Introduction
Adenomyosis is a common gynecological disorder
characterized by the presence of endometrial glands and stroma
within the myometrium (1). This
disease has been associated with a series of clinical symptoms,
including chronic pelvic pain, menorrhagia and infertility
(2); thus, adenomyosis can
negatively affect quality of life. In addition, the incidence of
adenomyosis at hysterectomy is ~20–30% (2); however, there is no therapy more
effective than hysterectomy for the irreversible progression of
this disease. At present, the pathogenesis of adenomyosis remains
unclear, yet numerous hypotheses exist. It is widely accepted that
adenomyosis is a benign disorder associated with malignant
behaviors, including enhanced invasive ability, metastasis
(3) and anti-apoptosis (4). It has been proposed that the invasion
of endometrial cells may be responsible for the development of
adenomyosis (5). Additionally,
epithelial-mesenchymal transition (EMT) has been reported to serve
vital roles in migration and metastasis of various types of cancer
(6).
EMT is a process by which epithelial cells undergo a
transition into mesenchymal cells, and frequently characterizes
embryonic development, tumor metastasis and fibrotic disease
(7). During EMT, epithelial cells
obtain the characteristics of mesenchymal cells by losing cell
polarity, remolding the cytoskeleton, and acquiring invasive
properties and resistance to apoptosis (8,9). In
addition, epithelial cells lose the expression of epithelial
markers, such as epithelial (E)-cadherin, and increase the
expression of mesenchymal markers, including neural (N)-cadherin
and vimentin (7). Downregulation
of E-cadherin, a key epithelial cell adhesion molecule, is a
characteristic molecular feature of EMT (10). Furthermore, EMT is regulated by
numerous transcription factors that suppress the expression of
E-cadherin, as well as Snail, Slug and Twist-related protein 1
(Twist1) (11). Previously,
E-cadherin expression was demonstrated to be significantly
downregulated in adenomyosis, indicating that EMT serves an
important role in the development of adenomyosis (12,13).
The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway
has also been associated with the pathogenesis of adenomyosis
(14).
Focal adhesion kinase (FAK) is a cytoplasmic protein
tyrosine kinase that mediates the signal transduction of integrins
and other cell surface receptors in a variety of cells (15). FAK has been reported to serve a
significant role in the regulation of cell adhesion, migration,
proliferation and survival (16).
Upregulation of FAK has been reported in numerous types of tumors
and fibrotic diseases (17–19).
Additionally, it has been suggested that FAK may serve a role in
the regulation of EMT (20). FAK
was also reported to induce the PI3K/AKT signaling cascade, leading
to the upregulation of EMT markers, including vimentin and Snail
(21).
Previous studies reported increased FAK expression
in the eutopic endometrium in adenomyosis (22,23)
and demonstrated that FAK facilitated the progression of the EMT;
however, the underlying mechanism by which FAK promotes EMT in
adenomyosis remains unknown. Thus, the present study aimed to
determine whether FAK regulates the EMT process in adenomyosis; the
expression levels of FAK and E-cadherin in human adenomyosis were
analyzed and compared with controls cells. In addition, the
function of FAK in the development of adenomyosis in the context of
the migration was investigated in the present study. Furthermore,
the association between FAK and its downstream target genes, PI3K,
AKT, E-cadherin and vimentin was determined; the involvement of FAK
and the PI3K/AKT signaling pathway in adenomyosis was also
investigated.
Materials and methods
Ethics statement
A total of 47 women of reproductive age (age, 38–50
years) were recruited for this study. Informed consent was obtained
from all participants prior to surgery. The present study was
approved by the Ethics Committee of Beijing Obstetrics and
Gynecology Hospital, Capital Medical University (approval no.
2016KY012; Beijing, China).
Sample collection
In the present study, endometrial tissue samples
from 24 female patients with adenomyosis (proliferative phase, 13
cases; secretory phase, 11 cases; age, 44.71±0.52 years) diagnosed
by pathological confirmation in a blind manner and 23 female
patients (proliferative phase, 13 cases; secretory phase, 10 cases;
age, 44.22±1.06 years) for hysterectomy for benign indications
without endometrial lesions, as matched controls, were used for
cell culture and subsequent experiments. In addition, the 26
samples (adenomyosis group, 13 cases; control group, 13 cases) that
were collected in the proliferative phase of the menstrual cycle
based on the patients' menstrual histories and histological
examinations were used for cell experiments. All patients underwent
hysterectomy at the Beijing Obstetrics and Gynecology Hospital,
Capital Medical University between January 2016 and July 2017. All
participants were of reproductive age (38–50 years old) with
regular menstrual cycles (28–32 days) and received no hormonal
therapy for ≥3 months prior to the surgery.
Immunohistochemistry (IHC)
Following surgery, the endometrial tissues were
immediately fixed in 4% formalin at room temperature for 48 h,
paraffin-embedded, cut into serial 5 µm sections and stained. All
IHC staining was performed using a PV-9000 two-step staining kit
(Beijing Zhongshan Golden Bridge Biotechnology, Co., Ltd., Beijing,
China), which includes three reagents [3% peroxidase, Polymer
Helper reagent and poly-peroxidase-anti-rabbit/mouse immunoglobulin
(Ig)G], according to the manufacturer's protocols. In addition, the
tissue samples were deparaffinized with xylene, rehydrated with
decreasing concentrations of ethanol (100, 100, 95, 95, 80 and 80%)
and incubated with 3% peroxidase for 10 min at room temperature.
Subsequently, the sections were rinsed with PBS three times and
incubated overnight using primary antibodies in a humidified
container at 4°C. The primary antibodies were as follows: Rabbit
anti-FAK monoclonal antibody (1:500; cat. no. ab40794; Abcam,
Cambridge, UK), rabbit anti-E-cadherin polyclonal antibody (1:200;
cat. no. sc-7870; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
mouse anti-vimentin monoclonal antibody (1:100; cat. no. BM0135;
Boster Biological Technology, Pleasanton, CA, USA), rabbit
anti-cytokeratin polyclonal antibody (1:100; cat. no. sc-15367;
Santa Cruz Biotechnology, Inc.) and rabbit anti-α-smooth muscle
actin (α-SMA) polyclonal antibody (1:200; cat. no. ab5694; Abcam).
Subsequently, the samples were sequentially incubated with Polymer
Helper reagent for 20 min at 37°C and rinsed with PBS, followed by
incubation with poly-peroxidase-anti-rabbit/mouse IgG
(ready-to-use) for 20 min at 37°C and rinsed again with PBS. The
sections were stained using a Diaminobenzidine Solution kit
(Beijing Zhongshan Golden Bridge Biotechnology, Co., Ltd.),
counterstained with hematoxylin for 1 min, dehydrated with
increasing concentrations of ethanol (80, 80, 95, 95, 100 and 100%)
and mounted for 1–3 min at room temperature. The staining was
observed under a light microscope (Olympus Corporation, Tokyo,
Japan) at magnification, ×100. In the negative control experiments,
PBS was used to replace the primary antibodies. A series of five
random images per section was collected. IHC localization of the
primary antibody was scored according to the intensity of staining
and proportion of positive cells by two experienced pathologists.
The proportion of positively stained cells was scored as follows:
0, <5%; 1, 5–25%; 2, 25–50%; 3, 50–75% and 4, >75%. The
average intensity of the staining was scored as follows: 0 for
negative, 1 for weak, 2 for moderate and 3 for strong. The total
IHC score was calculated by multiplying the percentage and
intensity scores.
Cell culture
Endometrium was obtained immediately following
hysterectomy of the patients, and placed in saline water prior to
analysis. Tissues were washed twice in PBS, minced into fragments
(<1 mm) and digested with collagenase I (2 mg/ml; cat. no.
