Introduction
Acute myocardial infarction (AMI) is a serious
disease that affects numerous people around the world (1). The incidence of AMI is ~208 cases per
100,000 a year (2). AMI is a
life-threatening disease and seriously influences patient quality
of life. Therefore, increased understanding of its pathogenesis may
shed new light on novel diagnostic methods and active
intervention.
The pathology of AMI mainly comprises persistent
acute ischemic hypoxia caused by vascular stenosis and increased
cardiac pressure. To date, investigations of ischemic hypoxia have
focused on endoplasmic reticulum stress (ERS) (3), autophagy (4) and protein synthesis (5). Alleviating ERS can mitigate cardiac
injury to a certain extent, however, the specific pathological
mechanism underlying acute ischemic hypoxia remains to be
elucidated.
Long non-coding RNAs (lncRNAs) are RNA molecules
which are >200 nucleotides in length and have no coding
potential. lncRNAs can be classified into intergenic lncRNAs,
intronic lncRNAs, antisense lncRNAs, promoter-associated lncRNAs
and UTR-associated lncRNAs. lncRNAs have been demonstrated to be
associated with several diseases, including obesity (6), tumorigenesis (7) and congenital heart disease (8). lncRNAs serve significant functions,
including structural or trafficking roles (9), cell differentiation and apoptosis
(8). lncRNAs also function via a
broad range of mechanisms, including regulating neighboring genes
(10), microRNA-sponge action
(11) and coding small peptides to
suppress colon cancer (12).
However, the lncRNA profile in neonatal rat cardiomyocytes exposed
to ischemic hypoxia remains to be elucidated. There is no doubt
that investigating the role and mechanism of lncRNAs in the
pathophysiology of acute ischemic hypoxia will increase the
understanding of AMI.
In the present study, a microarray profile was
performed to identify differential lncRNA expression in
cardiomyocytes. Altogether, 323 lncRNAs were identified, 168 of
which were upregulated and 155 of which were downregulated. A total
of 10 lncRNAs were randomly selected to verify the microarray
results. It was predicted that these dysregulated lncRNAs may
contribute to the process of AMI. Furthermore, an lncRNA termed
sloyfley attracted attention due to its neighboring gene Peg3,
which has been linked to brain ischemia hypoxia (13). Bioinformatics analysis was
performed and the coding potential and possible interaction
proteins and sequence of sloyfley were predicted. In summary, the
findings provide comprehensive data regarding dysregulated lncRNAs
in acute ischemic hypoxia and may provide further opportunities to
assist in the development of therapeutic strategies.
Materials and methods
Cell culture
Neonatal male Sprague-Dawley rats (2–3 days old; 5–8
g; obtained from Nanjing Medical University) were immediately
anaesthetized with 75% ethanol and their hearts were sheared and
placed in cold PBS. The hearts were then digested using 0.4% type 2
collagenase/0.6% pancreatin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Following digestion for 20 min, horse serum (HS;
Sigma-Aldrich; Merck KGaA) was used to terminate the process and
all the samples were centrifuged at 300 × g for 5 min at room
temperature. This process was repeated until all the tissue had
been completely digested. The cell pellets were resuspended in
Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal
bovine serum (FBS), 10% HS and 1.2% penicillin/streptomycin (all
from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
neonatal rat cardiomyocytes were cultured in 5% CO2 at
37°C. The present study was approved by the Animal Care and
Management Committee of Nanjing Medical University (Nanjing,
China). Cell purity was evaluated by indirect immunofluorescence
staining with a monoclonal anti-troponin T antibody under a
fluorescence microscope (cat. no. ab92546; 1:2,000; Abcam,
Cambridge, UK).
Acute ischemic hypoxia exposure
The neonatal rat cardiomyocytes were cultured for 4
days prior to exposure to acute ischemic hypoxia. The
cardiomyocytes were randomly assigned to two groups. The growth
media of the ischemic hypoxia group was replaced by serum-free DMEM
for 2 h, following which the cells were placed in a hypoxic
environment which was achieved using an AnaeroPack system anaerobic
jar. The AnaeroPack system can deplete the concentration of oxygen
to <1% within 30 min. The growth media of the ischemic hypoxia
group was replaced with serum-free DMEM for 2 h, and the cells were
placed in the hypoxic environment produced using the AnaeroPack
system anaerobic jar. The cardiomyocytes were placed in the hypoxic
environment. Following 3 h in hypoxia, the cells well collected in
TRIzol for further investigation.
