Introduction
Gastric cancer (GC) is the fifth most common cancer
worldwide and is considered a serious threat to human health, which
negatively affects quality of life. The main treatment options for
GC include surgery, chemotherapy and radiation therapy; however,
each treatment is associated with complications. Surgery is the
most common method of GC treatment and is a radical type of
treatment: The primary tumor is resected along with the metastatic
lymph nodes and the infiltrated tissue, with no tumor remaining
(1). Cardiovascular complications
are common in elderly patients with GC during the perioperative
period and are the main cause of mortality in elderly patients
following surgery. Acute myocardial infarction (AMI) is the most
serious cardiovascular complication that threatens patient
survival.
AMI, which is a serious cardiovascular disease
(2), is characterized by
inflammation, cardiomyocyte apoptosis and cardiac fibrosis
(3). AMI is a primary disease that
threatens human health due to its sudden onset, rapid progression
and high mortality rate (4,5). It
has previously been confirmed that there are no obvious clinical
features in the early stages of AMI (6). AMI can result in left ventricular
dilatation and heart failure, which can eventually lead to
mortality (7). Therefore, the
early discovery, diagnosis and treatment of this disease are
important for the prognosis of patients with AMI.
MicroRNAs (miRNAs/miRs) are conserved, small,
non-coding RNA molecules that negatively regulate the expression of
target genes (8). Abnormal miRNA
expression has been demonstrated in various diseases, particularly
in cancer (9,10). Numerous studies have indicated that
miRNAs participate in the occurrence and progression of
cardiovascular diseases, including AMI (11–16).
Liu et al reported that miR-150 serves a cardioprotective
role in AMI via regulating monocyte cell migration and
proinflammatory cytokine production (17). In addition, miR-92 is highly
expressed in patients with AMI, and is involved in the endothelial
injury process following AMI, whereas miR-92 inhibition may protect
endothelial cells after AMI (18).
Previous studies have suggested that miR-133a
expression is reduced in patients with GC (19), whereas it is highly expressed in
patients with AMI (20) compared
with in healthy controls. However, the expression of miR-133a in
patients with or without AMI following radical surgery for GC
remains unclear. The present study aimed to investigate the
expression levels of miR-133a in patients with or without AMI
following radical surgery for GC, and to explore its underlying
mechanisms.
Materials and methods
Clinical samples
The present study was approved by the Human Ethics
Committee Review Board at Cangzhou Central Hospital of Hebei
(Cangzhou, China), and informed consent was obtained from all
patients. A total of 20 blood samples were obtained from patients
(age range, 46–74 years; sex ratio, 1:1) that were diagnosed with
AMI 3 days after undergoing radical surgery for GC at Cangzhou
Central Hospital of Hebei between February 2015 and December 2016.
In addition, 20 blood samples were obtained from patients (age
range, 44–71 years; sex ratio, 1:1) that did not suffer from AMI 3
days after undergoing radical surgery for GC. No patients had
received any radiotherapy or chemotherapy prior to surgery. Blood
samples were used to detect miRNA-133a and endothelial injury
marker expression.
AMI rat model (21)
The rat study was approved by the Ethics Committee
at Cangzhou Central Hospital of Hebei. A total of 20 male Wistar
rats weighing 180–220 g were purchased from the Vital River Company
(Beijing, China) and bred in standard conditions (temperature,
21±1°C; humidity, 55–60%). They were given water and food ad
libitum. All the rats were anesthetized by intraperitoneal
administration of pentobarbital (50 mg/kg), and then AMI rat models
were established by ligating the left anterior descending coronary
artery (LAD), as previously described (21). The control rats underwent a sham
operation.
A miR-133a mimic (sense, 5′UUUGGUCCCCUUCAACCAGCUG3′
and antisense, 5′GCUGGUUGAAGGGGACCAAAUU3′); miR-133a inhibitor
(5′CAGCUGGUUGAAGGGGACCAAA3′) and negative control (NC; sense,
5′UUCUCCGAACGUGUCACGUTT3′ and antisense, 5′ACGUGACACGUUCGGAGAATT3′)
all obtained from Shanghai Pharmaceutical Technology Co., Ltd.; 2
µg mimic, inhibitor or NC in a total of 120 µl DMEM were injected
into three areas of the myocardium of anterior left ventricular
wall near the LAD during AMI surgery. The rats were then divided
into four groups (18): Control
group, in which 5 rats that underwent the sham operation were
injected with a vector; model group, in which 5 rats in the AMI
model group were injected with NC; miR-133a mimic group, in which 5
rats in the AMI model group were injected with a miR-133a mimic;
miR-133a inhibitor group, in which 5 rats in the AMI model group
were injected with a miR-133a inhibitor. All rats were sacrificed 3
days following LAD ligation. Then blood was collected from the
retroorbital plexus and immediately flash-frozen in liquid nitrogen
and stored at −80°C.
ELISA
To analyze the endothelial injury markers, including
cardiac troponin I (cTnI; cat. no. Ab200016; Abcam, Cambridge, UK),
heart-type fatty acid-binding protein (H-FABP; cat. no.
CSB-E09185h; Cusabio Biotech Co., Ltd., College Park, MD, USA) and
von Willebrand factor (vWF; cat. no. Ab108918; Abcam), blood
samples from patients and rats were collected. Subsequently, ELISA
analyses were performed according to the manufacturer's
protocols.
Cell culture and transfection
Human umbilical vein endothelial cells (HUVECs) and
the 293T cell line were obtained from the American Type Culture
Collection (Manassas, VA, USA). HUVECs were cultured in endothelial
growth medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) in a humidified atmosphere containing 5% CO2 at
37°C. The 293T cells were grown in high glucose Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) medium
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) at 5% CO2 and 37°C.