17100017; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
for 40 min at 37°C. The dispersed cells were filtered with a 100-µm
nylon mesh to remove undigested tissue. Subsequently, the cells
were centrifuged twice at 450 × g for 5 min at room temperature,
and the endometrial cells were incubated in Dulbecco's modified
Eagles medium (DMEM)/F12 (Gibco; Thermo Fisher Scientific, Inc.)
with 15% fetal bovine serum (FBS; Biological Industries, Kibbutz
Beit Haemek, Israel) and 1% penicillin/streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a humidified tissue culture
incubator containing 5% CO2.
Immunofluorescence
Cells (5×104 cells/well) were seeded onto
sterile fibronectin-coated glass coverslips in 6-well plates and
cultured on glass until 80% confluent. Cells were fixed in 4%
paraformaldehyde for 30 min at room temperature and blocked with
PBS containing 1% bovine serum albumin (Beijing Zhongshan Golden
Bridge Biotechnology, Co., Ltd.) at room temperature for 30 min.
Subsequently, the samples were incubated with the following primary
antibodies diluted in the aforementioned blocking solution
overnight at 4°C: Rabbit anti-FAK monoclonal antibody (1:500),
rabbit anti-E-cadherin polyclonal antibody (1:500) and mouse
anti-vimentin monoclonal antibody (1:500). Cells were subsequently
washed in PBS three times and incubated with secondary antibody
separately at 37°C for 1 h in the dark. The secondary antibodies
were: Fluorescein isothiocyanate-conjugated goat anti-rabbit IgG
(1:100; cat. no. ZF0311; OriGene Technologies, Inc.) and
tetramethylrhodamine-conjugated goat anti-mouse IgG (1:100; cat.
no. ZF0313; OriGene Technologies, Inc.). Following thorough washing
in PBS, coverslips were mounted with anti-fade mounting medium with
DAPI (OriGene Technologies, Inc.) for 2 min at room temperature,
observed under a fluorescence microscope (Olympus Corporation,
Tokyo, Japan) at magnification, ×100 and 50 cells were counted in
each field.
Reverse-transcription polymerase chain
reaction (RT-qPCR)
RT-qPCR was used to examine the mRNA expression
levels of FAK, PI3K, AKT, Snail, Slug, Twist1, E-cadherin,
cytokeratin, N-cadherin and vimentin at the mRNA level. Total RNA
was extracted from cultured endometrial cells (1×105
cells/ml) using RNAiso plus (Takara Bio, Inc., Otsu, Japan), and
RNA quality was confirmed using a NanoDrop 2000 spectrophotometer
(NanoDrop Technologies; Thermo Fisher Scientific, Inc., Wilmington,
DE, USA). Subsequently, RNA (1 µg) was reverse transcribed into
cDNA using the FastQuant RT kit (Tiangen Biotech Co., Ltd.,
Beijing, China), according to the manufacturer's protocols. qPCR
was performed using an ABI7500 PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.); reactions were performed using the
SuperReal PreMix Plus kit (Tiangen Biotech Co., Ltd.). The
thermocycling conditions were as follows: Initial denaturation at
95°C for 15 min, followed by 40 cycles of denaturation at 95°C for
10 sec and annealing/extension at 60°C for 32 sec. To ensure
technical reliability, experiments were performed in triplicate.
All primers used for qPCR were synthesized by Sangon Biotech Co.,
Ltd. (Shanghai, China), and GAPDH was used as an internal
normalization control; the sequences are presented in Table I. Relative mRNA expression levels
were determined using the 2−∆∆Cq method (24).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequences
(5′→3′) |
|---|
| FAK | F:
ATCCCACACATCTTGCTGACTT |
|
| R:
GCATTCCTTTTCTGTCCTTGTC |
| PI3K | F:
CCCTGCTCATCAACTAGGAAACC |
|
| R:
TTGCCGTAAATCATCCCCATT |
| AKT | F:
TGCCCTTCTACAACCAGGAC |
|
| R:
ACACGATACCGGCAAAGAAG |
| Snail | F:
TCCTTCGTCCTTCTCCTCTACTT |
|
| R:
TGTTGCAGTATTTGCAGTTGAAG |
| Slug | F:
AGATGCATATTCGGACCCACA |
|
| R:
CCTCATGTTTGTGCAGGAGAG |
| Twist1 | F:
CAAGTCTGCAGCTCTCGCCA |
|
| R:
CCAACGGCTGGCGCACAC |
| E-cadherin | F:
ATTGCTCACATTTCCCAACTCC |
|
| R:
CTCTGTCACCTTCAGCCATCCT |
| Cytokeratin | F:
ACATCGAGATCGCCACCTAC |
|
| R:
CCAGAGCCAAAGCTGGAG |
| N-cadherin | F:
AGCCAACCTTAACTGAGGAGT |
|
| R:
GGCAAGTTGATTGGAGGGATG |
| Vimentin | F:
TACAGGAAGCTGCTGGAAGG |
|
| R:
ACCAGAGGGAGTGAATCCAG |
| GAPDH | F:
CTCCTCCACCTTTGACGCTG |
|
| R:
TCCTCTTGTGCTCTTGCTGG |
Western blotting
Each experiment was repeated three times.
Endometrial cells were washed with cold PBS; protein was extracted
from endometrial cells (1×105 cells/ml) with
radioimmunoprecipitation assay lysis buffer (Beijing Solarbio
Science and Technology Co., Ltd., Beijing, China). Total protein
concentration was measured with a Bicinchoninic Acid protein assay
kit (Beijing Solarbio Science and Technology Co., Ltd.). An equal
amount of protein (30 µg) was loaded into each lane and separated
by 10% SDS-PAGE, which were then transferred to a polyvinylidene
fluoride membrane (EMD Millipore, Billerica, MA, USA). Following
blocking in 5% skim milk at room temperature for 30 min, the
membrane was incubated with a mouse anti-vimentin monoclonal
antibody (1:250), rabbit anti-E-cadherin polyclonal antibody
(1:200), anti-FAK monoclonal antibody (1:500), anti-PI3K monoclonal
antibody (ab151549, 1:500), anti-AKT monoclonal antibody (ab200195,
1:1,000) and GAPDH (ab181602, 1:1,000; all Abcam) at 4°C overnight.
Following incubation at room temperature for 20 min with
appropriate horseradish peroxidase-conjugated secondary IgG
antibodies (1:100; cat. nos. AS003 and AS014; ABclonal Biotech Co.,
Ltd., Wuhan, China); antigen detection was performed using an
Enhanced Chemiluminescence Detection System (WBKLS100; EMD
Millipore) according to the manufacture's protocols. Images were
acquired using a ChemiDoc XRS+ system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and subsequently analyzed with ImageJ software
v1.8.0 (National Institutes of Health, Bethesda, MD, USA). Western
blotting experiments were replicated three times for each
sample.
RNA interference
FAK-directed small interfering (si)RNA and negative
controls were synthesized by Sangon Biotech Co., Ltd. The target
sequences were as follows: FAK siRNA1 sense,
5′-GGUUCAAGCUGGAUUAUUUTT-3′and antisense,
5′-AAAUAAUCCAGCUUGAACCTT-3′; FAK siRNA2 sense,
5′-CCGGUCGAAUGAUAAGGUGUA-3′ and antisense,
5′-UACACCUUAUCAUUCGACCGG-3′; FAK siRNA3 sense,
5′-GGAAAUACAGUUUGGAUCUTT-3′ and antisense,
5′-AGAUCCAAACUGUAUUUCCTT-3′ and negative control siRNA sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. Endometrial cells were cultured in
6-well plates until 70% confluence, and transfected for 24 h with
100 nM FAK siRNAs or negative control siRNA using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols.