Reactive oxygen species (ROS)
detection
The cardiomyocytes were incubated with H2DCF-DA (10
µmol/l) for 30 min at 37°C and then rinsed with PBS three times.
DCFH-DA is a probe which can enter cells to measure the ROS
production (14). DCFH-DA is not
fluorescent and can pass through the cell membrane freely. On
entering the cell, it can be hydrolyzed by the intracellular
esterase to form DCFH, making it easy for the probe to be loaded
into the cell. Intracellular ROS can oxidize non-fluorescent DCFH
to produce fluorescent DCF. Detecting DCF fluorescence can be used
to measure the levels of intracellular ROS. The fluorescence was
observed with a fluorescence microscope (BX61; Olympus Corporation,
Tokyo, Japan).
Lactate dehydrogenase (LDH)
detection
The cell supernatant was collected for LDH analysis.
Destruction of the cell membrane structure caused by apoptosis or
necrosis results in the release of enzymes in the cytoplasm into
the culture medium, including LDH with stable enzyme activity
(15). Under the action of LDH,
nicotinamide adenine dinucleotide (NAD+) is reduced to
produce NADH. NADH and
2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium are
catalyzed by lipoamide dehydrogenase to form NAD+ and
chromophorin, and an absorption peak is generated at a wavelength
of 490 nm; therefore, the activity of LDH can be quantified by
colorimetry. The release of LDH was spectrophotometrically measured
using the CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (cat.
no. G1780; Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocol.
Cell survival calculation
The cardiomyocytes were digested for 3 min and then
pipetting completely. Trypan blue staining was used to assess the
cell survival rates, under a light microscope.
Microarray analysis
Rat Transcriptome Assay 1.0 was designed for the
global profiling of differential lncRNA in acute ischemic hypoxia
in rat models, providing a rich data set sufficient to decipher
gene expression changes. The lncRNAs were collected from Ensembl
(asia.ensembl.org/index.html), RefSeq
(www.ncbi.nlm.nih.gov/refseq), Aceview
(www.ncbi.nlm.nih.gov/ieb/research/acembly), and
NONCODE (www.noncode.org). Positive probes for
housekeeping genes and negative probes were also printed onto the
array for hybridization quality control. The microarray
hybridization was performed according to the manufacturer's
protocol.
Gene Ontology (GO) and pathway
analysis
The Gene Ontology (http://www.geneontology.org) program is a cooperative
effort to describe the requirement for gene products in different
databases. The present study analyzed the biological processes that
differential lncRNAs may contribute to; the data were analyzed
based on GO terms. Fisher's exact test and the v2 test were used to
classify the GO category. Pathway analysis is a functional analysis
for mapping genes to Kyoto Encyclopedia of Genes and Genomes
pathways (david.ncifcrf.gov). The lower the
P-value, the more important the pathway.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The National Center for Biotechnology Information
(NCBI; www.ncbi.nlm.nih.gov/pubmed) was used to design the
primers and they were synthesized by Generay Biotech Co., Ltd.
(Shanghai, China) (Table I).
Briefly, total RNA was extracted from cardiomyocytes using TRIzol
reagent (Thermo Fisher Scientific, Inc.). RNA purity was determined
by measuring the absorbance ratio at 260/280 nm using a NanoDrop
ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc.). RNA was
reverse transcribed using a PrimeScript™ RT reagent kit
with gDNA eraser (cat. no. RR047A; Takara Bio, Inc., Otsu, Japan)
at 37°C for 15 min, 85°C for 5 sec and 4°C for 5 min. qPCR was
performed using SYBR® Premix Ex Taq™ (cat. no. RR420A;
Takara Bio, Inc.), using the following thermocycling conditions:
95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C
for 30 sec. Data were normalized to GAPDH levels and further
analyzed by the 2−ΔΔCq method (16). All the primers used for qPCR were
listed in Table I.
 | Table I.Primers used in the present study for
quantitative polymerase chain reaction analysis. |
Table I.