A miR-133a inhibitor (5′CAGCUGGUUGAAGGGGACCAAA3′;
100 nM), NC (sense, 5′UUCUCCGAACGUGUCACGUTT3′ and antisense,
5′ACGUGACACGUUCGGAGAATT3′; 50 nM) or 2 µl B-cell lymphoma 2
(Bcl-2)-like 1 (Bcl2l1) small interfering (si)RNA (cat. no.
sc-43630; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) were
transfected into HUVECs (5×104 cells/well) using the
Lipofectamine® LTX kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
HUVECs were divided into four groups: The control group, in which
cells were not transfected; the NC group, in which cells were
transfected with NC; the inhibitor group, in which cells were
transfected with a miR-133a inhibitor; and the inhibitor + Bcl2l1
siRNA group, in which cells were cotransfected with a miR-133a
inhibitor and Bcl2l1 siRNA. Pre-experimental results demonstrated
that Blc2l1 siRNA alone significantly decreased Bcl2l1 expression.
Thus, the present study did not examine cells transfected with the
Bcl2l1 siRNA alone.
Luciferase activity assay
To verify whether miR-133a directly targets the 3′
untranslated region (3′UTR) of Bcl2l1, Bcl2l1-3′UTR-wild type (WT)
and Bcl2l1-3′UTR-mutant (MUT) vectors, containing wild type and
mutated 3′UTR of Bcl2l1 mRNA respectively, were established as
previously described (22). 293T
cells were seeded in a 24-well plate (5×104 cells/well)
and were then co-transfected with Bcl2l1-3′UTR-WT or
Bcl2l1-3′UTR-MUT and 50 nM miR-133a or 100 nM NC vector using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Following transfection for 48 h, Luciferase activity was
analyzed using a Dual-Luciferase Reporter Assay kit (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol.
Cell proliferation and apoptosis
assays
A total of 48 h post-transfection, an MTT assay was
applied to evaluate cell proliferation. Cells (3×103
cells per well) were seeded in a 96-well plate, and were incubated
for 48 h followed by staining with 20 µl MTT (5 g/l) at 37°C for 4
h. Subsequently, the supernatant was discarded and the precipitate
was dissolved following the addition of 200 µl dimethyl sulfoxide.
The optical density value of each sample was measured at 490 nm
using a spectrophotometer. Experiments were performed in
triplicate. Cell apoptosis was determined using an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis
Detection kit (BD Biosciences, Franklin Lakes, NJ, USA) using flow
cytometry. In brief, 48 h following cell transfection, cells were
rinsed with cold PBS. Cells (5×105 cells/well) were then
labeled with Annexin V-FITC and propidium iodide, according to the
manufacturer's protocol. Flow cytometry (BD Biosciences, Franklin
Lakes. NJ, USA) was used for cell apoptosis analysis. WinMDI
version 2.5 (Purdue University Cytometry Laboratories; www.cyto.purdue.edu/flowcyt/software/Catalog.htm) was
used for data analysis and each test was repeated in
triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from blood or cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. GAPDH or U6 were used as
internal controls. The PrimeScript RT reagent kit (Takara Bio,
Inc., Otsu, Japan) was used to transcribe total RNA into cDNA
according to the manufacturer's protocol. Amplification conditions
for RT-PCR were as follows: 16°C for 30 min, 42°C for 30 min, 85°C
for 5 min and holding at 4°C. qPCR analysis was conducted using
SYBR Premix Ex Taq (Takara, Bio, Inc.). qPCR was conducted with
following conditions: 95°C for 10 min, followed by 37 cycles at
95°C for 15 sec and 72°C for 30 sec. Relative gene expression was
calculated using the 2−ΔΔCq method (23). Each experiment was performed in
triplicate. The PCR primer sequences are listed in Table I.
 | Table I.Primer sequences for quantitative
polymerase chain reaction. |
Table I.
Primer sequences for quantitative
polymerase chain reaction.
| Gene | Sequence (5′-3′) |
|---|
| Bcl2l1-F |
GGTGGTTGACTTTCTCTCCT |
| Bcl2l1-R |
GCATCTCCTTGTCTACGCTT |
| GAPDH-F |
CTTTGGTATCGTGGAAGGACTC |
| GAPDH-R |
GTAGAGGCAGGGATGATGTTCT |
| miR-133a-F |
TTTGGTCCCCTTCAACCAGCTG |
| miR-133a-R |
TAAACCAAGGTAAAATGGTCGA |
| U6-F |
CTCGCTTCGGCAGCACA |
| U6-R |
AACGCTTCACGAATTTGCGT |
Western blot analysis
Cells were harvested and lysed using
radioimmunoprecipitation assay butter (Cell Signaling Technology,
Inc., Danvers, MA, USA). A bicinchoninic acid protein assay kit
(Beyotime Institute of Biotechnology, Haimen, China) was used for
protein quantification. Protein samples (25 µg/lane) were separated
by 12% SDS-PAGE and were then transferred to a polyvinylidene
fluoride membrane. The membranes were blocked in Tris-buffered
saline with 0.1% Tween® (TBST) with 5% skimmed milk for
1.5 h at room temperature. Subsequently, the membranes were
incubated with primary antibodies against Bcl2l1 (cat. no. 2764;
1:1,000; Cell Signaling Technology, Inc.) and GAPDH (cat. no. 8884;
1:2,000; Cell Signaling Technology, Inc.) at 4°C overnight. After
washing with TBST, the membranes were incubated with anti-rabbit
IgG, HRP-linked Antibody (cat. no. 7074; 1:5,000; Cell Signaling
Technology, Inc.) at room temperature for 4 h. Protein bands were
visualized using enhanced chemiluminescence substrates (EMD
Millipore, Billerica, MA, USA) and images were then captured.
