Introduction
Mesencephalic astrocyte-derived neurotrophic factor
(MANF) is a recently discovered neurotrophic factor, which was
initially isolated from astrocyte culture medium and was revealed
to selectively exert protective effects on dopaminergic neurons
(1,2). Subsequent studies indicated that MANF
is induced and secreted in response to experimental endoplasmic
reticulum (ER) stress in vitro and in vivo (3–6).
MANF has a protective role under physiological and
pathophysiological conditions, including in neurodegenerative
diseases, ischemic heart disease, diabetes mellitus,
chondrodysplasia and polycystic ovary syndrome (4,6–15).
Further studies have suggested that MANF protects against various
forms of ER stress-induced cell damage via its C-terminal domain.
Our previous studies also confirmed that MANF protects neurons in
rats with middle cerebral artery occlusion-induced focal cerebral
ischemia via alleviating ER stress (5,7,16,17).
A recent study identified MANF as an immune modulator that serves a
critical role in mediating tissue repair in the retina (18). Our previous study also indicated
that MANF is involved in regulation of inflammation. Notably, it is
upregulated in patients with rheumatoid arthritis and systemic
lupus erythematosus, and in rabbits with antigen-induced arthritis
(19). Further studies have
demonstrated that MANF is a novel negative regulator of
inflammation, functioning as an inhibitor of the nuclear factor
(NF)-κB signaling pathway by blocking the binding of p65 to the
promoter of its target genes (20,21).
In addition, it has been reported that MANF inhibits oxygen-glucose
deprivation-induced cell damage and inflammatory cytokine secretion
via suppressing ER stress in rat astrocytes in primary culture
(22). Furthermore, MANF may
decrease lipopolysaccharide (LPS)-induced proinflammatory
cytokines, interleukin (IL)-1β, tumor necrosis factor (TNF)-α and
interferon-γ, through regulating the NF-κB signaling pathway and
phosphorylation of p38-mitogen-activated protein kinases in neural
stem cells (23). However, how
inflammation regulates MANF expression in inflammatory diseases
remains unknown.
Activator protein-1 (AP-1) is a transcription factor
complex composed of heterodimers or homodimers of members of the
Fos, Jun, activating transcription factor and MAF protein families
(24). The c-Fos/c-Jun heterodimer
is the prototypic form of the AP-1 complex, which binds to its
target DNA motifs to transcriptionally activate target genes
(24,25). AP-1 and NF-κB are the principal
components of two critical inflammatory signaling pathways involved
in the response to inflammatory stimuli (26,27).
Proinflammatory factors, such as TNF-α and IL-1, can activate the
AP-1 signaling pathway (28,29).
Using the Database of Transcriptional Start Sites (DBTSS) and
Transcription Element Search Software (TESS), three putative
binding sites of AP-1 were detected in the 5′-flanking region
(−1,239 to +176 bp) of the human MANF gene in the present
study.
Since the effects of AP-1 on MANF expression in
inflammatory diseases remain to be elucidated, it was hypothesized
that MANF may be a potential target gene of transcriptional
activation by AP-1. The present study investigated the association
between AP-1 and MANF expression in human liver tissues and a mouse
liver injury model. In addition, the binding of AP-1 to the MANF
promoter region was analyzed using chromatin immunoprecipitation
(ChIP) assays.
Materials and methods
Collection of human liver tissues
Human liver tissues were obtained from 10 patients
with hepatocellular carcinoma (HCC) and HBV infection and four
control patients with hepatic hemangioma (HHG); these patients were
admitted to the First Affiliated Hospital of Anhui Medical
University (Hefei, China) and the Chinese People's Liberation Army
123 Hospital (Bengbu, China) between January and December 2014.
General patient information is presented in Table I. All patients received partial
hepatectomy. Final diagnosis of the hepatic nodules was confirmed
by histological examination of biopsy specimens. All tissue samples
were fixed in 4% neutral buffered formaldehyde at 4°C for 24 h and
were embedded in paraffin. The use of human liver tissues was in
accordance with the ethical standards of the Declaration of
Helsinki. Written informed consent was obtained from all patients
and the present study was approved by the Human Research Ethics
Committee of Anhui Medical University (license number:
20131359).
 | Table I.Clinical characteristics of the
selected subjects. |
Table I.
Clinical characteristics of the
selected subjects.
| Characteristic | HBV (n=10) | HHG (n=4) |
|---|
| Sex, n (%) |
|
|
|
Male | 9 (90) | 3 (75) |
|
Female | 1 (10) | 1 (25) |
| Age, years (means ±
standard deviation) | 51.2±3.9 | 53.0±6.7 |
| Etiology, n
(%) |
|
|
|
HBV | 10 (100%) | – |
|
HCV | 0 | – |
|
Others | 0 | – |
| Liver injury, U/l
(means ± standard deviation) |
|
|
| Serum
ALT | 74.2±69.2 | 13.8±4.9 |
| Serum
AST | 52.8±42.6 | 18.5±1.7 |
Preparation of a murine model of liver
fibrosis
A total of 12 C57BL/6J mice (age, 6 weeks; weight,
~20 g) were obtained from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China) and were individually housed
in ventilated cages at the Anhui Medical University animal
facility. The mice were kept in a controlled temperature (21±1°C)
and humidity (50±5%) environment, under a 12 h light/dark cycle,
with ad libitum access to food and water. To induce liver
fibrosis, six male mice received an intraperitoneal injection of 2
µl/g carbon tetrachloride (CCl4) in sterile olive oil
three times per week for 4 weeks. The six control mice were
injected with same volume of sterile olive oil three times per week
for 4 weeks. The body weight of the mice was monitored each week.
The mice were sacrificed after 4 weeks, and livers were removed and
were immediately fixed in 4% paraformaldehyde at 4°C for 24 h. All
mouse studies were conducted according to protocols approved by the
Animal Ethics Committee of Anhui Medical University (license
number: 20160329).
Histological analysis of liver
fibrosis
Mouse liver sections were stained with hematoxylin
at 25°C for 8 min and eosin at 25°C for 3 sec (H&E), and Sirius
red at 25°C for 1 h (SR), in order to evaluate the degree of
inflammation and collagen deposition, respectively. The degree of
mouse hepatic fibrosis was determined by Masson trichrome staining
according to the manufacturer's protocol (cat. no. MST-8003; Fuzhou
Maixin Biotechnology Development Co., Ltd., Fuzhou, Fujian,
China).