Primers used in the present study for
quantitative polymerase chain reaction analysis.
| Primer | Sequence
(5′-3′) |
|---|
| stodee | F:
TGGAAAAACGTTAGAGCCTGAGG |
|
| R:
CCCAAAGAAGGGGAGTAAGCT |
| RGD735140 | F:
GCATGAAGCACAAGAGGG |
|
| R:
AGTTTTTCATCATTGAAGATG |
| smaler | F:
TGCTCGCAGTGGACTCTCTGTTG |
|
| R:
TGCTCGCAGTGGACTCTCTGTTG |
| LOC102548059 | F:
GAGAAGGAGTCAGATGGTAC |
|
| R:
GCGGCTTCTCAAAGCTGATC |
| XLOC028424 | F:
TTTTTTTGGGATTTGTATTTGATTT |
|
| R:
AAAACTTACCAACCTCTAATTCCAC |
| sleyko | F:
CAGAGAAGAAGGGCTGGGG |
|
| R:
GCCTCTCTTTTGCACTGCTGGTTCA |
| dakla | F:
CAGGCTTCAGGCTCTGCTTCC |
|
| R:
CCCACTCTTCGCCCAGTGCT |
| loydey | F:
AGGGCTATTGAATCTCAGAGG |
|
| R:
GGAGATGACCCCTCCATCTCC |
| sleegler | F:
CTGCAATGTTTAAGCATTACTA |
|
| R:
GCTGGCTGAAATTCCAAATTTC |
| slorlar | F:
GAGGTGTGGGGCCCGCAGC |
|
| R:
ACGCCCTCACTAGAATGT |
| GAPDH | F:
ACCACAGTCCATGCCATCAC |
|
| R:
TCCACCACCCTGTTGCTGTA |
Bioinformatics analysis
The neighboring genes were detected using NCBI. In
addition, the location of sloyfley was labeled in red. Coding
potential was calculated using calculator analyses (Peking
University, Beijing, China). To identify the potential proteins
that may interact with the lncRNA sloyfley, catRAPID (service.tartaglialab.com/page/catrapid_group) was
used to simulate the candidate proteins. Computational predictions
of ribonucleoprotein complex structures facilitate the detection of
protein-RNA interactions and their molecular function. The
potential binding sequence was also supplied using the RNA-Binding