Statistical analysis
SPSS 18.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used to conduct all statistical analyses. Each
experiment was repeated in triplicate. Data are presented as the
mean ± standard deviation. Comparisons between two groups were
performed using Student's t-test. Comparisons between multiple
groups were performed using one-way analysis of variance followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients with AMI exhibit higher
miR-133a and endothelial injury marker expression
The expression levels of miR-133a and endothelial
injury markers were detected in patients with or without AMI
following radical surgery for GC using RT-qPCR and ELISA,
respectively. The results demonstrated that compared with the
control patients, patients with AMI exhibited higher miR-133a and
endothelial injury marker expression (Fig. 1).
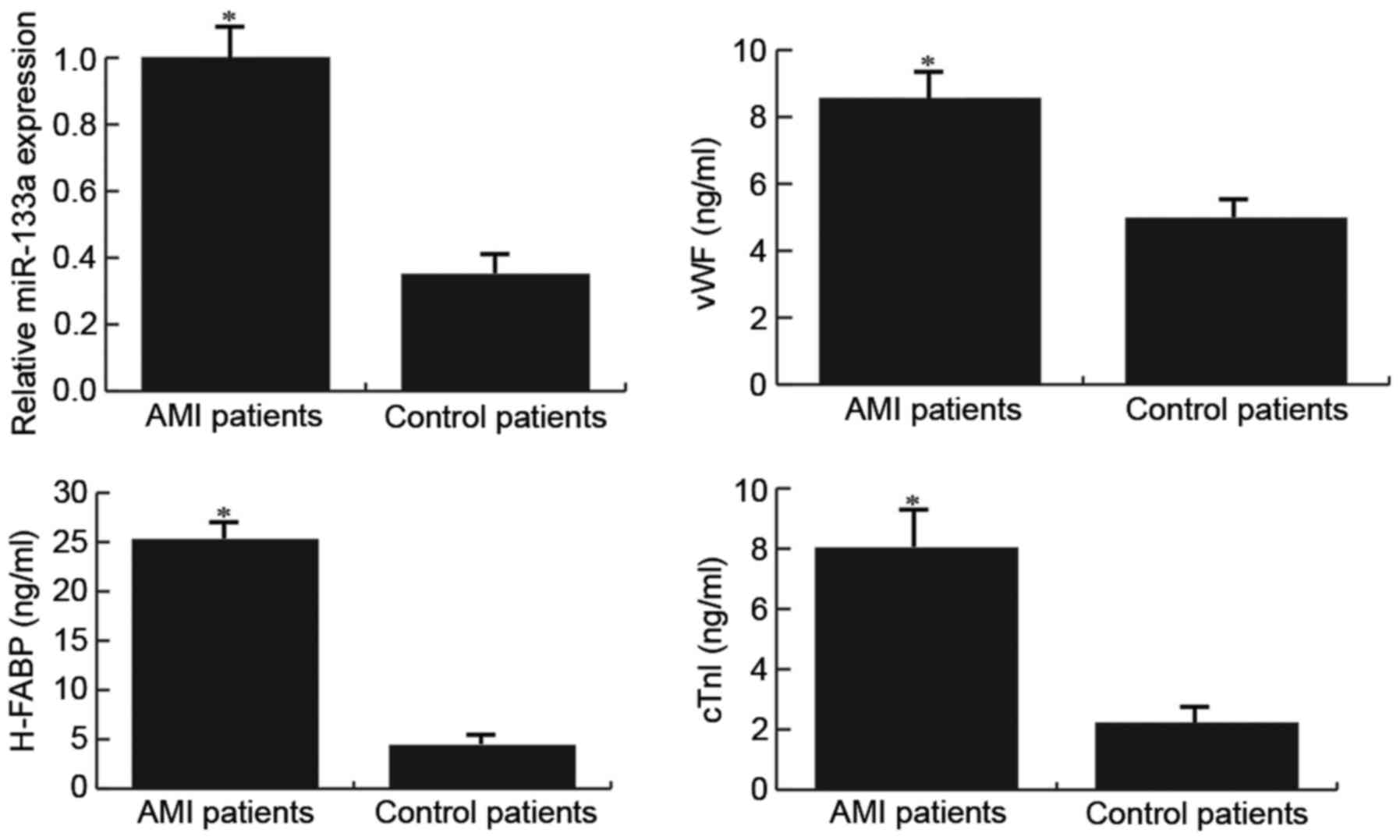 | Figure 1.Expression levels of miR-133a and
endothelial injury markers (vWF, H-FABP and cTnI) in patients with
or without AMI. The expression levels of miR-133a, and vWF, H-FABP
and cTnI, were detected in patients using reverse
transcription-quantitative polymerase chain reaction and ELISA,
respectively. AMI patients, patients with AMI following radical
surgery for gastric cancer; Control patients, patients without AMI
following radical surgery for gastric cancer. Data are presented as
the mean ± standard deviation. *P<0.05 vs. the Control patients.
AMI, acute myocardial infarction; cTnI, cardiac troponin I; H-FABP,
heart-type fatty acid-binding protein; miR-133a, microRNA-133a;
vWF, Von Willebrand factor. |
miR-133a regulates endothelial injury
marker expression
The present study also investigated the effects of
miR-133a on endothelial injury marker expression in rats, following
the establishment of an AMI rat model (21). As presented in Fig. 2, miR-133a expression was
significantly increased in the AMI rat model; treatment with a
miR-133a mimic further enhanced the expression of miR-133a, whereas
miR-133a expression was decreased in the miR-133a inhibitor group.
Compared with the control group, the expression levels of the
endothelial injury markers (vWF, H-FABP and cTnI) were
significantly increased in the AMI rat model. In addition,
treatment with the miR-133a mimic enhanced the expression levels of
endothelial injury markers (vWF, H-FABP and cTnI) in the AMI rat
model, whereas the miR-133a inhibitor had the opposite effect.