Immunohistochemistry (IHC)
Paraffin-embedded mouse and human tissues sections
(3–5 µm) were deparaffinized and rehydrated prior to IHC for the
detection of MANF, c-Fos and c-Jun proteins, as previously
described (30). Briefly, antigen
retrieval was performed by pressure cooking slides for 5 min in
0.01 M citrate buffer. Slides were incubated for 30 min at 37°C in
20% (vol/vol) hydrogen peroxide to block endogenous peroxidase
activity and then washed in phosphate-buffered saline (PBS).
Following blocking with 10% normal goat serum, the sections were
incubated with primary antibodies, including rabbit anti-MANF
(dilution 1:1,000; cat. no. ab126321; Abcam, Cambridge, MA, USA),
rabbit anti-c-Fos (dilution 1:100; cat. no. 2250; Cell Signaling
Technology, Inc., Danvers, MA, USA) and rabbit anti-c-Jun (dilution
1:250; cat. no. ab32137; Abcam), at 4°C overnight, followed by
horseradish peroxidase-conjugated secondary antibody at 37°C
(dilution 1:2,000; cat. no. TA140003, OriGene Technologies, Inc.,
Beijing, China) for 30 min. Normal IgG (dilution 1:100; cat. no.
3900; Cell Signaling Technology, Inc.) was used as a control. DAB
was used for visualization and dark-brown staining was considered
positive. Immunohistochemical staining was visualized and images
were captured using an Olympus BX35 microscope (Olympus
Corporation, Tokyo, Japan); staining was analyzed by a blinded
independent pathologist. For analysis, the total cells were counted
in >5 views from one slide and the positive intensity was
measured by ImageJ (version 1.8.0, National Institutes of Health,
Bethesda, MD, USA). Briefly, positive staining for each antibody
was determined based on the percentage of positive cells (0,
negative; 1+, weak; 2+, moderate; 3+, strong). Representative
images were collected using the Olympus BX35 microscope.
Cell culture and transfection
The HepG2 human liver cancer cell line and 293
cells, selected for ease of transfection, were obtained from CHI
Scientific, Ltd. (Jiangyin, Jiangsu, China). All cells were
maintained in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Tianjin Ankangyuan Technology Development Co.,
Ltd., Tianjin, China) at 37°C in a humidified atmosphere containing
5% CO2. The HepG2 human liver cancer cells
(0.8×105 cells per well in 24-well plates in serum-free
medium) were transiently transfected with the pGL3-hMANF plasmids
and pcDNA3.1/c-Fos or pcDNA3.1/c-Jun plasmids with 800 ng/ml at
37°C for 24 h using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol.
Prediction of transcription factor
binding sites
The putative binding sites for AP-1 in the
5′-flanking region (−1,239 to +176 bp) of the human MANF gene were
predicted using the DBTSS (http://dbtss.hgc.jp/index.html, Human Genome Center,
Institute of Medical Science, The University of Tokyo, Tokyo,
Japan) and TESS version 10.0 beta (http://www.cbil.upenn.edu/tess/, Computational Biology
and Informatics Laboratory, University of Pennsylvania,
Philadelphia, PA, USA).
Cloning and mutagenesis
The 1,415-bp human MANF promoter region spanning
−1,239 to +176 bp was amplified by polymerase chain reaction (PCR)
from human genomic DNA extracted from 293 cells using the primer
sets 5′-CCATTGTCCCAAGAGGTATTTT-3′ (forward) and
5′-CTATCCCGCACCTTCGCAG-3′ (reverse). PCR products were then ligated
into a pMD-18-T vector (cat. no. 6011; Takara Biomedical Technology
Co., Ltd, Beijing, China) using KpnI and HindIII,
followed by subcloning into the dual luciferase expression vector
pGL3-Basic (Promega Corporation, Madison, WI, USA), in order to
prepare the recombinant plasmid pGL3-hMANF (−1,293/+176) expressing
the entire promoter region of human MANF. The sequencing results
were compared with the human MANF cDNA sequence reported in the
GenBank database (https://www.ncbi.nlm.nih.gov/genbank/?). Three
truncates, pGL3-hMANF (−880/+83), pGL3-hMANF (−423/+83), and
pGL3-hMANF (−265/+83), expressing 963-, 506- and 348-bp fragments
of the human MANF promoter region, respectively, were generated
using pGL3-hMANF (−1,293/+176) as a template and the following
primer sets: pGL3-hMANF (−880/+83), forward
5′-CGGGGTACCCAGTGCTTCTCTGGTGATTCCC-3′, reverse
5′-GGGAAGCTTCATCCTCCTCATCCTCCTCATC-3′; pGL3-hMANF (−423/+83),
forward 5′-CGGGGTACCGTCTTGGCTGACCCCAGAACTC-3′, reverse
5′-GGGAAGCTTCATCCTCCTCATCCTCCTCATC-3′; and pGL3-hMANF (−265/+83),
forward 5′-CGGGGTACCCCACACCGCTTCCGTCG-3′ and reverse
5′-GGGAAGCTTCATCCTCCTCATCCTCCTCATC-3′. The deletion mutants were
subcloned into the luciferase expression vector pGL3-Basic to
generate pGL3-hMANF (−880/+83), pGL3-hMANF (−423/+83) and
pGL3-hMANF (−265/+83) plasmids.
Mutagenesis of putative AP-1 binding sites located
at position −421 and −326 of the MANF promoter was performed using
a Multipoints Mutagenesis kit (Takara Bio, Inc., Otsu, Japan),
according to the manufacturer's protocol. All
mutations/substitutions in the DNA were confirmed by Sanger
sequencing (Sangon Biotech Co., Ltd., Shanghai, China).
Luciferase assay
A luciferase assay was performed using a Dual
Luciferase Reporter Assay system (Promega Corporation), according
to the manufacturer's protocol. Briefly, HepG2 cells were seeded in
24-well plates at a density of 0.8×105 cells/well and
were cotransfected with the pGL3-hMANF plasmids and c-Fos or
c-Jun-expressing plasmids with 800 ng/ml at 37°C for 24 h. The
Renilla luciferase reporter plasmid pRL-TK (Promega
Corporation) was used as an internal control. Cells were lysed 24 h
post-transfection using lysis buffer (Promega Corporation) and
luciferase activity was immediately determined using a GloMax™20/20
luminometer (Promega Corporation). The results were normalized to
corresponding pRL-TK activity. Independent experiments were
performed at least three times.
