Introduction
Varicocele (VC) is considered to be the main cause
of male infertility (1). It has
been demonstrated that VCs adversely affect sperm quality (2,3),
sperm function (4,5), testicular histology (6,7) and
reproductive hormones (8). VCs are
considered to exert a negative effect on the function of Sertoli
and Leydig cells. The imbalanced hypothalamus-pituitary-gonadal
axis ultimately affects spermatogenesis due to the decreased
testosterone levels (9).
The blood-testis barrier (BTB) consists of
junctional and cytoskeletal structures, and it is one of the
tightest blood-tissue barriers in mammals (10,11).
Tight junctions (TJs), gap junctions (GJs) and desmosomes form this
barrier. Claudins are proteins that interact with cytoskeletal
proteins and are important components of tight junctions (12). A variety of claudins are expressed
in rodent testes (13) and several
participate in the formation of the BTB. Claudin-11 is detected at
day 12 post-coitus (14), and
claudin-11 knockout is not lethal in mice, but they are sterile
(13). Claudin-11−/−
Sertoli cells exhibit a unique phenotype by loss of tight junction
connection integrity, leading to loss of the epithelial phenotype
(15). The BTB has a crucial role
in the process of spermatogenesis, and claudin-11 is an important
tight junction protein expressed by Sertoli cells that is involved
in the formation of the BTB. Abnormal expression of claudin-11
affects the function of the BTB; however, precisely how VC affects
claudin-11 expression resulting in further alterations of the BTB
remains unknown.
Recent studies have demonstrated that transforming
growth factor (TGF)-β secreted by testicular cells can alter the
tightness of the tight junctions via the p38-mitogen-activated
protein kinase pathway (16,17).
Therefore, it would be interesting to elucidate whether TGF-β is
involved in the pathogenesis of VC, as preventing the testicular
cells from synthesizing TGF-β may affect the tightness of the
junctions between supporting cells and prevent spermatogenesis. To
address these questions, a VC rat model was established and the
molecular and histological changes in the testes were observed, in
order to provide evidence on the pathogenic mechanisms of VC and
determine the potential value in development of non-hormonal male
contraceptives.
To the best of our knowledge, this study is the
first to measure claudin-11 expression levels in ipsilateral and
contralateral testicular Sertoli cells in a VC model, and to
investigate its characteristics over time and the effects on BTB
function from the perspective of VC-induced male infertility. The
research results may further establish the inclusion of claudin-11
in BTB-associated studies and provide a basis for the development
of potential non-hormonal male contraceptives.
Materials and methods
Animals and groups
A total of 32 mature male (12 weeks) Sprague-Dawley
rats, weighing 200±20 g, were purchased from the Shanghai
Laboratory Animal Centre (Shanghai, China). The animal experiments
were performed in accordance with the guidelines of the ethics
committee of Zhejiang University, and the animals were handled
according to the Guide for the Care and Use of Laboratory Animals
published by the US National Institutes of Health (18). The experiment was approved by the
Animal Care and Use Committee of Zhejiang University
(ZDSX2017-0012).
The rats were randomly divided into the following 7
groups: Normal control group (NC), 4 weeks VC (4W-VC), 4 weeks sham
surgery (4W-SHAM), 6 weeks VC (6W-VC), 6 weeks sham surgery
(6W-SHAM), 8 weeks VC (8W-VC) and 8 weeks sham surgery (8W-SHAM).
The VC model was established by partial ligation of the left renal
vein according to the method described by Hurt et al
(19,20). Briefly, 1% pentobarbital sodium (35
mg/kg) was administered via intraperitoneal injection to induce
anaesthesia. The left renal vein was isolated at its junction with
the inferior vena cava, and a 0.8-mm metal ligature wire was placed
to narrow the left renal vein to half of its original diameter. The
branch veins of the spermatic vein were also ligated. Successful
modelling criteria included: i) Diameter of the spermatic vein
>1 mm; and ii) no difference in size between the left and right
kidneys. Isolation of the left renal vein with no ligation was
performed in the sham surgery control group. The rats were
euthanized by cervical dislocation following anaesthesia with
intraperitoneal injection of 10% chloral hydrate (200 mg/kg). The
testicles were collected at 4, 6 and 8 weeks after model
establishment and weighed. The structure of the spermatic vein was
observed under a microscope, and the diameter was measured with
scales.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from testicular tissues
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific Inc., Waltham, MA, USA). RNA quantity and quality were
tested with NanoDrop 1000 (NanoDrop; Thermo Fisher Scientific Inc.)