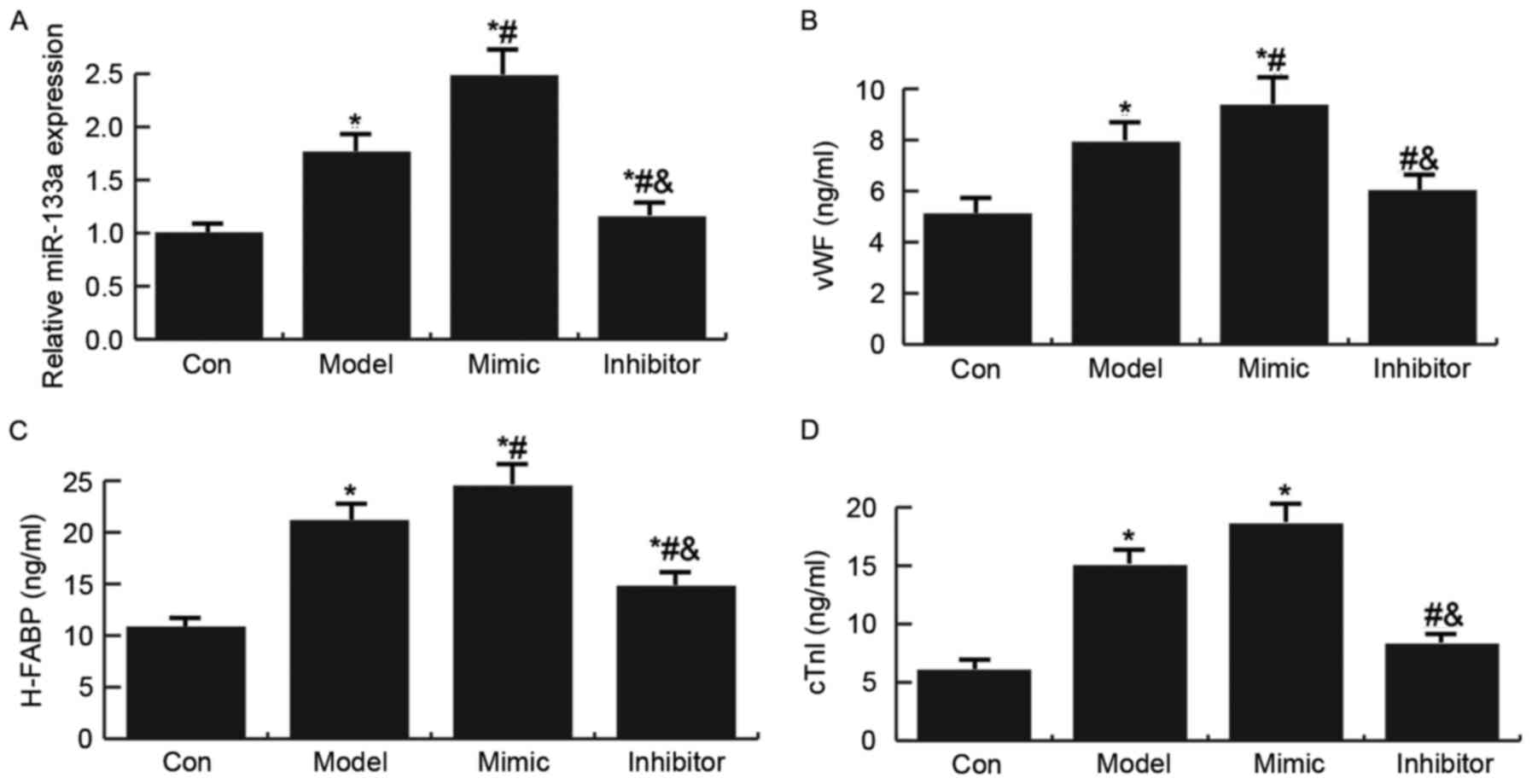 | Figure 2.Expression levels of miR-133a and
endothelial injury markers (vWF, H-FABP and cTnI) in a rat model of
acute myocardial infarction. (A) Quantification of the expression
levels of miR-133a in model rats, as determined by RT-qPCR.
Expression levels of (B) vWF, (C) H-FABP and (D) cTnI in model
rats, as determined by ELISA. Data are presented as the mean ±
standard deviation. *P<0.05 vs. the Con group;
#P<0.05 vs. the Model group;
&P<0.05 vs. the Mimic group. Con: Control group,
rats that underwent the sham operation were injected with a vector;
Model: Model group, rats in the AMI model group were injected with
NC; mimic, miR-133a mimic group, rats in the AMI model group were
injected with a miR-133a mimic; inhibitor, miR-133a inhibitor
group, rats in the AMI model group were injected with a miR-133a
inhibitor; cTnI, cardiac troponin I; H-FABP, heart-type fatty
acid-binding protein; miR-133a, microRNA-133a; vWF, Von Willebrand
factor. |