Protein Database (rbpdb.ccbr.utoronto.ca/index.php), and the
transcription factor motifs which combined with the lncRNA sloyfley
were predicted.
Statistical analysis
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used to
calculate all values. Values are presented as the mean ± standard
error of the mean. Statistical analyses were performed using
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of differentially
expressed lncRNAs
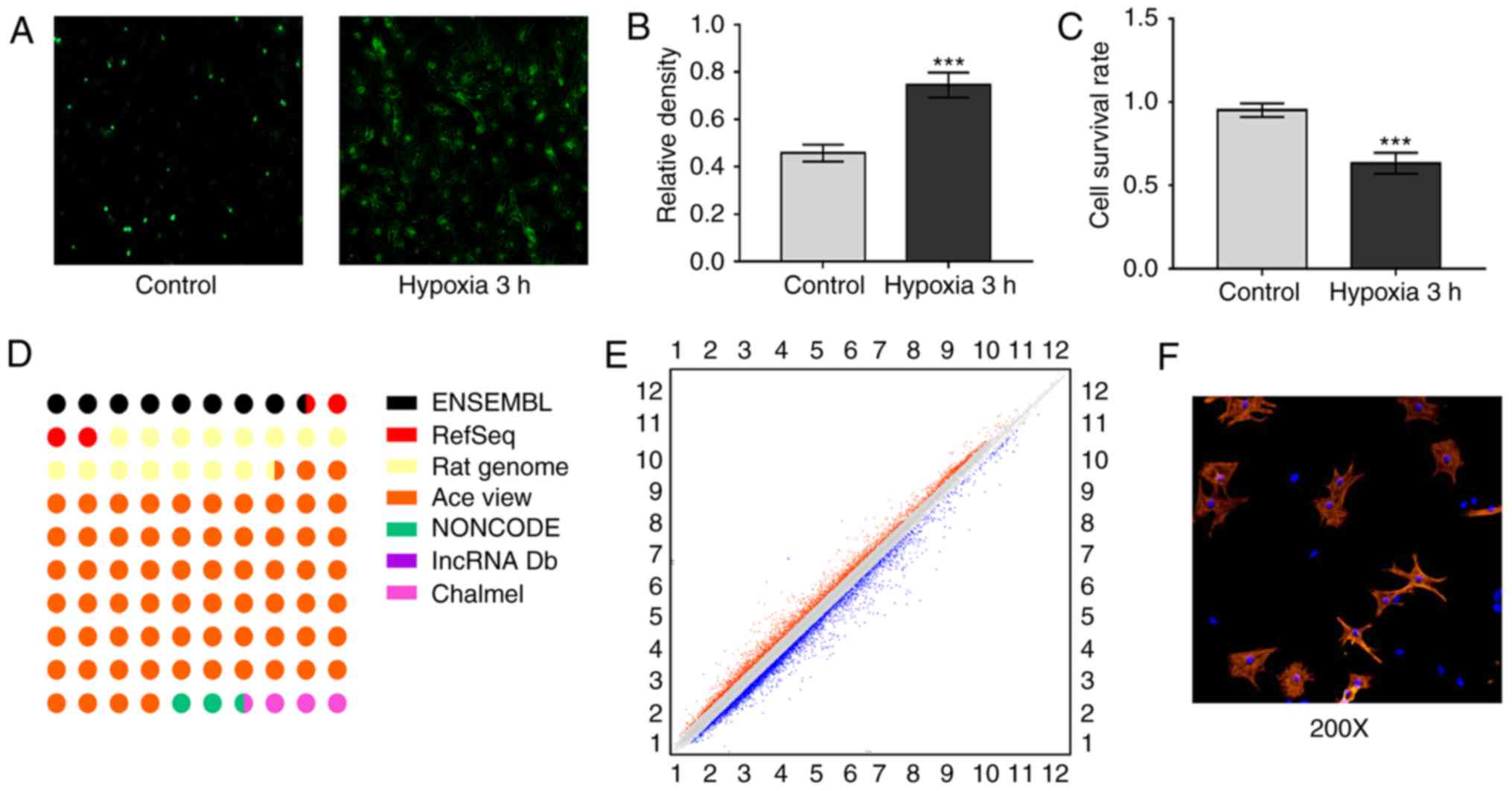
ROS and LDH were selected to verify the acute
ischemic hypoxia model (17).
Following hypoxia for 3 h, ROS and LDH levels were significantly
increased compared with levels in the control group (Fig. 1A and B). The cell survival rates
were also measured using trypan staining. The cell survival rates
in the hypoxia group were decreased compared with those in the
control group (Fig. 1C),
suggesting that the acute ischemic hypoxia model had been
established successfully. The Affymetrix rat lncRNA microarray was
designed for the global profiling of lncRNA and protein-coding
transcripts. The differentially expressed lncRNAs were carefully
selected from the Ensembl, RefSeq RatGenome, AceView, NONCODE and
lncRNA database (Fig. 1D). Scatter
plots were used for visualization and assessment of the variation
of lncRNA expression (Fig. 1E). By
setting the threshold for differential expression at P<0.05 and
a fold change of ≥1.5, a total of 323 lncRNAs were identified, 168
of which were upregulated and 155 of which were downregulated.