Following transfection, immunofluorescence, RT-qPCR, western
blotting (all as aforementioned) and migration assays were
conducted to determine alterations in mRNA and protein expression
levels, and the migration potential of cells. The silencing
efficiencies of FAK siRNA1-siRNA3 were determined by RT-qPCR; FAK
siRNA2 exhibited the highest efficiency of silencing and was
selected for subsequent analysis (Table II).
 | Table II.Efficiency of FAK silencing by
transfection with FAK siRNAs. |
Table II.
Efficiency of FAK silencing by
transfection with FAK siRNAs.
|
| Relative FAK mRNA
expression |
|
|---|
|
|
|
|
|---|
| siRNA | FAK siRNA | Negative
controla | Silencing
efficiency(%) |
|---|
| 1 | 0.35±0.02 | 0.94±0.07 | 62.7 |
| 2 | 0.27±0.00 | 1.00±0.06 | 73.0 |
| 3 | 0.30±0.10 | 0.95±0.18 | 68.4 |
Migration assay
Migration was assayed using 6.5-mm Transwell inserts
with an 8.0-µm pore-size polycarbonate membrane filter (Corning
Incorporated, Corning, NY, USA), according to the manufacturer's
protocols. At 24 h post-transfection, cells (1×105
cells/well) were seeded into the upper chambers in serum-free
DMEM/F12 medium, and 20% FBS-DMEM/F12 medium was added to the lower
chambers. At 24, 36, 48, 60 and 72 h of culture, non-migratory
cells were removed using a cotton swab, and the migrated cells on
the underside of the filter were fixed with 4% paraformaldehyde for
30 min and stained with 1% crystal violet (Genia Life Sciences,
Inc., Beijing, China) for 20 min at room temperature. The number of
migrating cells was observed and quantified in five random fields
using a light microscope (magnification, ×100).
Statistical analysis
All data were presented as the mean ± standard
deviation of at least three replicates. Data were analyzed using
GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA).
Differences between two groups were analyzed using a Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
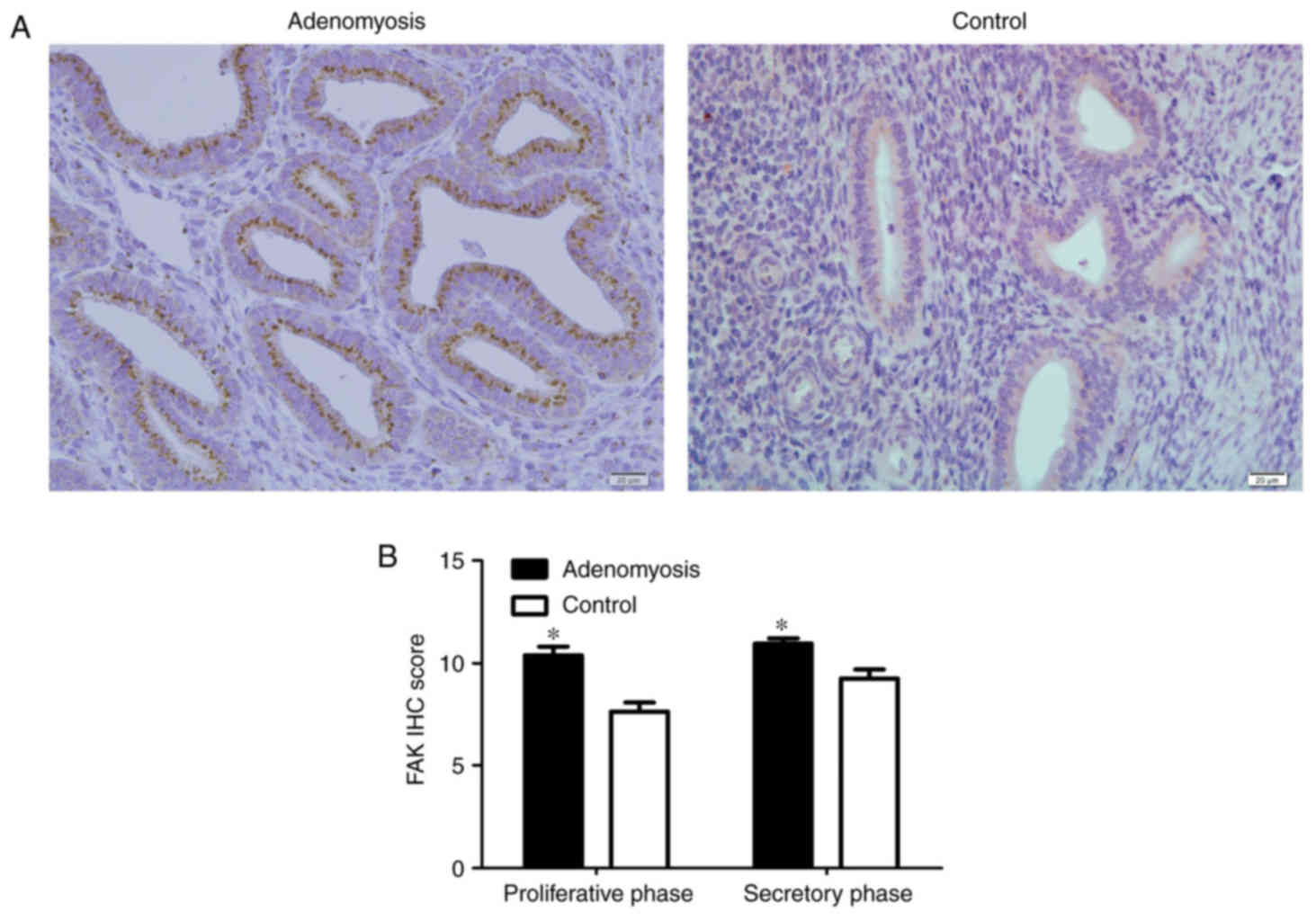
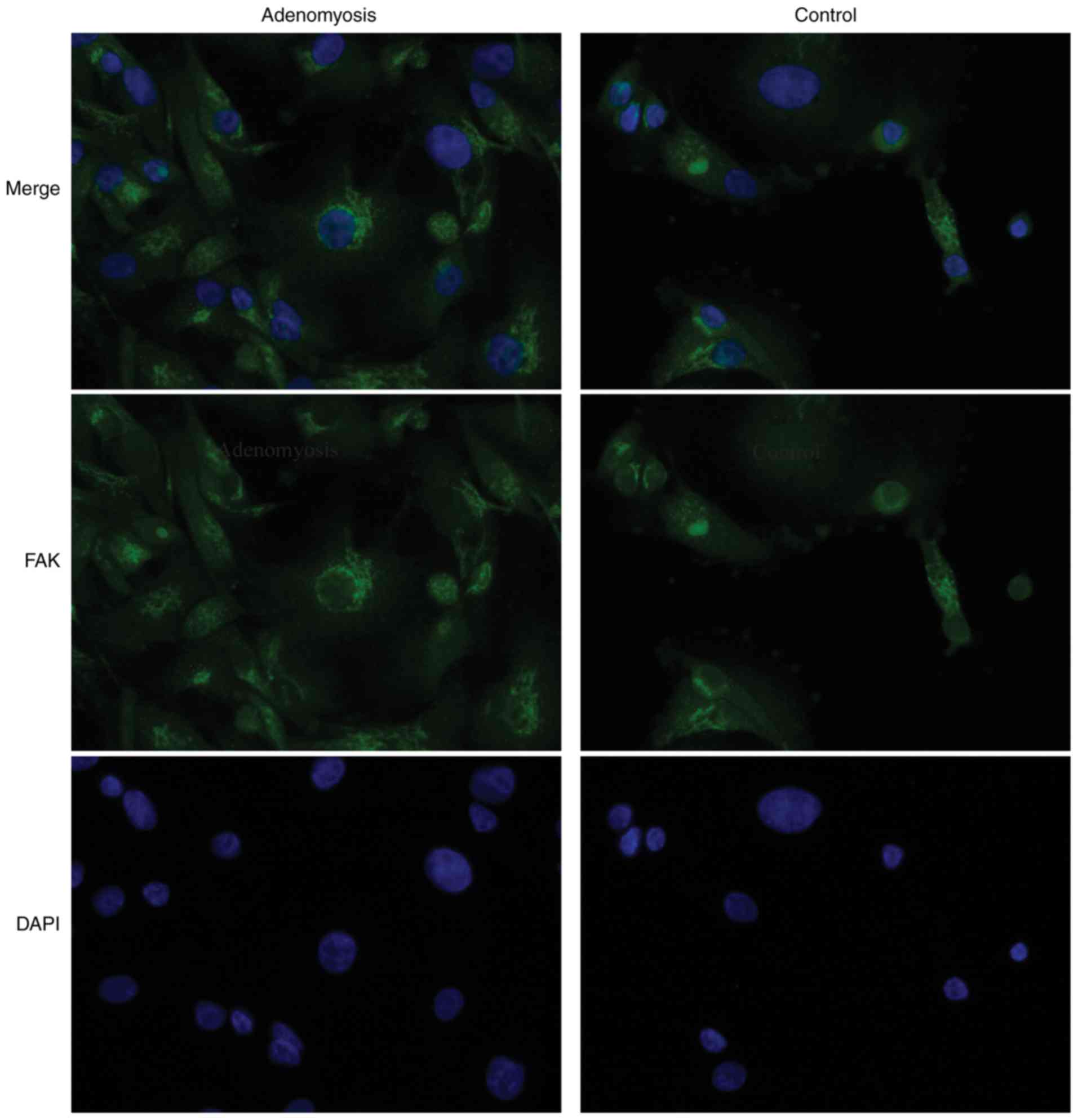
Elevated expression levels of FAK and
EMT markers in adenomyosis
To determine whether FAK is dysregulated in
adenomyosis, FAK expression was analyzed by IHC,