ChIP assay
ChIP was performed using a Agarose ChIP assay kit
(cat. no. 26156; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The nuclei were isolated from HepG2 liver
cancer cells following treatment with
phorbol-12-myristate-13-acetate (10 nM) at 37°C for 2 h. Chromatin
complexes were immunoprecipitated with 4 µg anti-c-Jun (cat. no.
ab32137; Abcam, Cambridge, MA, USA) or anti-c-Fos (cat. no. ab7963;
Abcam), according to the manufacturer's protocol. A parallel
immunoprecipitation was carried out using 2 µl normal rabbit IgG in
500 µl diluted lysate (cat. no. 26156, Thermo Fisher Scientific,
Inc.) as a negative control and 10 µl anti-RNA polymerase II
antibody in 500 µl diluted lysate (cat. no. 26156, Thermo Fisher
Scientific, Inc.) as a positive control. PCR was performed to
amplify a 236-bp fragment containing the putative AP-1 binding
sites using appropriate primers (forward, TCACATTCTCACCAGCCACT;
reverse, CAGGTCGATCTGCTTGTCATAC), as previously described (21). PCR products were resolved on a 1.5%
agarose gel and were visualized by ethidium bromide (cat. no.
123945-8, Sangon Biotech Co., Ltd.) staining.
Statistical analysis
Data are presented as the means ± standard
deviation. All statistical analyses were performed using GraphPad
Prism (GraphPad Software, Inc., La Jolla, CA, USA). Two-way
analysis of variance with Tukey's post hoc correction was conducted
for multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
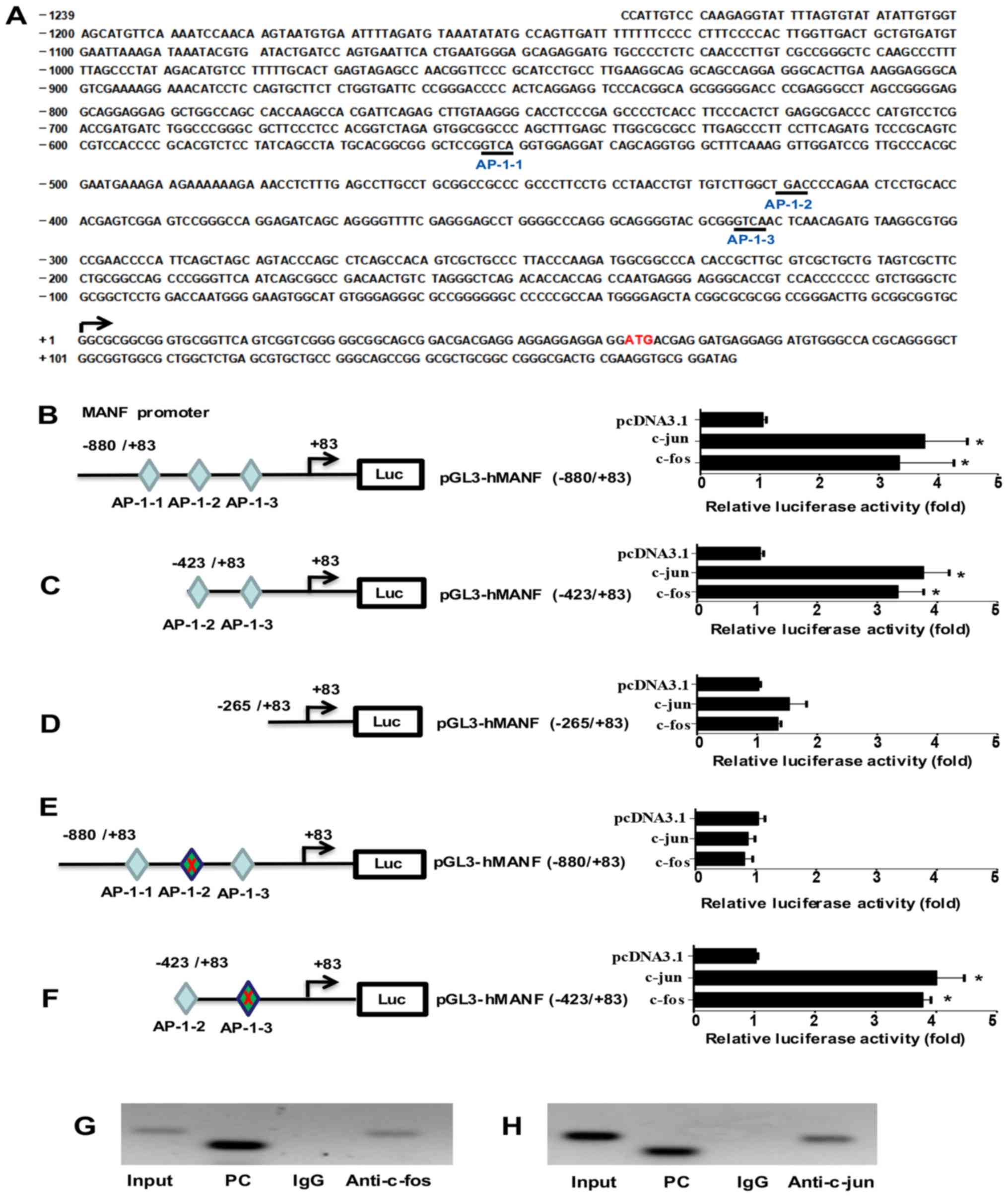
Screening of putative AP-1 binding
sites in the MANF promoter region
To screen the transcriptional regulators of MANF,
the promoter region of the human MANF gene within a sequence
located 1,239 bp upstream of the transcription start site was
analyzed using DBTSS and TESS. Three putative AP-1 binding sites
were identified as follows: At −554 bp (5′-GTCA-3′; AP-1-1), at
−421 bp (5′-TGAC-3′; AP-1-2) and at −326 bp (5′-TGCA-3′; AP-1-3)
(Fig. 1A).