Expression signatures of
differentially expressed lncRNAs
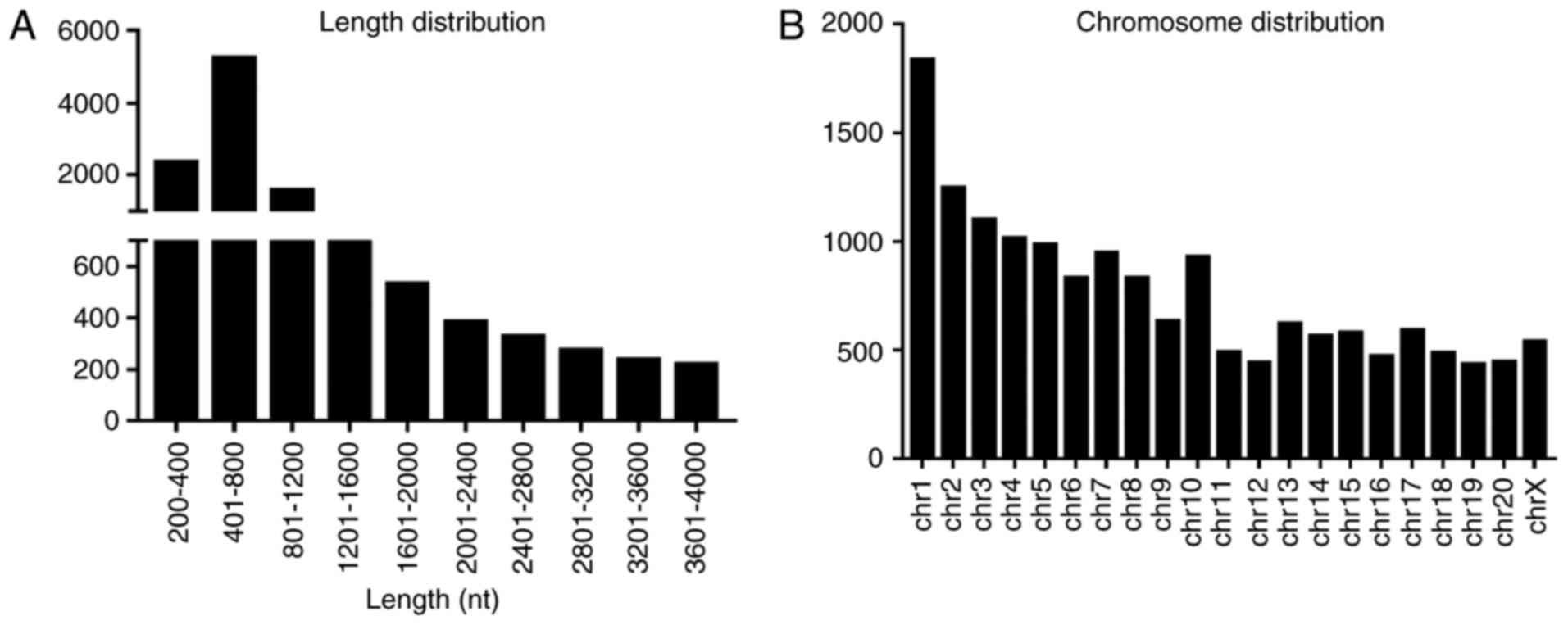
Following strident selection, the general signatures
of distinctly expressed lncRNAs, including length distribution and
chromosome distribution, were summarized. The lncRNAs were mainly
400–800 nucleotides in length (Fig.
2A). Chromosomal distribution showed the numbers of
dysregulated lncRNAs located on different chromosomes (Fig. 2B).
GO and pathway analyses
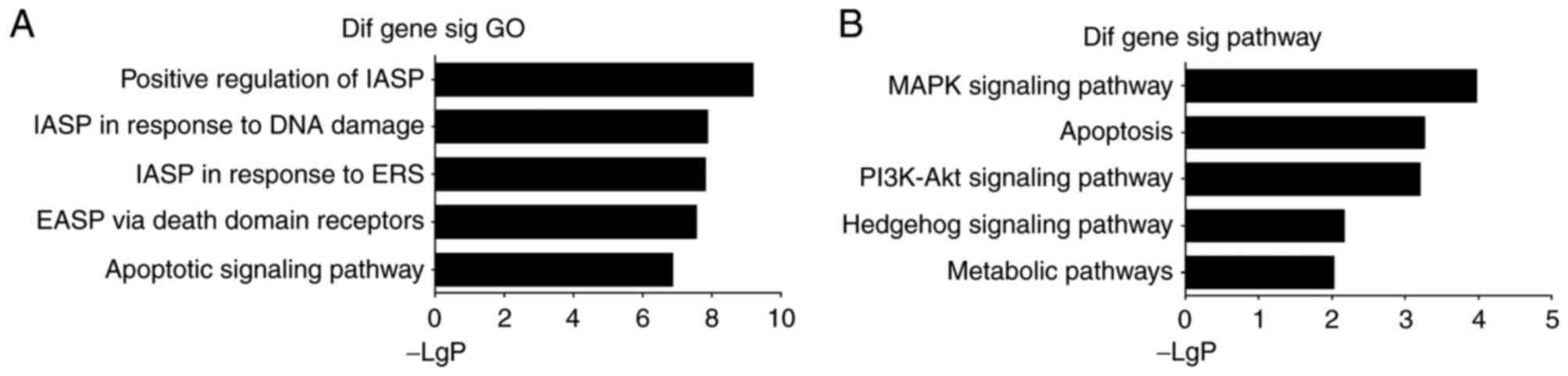
GO analysis accessible online (http://www.geneontology.org). GO analysis was
performed in the present study to discern the possible biological
processes in which the lncRNAs may be involved. The genes
potentially relevant were as follows: i) GO:2001244, positive
regulation of intrinsic apoptotic signaling pathway; ii)
GO:0042771, intrinsic apoptotic signaling pathway in response to
DNA damage by p53 class mediator; iii) GO:0070059, intrinsic
apoptotic signaling pathway in response to endoplasmic reticulum
stress; iv) GO:0008625, extrinsic apoptotic signaling pathway via
death domain receptors; v) GO:0097190, apoptotic signaling pathway
(Fig. 3A). Of note, the biological
processes acquired were relevant to the apoptotic signaling pathway
to a large extent. The apoptotic signaling pathway has been
investigated extensively, particularly the intrinsic pathway.