immunofluorescence, RT-qPCR and western blotting in endometrial
tissue from patients with and without adenomyosis. FAK localization
was observed in the cytoplasm of endometrial cells, which appeared
to be enhanced in the adenomyosis group compared with the control
group (Figs. 1 and 2). Additionally, the IHC scores of FAK in
the proliferative and secretory phases were significantly higher in
the adenomyosis group compared with in the control (P<0.05;
Fig. 1B).
To further understand the possible role of FAK in
EMT, IHC analysis on adenomyosis and control endometrial samples
was conducted using antibodies against E-cadherin, cytokeratin,
vimentin and α-SMA (Fig. 3).
E-cadherin and cytokeratin expressions were notably lower in
adenomyosis group tissues compared with the expressions in the
control group, whereas vimentin and α-SMA expression were notably
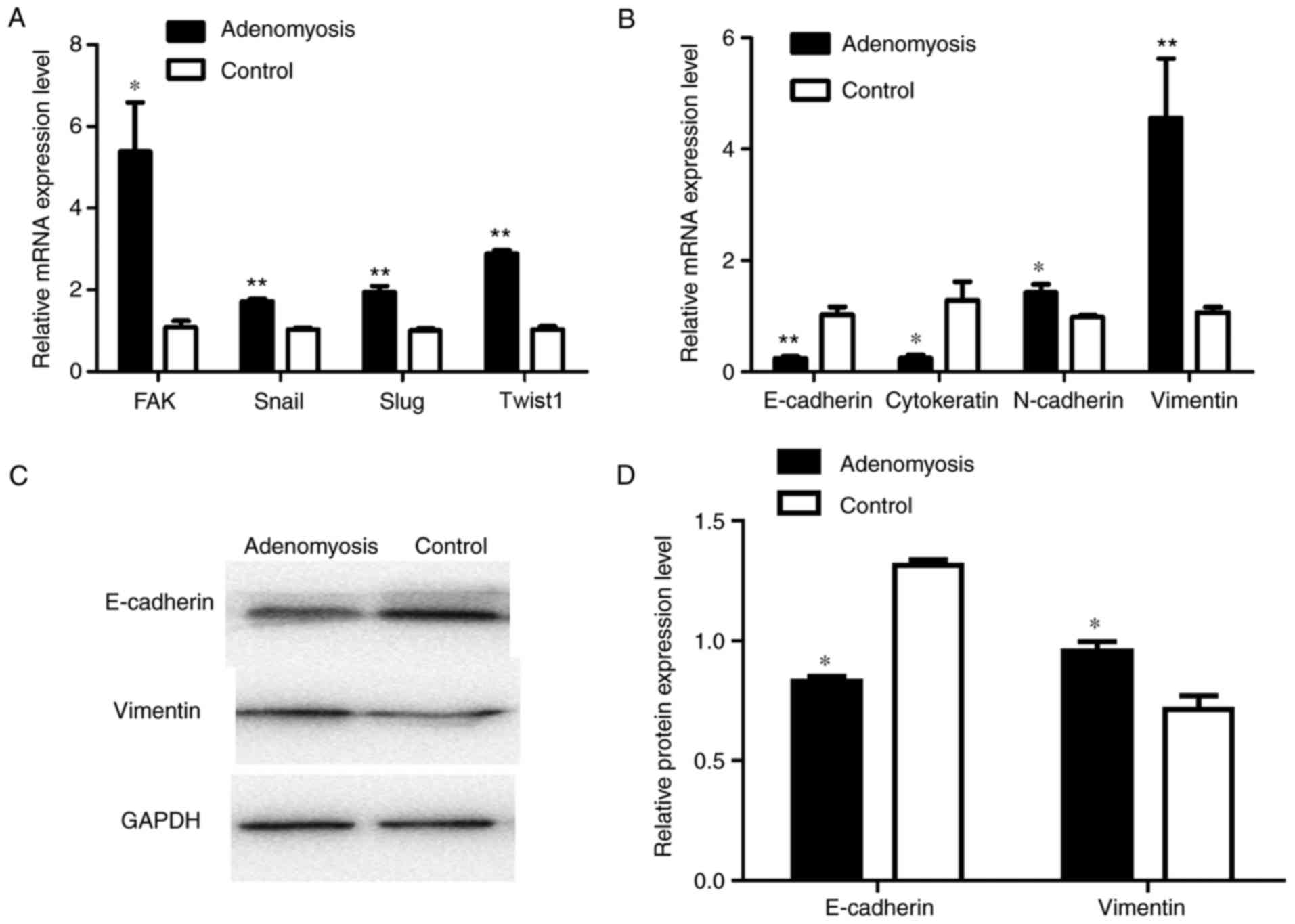
higher in adenomyosis group than that in the control group. RT-qPCR
was performed to determine the mRNA expression levels of
EMT-associated markers, including FAK, Snail, Slug, Twist1,
E-cadherin, cytokeratin, N-cadherin and vimentin in endometrial
cells (Fig. 4A and B). The mRNA
expression levels of FAK, Snai1, Slug and Twist1 were significantly
increased in adenomyosis endometrial tissue compared with the
respective expression levels in the control. E-cadherin and
cytokeratin expression levels were significantly decreased in the
endometrial cells of the adenomyosis group, whereas those of
N-cadherin and vimentin were significantly increased compared with
in the control. Western blotting was also performed to analyze the
expression of E-cadherin and vimentin (Fig. 4C and D); compared with the protein
expression levels in the control group, the expression levels of
E-cadherin protein were significantly decreased and that of
vimentin were increased in the adenomyosis group. The results
demonstrated that the expression levels of mesenchymal markers were
upregulated, whereas the expression levels of epithelial markers
were decreased in the adenomyosis group, which indicated the EMT
process may serve a role in the progress of adenomyosis.