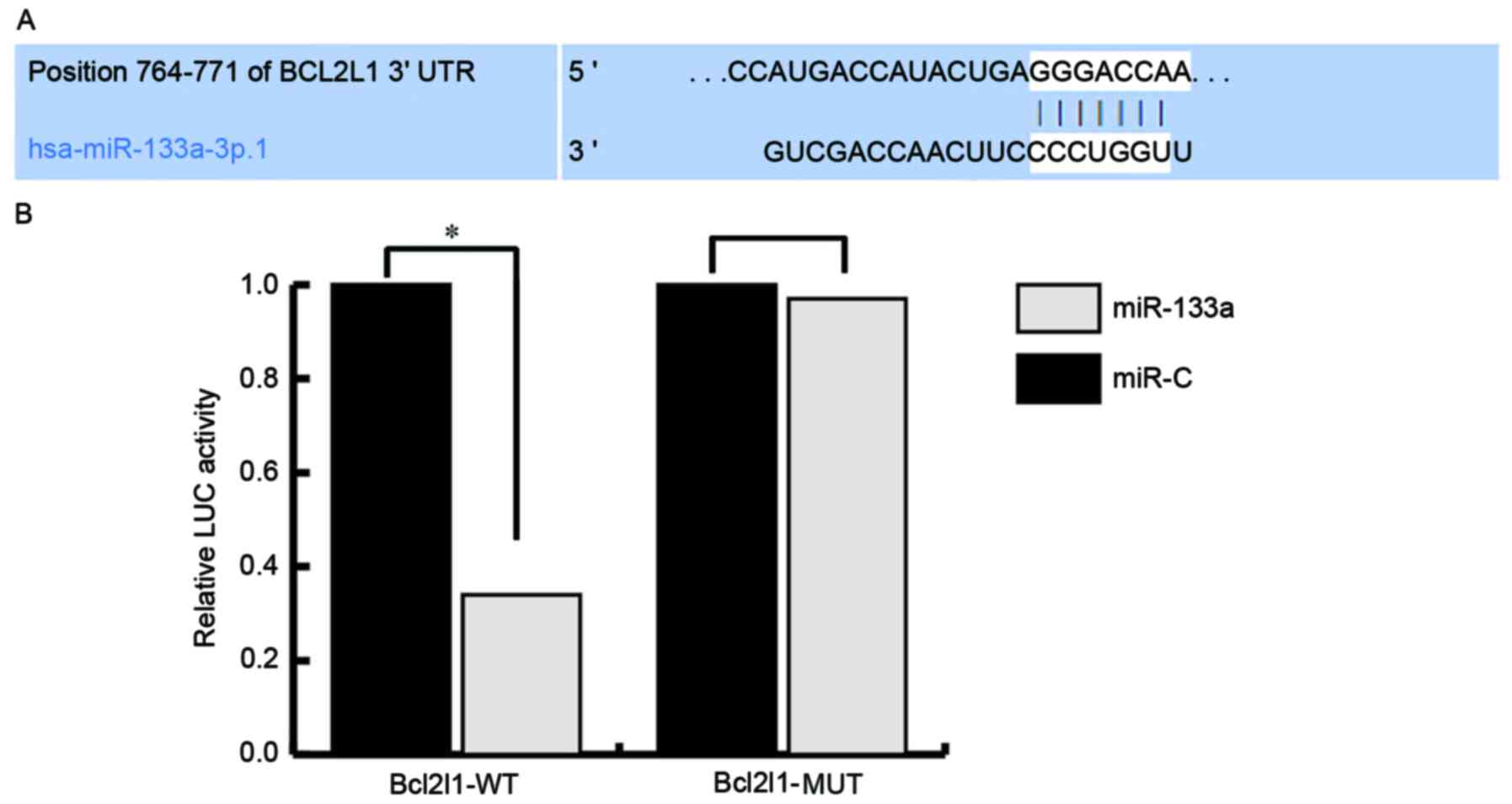
miR-133a directly targets Bcl2l1 and
regulates Bcl2l1 expression
To investigate the mechanisms underlying the effects
of miR-133a on the regulation of endothelial injury, the target
genes of miR-133a were investigated using TargetScan (http://www.targetscan.org/vert_71/) and miRanda
(http://www.microrna.org/microrna/home.do) databases.
Numerous target genes were discovered, including Bcl2l1. As Bcl2l1
serves critical roles in cell proliferation and apoptosis, Bcl2l1
was selected for further analysis. Subsequently, a luciferase
activity assay was used to confirm the predictions. The results
indicated that miR-133a directly targets Bcl2l1 (Fig. 3).
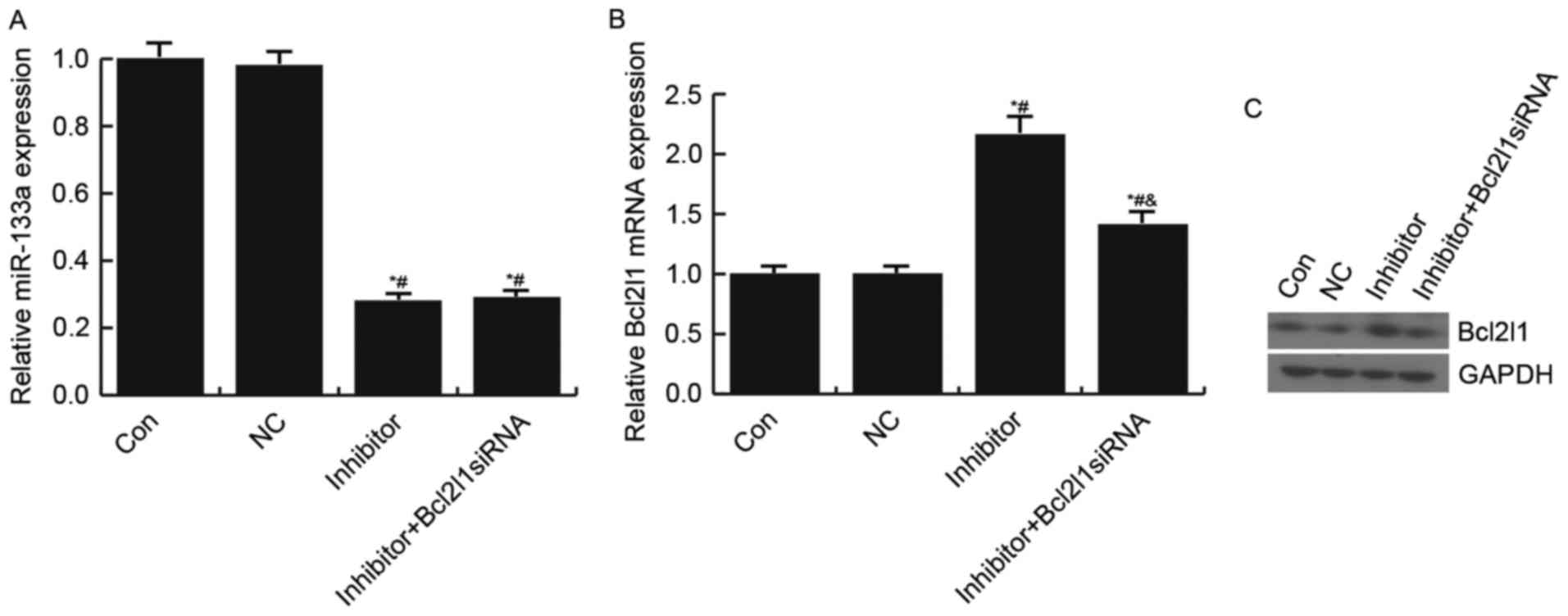
The present study also confirmed that miR-133a
negatively regulated Bcl2l1 expression in HUVECs. Transfection with
the miR-133a inhibitor significantly increased Bcl2l1 expression;
however, this increase was reduced following transfection with the
Bcl2l1 siRNA (Fig. 4).