However, whether lncRNAs can influence cell death through the
apoptotic signaling pathway remains to be elucidated.
To further characterize the functional significance
of the differentially expressed lncRNAs, pathway analysis was
performed and the following five enriched pathways were identified:
i) MAPK signaling pathway; ii) Apoptosis; iii) PI3K-Akt signaling
pathway; iv) Hedgehog signaling pathway; v) Metabolic pathways
(Fig. 3B). Based on this computed
signaling network, it was found that multiple signaling procedures,
including apoptosis and the MAPK signaling pathway, were
significant to the network.
Confirmation of candidate lncRNAs
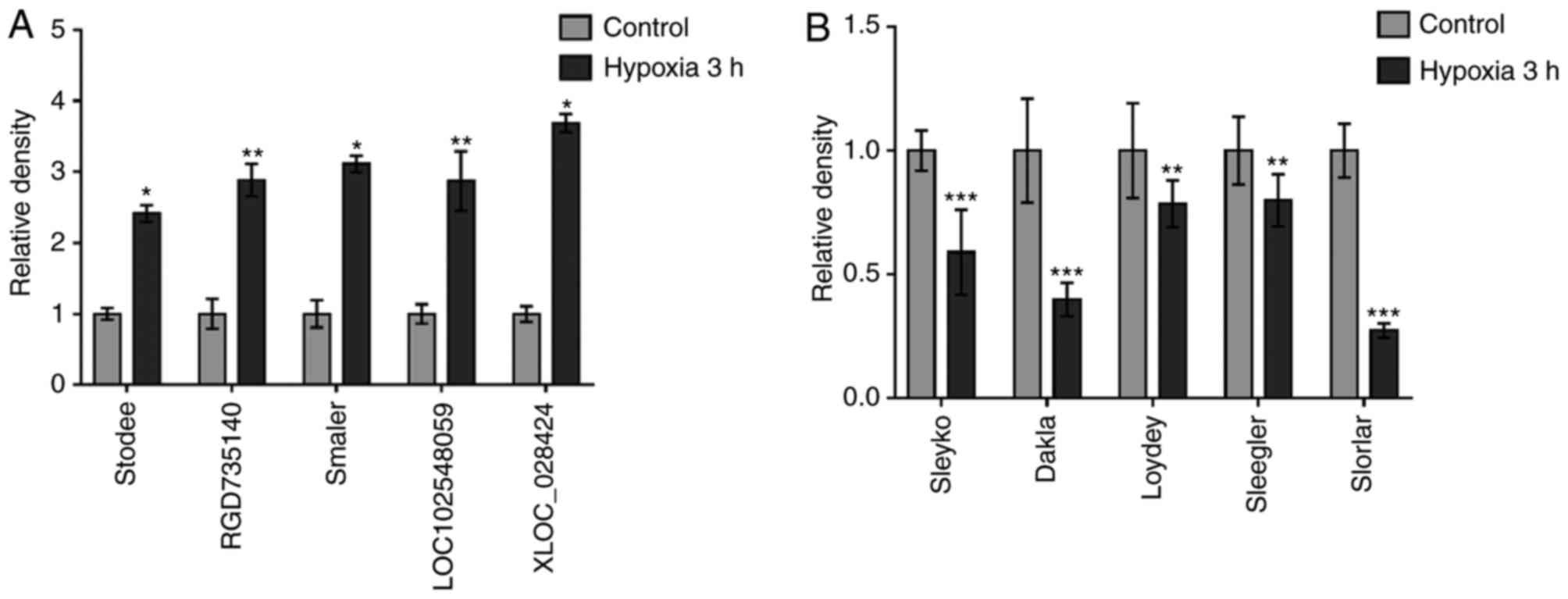
To validate the microarray results, five upregulated
lncRNAs (stodee, RGD735140, smaler, LOC102548059 and XLOC_028424)
and five downregulated lncRNAs (sleyko, dakla, Loydey, sleegler and
slorlar) were randomly selected for verification. The five lncRNAs
selected were upregulated compared with the control group (Fig. 4A). The downregulated lncRNAs were
also verified using RT-qPCR analysis and the five lncRNAs were
downregulated compared with the control group (Fig. 4B). Therefore, a series of lncRNAs
were acquired that were constantly differentially expressed in
acute ischemic hypoxia.