and gel electrophoresis. The primers (Table I) for qPCR were designed using
Primer Premier 5.0 software and synthesized by Generay Biotech Co.,
Ltd. (Shanghai, China). cDNA synthesis was performed using the
ReverTra Ace® qPCR RT kit (Toyobo Life Science, Osaka,
Japan) according to the manufacturer's protocol. The reverse
transcription reaction was performed at 65°C for 5 min, 37°C for 15
min and 98°C for 5 min. qPCR was performed using the KAPA SYBR
Green Supermix PCR kit (Kapa Biosystems; Roche Diagnostics,
Indianapolis, IN, USA) according to the kit protocol, with AriaMx
Real-Time PCR System (Agilent Technologies, Inc., Santa Clara, CA,
USA). The thermocycling conditions were as follows: 95°C for 30
sec, followed by 40 cycles of 95°C for 20 sec and 61°C for 30 sec.
The relative expression of different genes was determined using the
2−ΔΔCq algorithm (21).
 | Table I.Primer sequences used in the reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used in the reverse
transcription-quantitative polymerase chain reaction.
| Primer | Direction | Sequence (5′→3′) |
|---|
| Claudin-11 | F |
TTAGACATGGGCACTCTTGG |
|
| R |
ATGGTAGCCACTTGCCTTC |
| GAPDH | F |
CAAGTTCAACGGCACAGTCAAG |
|
| R |
ACATACTCAGCACCAGCATCAC |
Immunohistochemistry (IHC)
Testicular tissues were fixed with 4%
paraformaldehyde for 24 h at 4°C, embedded in paraffin and cut into
5 µm thick sections by Servicebio (Shanghai, China). Sections were
blocked in 5% BSA for 1.5 h at 37°C (Beyotime Institute of
Biotechnology, Shanghai, China). The antibodies were purchased from
Abcam (Cambridge, UK) and included anti-TGF-β1 (cat. no. ab9758;
1:1,000), anti-claudin-11 (cat. no. ab7474; 1:1,000) and
horseradish peroxidase-conjugated secondary antibody (cat. no.
ab6721; 1:1,000). Detailed experimental steps were performed as
described by Jasinski-Bergner et al (22). Scoring of the expression of
claudin-11 and TGF-β1 was performed by two independent pathologists
as described by Sewify et al (23).
Statistical analysis
All data are expressed as the mean ± standard
deviation unless otherwise specified. GraphPad Prism 5 (GraphPad
Software, Inc., La Jolla, CA, USA) was used to perform statistical
analysis for all results. Significance between groups was evaluated
by one-way analysis of variance. Each experiment of RT-qPCR and IHC
was performed three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
VC modelling
The diameter of the spermatic vein in the VC model
group was significantly larger compared with that in the NC group,
and further increased with time (P<0.001; Fig. 1). The left testicular weight in the
6W-VC group was significantly decreased compared with the right
testicular weight (1.36±0.05 vs. 1.46±0.08 g, respectively;
P<0.05), and the left testicular weight in the 8W-VC group was
significantly decreased compared with the right testicular weight
(1.35±0.05 vs. 1.50±0.06 g, respectively; P<0.001; Fig. 2). These results indicated
successful construction of the VC model in rats.
RT-qPCR of claudin-11
There was no statistically significant difference in
mRNA levels of claudin-11 between the SHAM and VC groups at 4
weeks. However, the expression of claudin-11 was significantly
downregulated in 6W-VC-L compared with 6W-SHAM-L (P<0.05). In
addition, the expression of the claudin-11 was more significantly
downregulated in 8W-VC-L compared with the 8W-SHAM-L (P<0.001).
Notably, there was a statistically significant difference between
8W-VC-L and 8W-VC-R, and between 8W-SHAM-R and 8W-VC-R (P<0.01;
Fig. 3).