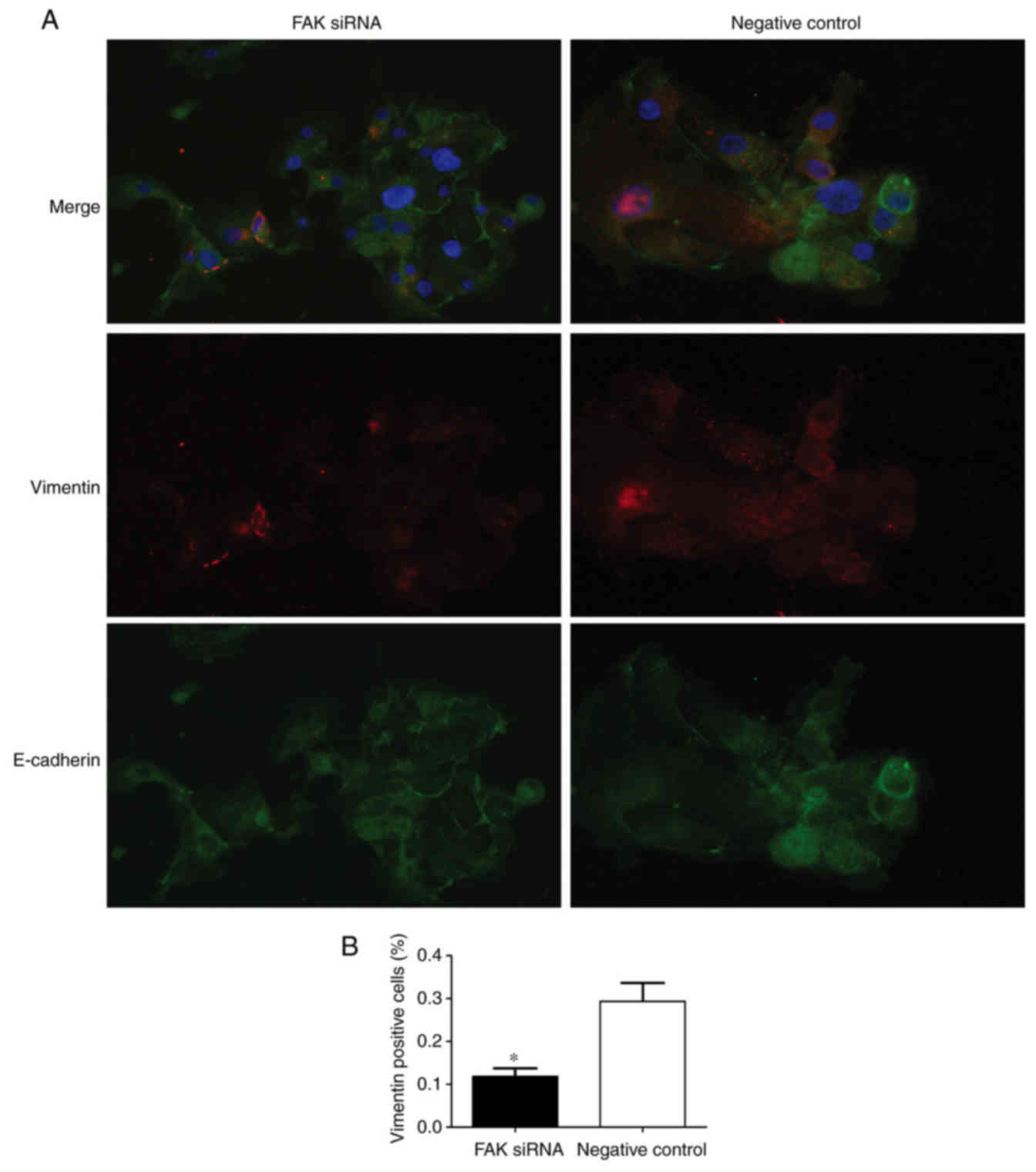
FAK silencing inhibits EMT
To further confirm the function of FAK in EMT within
adenomyosis, FAK-targeting siRNAs and a negative control siRNA were
transfected into the endometrial cells. Immunofluorescence and
RT-qPCR analyses were conducted to detect the expression of
EMT-associated markers 24 h post-transfection. Vimentin is a
mesenchymal marker and E-cadherin is an epithelial marker.
Therefore, vimentin and E-cadherin expressions were used to examine
the state of EMT process in two groups. The results demonstrated
that vimentin expression was lower and E-cadherin expression was
higher in FAK siRNA-treated group compared with the respective
expression levels in the negative control group (Fig. 5A), which indicated that FAK may
regulate the EMT process by affecting the expression of EMT
markers. The number of vimentin-positive cells was significantly
lower in the FAK siRNA-transfected group compared with the negative
control-transfected group (Fig.
5B). RT-qPCR was performed to analyze the mRNA expression
levels of the transcription factors Slug, Snail and Twist1, the EMT
epithelial markers E-cadherin and cytokeratin, and the mesenchymal
markers N-cadherin and vimentin, in transfected endometrial cells;
successful knockdown of FAK mRNA expression was observed in FAK
siRNA-transfected cells (Fig. 6A).
The results revealed the expression levels of Slug, Snail and
Twist1 were significantly reduced in the FAK-siRNA group compared
with expression levels in the control group (Fig. 6A). Conversely, FAK silencing was
associated with a significant increase in the expression levels of
the epithelial phenotypic markers, E-cadherin and cytokeratin, and
a significant decrease in the mesenchymal phenotypic markers,
N-cadherin and vimentin, compared with expressions in the control
cells (Fig. 6B). Western blotting
demonstrated a significant increase and decrease in the protein
expression levels of E-cadherin and vimentin, respectively, in
response to FAK silencing, compared with in the control (Fig. 6C and D). These data suggested that
FAK may be involved in the EMT process of adenomyosis.
FAK interference inhibits cell
migration
As previously reported, FAK expression was strongly
correlated with the metastasis in numerous types of cancer
(15). It has been widely
demonstrated that EMT participates in the development of
adenomyosis (12); FAK protein
overexpression was observed in adenomyosis endometrial cells
(22). Thus, the present study
hypothesized that FAK controls the migration of endometrial cells
in adenomyosis. To determine the potential mechanism underlying the
promotion of migration by FAK overexpression, endometrial cells
were transfected with siRNA targeting FAK or a negative control
siRNA. FAK siRNA effectively suppressed the mRNA expression of FAK
as demonstrated by RT-qPCR (Fig.
6A). To assess the biological significance of FAK on cell
mobility, Transwell migration assays were conducted (Fig. 7A). The results revealed that,
compared with the control, the siRNA-mediated knockdown of FAK
significantly decreased the migratory ability of endometrial cells
in adenomyosis in a time-dependent manner (Fig. 7B).
FAK participates in EMT via the
PI3K/AKT signaling pathway
FAK was reported to be associated with EMT,
primarily via the PI3K/AKT signaling pathway (21,25).
Results from present study demonstrated significantly increased
PI3K and AKT expression levels in adenomyosis endometrial cells at
the mRNA and protein expression levels compared with the control
(Fig. 8A-C). The results suggested
that the FAK/PI3K/AKT signaling pathway may mediate EMT following
the upregulation of FAK. To investigate the role of PI3K/AKT in
FAK-induced EMT, FAK siRNA was transfected into endometrial cells.