Identification of the AP-1 binding
site involved in MANF transcriptional regulation
To confirm the aforementioned sites as AP-1 binding
sites, the MANF promoter region and the truncates were cloned into
the pGL3-Basic vector to form pGL3-hMANF (−880/+83) (Fig. 1B), pGL3-hMANF (−423/+83) (Fig. 1C) and pGL3-hMANF (−256/+83)
(Fig. 1D). Subsequently,
luciferase activity was detected after co-transfecting HepG2 liver
cells with the pGL3-hMANF plasmids and c-Fos or c-Jun-expressing
plasmids. The plasmids were successfully transfected, as verified
using immunofluorescence and western blotting (data not shown). As
shown in Fig. 1B-D, c-Jun and
c-Fos were able to significantly increase the luciferase activity
of cells transfected with plasmids containing the MANF promoter and
the −423/+83 truncate, but not the −256/+83 truncate, which does
not contain the putative AP-1 binding site. In addition, there was
no significant difference in luciferase activity between pGL3-hMANF
(−880/+83) and pGL3-hMANF (−423/+83) truncates was observed
(Fig. 1B and C), thus suggesting
that the AP-1-1 binding site may not be required for regulating
MANF transcription. These findings indicated that the two putative
binding sites AP-1-2 and AP-1-3 are required for the enhancement of
MANF promoter activity by AP-1.
To further confirm the essential binding sites for
AP-1 in the MANF promoter region, mutations were introduced into
the putative binding sites AP-1-2 (TGAC→CAGC) (Fig. 1E) and AP-1-3 (GTCA→CAGC) (Fig. 1F). HepG2 liver cells were
co-transfected with the mutated plasmids and c-Fos or c-Jun, and
the reporter activity was detected. The results revealed that the
AP-1-2 mutation abolished the enhancement of MANF promoter activity
by c-Fos or c-Jun (Fig. 1E).
However, the AP-1-3 mutation did not alter the effects of c-Fos and
c-Jun on MANF promoter activity (Fig.
1F), thus suggesting that AP-1-2 (5′-TGAC-3′) is the essential
binding site of AP-1 required for MANF transcriptional
regulation.
AP-1 directly binds to the promoter
region of the MANF gene
The present study performed ChIP analysis to
validate the direct binding of AP-1 to the MANF promoter region.
For this experiment, ChIP with anti-c-Fos and c-Jun antibodies was
performed. The pulled down DNA was subjected to PCR (Fig. 1G, lane 4 and Fig. 1H, lane 4) to amplify the MANF
promoter region containing the AP-1 binding site. As shown in
Fig. 1G and H, c-Fos and c-Jun
were revealed to bind to the MANF promoter, thus suggesting that
upregulation of MANF may be caused by the direct binding of AP-1 to
the promoter region of the MANF gene.
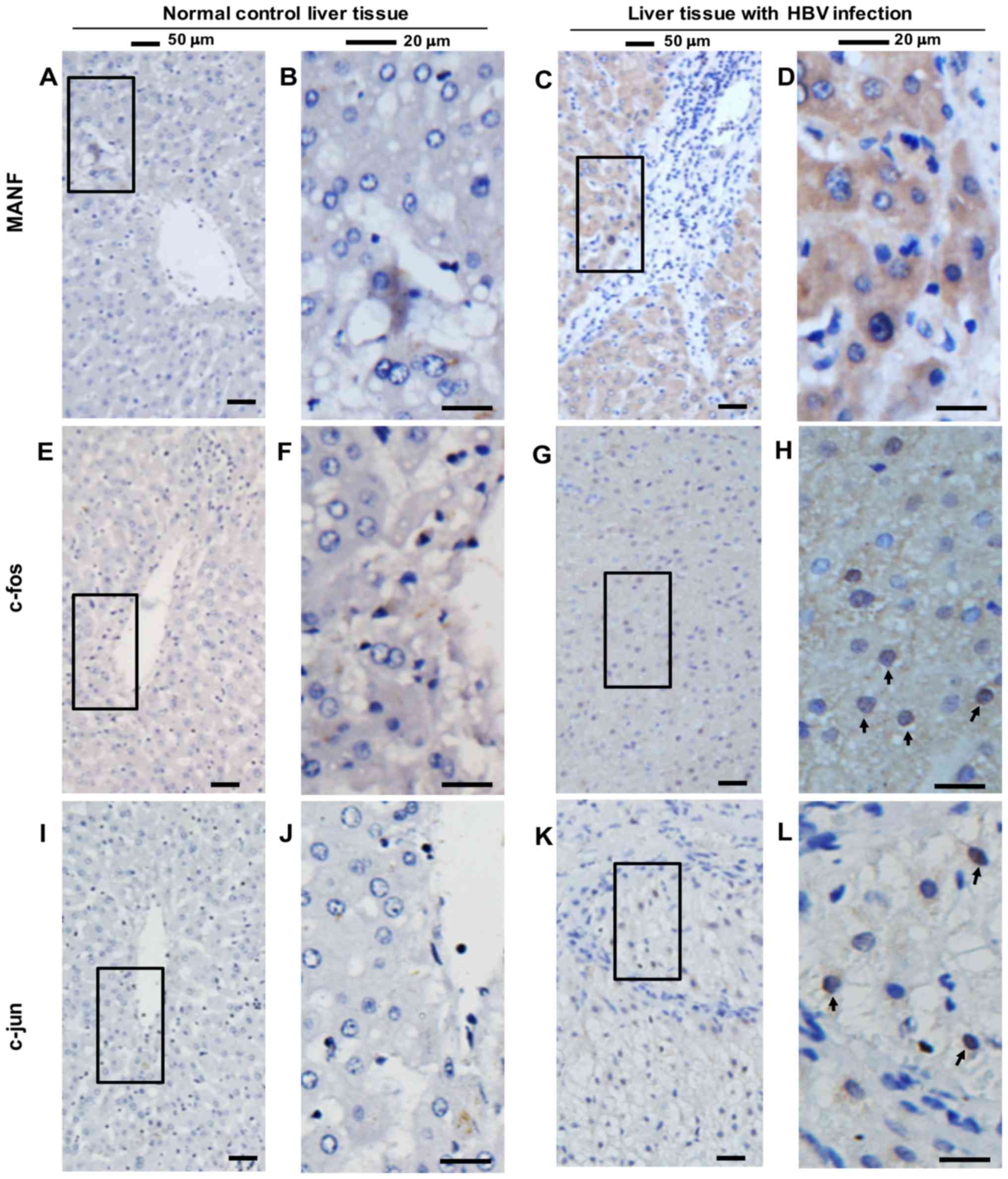
Expression of MANF and AP-1 in human
and murine liver samples
Previous studies have demonstrated that MANF is an
ER stress-inducible protein (3),
which is upregulated in inflammatory diseases (19,21).