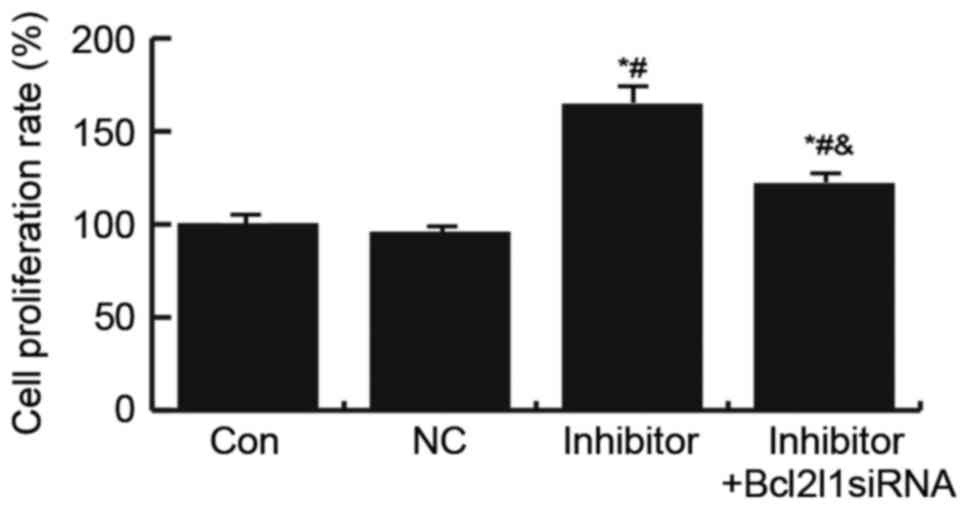
Effects of miR-133a on HUVEC cell
proliferation
To explore the underlying mechanisms of miR-133a in
the regulation of endothelial injury, the present study determined
the effects of miR-133a on HUVEC cell proliferation using an MTT
assay. The results suggested that transfection with the miR-133a
inhibitor significantly increased HUVEC cell proliferation, whereas
this increase was abrogated by Bcl2l1 siRNA (Fig. 5).
Effects of miR-133a on HUVEC
apoptosis
Finally, an Annexin V-FITC/PI Apoptosis Detection
kit was used to analyze the effects of miR-133a on apoptosis of
HUVECs. As presented in Fig. 6,
compared with in the control and NC groups, the apoptotic rate of
HUVECs was reduced in the miR-133a inhibitor group, whereas
transfection with Bcl2l1 siRNA was able to reverse the miR-133a
inhibitor-induced reduction in cell apoptosis.
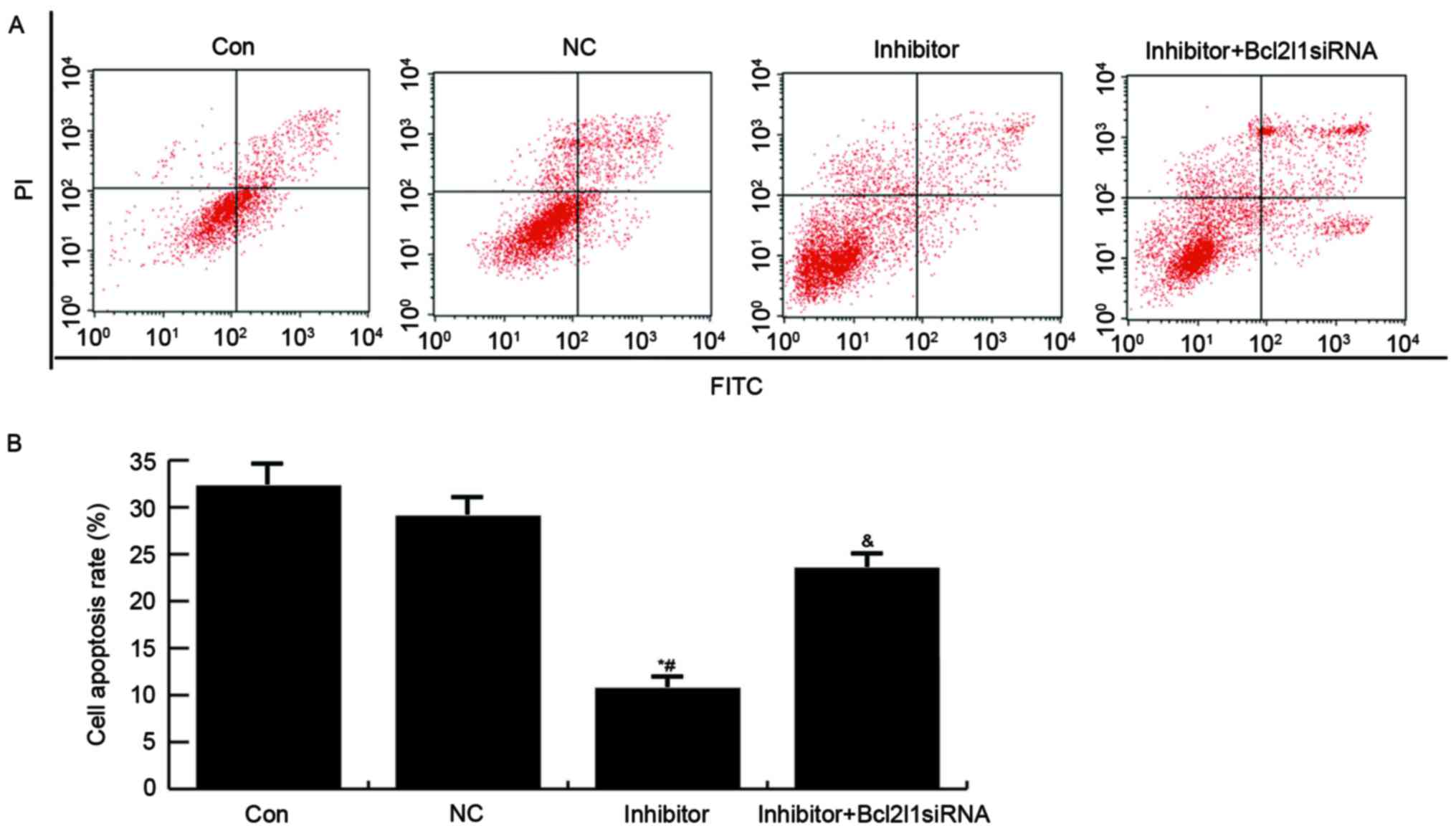 | Figure 6.Effects of miR-133a on HUVEC
apoptosis. (A) A total of 48 h post-transfection, HUVEC apoptosis
was determined by flow cytometry. Con, untreated HUVECs; NC: HUVECs
transfected with NC; inhibitor, HUVECs transfected with a miR-133a
inhibitor; inhibitor + Bcl2l1 siRNA, HUVECs cotransfected with a
miR-133a inhibitor and Bcll12 siRNA. (B) Data are presented as the
mean ± standard deviation. *P<0.05 vs. the Con group;
#P<0.05 vs. the NC group; &P<0.05
vs. the inhibitor group. Bcl2l1, B-cell lymphoma 2-like 1; FITC,
fluorescein isothiocyanate; HUVECs, human umbilical vein
endothelial cells; miR-133a, microRNA-133a; NC, negative control;
PI, propidium iodide; siRNA, small interfering RNA. |
Discussion
Taken together, the present study demonstrated that
compared with the control patients, patients with AMI following
radical surgery for GC exhibited higher miR-133a and endothelial
injury marker expression. In addition, miR-133a could promote the
expression of endothelial injury markers (vWF, H-FABP and cTnI),
whereas miR-133a inhibition could promote HUVEC proliferation and
reduce cell apoptosis by targeting Bcl2l1. These results indicated
that miR-133a was associated with endothelial injury following AMI
by targeting Bcl2l1.
With the extensive application of
electrocardiograms, perioperative cardiovascular complications
during surgery for gastrointestinal tumors have garnered attention.
However, the causes of perioperative cardiovascular complications
of gastrointestinal tumors are very complex. Elderly patients often
have the pathological basis of atherosclerosis, which increases the
risk of AMI (24). AMI seriously
threatens human survival, particularly in patients with AMI