Similarly, compared with the expression levels in control cells,
FAK siRNA-transfected cells exhibited significantly lower mRNA and
protein expression levels of PI3K and AKT (Fig. 8D-F). Additionally, FAK siRNA
suppressed the expression of various EMT markers in association
with downregulation of the transcription factors Snail, Slug and
Twist1 (Fig. 6). Consistent with
the downregulation of FAK, the expression levels of the mesenchymal
markers N-cadherin and vimentin were decreased, whereas the
expression levels of the epithelial markers E-cadherin and
cytokeratin increased (Fig. 6).
These results further indicated that the FAK/PI3K/AKT signaling
pathway may participate in the EMT of endometrial cells in
adenomyosis.
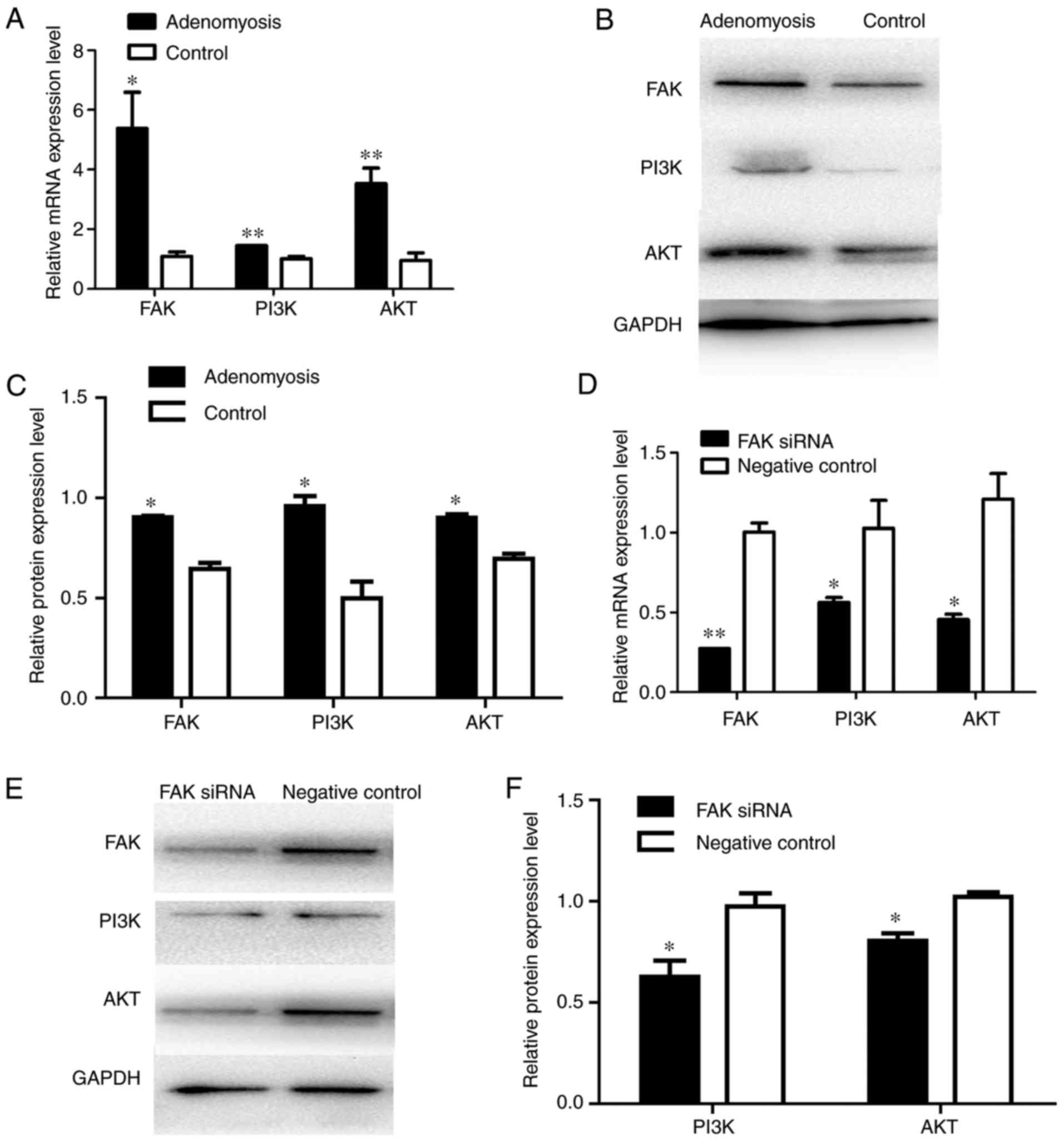 | Figure 8.Alterations in the expression of PI3K
and AKT in adenomyosis and FAK siRNA-treated cells. (A) RT-qPCR
analysis of the mRNA expression levels of FAK, PI3K and AKT in
cells of the adenomyosis and control groups. (B) Protein expression
levels of FAK, PI3K and AKT in endometrial cells of the adenomyosis
and control group were detected by western blotting. (C) Relative
protein expression levels of FAK, PI3K and AKT in endometrial cells
of the adenomyosis and control groups. (D) RT-qPCR analysis of the
mRNA expression levels of FAK, PI3K and AKT in the FAK siRNA and
negative control groups. (E and F) Western blot analysis of protein
expression levels of FAK, PI3K and AKT in the FAK siRNA and
negative control groups. The mRNA and protein expression levels
were normalized to GAPDH; *P<0.05 and **P<0.01 vs. control
group. AKT, protein kinase B; FAK, focal adhesion kinase; PI3K,
phosphoinositide 3-kinase; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; siRNA, small
interfering RNA. |
Discussion
Previous studies suggested that FAK is overexpressed
in a variety of human diseases, including cancer and fibrosis; this
overexpression was reported to promote cellular migration and
invasion (26,27). Adenomyosis exhibits numerous
characteristics similar to malignant diseases (2), yet the role of FAK in adenomyosis
remains unknown. A recent study reported significantly higher
expression levels of FAK in the eutopic endometrium of adenomyosis
(22); however, the effects of FAK
on the endometrial cells of adenomyosis require further
investigation. In the present study, the effects of FAK on the EMT
of endometrial cells in adenomyosis and the underlying pathway
associated with EMT were investigated.
FAK is a cytoplasmic protein tyrosine kinase that is
a vital component of integrin complexes and its numerous downstream
signaling pathways (28). FAK
regulates the cytoskeleton and cell motility, and participates in
numerous cell processes (16).
Recent studies have revealed important functions of FAK in EMT,
whereby epithelial cells transition to a mesenchymal state
(20,21,27,29).
During EMT, epithelial cells undergo intricate alterations in
cell-cell contacts, cell-matrix interactions and cell signaling
(7). There are three main
characteristic alterations associated with EMT: i) Morphological
changes, including the downregulation of cell adhesive structures,
changes in cell polarity and remolding of the cytoskeleton; ii)
molecular marker expression changes, including upregulated
mesenchymal marker and decreased epithelial marker expression, as
well as upregulation of several transcription factors, including
zing-finger E-box binding homeobox 1, Snail, Slug and Twist1; and
iii) changes in biological behaviors, including increased abilities
to migrate, invade and resist apoptosis (9). In addition, FAK overexpression
appears to be involved in numerous signaling pathways, including
the PI3K/AKT, transforming growth factor β (TGF-β), hepatocyte
growth factor and mitogen-activated protein kinase
(MAPK)/extracellular signal-regulated kinase ERK pathways, which
participate in the EMT process (29–32).