To determine the expression of MANF in human liver samples, liver
tissues were collected from 10 patients with hepatocellular
carcinoma (HCC) and HBV infection, and from four patients with HHG
(Fig. 2). It was revealed that
MANF was strongly expressed in the paracancerous liver tissue of
five patients with HCC and HBV infection (Fig. 2C and D), compared with in normal
liver tissues from patients with HHG (Fig. 2A and B). Additionally, it was
revealed that c-Fos and c-Jun were upregulated in the nuclei of
hepatocytes of patients with HBV infection (Fig. 2E-L, indicated by arrows).
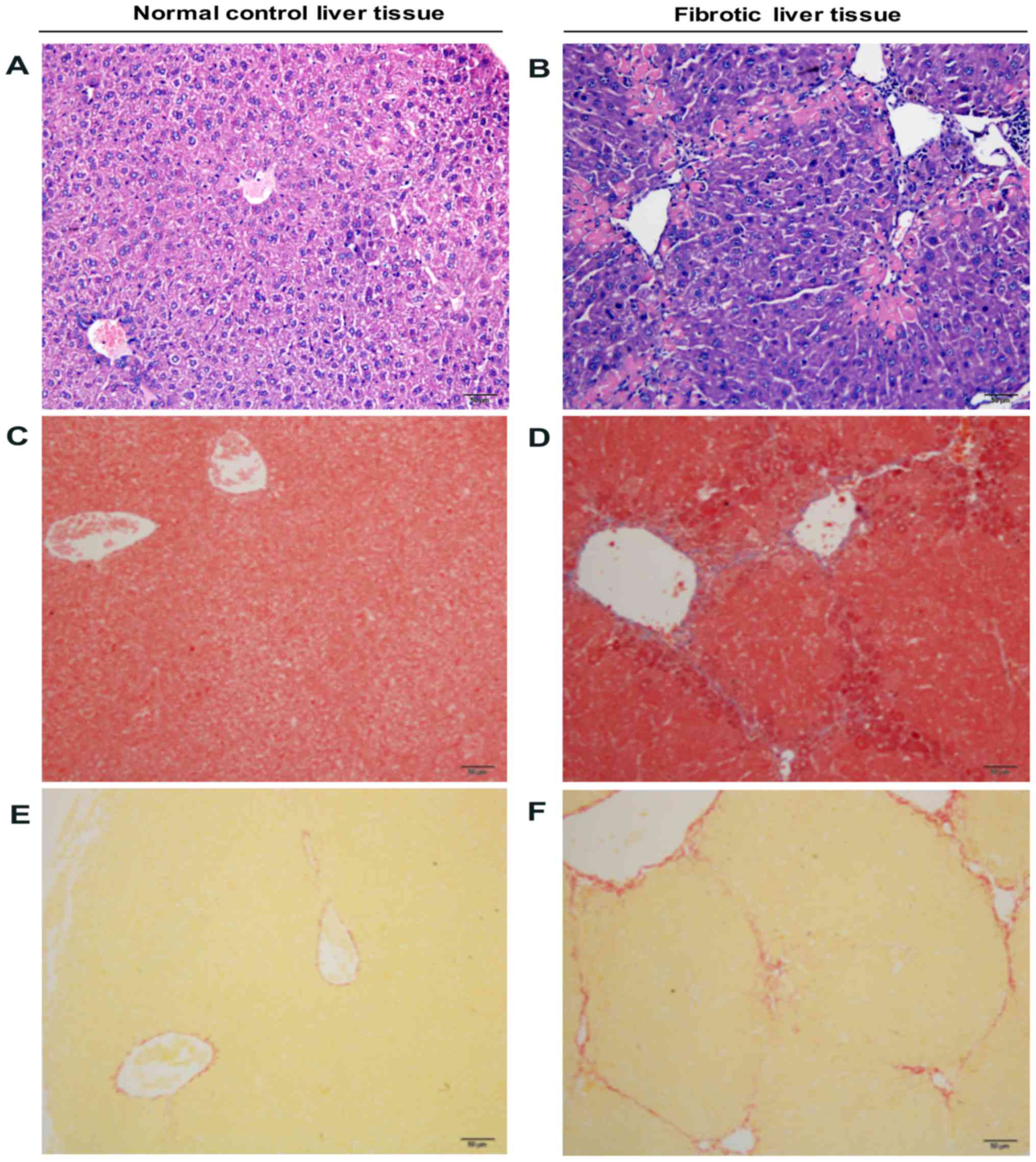
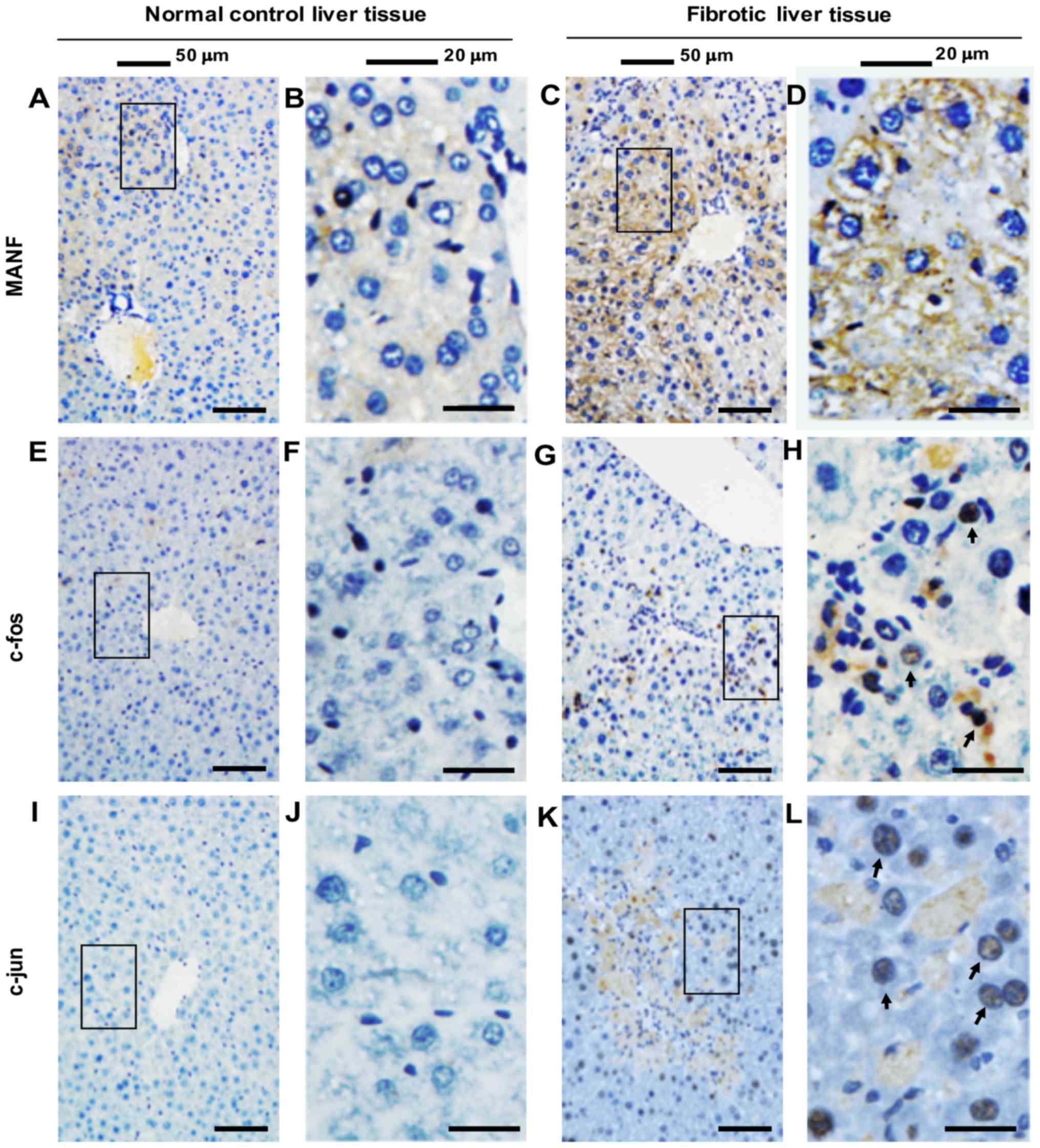
MANF expression was also detected in liver tissues
from mice treated with CCl4. In the mouse liver injury
model, CCl4-induced liver fibrosis was initially
evaluated via H&E, SR and Masson trichrome staining. As shown
in Fig. 3, CCl4
treatment for 4 weeks resulted in liver inflammation and fibrosis.
Furthermore, it was revealed that few MANF-positive cells were
detected in the liver samples obtained from the control group.
Conversely, in CCl4-treated mice, the majority of
hepatocytes were MANF-positive (Fig.
4A-D). Coincidentally, c-Fos and c-Jun were also strongly
expressed in the nuclei of hepatocytes in CCl4-treated
mice, compared with in the control mice (Fig. 4E-L). These results suggested that
MANF and AP-1 may be involved in liver inflammation, and that MANF
expression may be associated with AP-1 under such a condition.
Discussion
Our previous studies demonstrated that MANF is
upregulated under various conditions, including ischemia and
hypoxia, and in response to inflammatory stimuli, all of which are
associated with ER stress (5,7,19,21).
However, how MANF is upregulated in response to ER stress,
particularly in an inflammatory state, remains unclear. A previous
study demonstrated that the unfolded protein response is able to
induce mouse MANF expression via ER stress-responsive element
(ERSE) II (31). Recently, it was
reported that ERSE I serves a dominant role in mediating X-box
binding protein 1-induced MANF expression, whereas ERSE II exerts
little impact on MANF transcription (32). The present study demonstrated that
the AP-1 complex, comprised of c-Fos and c-Jun subunits, may
enhance MANF transcription through binding to the sequence TGAC
(−421/−418 bp) within the MANF promoter region. Collectively these
findings suggested that MANF transcription may be regulated by
numerous mechanisms. The current findings may aid to understand how
MANF is regulated under inflammatory conditions.