following tumor surgery; therefore, it is necessary to explore AMI
pathogenesis, and to research novel diagnostic markers and
treatment methods.
miRNAs have been reported to be associated with
cardiovascular diseases, due to their involvement in the regulation
of a wide range of processes, including gene expression control
(25). Numerous studies have
reported that miRNAs participate in endothelial injury (26,27).
Although a previous study has demonstrated that miR-133a expression
is increased in the plasma of patients with AMI (20), the mechanism remained to be
elucidated. The present study detected the upregulation of miR-133a
and endothelial injury markers (vWF, H-FABP and cTnI) in patients
with AMI. To elucidate the functional roles of miR-133a in AMI, a
rat model of AMI was generated. The results demonstrated that
miR-133a was able to promote the expression of endothelial injury
markers (vWF, H-FABP and cTnI) in a rat model of AMI, whereas
treatment with the miR-133a inhibitor had the opposite effect.
These data indicated that miR-133a may participate in endothelial
injury.
The original indication of improvement following MI
is endothelial activation, determined by the enhanced cell
proliferation ability and reduced apoptosis (28). To further investigate the
underlying mechanisms of miR-133a in the regulation of endothelial
injury in AMI, Bcl2l1 was initially identified as a direct target
gene of miR-133a. The protein encoded by the Bcl2l1 gene belongs to
the Bcl-2 protein family and functions as an anti-apoptotic
molecule. The present study subsequently determined the effects of
miR-133a on HUVEC cell proliferation and apoptosis. The results
demonstrated that compared with in the control and NC groups,
transfection with a miR-133a inhibitor was able to promote HUVEC
proliferation and reduce cell apoptosis by upregulating Bcl2l1 gene
expression.
In conclusion, the present study is the first, to
the best of the authors' knowledge, to demonstrate that patients
with AMI following radical surgery for GC had higher miR-133a
expression compared with patients without AMI. In addition, the
results indicated that miR-133a could regulate the endothelial
injury process following AMI though the modulation of endothelial
cells via targeting Bcl2l1. Therefore, miR-133a may be considered a
novel therapeutic target in the future treatment of AMI; however,
whether miR-133a may be targeted as a preventative intervention for
AMI following radical surgery for GC requires further
investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY collaborated in the design of the present study.