For example, FAK has been associated with the process by which
TGF-β promotes the migration of human oral squamous cell carcinoma
cells (29). Previous studies have
suggested that FAK may be a critical mediator in TGF-β-induced EMT
of hepatocytes and renal tubular epithelial cells (30,31).
FAK has also been associated with the regulation of transcription
factor expression (32), which
requires the activity of MAPK and PI3K (33,34).
In gynecological tissues, FAK may contribute to alterations in
physiological functions, including decidualization and embryo
implantation (35); however, in
endometrial hyperplasia and carcinoma, FAK overexpression has been
reported to potentially correlate with disease grade (36). Elevated FAK expression has also
been observed in ovarian endometriotic tissues (37). In the present study, FAK expression
was upregulated in adenomyosis endometrial tissues, which was
associated with alterations in the expression of EMT-associated
markers, consistent with a report by Mu et al (22). Following treatment with FAK siRNA,
endometrial cells exhibited fewer mesenchymal characteristics in
the present study. Therefore, it was proposed that FAK may be a
mediator of EMT in adenomyosis.
The results of the present study demonstrated that
FAK may be a critical mediator of EMT in adenomyosis, which is
supported by at least three lines of evidence. FAK may regulate the
expression of epithelial-associated markers (E-cadherin and
cytokeratin) and mesenchymal-associated markers (N-cadherin and
vimentin). EMT is characterized by the downregulation of E-cadherin
with the concomitant acquisition of vimentin (8). The loss of E-cadherin may result in
the weakening of cell-cell adhesion and reorganization of the
cytoskeleton (38). Elevated
N-cadherin expression may reduce the stability of cell-adhesion
complexes and enhance the motility of cells (38–40).
FAK has been reported to affect E-cadherin expression by regulating
that of transcription factors, including Snail, Slug and Twist1
(41). These transcription factors
suppress the expression of epithelial markers, such as E-cadherin,
by binding to the common box sequences of the promoter region of
the E-cadherin gene (42),
maintaining the mesenchymal phenotype (40). FAK may promote cellular migration,
which has been associated with the development of the numerous
types of disease (16). FAK can
regulate the expression and secretion of matrix metalloproteinases
(MMPs), which degrades the protein components of the extracellular
matrix to facilitate migration (31). During EMT, epithelial cells exhibit
reduced adhesion to adjacent cells and the basement membrane,
resulting in the promotion of migration (9). In addition, FAK may promote the
survival of endometrial cells and anti-apoptotic potential
(28). A previous study reported
the survival function of FAK and demonstrated that interactions
between FAK and p53 can affect the transcriptional and apoptotic
activity of p53 (43).
Collectively, these findings suggested that FAK may contribute to
EMT by promoting the expression of mesenchymal-associated markers,
cell migration and cell survival.
Adenomyosis results from the disruption of normal
uterine boundaries following the growth of endometrial glands and
stroma within the myometrium (44). The present study reported that,
compared with control cells, adenomyosis endometrial cells
exhibited greater migration potential. This observation supports
the hypothesis that adenomyosis proceeds from the migration of
endometrial cells into the myometrium secondary to the impairment
of the uterine endometrial-myometrial interface (45). The present study also proposed that
EMT occurs during the development of adenomyosis via altering the
microenvironment of the uterine endometrium and promoting the
migration of endometrial cells.
Notable evidence has suggested that FAK serves an
important role in regulating EMT (30). A recent study demonstrated that the
PI3K/AKT signaling pathway participates in FAK-promoted cell
migration (25,46). FAK/PI3K/AKT signaling has been
associated with EMT in hepatocellular carcinoma (25) and renal cancer cells (46). The PI3K/AKT signaling cascade may
mediate EMT by promoting the expression of Snail (47,48)
and MMPs (49). PI3K and AKT can
also increase the expression of nuclear factor-κB, which is
translocated to the nucleus and facilitates the expression of
EMT-associated markers, including vimentin and Snail (21). Downstream of PI3K/AKT signaling
pathway activation, the suppression of E-cadherin was reported to
alter cell-cell adhesion and the suppression of apoptosis (50). In the present study, it was
observed that FAK expression was increased in accordance with the
upregulation of PI3K and AKT in adenomyosis endometrial cells.
Additionally, following treatment with FAK siRNA, the expression
levels of PI3K, AKT, transcription factors and EMT-associated
molecules in endometrial cells were significantly reduced.
Therefore, it was proposed that the FAK/PI3K/AKT signaling pathway
may serve an important role in EMT progression; however, as a
limitation of the present study, alterations in protein
phosphorylation were not analyzed. Further investigation is
required for the activation of proteins, including their
phosphorylation.
In summary, the present study revealed that FAK may
mediate the EMT of endometrial cells in adenomyosis by affecting
the expression of EMT-associated molecules and promoting the
migration of endometrial cells. This process may be achieved
downstream of FAK/PI3K/AKT signaling in endometrial cells.
Consequently, our study provided insight into the possible
mechanisms underlying FAK regulation of EMT in adenomyosis and
suggested that FAK may be a potential target for preventing the
progression of adenomyosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81571412), the
Beijing Municipal Administration of Hospitals Clinical Medicine
Development of Special Funding Support (grant no. ZYLX201406) and
the Basic-Clinic Cooperation Fund of Capital Medical University
(grant no. 16JL44).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DZ performed the experiments and wrote the
manuscript. HD and SW made substantial contributions to conception
and experimental design. QX and LG were involved in performing the
cell experiments, drafting the manuscript and revising it
critically for important intellectual content. JL and QD acquired,
analyzed and interpreted the data. The manuscript has been approved
by all the authors for publication.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing Obstetrics and Gynecology Hospital, Capital
Medical University (approval no. 2016KY012). Informed consent was
obtained from all participants prior to surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Senturk LM and Imamoglu M: Adenomyosis:
What is new? Womens Health (Lond). 11:717–724. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garcia L and Isaacson K: Adenomyosis:
Review of the literature. J Minim Invasive Gyneco. l18:428–437.
2011. View Article : Google Scholar
|
|
3
|
Zhou S, Yi T, Liu R, Bian C, Qi X, He X,
Wang K, Li J, Zhao X, Huang C and Wei Y: Proteomics identification
of annexin A2 as a key mediator in the metastasis and
proangiogenesis of endometrial cells in human adenomyosis. Mol Cell
Proteomics. 11(M112): 0179882012.PubMed/NCBI
|
|
4
|
Yang JH, Wu MY, Chen CD, Chen MJ, Yang YS
and Ho HN: Altered apoptosis and proliferation in endometrial
stromal cells of women with adenomyosis. Hum Reprod. 22:945–952.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parrott E, Butterworth M, Green A, White
IN and Greaves P: Adenomyosis-a result of disordered stromal
differentiation. Am J Pathol. 159:623–630. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Franco-Chuaire ML, Carolina Magda SC and
Chuaire-Noack L: Epithelial-mesenchymal transition (EMT):
Principles and clinical impact in cancer therapy. Invest Clin.
54:186–205. 2013.PubMed/NCBI
|
|
7
|
Hay ED and Zuk A: Transformations between
epithelium and mesenchyme: Normal, pathological, and experimentally
induced. Am J Kidney Dis. 26:678–690. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen YJ, Li HY, Huang CH, Twu NF, Yen MS,
Wang PH, Chou TY, Liu YN, Chao KC and Yang MH: Oestrogen-induced
epithelial-mesenchymal transition of endometrial epithelial cells
contributes to the development of adenomyosis. J Pathol.