AP-1 is part of an important inflammatory signaling
pathway and is activated by LPS and TNF-α via the signaling
pathways of TNF receptor-associated factor 6 and transforming
growth factor-β-activated kinase 1 (33,34).
Activation of the AP-1 signaling pathway promotes the expression of
inflammatory factors, including IL-1β, IL-6 and inducible nitric
oxide synthase (35). The present
study revealed that MANF was a downstream target, which was
upregulated by c-Fos and c-Jun as part of the AP-1 complex, via a
direct binding interaction with the MANF gene promoter. In
addition, only one binding site was effective in enhancing MANF
transcription among the three putative AP-1 binding sites
identified. Unlike other AP-1 targets, MANF reportedly exerts
anti-inflammatory activity by negatively regulating the NF-κB
signaling pathway (21), thus
suggesting that the AP-1 signaling pathway may be involved in
dual-directional regulation of inflammation.
Previous studies have reported that HBV replication
and expression of hepatoviral proteins, such as HBV protein X, are
associated with potent activation of AP-1 (36–39),
and that AP-1 expression is increased in HBV (40,41).
Upregulation of AP-1 has also been reported in mouse liver fibrosis
induced by CCl4 treatment and common bile duct ligation
(42,43), which is consistent with the present
findings. Although c-Fos and c-Jun were initially considered as
oncogenes, it has been revealed that c-Fos can suppress the growth
of murine hepatocytes by inducing cell cycle inhibition and cell
death (44). Regarding the
mechanisms underlying the effects of AP-1 activation on liver
fibrosis, this likely occurs via the ER stress
response/extracellular signal-regulated kinase (ERK)/AP-1 signaling
pathway, as it has been reported that ER stress inducers activate
AP-1-associated genes, including c-Fos and c-Jun, in an
ERK-dependent manner in hepatic cells and murine livers (45). In the present study, MANF
expression was increased in the liver tissues of patients with HBV
infection and of mice with CCl4-induced liver fibrosis.
Therefore, it may be hypothesized that the increase in MANF
expression in the liver may partially be stimulated by AP-1.
However, the impact of MANF on liver inflammation requires further
investigation. We have prepared hepatocyte-specific MANF knockout
mice and mono-macrophage-specific MANF knockout mice, and aim to
compare liver inflammation and fibrosis in wild-type mice and MANF
knockout mice in the future; in addition, the underlying mechanisms
will be investigated.