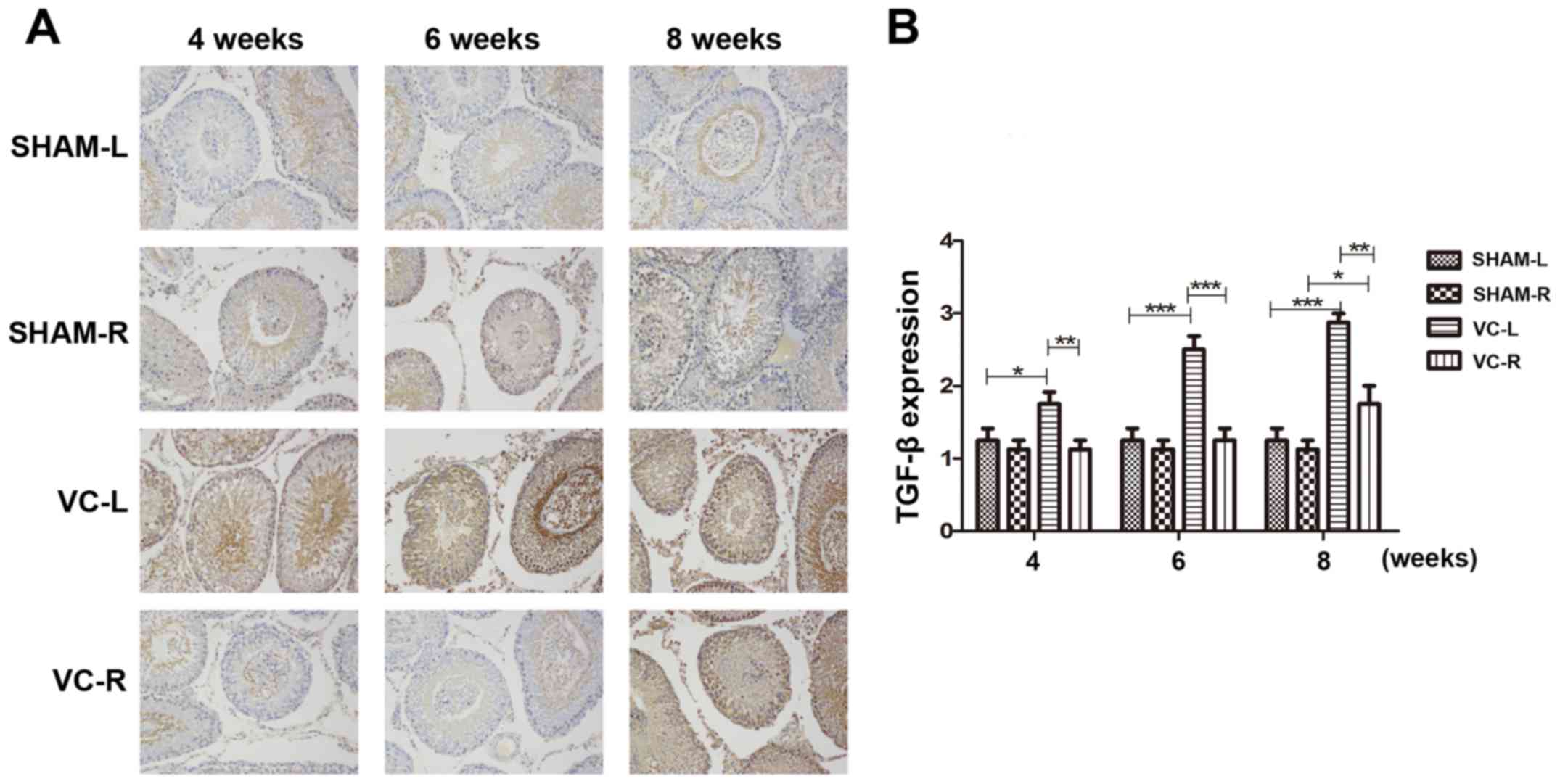
IHC of claudin-11 and TGF-β
The expression of claudin-11 was further analysed in
32 stained paraffin-embedded tissue samples of rat testicular
tissue (Fig. 4). The expression of
claudin-11 was downregulated in the VC model compared with the NC
group (P<0.01), and there was a significant difference between
the diseased side (left) and the healthy side (right). The
expression level of TGF-β in the VC model group was significantly
higher compared with that in the SHAM group at week 6 (P<0.01),
and more significantly increased at week 8 (P<0.001). Compared
with the normal side (VC-R), the operated side (VC-L) also
exhibited significantly higher expression of TGF-β in all three
time points (P<0.05; Fig.
5).
Discussion
In the present study, a VC rat model was established
to study the molecular and histological changes in the testes in
the early phases of VC development. The pathogenic process was
initiated by disruption of the BTB.
