs in the presence of RPM, which has
been investigated in the present study.
Since lncRNAs MALAT1 and SENCR have demonstrated
enhancement of endothelial cell proliferation and migration
(14,16,17,14), we hypothesized that
overexpression of them may alleviate the inhibitory effects of RPM
on HUVEC. The work described here studied the expression of lncRNA
SENCR and MALAT1 in HUVECs after RPM treatment and found that
lncRNA SENCR expression decreased more significantly than that of
lncRNA MALAT1 when exposed to RPM. This result implied that the
lncRNA SENCR might serve as a sensitive indicator of endothelial
cell function judgement in patients treated with RPM-eluting
stents. We further demonstrated that transfection of lncRNA SENCR
abolished the inhibitory effects of RPM on the proliferation, cell
migration, sprouting and cell cycle progression of HUVECs. Besides
the regulation of endothelial proliferation, migration, and
angiogenesis, SENCR induced endothelial development and function
were also evidenced by Boulberdaa and co-wokers (17). To understand the targeted gene
modulated by lncRNA SENCR during angiogenesis-related function,
some pro-angiogenic genes can be focused on. Boulberdaa's work
studied CCL5, CEACAM1 and CX3CL1 as targeted genes. They confirmed
that knockdown of SENCR resulted in a decrease of these genes and
its overexpression induced an upregulation of CCL5 and CX3CL1.
Similarly, we focused on VEGFA and VCAM-1, and p21
and data indicated that lncRNA SENCR overexpression effectively
restored the transcriptional level of VEGFA and VCAM-1, and
inhibited p21 post-RPM treatment, which at least partly contributed
to the augmentation of HUVEC function.
Besides regulatory proteins and microRNAs, lncRNAs
are emerging critical regulatory moleculars for the control of the
mTOR signaling circuit. Further, because RPM is a famous mTOR
inhibitor, we try to assess related proteins in the ERK1/2 and mTOR
pathways. Consistent with previous reports (28,29),
the results revealed that both p-ERK1/2/ERK1/2 and p-mTOR/mTOR
exhibited increased levels following lncRNA SENCR upregulation.
Based on the above data and analysis, we conclude the likely
mechanism of action of lncRNA SENCR via relieving the inhibitory
effect of RPM on ERK1/2 and mTOR pathways and promoting HUVEC
proliferation and migration.
In conclusion, this work gives novel insight into
the regulatory of vascular endothelial functions by lncRNAs after
RPM-eluting grafts implantation. In patients carrying RPM-eluting
stent grafts, lncRNA SENCR might be used to evaluate the
endothelial cell performance. Additionally, the nice enhancement of
endothelial cell function made lncRNA SENCR a promising agent for
improving the efficacy of RPM-eluting stents after implantation. In
light of the diversity of the mode of action of lncRNAs, more
RPM-related lncRNAs and their underlying mechanisms need to be
explored in future research.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All the data used in the current study are available
from the corresponding author on reasonable request.
Authors' contributions
HT conceived and designed the study. Construction of
overexpression plasmid and cellular experiments were performed by
SY. mRNA and protein related experiments were performed by MS. All
the authors participated in the data analysis and manuscript
writing.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Werner M: Drug eluting stents and modern
stent technologies for in-stent restenosis. J Cardiovasc Surg
(Torino). 58:497–500. 2017.PubMed/NCBI
|
|
2
|
Guo N, Chen F, Zhou J, Fang Y, Li H, Luo Y
and Zhang Y: Curcumin attenuates rapamycin-induced cell injury of
vascular endothelial cells. J Cardiovasc Pharmacol. 66:338–346.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matter CM, Rozenberg I, Jaschko A,
Greutert H, Kurz DJ, Wnendt S, Kuttler B, Joch H, Grünenfelder J,
Zünd G, et al: Effects of tacrolimus or sirolimus on proliferation
of vascular smooth muscle and endothelial cells. J Cardiovasc
Pharmacol. 48:286–292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chong E, Poh KK, Liang S, Lee RC, Low A,
Teo SG and Tan HC: Two-year clinical registry follow-up of
endothelial progenitor cell capture stent versus sirolimus-eluting
bioabsorbable polymer-coated stent versus bare metal stents in
patients undergoing primary percutaneous coronary intervention for
ST elevation myocardial infarction. J Interv Cardiol. 23:101–108.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu HT, Li F, Wang WY, Li XJ, Liu YM, Wang
RA, Guo WY and Wang HC: Rapamycin inhibits re-endothelialization
after percutaneous coronary intervention by impeding the
proliferation and migration of endothelial cells and inducing
apoptosis of endothelial progenitor cells. Tex Heart Inst J.
37:194–201. 2010.PubMed/NCBI
|
|
6
|
Booy EP, McRae EK, Koul A, Lin F and
McKenna SA: The long non-coding RNA BC200 (BCYRN1) is critical for
cancer cell survival and proliferation. Mol Cancer. 16:1092017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hua F, Liu S, Zhu L, Ma N, Jiang S and
Yang J: Highly expressed long non-coding RNA NNT-AS1 promotes cell
proliferation and invasion through Wnt/β-catenin signaling pathway
in cervical cancer. Biomed Pharmacother. 92:1128–1134. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Chen Y, Chen Z, He A, Xie H, Zhang
Q, Cai Z, Liu Y and Huang W: SPRY4-IT1: A novel oncogenic long
non-coding RNA in human cancers. Tumor Biol.