In conclusion, the present study demonstrated that
the AP-1 complex may be a novel regulator of MANF transcriptional
enhancement, and that MANF is a novel downstream target of AP-1,
which may indicate a novel role of AP-1 in regulating inflammatory
pathways. Therefore, targeting the AP-1/MANF signaling pathway may
be a potential therapeutic strategy for the treatment of
inflammation and liver injury.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 91129729, 81372576
and 81673438 awarded to YXS) and the Youth Fund Project of the
First Affiliated Hospital of Anhui Medical University (grant no.
2009kj19 awarded to CHW).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CHW performed the experiments and wrote the
manuscript; TCJ, WMQ and LZ performed some of the experiments; YJS
and LJF helped to analyze the data and revised the manuscript; YXS
designed the experiments and wrote the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The use of human liver tissues was in accordance
with the ethical standards of the Declaration of Helsinki. Written
informed consent was obtained from all patients and the present
study was approved by the Human Research Ethics Committee of Anhui
Medical University (license number: 20131359). All mouse studies
were conducted according to protocols approved by the Animal Ethics
Committee of Anhui Medical University (license number:
20160329).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have competing
interests.
References
|
1
|
Petrova P, Raibekas A, Pevsner J, Vigo N,
Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, et al:
MANF: A new mesencephalic, astrocyte-derived neurotrophic factor
with selectivity for dopaminergic neurons. J Mol Neurosci.
20:173–188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Airavaara M, Shen H, Kuo CC, Peränen J,
Saarma M, Hoffer B and Wang Y: Mesencephalic astrocyte-derived
neurotrophic factor reduces ischemic brain injury and promotes
behavioral recovery in rats. J Comp Neurol. 515:116–124. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Apostolou A, Shen Y, Liang Y, Luo J and
Fang S: Armet, a UPR-upregulated protein, inhibits cell
proliferation and ER stress-induced cell death. Exp Cell Res.
314:2454–2467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tadimalla A, Belmont PJ, Thuerauf DJ,
Glassy MS, Martindale JJ, Gude N, Sussman MA and Glembotski CC:
Mesencephalic astrocyte-derived neurotrophic factor is an
ischemia-inducible secreted endoplasmic reticulum stress response
protein in the heart. Circ Res. 103:1249–1258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu YQ, Liu LC, Wang FC, Liang Y, Cha DQ,
Zhang JJ, Shen YJ, Wang HP, Fang S and Shen YX: Induction profile
of MANF/ARMET by cerebral ischemia and its implication for neuron
protection. J Cereb Blood Flow Metab. 30:79–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glembotski CC, Thuerauf DJ, Huang C,
Vekich JA, Gottlieb RA and Doroudgar S: Mesencephalic
astrocyte-derived neurotrophic factor protects the heart from
ischemic damage and is selectively secreted upon sarco/endoplasmic
reticulum calcium depletion. J Biol Chem. 287:25893–25904. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang W and Shen Y, Chen Y, Chen L, Wang L,
Wang H, Xu S, Fang S, Fu Y, Yu Y and Shen Y: Mesencephalic
astrocyte-derived neurotrophic factor prevents neuron loss via
inhibiting ischemia-induced apoptosis. J Neurol Sci. 344:129–138.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stratoulias V and Heino TI: MANF
silencing, immunity induction or autophagy trigger an unusual cell
type in metamorphosing Drosophila brain. Cell Mol Life Sci.
72:1989–2004. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stratoulias V and Heino TI: Analysis of
the conserved neurotrophic factor MANF in the Drosophila
adult brain. Gene Expr Patterns. 18:8–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palgi M, Greco D, Lindström R, Auvinen P
and Heino TI: Gene expression analysis of Drosophila Manf
mutants reveals perturbations in membrane traffic and major
metabolic changes. BMC Genomics. 13:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palgi M, Lindström R, Peränen J, Piepponen
TP, Saarma M and Heino TI: Evidence that DmMANF is an invertebrate
neurotrophic factor supporting dopaminergic neurons. Proc Natl Acad
Sci USA. 106:2429–2434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lindholm P, Voutilainen MH, Laurén J,
Peränen J, Leppänen VM, Andressoo JO, Lindahl M, Janhunen S,
Kalkkinen N, Timmusk T, et al: Novel neurotrophic factor CDNF
protects and rescues midbrain dopamine neurons in vivo. Nature.
448:73–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindahl M, Danilova T, Palm E, Lindholm P,
Võikar V, Hakonen E, Ustinov J, Andressoo JO, Harvey BK, Otonkoski
T, et al: MANF is indispensable for the proliferation and survival
of pancreatic β cells. Cell Rep. 7:366–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang N, Wang L, Le F, Zhan Q, Zheng Y,
Ding G, Chen X, Sheng J, Dong M, Huang H and Jin F: Altered
expression of Armet and Mrlp51 in the oocyte, preimplantation
embryo, and brain of mice following oocyte in vitro maturation but
postnatal brain development and cognitive function are normal.
Reproduction. 142:401–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cameron TL, Bell KM, Tatarczuch L, Mackie
EJ, Rajpar MH, McDermott BT, Boot-Handford RP and Bateman JF:
Transcriptional profiling of chondrodysplasia growth plate
cartilage reveals adaptive ER-stress networks that allow survival
but disrupt hypertrophy. PLoS One. 6:e246002011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen YX, Sun AM, Fang S, Feng LJ, Li Q,
Hou HL, Liu C, Wang HP, Shen JL, Luo J and Zhou JN: Hrd1
facilitates tau degradation and promotes neuron survival. Curr Mol
Med. 12:138–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang XY, Song MM, Bi SX, Shen YJ, Shen YX
and Yu YQ: MRI dynamically evaluates the therapeutic effect of
recombinant human MANF on ischemia/reperfusion injury in rats. Int
J Mol Sci. 17:E14762016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neves J, Zhu J, Sousa-Victor P, Konjikusic
M, Riley R, Chew S, Qi Y, Jasper H and Lamba DA: Immune modulation
by MANF promotes tissue repair and regenerative success in the
retina. Science. 353:aaf36462016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Cheng Q, Wang X, Zu B, Xu J, Xu Y,
Zuo X and Shen Y, Wang J and Shen Y: Deficiency of IRE1 and PERK
signal pathways in systemic lupus erythematosus. Am J Med Sci.