JY, XC and YZ were responsible for data accession and analysis. JY,
XC, YZ, LY and JW collaborated to interpret results and develop the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Ethics
Committee Review Board at Cangzhou Central Hospital of Hebei
(Cangzhou, China), and informed consent was obtained from all
patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shi Y and Zhou Y: The role of surgery in
the treatment of gastric cancer. J Surg Oncol. 101:687–692. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
White HD and Chew DP: Acute myocardial
infarction. Lancet. 372:570–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bogomolov AN, Kozlov KL, Kurochkina ON and
Olesiuk IB: Coronary stenting in elderly patients with acute
myocardial infarction (review). Adv Gerontol. 26:151–160. 2013.(In
Russian). PubMed/NCBI
|
|
4
|
Lipinski MJ, Escárcega RO, D'Ascenzo F,
Magalhães MA, Baker NC, Torguson R, Chen F, Epstein SE, Miró O,
Llorens P, et al: A systematic review and collaborative
metaanalysis to determine the incremental value of copeptin for
rapid rule-out of acute myocardial infarction. Am J Cardiol.
113:1581–1591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Velibey Y, Erbay A, Ozkurt E, Usta E and
Akin F: Acute myocardial infarction associated with blood
transfusion: Case report and literature review. Transfus Apher Sci.
50:260–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yahalom M, Roguin N, Suleiman K and
Turgeman Y: Clinical signifcance of conditions presenting with ECG
changes mimicking acute myocardial infarction. Int J Angiol.
22:115–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mozarian D, Benjamin EJ, Go AS, Arnett DK,
Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ,
Howard VJ, et al: Heart disease and stroke statistics update: A
report from the American Heart Association. Circulation.
131:e29–e322. 2015.PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Z, Han Y, Cheng K, Zhang G and Wang
X: miR-99a directly targets the mTOR signalling pathway in breast
cancer side population cells. Cell Prolif. 47:587–595. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niu G, Li B, Sun J and Sun L: miR-454 is
down-regulated in osteosarcomas and suppresses cell proliferation
and invasion by directly targeting c-Met. Cell Prolif. 48:348–355.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sala V, Bergerone S, Gatti S, Gallo S,
Ponzetto A, Ponzetto C and Crepaldi T: MicroRNAs in myocardial
ischemia: Identifying new targets and tools for treating heart
disease. New frontiers for miR-medicine. Cell Mol Life Sci.
71:1439–1452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thum T, Gross C, Fiedler J, Fischer T,
Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W and Frantz S:
MicroRNA-21 contributes to myocardial disease by stimulating MAP
kinase signalling in fbroblasts. Nature. 456:980–984. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Zhang X, Ren XP, Chen J, Liu H,
Yang J, Medvedovic M, Hu Z and Fan GC: MicroRNA-494 targeting both
proapoptotic and antiapoptotic proteins protects against
ischemia/reperfusion-induced cardiac injury. Circulation.
122:1308–1318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fiedler J, Jazbutyte V, Kirchmaier BC,
Gupta SK, Lorenzen J, Hartmann D, Galuppo P, Kneitz S, Pena JT,
Sohn-Lee C, et al: MicroRNA-24 regulates vascularity after
myocardial infarction. Circulation. 124:720–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hinkel R, Penzkofer D, Zühlke S, Fischer
A, Husada W, Xu QF, Baloch E, van Rooij E, Zeiher AM, Kupatt C and
Dimmeler S: Inhibition of microRNA-92a protects against
ischemia/reperfusion injury in a large-animal model. Circulation.
128:1066–1075. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Devaux Y, Vausort M, McCann GP, Zangrando
J, Kelly D, Razvi N, Zhang L, Ng LL, Wagner DR and Squire IB:
MicroRNA-150: A novel marker of left ventricular remodeling after
acute myocardial infarction. Circ Cardiovasc Genet. 6:290–298.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Ye P, Wang S, Wu J, Sun Y, Zhang A,
Ren L, Cheng C, Huang X, Wang K, et al: MicroRNA-150 protects the
heart from injury by inhibiting monocyte accumulation in a mouse
model of acute myocardial infarction. Circ Cardiovasc Genet.
8:11–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu H, Li G, Zhao W and Hu Y: Inhibition
of MiR-92a may protect endothelial cells after acute myocardial
infarction in rats: Role of KLF2/4. Med Sci Monit. 22:2451–2462.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong Y, Ren J, Liu K and Tang LM: Tumor
suppressor role miR-133a in gastric cancer by repressing IGF1R.
World J Gastroenterol. 21:2949–2958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang F, Long G, Zhao C, Li H, Chaugai S,
Wang Y, Chen C and Wang DW: Plasma microRNA-133a is a new marker
for both acute myocardial infarction and underlying coronary artery
stenosis. J Transl Med. 11:2222013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang D, Zhu L, Li C, Mu J, Fu Y, Zhu Q,
Zhou Z, Liu P and Han C: Sialyltransferase7A, a Klf4-responsive
gene, promotes cardiomyocyte apoptosis during myocardial
infarction. Basic Res Cardiol. 110:282015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang GJ, Zhou H, Xiao HX, Li Y and Zhou
T: Up-regulation of miR-224 promotes cancer cell proliferation and
invasion and predicts relapse of colorectal cancer. Cancer Cell
Int. 13:1042013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agarwal S, Sud K, Thakkar B, Menon V,
Jaber WA and Kapadia SR: Changing trends of atherosclerotic risk
factors among patients with acute myocardial infarction and acute
ischemic stroke. Am J Cardiol. 119:1532–1541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Small EM and Olson EN: Pervasive roles of
microRNAs in cardiovascular biology. Nature. 469:336–342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bonauer A, Carmona G, Iwasaki M, Mione M,
Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et
al: MicroRNA-92a controls angiogenesis and functional recovery of
ischemic tissues in mice. Science. 324:1710–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou J, Wang KC, Wu W, Subramaniam S, Shyy
JY, Chiu JJ, Li JY and Chien S: MicroRNA-21 targets peroxisome
proliferators-activated receptor-alpha in an autoregulatory loop to
modulate flow-induced endothelial inflammation. Proc Natl Acad Sci
USA. 108:10355–10360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tedgui A and Mallat Z: Cytokines in
atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev.
86:515–581. 2006. View Article : Google Scholar : PubMed/NCBI
|