39:10104283177114062017. View Article : Google Scholar
|
|
9
|
Li Y, Jiang B, Zhu H, Qu X, Zhao L, Tan Y,
Jiang Y, Liao M and Wu X: Inhibition of long non-coding RNA ROR
reverses resistance to Tamoxifen by inducing autophagy in breast
cancer. Tumor Biol. 39:10104283177057902017. View Article : Google Scholar
|
|
10
|
Zhang CY, Yu MS, Li X, Zhang Z, Han CR and
Yan B: Overexpression of long non-coding RNA MEG3 suppresses breast
cancer cell proliferation, invasion, and angiogenesis through AKT
pathway. Tumor Biol. 39:10104283177013112017.
|
|
11
|
Zhang R, Xia Y, Wang Z, Zheng J, Chen Y,
Li X, Wang Y and Ming H: Serum long non coding RNA MALAT-1
protected by exosomes is up-regulated and promotes cell
proliferation and migration in non-small cell lung cancer. Biochem
Biophys Res Commun. 490:406–414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y and Hu H: Long non-coding RNA
CCAT1/miR-218/ZFX axis modulates the progression of laryngeal
squamous cell cancer. Tumor Biol. 39:10104283176994172017.
|
|
13
|
Xu JH, Chang WH, Fu HW, Shu WQ, Yuan T and
Chen P: Upregulated long non-coding RNA LOC90784 promotes cell
proliferation and invasion and is associated with poor clinical
features in HCC. Biochem Biophys Res Commun. 490:920–926. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thum T and Kumarswamy R: The smooth long
noncoding RNA SENCR. Arterioscler Thromb Vasc Biol. 34:1124–1125.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michalik KM, You X, Manavski Y,
Doddaballapur A, Zörnig M, Braun T, John D, Ponomareva Y, Chen W,
Uchida S, et al: Long noncoding RNA MALAT1 regulates endothelial
cell function and vessel growth. Circ Res. 114:1389–1397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bell RD, Long X, Lin M, Bergmann JH, Nanda
V, Cowan SL, Zhou Q, Han Y, Spector DL, Zheng D and Miano JM:
Identification and initial functional characterization of a human
vascular cell-enriched long noncoding RNA. Arterioscler Thromb Vasc
Biol. 34:1249–1259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boulberdaa M, Scott E, Ballantyne M,
Garcia R, Descamps B, Angelini GD, Brittan M, Hunter A, McBride M,
McClure J, et al: A role for the long noncoding RNA SENCR in
commitment and function of endothelial cells. Mol Ther. 24:978–990.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jen J, Tang YA, Lu YH, Lin CC, Lai WW and
Wang YC: Oct4 transcriptionally regulates the expression of long
non-coding RNAs NEAT1 and MALAT1 to promote lung cancer
progression. Mol Cancer. 16:1042017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li C, Cui Y, Liu LF, Ren WB, Li QQ, Zhou
X, Li YL, Li Y, Bai XY and Zu XB: High expression of long noncoding
RNA MALAT1 indicates a poor prognosis and promotes clinical
progression and metastasis in bladder cancer. Clin Genitourin Canc.
15:570–576. 2017. View Article : Google Scholar
|
|
21
|
Pruszko M, Milano E, Forcato M, Donzelli
S, Ganci F, Di Agostino S, De Panfilis S, Fazi F, Bates DO,
Bicciato S, et al: The mutant p53-ID4 complex controls VEGFA
isoforms by recruiting lncRNA MALAT1. EMBO Rep. 18:1331–1351. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun JY, Zhao ZW, Li WM, Yang G, Jing PY,
Li P, Dang HZ, Chen Z, Zhou YA and Li XF: Knockdown of MALAT1
expression inhibits HUVEC proliferation by upregulation of miR-320a
and downregulation of FOXM1 expression. Oncotarget. 8:61499–61509.
2017.PubMed/NCBI
|
|
23
|
Wang C, Mao ZP, Wang L, Wu GH, Zhang FH,
Wang DY and Shi JL: Long non-coding RNA MALAT1 promotes
cholangiocarcinoma cell proliferation and invasion by activating
PI3K/Akt pathway. Neoplasma. 64:725–731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu L, Wang X and Guo Y: Long non-coding
RNA MALAT1 is upregulated and involved in cell proliferation,
migration and apoptosis in ovarian cancer. Exp Ther Med.
13:3055–3060. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Gonzalo-Calvo D, Kenneweg F, Bang C,
Toro R, van der Meer RW, Rijzewijk LJ, Smit JW, Lamb HJ,
Llorente-Cortes V and Thum T: Circulating long-non coding RNAs as
biomarkers of left ventricular diastolic function and remodelling
in patients with well-controlled type 2 diabetes. Sci Rep.
6:373542016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou ZQ, Xu J, Li L and Han YS:
Down-regulation of SENCR promotes smooth muscle cells proliferation
and migration in db/db mice through up-regulation of FoxO1 and
TRPC6. Biomed Pharmacother. 74:35–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao J, Zhang W, Lin M, Wu W, Jiang P, Tou
E, Xue M, Richards A, Jourd'heuil D, Asif A, et al: MYOSLID is a
novel serum response factor-dependent long noncoding RNA that
amplifies the vascular smooth muscle differentiation program.
Arterioscler Thromb Vasc Biol. 36:2088–2099. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu C, Zhang H, Liu C, Zhu Y, Wang X, Gao
W, Huang S and Chen L: Rapamycin inhibits Erk1/2-mediated neuronal
apoptosis caused by cadmium. Oncotarget. 6:21452–21467.
2015.PubMed/NCBI
|
|
29
|
Liu P, Yang X, Hei C, Meli Y, Niu J, Sun T
and Li PA: Rapamycin reduced ischemic brain damage in diabetic
animals is associated with suppressions of mTOR and ERK1/2
signaling. Int J Biol Sci. 12:1032–1040. 2016. View Article : Google Scholar : PubMed/NCBI
|