Bioinformatics and coding potential of
sloyfley
Non-coding genes can be located within the intronic
area and can overlap with the sense gene or antisense gene. The
coding pattern is important in the function and mechanism of a
candidate lncRNA. Differing locations often decide the fate of
lncRNAs, which may function through the cis-mechanism or
trans-mechanism. Among the dysregulated lncRNAs, a candidate
was selected with a relatively high expression and an associated
neighboring gene with possible functions in apoptosis and relevant
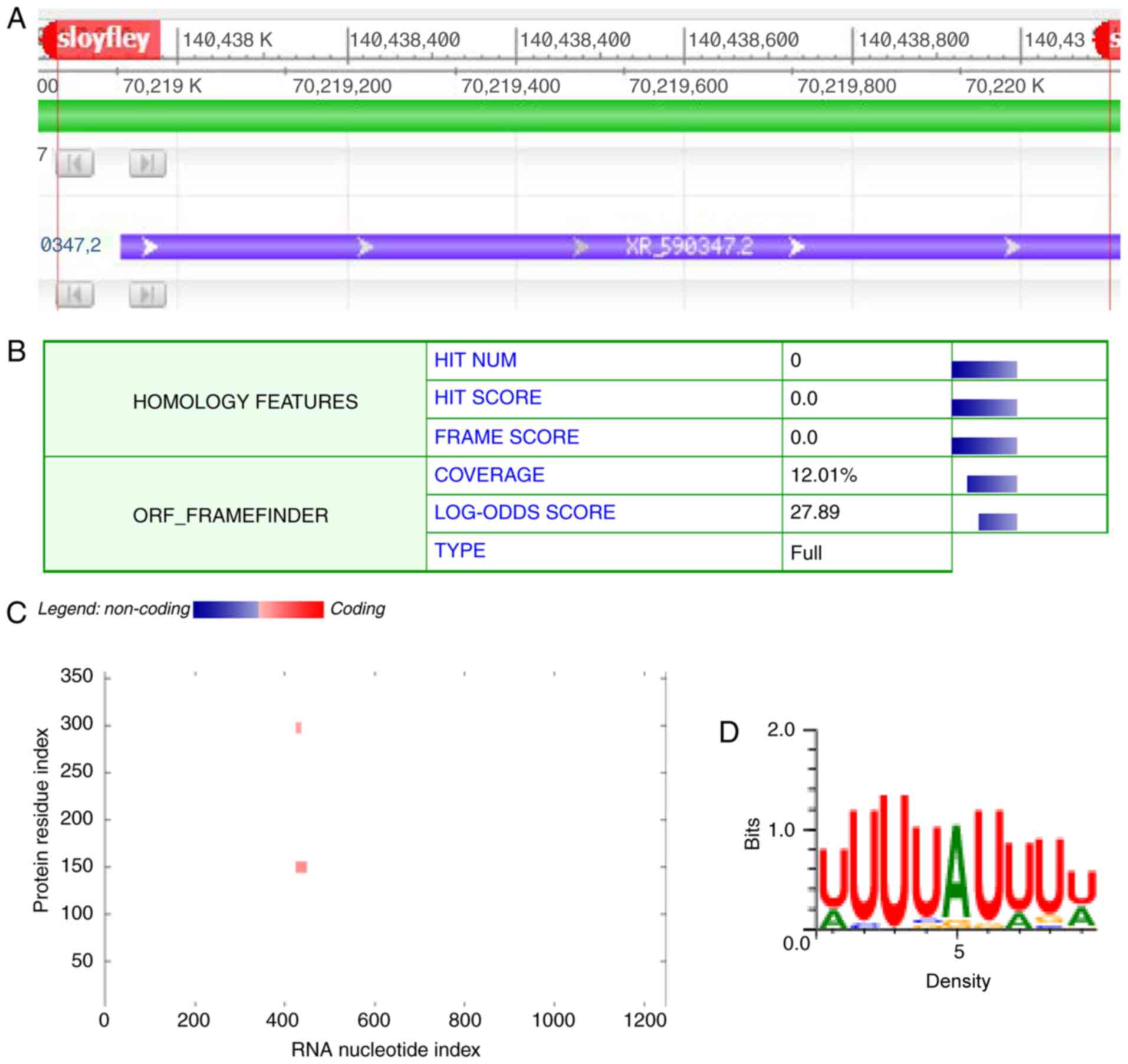
pathways. An lncRNA named sloyfley attracted attention due to its
high expression level and its neighboring gene Peg3 (Fig. 5A), which has been demonstrated to
contribute to the process of brain ischemic hypoxia (13). The coding potential of lncRNA
sloyfley was the calculated using Coding Potential Calculator
analyses (Peking University, Beijing, China). Sloyfley had no
credible protein-coding open reading frame with a coding potential
score of 1.21214 (Fig. 5B). To
further identify the potential protein that may interact with
lncRNA sloyfley, catRAPID analysis was performed to calculate the
interaction with potential protein ELAVL2 (Fig. 5C). The prediction of transcription
factor binding sites indicated the possible interaction sequence
(Fig. 5D).
Discussion
In the present study, differential lncRNAs were
identified in cardiomyocytes exposed to acute ischemic hypoxia. GO
and Pathway analyses were performed to assess the potential
function of dysregulated lncRNA. Analysis was also performed of the
bioinformatics information for lncRNA sloyfley, with neighboring
gene Peg3, which has previously been demonstrated to be associated
with brain ischemic hypoxia. The relative potential binding
sequence of sloyfley was also identified. The findings of the
present study provided comprehensive data regarding dysregulated