222:261–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oh SJ, Shin JH, Kim TH, Lee HS, Yoo JY,
Ahn JY, Broaddus RR, Taketo MM, Lydon JP, Leach RE, et al:
β-catenin activation contributes to the pathogenesis of adenomyosis
through epithelial-mesenchymal transition. J Pathol. 231:210–222.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xue J, Zhang H, Liu W, Liu M, Shi M, Wen Z
and Li C: Metformin inhibits growth of eutopic stromal cells from
adenomyotic endometrium via AMPK activation and subsequent
inhibition of AKT phosphorylation: A possible role in the treatment
of adenomyosis. Reproduction. 146:397–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J and Guan JL: Signal transduction by
focal adhesion kinase in cancer. Cancer Metastasis Rev. 28:35–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schaller MD: Cellular functions of FAK
kinases: Insight into molecular mechanisms and novel functions. J
Cell Sci. 123:1007–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang LL, Liu J, Lei S, Zhang J, Zhou W
and Yu HG: PTEN inhibits the invasion and metastasis of gastric
cancer via downregulation of FAK expression. Cell Signal.
26:1011–1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Golubovskaya VM, Ylagan L, Miller A,
Hughes M, Wilson J, Wang D, Brese E, Bshara W, Edge S, Morrison C
and Cance WG: High focal adhesion kinase expression in breast
carcinoma is associated with lymphovascular invasion and
triple-negative phenotype. BMC Cancer. 14:7692014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mael-Ainin M, Abed A, Conway SJ, Dussaule
JC and Chatziantoniou C: Inhibition of periostin expression
protects against the development of renal inflammation and
fibrosis. J Am Soc Nephrol. 25:1724–1736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Avizienyte E and Frame MC: Src and FAK
signalling controls adhesion fate and the epithelial-to-mesenchymal
transition. Curr Opin Cell Biol. 17:542–547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bouchard V, Demers MJ, Thibodeau S,
Laquerre V, Fujita N, Tsuruo T, Beaulieu JF, Gauthier R, Vézina A,
Villeneuve L, et al: Fak/src signaling in human intestinal
epithelial cell survival and anoikis: Differentiation
state-specific uncoupling with the PI3K/AKT1 and MEK/ERK pathways.
J Cell Physiol. 212:717–728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mu L, Chen W, Ma Y and Zheng W: Expression
of focal adhesion kinase in the eutopic endometrium of women with
adenomyosis varies with dysmenorrhea and pelvic pain. Exp Ther Med.
10:1903–1907. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L, Zhou S, Zou L and Zhao X: The
expression and functionality of stromal caveolin 1 in human
adenomyosis. Hum Reprod. 28:1324–1338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR,
Cai JB, Zhang C, Huang XY, Tian MX, Hu ZQ, et al: Galectin-1
induces hepatocellular carcinoma EMT and sorafenib resistance by
activating FAK/PI3K/AKT signaling. Cell Death Dis. 7:e22012016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roy-Luzarraga M and Hodivala-Dilke K:
Molecular pathways: Endothelial cell FAK-A target for cancer
treatment. Clin Cancer Res. 22:3718–3724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parsons JT: Focal adhesion kinase: The
first ten years. J Cell Sci. 116:1409–1416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mehta D: Focal adhesion kinase regulation
of endothelial barrier function, apoptosis, and neovascularization.
Microvasc Res. 83:1–2. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saito D, Kyakumoto S, Chosa N, Ibi M,
Takahashi N, Okubo N, Sawada S, Ishisaki A and Kamo M: Transforming
growth factor-β1 induces epithelial-mesenchymal transition and
integrin α3β1-mediated cell migration of HSC-4 human squamous cell
carcinoma cells through Slug. J Biochem. 153:303–315. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cicchini C, Laudadio I, Citarella F,
Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L and
Tripodi M: TGFbeta-induced EMT requires focal adhesion kinase (FAK)
signaling. Exp Cell Res. 314:143–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deng B, Yang X, Liu J, He F, Zhu Z and
Zhang C: Focal adhesion kinase mediates TGF-β1-induced renal
tubular epithelial-to-mesenchymal transition in vitro. Mol Cell
Biochem. 340:21–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li XY, Zhou X, Rowe RG, Hu Y, Schlaepfer
DD, Ilić D, Dressler G, Park A, Guan JL and Weiss SJ: Snail1
controls epithelial-mesenchymal lineage commitment in focal
adhesion kinase-null embryonic cells. J Cell Biol. 195:729–738.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Slack-Davis JK, Eblen ST, Zecevic M,
Boerner SA, Tarcsafalvi A, Diaz HB, Marshall MS, Weber MJ, Parsons
JT and Catling AD: PAK1 phosphorylation of MEK1 regulates
fibronectin-stimulated MAPK activation. J Cell Biol. 162:281–291.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Julien S, Puig I, Caretti E, Bonaventure
J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A
and Larue L: Activation of NF-kappaB by Akt upregulates Snail
expression and induces epithelium mesenchyme transition. Oncogene.
26:7445–7456. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hanashi H, Shiokawa S, Akimoto Y, Sakai K,
Sakai K, Suzuki N, Kabir-Salmani M, Nagamatsu S, Iwashita M and
Nakamura Y: Physiologic role of decidual beta1 integrin and focal
adhesion kinase in embryonic implantation. Endocr J. 50:189–198.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livasy CA, Moore D, Cance WG and Lininger
RA: Focal adhesion kinase overexpression in endometrial neoplasia.
Appl Immunohistochem Mol Morphol. 12:342–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mu L, Zheng W, Wang L, Chen X and Yang J:
Focal adhesion kinase expression in ovarian endometriosis. Int J
Gynaecol Obstet. 101:161–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gumbiner BM: Regulation of
cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol.
6:622–634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shapiro L and Weis WI: Structure and
biochemistry of cadherins and catenins. Cold Spring Harb Perspect
Biol. 1:a0030532009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mirantes C, Espinosa I, Ferrer I, Dolcet
X, Prat J and Matias-Guiu X: Epithelial-to-mesenchymal transition
and stem cells in endometrial cancer. Hum Pathol. 44:1973–1981.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thompson EW and Williams ED: EMT and MET
in carcinoma-clinical observations, regulatory pathways and new
models. Clin Exp Metastasis. 25:591–592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Golubovskaya VM, Finch R and Cance WG:
Direct Interaction of the N-terminal domain of focal adhesion
kinase with the N-terminal transactivation domain of p53. J Biol
Chem. 280:25008–25021. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ferenczy A: Pathophysiology of
adenomyosis. Hum Reprod Update. 4:312–322. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Curtis KM, Hillis SD, Marchbanks PA and
Peterson HB: Disruption of the endometrial-myometrial border during
pregnancy as a risk factor for adenomyosis. Am J Obstet Gynecol.
187:543–544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yuan H, Meng X, Guo W, Cai P, Li W, Li Q,
Wang W, Sun Y, Xu Q and Gu Y: Transmembrane-bound IL-15-promoted
epithelial-mesenchymal transition in renal cancer cells requires
the Src-dependent Akt/GSK-3β/β-catenin pathway. Neoplasia.
17:410–420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 30′kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grille SJ, Bellacosa A, Upson J,
Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and
Larue L: The protein kinase Akt induces epithelial mesenchymal
transition and promotes enhanced motility and invasiveness of
squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
2003.PubMed/NCBI
|
|
49
|
Lee KR, Lee JS, Song JE, Ha SJ and Hong
EK: Inonotus obliquus-derived polysaccharide inhibits the migration
and invasion of human non-small cell lung carcinoma cells via
suppression of MMP-2 and MMP-9. Int J Oncol. 45:2533–2540. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lu M, Marsters S, Ye X, Luis E, Gonzalez L
and Ashkenazi A: E-cadherin couples death receptors to the
cytoskeleton to regulate apoptosis. Mol Cell. 54:987–998. 2014.
View Article : Google Scholar : PubMed/NCBI
|