As rats are commonly used in BTB disruption studies
(24,25), a classic VC rat model was
established to mimic damage to the BTB in humans, and determine the
effect in claudin-11 in the variceal and contralateral testis at
different time points postoperatively (12).
RT-PCR revealed that the expression of claudin-11
was downregulated in VC rats at 6 and 8 weeks, and the diseased
side was more significantly affected compared with the healthy side
at week 8. The same trends were observed on IHC examination. In
addition, the expression of claudin-11 decreased gradually over
time.
The histopathological examination revealed
increasing disruption of the integrity of the BTB over time, and
unilateral VC also affected contralateral testicular function. Oh
et al (26) reported that
an increase in the levels of pro-inflammatory cytokines may be
caused by deregulation of claudin-11 expression in the Sertoli
cells of VC testes, leading to alterations in the permeability of
the BTB and immunological barriers to normal spermatogenesis. The
results of previous studies (24,27)
suggest that VC-induced male infertility is mediated by damage to
the BTB.
The results of the current study also demonstrated
that the expression of TGF-β increased in VC rats, which was not
limited to the diseased side, but also gradually affected the
healthy side over time. TGF-β was reported to be associated with
fibrosis of the seminiferous tubules and disruption of
spermatogenesis. In the testis, TGF-β is known to stimulate
collagen and fibronectin, further triggering the production of
extracellular matrix (28). During
puberty, the expression of TGF-β is regulated by hormonal
influences and is involved in steroidogenesis and spermatogenesis
(29,30).
In conclusion, the present study partially explained
the histopathological and molecular mechanisms underlying the
pathogenesis of VC, and the findings may be useful in the treatment
of male infertility.
Acknowledgements
The authors acknowledge the valuable suggestions and
technical assistance provided by the laboratory staff of the
Department of Urology at Shaoxing People's Hospital (Shaoxing,
China).
Funding
The present study was funded by the Shaoxing
Municipal Bureau of Science and Technology in China (grant no.
2015B70054).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JP was involved in all aspects of the research. ZZ
and JY were involved in the animal experiments and data analysis.
GX, LN, LY and ZGL were involved in RT-qPCR and IHC. All the
authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
The animal experiments were performed in accordance
with the guidelines of the Ethics Committee of Zhejiang University
(Shaoxing, China), and the animals were handled according to the
Guide for the Care and Use of Laboratory Animals published by the
US National Institutes of Health. The experiment was approved by
the Animal Care and Use Committee of Zhejiang University
(ZDSX2017-0012).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dubin L and Amelar RD: Etiologic factors
in 1294 consecutive cases of male infertility. Fertil Steril.
22:469–474. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Damsgaard J, Joensen UN, Carlsen E,
Erenpreiss J, Jensen Blomberg M, Matulevicius V, Zilaitiene B,
Olesen IA, Perheentupa A, Punab M, et al: Varicocele is associated
with impaired semen quality and reproductive hormone Levels: A
study of 7035 healthy young men from six european countries. Eur
Urol. 70:1019–1029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
The influence of varicocele on parameters
of fertility in a large group of men presenting to infertility
clinics. World Health Organization. Fertil Steril. 57:1289–1293.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang YJ, Zhang RQ, Lin YJ, Zhang RG and
Zhang WL: Relationship between varicocele and sperm DNA damage and
the effect of varicocele repair: A meta-analysis. Reprod Biomed
Online. 25:307–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vigil P, Wöhler C, Bustos-Obregón E,
Comhaire F and Morales P: Assessment of sperm function in fertile
and infertile men. Andrologia. 26:55–60. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdelrahim F, Mostafa A, Hamdy A, Mabrouk
M, el-Kholy M and Hassan O: Testicular morphology and function in
varicocele patients: Pre-operative and post-operative
histopathology. Br J Urol. 72:643–647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Etriby A, Girgis SM, Hefnawy H and Ibrahim
AA: Testicular changes in subfertile males with varicocele. Fertil
Steril. 18:666–671. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cayan S, Kadioglu A, Orhan I, Kandirali E,
Tefekli A and Tellaloglu S: The effect of microsurgical
varicocelectomy on serum follicle stimulating hormone, testosterone
and free testosterone levels in infertile men with varicocele. BJU
Int. 84:1046–1049. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pastuszak AW and Wang R: Varicocele and
testicular function. Asian J Androl. 17:659–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng CY and Mruk DD: The blood-testis
barrier and its implications for male contraception. Pharmacol Rev.