348:465–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Ke Z, Alimov A, Xu M, Frank JA,
Fang S and Luo J: Spatiotemporal expression of MANF in the
developing rat brain. PLoS One. 9:e904332014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen L, Feng L, Wang X, Du J, Chen Y, Yang
W, Zhou C, Cheng L, Shen Y, Fang S, et al: Mesencephalic
astrocyte-derived neurotrophic factor is involved in inflammation
by negatively regulating the NF-kappaB pathway. Sci Rep.
5:81332015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao H, Liu Y, Cheng L, Liu B, Zhang W,
Guo YJ and Nie L: Mesencephalic astrocyte-derived neurotrophic
factor inhibits oxygen-glucose deprivation-induced cell damage and
inflammation by suppressing endoplasmic reticulum stress in rat
primary astrocytes. J Mol Neurosci. 51:671–678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu W, Li J, Liu Y, Xie K, Wang L and Fang
J: Mesencephalic astrocyte-derived neurotrophic factor attenuates
inflammatory responses in lipopolysaccharide-induced neural stem
cells by regulating NF-kappaB and phosphorylation of p38-MAPKs
pathways. Immunopharmacol Immunotoxicol. 38:205–213. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eferl R and Wagner EF: AP-1: A
double-edged sword in tumorigenesis. Nat Rev Cancer. 3:859–868.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gustems M, Woellmer A, Rothbauer U, Eck
SH, Wieland T, Lutter D and Hammerschmidt W: c-Jun/c-Fos
heterodimers regulate cellular genes via a newly identified class
of methylated DNA sequence motifs. Nucleic Acids Res. 42:3059–3072.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Adcock IM: Transcription factors as
activators of gene transcription: AP-1 and NF-kappa B. Monaldi Arch
Chest Dis. 52:178–186. 1997.PubMed/NCBI
|
|
27
|
Hsu TC, Young MR, Cmarik J and Colburn NH:
Activator protein 1 (AP-1)- and nuclear factor kappaB
(NF-kappaB)-dependent transcriptional events in carcinogenesis.
Free Radic Biol Med. 28:1338–1348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brenner DA, O'Hara M, Angel P, Chojkier M
and Karin M: Prolonged activation of jun and collagenase genes by
tumour necrosis factor-alpha. Nature. 337:661–663. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu Y, Ge N, Xie M, Sun W, Burlingame S,
Pass AK, Nuchtern JG, Zhang D, Fu S, Schneider MD, et al:
Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is
required for interleukin (IL)-1-mediated optimal NFkappaB and AP-1
activation as well as IL-6 gene expression. J Biol Chem.
283:24497–24505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Sha M, Wang Q, Ma Y, Geng X, Gao Y,
Feng L and Shen Y and Shen Y: Small ubiquitin-related modifier 2/3
interacts with p65 and stabilizes it in the cytoplasm in
HBV-associated hepatocellular carcinoma. BMC Cancer. 15:6752015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mizobuchi N, Hoseki J, Kubota H, Toyokuni
S, Nozaki J, Naitoh M, Koizumi A and Nagata K: ARMET is a soluble
ER protein induced by the unfolded protein response via ERSE-II
element. Cell Struct Funct. 32:41–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang D, Hou C, Cao Y, Cheng Q, Zhang L, Li
H, Feng L and Shen Y: XBP1 activation enhances MANF expression via
binding to endoplasmic reticulum stress response elements within
MANF promoter region in hepatitis B. Int J Biochem Cell Biol.
99:140–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mackman N, Brand K and Edgington TS:
Lipopolysaccharide- mediated transcriptional activation of the
human tissue factor gene in THP-1 monocytic cells requires both
activator protein 1 and nuclear factor kappa B binding sites. J Exp
Med. 174:1517–1526. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mao R, Fan Y, Mou Y, Zhang H, Fu S and
Yang J: TAK1 lysine 158 is required for TGF-β-induced
TRAF6-mediated Smad-independent IKK/NF-kappaB and JNK/AP-1
activation. Cell Signal. 23:222–227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arzumanyan A, Reis HM and Feitelson MA:
Pathogenic mechanisms in HBV- and HCV-associated hepatocellular
carcinoma. Nat Rev Cancer. 13:123–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fuest M, Willim K, MacNelly S, Fellner N,
Resch GP, Blum HE and Hasselblatt P: The transcription factor c-Jun
protects against sustained hepatic endoplasmic reticulum stress
thereby promoting hepatocyte survival. Hepatology. 55:408–418.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Twu JS, Lai MY, Chen DS and Robinson WS:
Activation of protooncogene c-jun by the X protein of hepatitis B
virus. Virology. 192:346–350. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Benn J, Su F, Doria M and Schneider RJ:
Hepatitis B virus HBx protein induces transcription factor AP-1 by
activation of extracellular signal-regulated and c-Jun N-terminal
mitogen-activated protein kinases. J Virol. 70:4978–4985.
1996.PubMed/NCBI
|
|
40
|
Kanda T, Yokosuka O, Nagao K and Saisho H:
State of hepatitis C viral replication enhances activation of
NF-κB- and AP-1-signaling induced by hepatitis B virus X. Cancer
Lett. 234:143–148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo L, Guo Y, Xiao S and Shi X: Protein
kinase p-JNK is correlated with the activation of AP-1 and its
associated Jun family proteins in hepatocellular carcinoma. Life
Sci. 77:1869–1878. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chu X, Wang H, Jiang YM, Zhang YY, Bao YF,
Zhang X, Zhang JP, Guo H, Yang F, Luan YC and Dong YS: Ameliorative
effects of tannic acid on carbon tetrachloride-induced liver
fibrosis in vivo and in vitro. J Pharmacol Sci. 130:15–23. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Y, Ma J, Chen L, Xie XL and Jiang H:
Inhibition of focal adhesion kinase on hepatic stellate-cell
adhesion and migration. Am J Med Sci. 353:41–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mikula M, Gotzmann J, Fischer AN, Wolschek
MF, Thallinger C, Schulte-Hermann R, Beug H and Mikulits W: The
proto-oncoprotein c-Fos negatively regulates hepatocellular
tumorigenesis. Oncogene. 22:6725–6738. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Olivares S, Green RM and Henkel AS:
Endoplasmic reticulum stress activates the hepatic activator
protein 1 complex via mitogen activated protein kinase-dependent
signaling pathways. PLoS One. 9:e1038282014. View Article : Google Scholar : PubMed/NCBI
|