64:16–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Franca LR, Auharek SA, Hess RA, Dufour JM
and Hinton BT: Blood-tissue barriers: Morphofunctional and
immunological aspects of the blood-testis and blood-epididymal
barriers. Adv Exp Med Biol. 763:237–259. 2012.PubMed/NCBI
|
|
12
|
Morrow CM, Mruk D, Cheng CY and Hess RA:
Claudin and occludin expression and function in the seminiferous
epithelium. Philos Trans R Soc Lond B Biol Sci. 365:1679–1696.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morita K, Sasaki H, Fujimoto K, Furuse M
and Tsukita S: Claudin-11/OSP-based tight junctions of myelin
sheaths in brain and Sertoli cells in testis. J Cell Biol.
145:579–588. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hellani A, Ji J, Mauduit C, Deschildre C,
Tabone E and Benahmed M: Developmental and hormonal regulation of
the expression of oligodendrocyte-specific protein/claudin 11 in
mouse testis. Endocrinology. 141:3012–3019. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mazaud-Guittot S, Meugnier E, Pesenti S,
Wu X, Vidal H, Gow A and Le Magueresse-Battistoni B: Claudin 11
deficiency in mice results in loss of the Sertoli cell epithelial
phenotype in the testis. Biol Reprod. 82:202–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tossetta G, Paolinelli F, Avellini C,
Salvolini E, Ciarmela P, Lorenzi T, Emanuelli M, Toti P, Giuliante
R, Gesuita R, et al: IL-1β and TGF-β weaken the placental barrier
through destruction of tight junctions: An in vivo and in vitro
study. Placenta. 35:509–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rachakonda G, Vu T, Jin L, Samanta D and
Datta PK: Role of TGF-β-induced Claudin-4 expression through c-Jun
signaling in non-small cell lung cancer. Cell Signal. 28:1537–1544.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Research Council, . Guide for the
care and use of laboratory animals. 8th edition. Washington (DC):
National Academies Press (US); 2011, PubMed/NCBI
|
|
19
|
Hurt GS, Howards SS and Turner TT: The
effects of unilateral, experimental varicocele are not mediated
through the ipsilateral testis. J Androl. 8:403–408. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hurt GS, Howards SS and Turner TT: Repair
of experimental varicoceles in the rat. Long-term effects on
testicular blood flow and temperature and cauda epididymidal sperm
concentration and motility. J Androl. 7:271–276. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jasinski-Bergner S, Büttner M, Quandt D,
Seliger B and Kielstein H: Adiponectin and its receptors are
differentially expressed in human tissues and cell lines of
distinct origin. Obes Facts. 10:569–583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sewify EM, Afifi OA, Mosad E, Zaki AH and
El Gammal SA: Cyclin D1 amplification in multiple myeloma is
associated with multidrug resistance expression. Clin Lymphoma
Myeloma Leuk. 14:215–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ha HK, Park HJ and Park NC: Expression of
E-cadherin and α-catenin in a varicocele-induced infertility rat
model. Asian J Androl. 13:470–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Ding D and Liu J: Varicocele-caused
progressive damage in bilateral testis and sertoli cell-only
syndrome in homolateral testis in rats. Med Sci Monit.
20:1931–1936. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oh YS, Jo NH, Park JK and Gye MC: Changes
in inflammatory cytokines accompany deregulation of claudin-11,
resulting in inter-sertoli tight junctions in varicocele rat
testes. J Urol. 196:1303–1312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koksal IT, Ishak Y, Usta M, Danisman A,
Guntekin E, Bassorgun IC and Ciftcioglu A: Varicocele-induced
testicular dysfunction may be associated with disruption of
blood-testis barrier. Arch Androl. 53:43–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Skinner MK: Cell-cell interactions in the
testis. Endocr Rev. 12:45–77. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kimelman D: Peptide growth factors and the
regulation of early amphibian development. Biochim Biophys Acta.
1155:227–237. 1993.PubMed/NCBI
|
|
30
|
Dobashi M, Fujisawa M, Yamazaki T, Okada H
and Kamidono S: Distribution of intracellular and extracellular
expression of transforming growth factor-beta1 (TGF-beta1) in human
testis and their association with spermatogenesis. Asian J Androl.
4:105–109. 2002.PubMed/NCBI
|