lncRNAs in acute ischemic hypoxia, and may provide further
opportunities for therapeutic strategies.
lncRNAs are known to regulate the expression of
neighboring genes through a cis-mechanism (18), or to function through a distinct
location instead of the transcription location as a
trans-mechanism (9). In
previous years, thousands of lncRNAs have been identified in
numerous diseases (19). A
persistent challenge in the investigation of lncRNAs is identifying
the association between candidate lncRNAs and neighboring genes
(20). To examine the function of
candidate lncRNAs, the function of adjacent genes is an important
element of the investigation. One possible mechanism is that
lncRNAs may act locally, regulating the expression of neighboring
gene transcripts (21). In the
present study, the distinct lncRNA expression in cardiomyocytes
exposed to acute ischemic hypoxia was investigated. It was
predicted that the dysregulated lncRNAs may contribute to acute
myocardial infarction. It was demonstrated that sloyfley may
influence acute ischemic hypoxia, and the neighboring gene Peg3 has
been demonstrated to be associated with brain ischemic hypoxia.
Peg3 is involved in the p53-mediated cell death pathway as a
downstream effector of p53 in brain ischemia hypoxia (13). However, the association between
sloyfley and Peg3 remains to be elucidated. The results of the
present study showed the potential binding sequence of sloyfley;
specific experiments of the potential mechanism and its effect in
acute ischemic hypoxia are clearly warranted.
GO and Pathway analyses were performed to identify
the potential functions to which lncRNA may contribute (22). The results revealed that the
dysregulated lncRNA was associated with the apoptosis process. The
apoptotic pathway can be classified into two pathways (23); the extrinsic pathway involves cell
surface receptors, whereas the intrinsic pathway utilizes the
mitochondria activity and endoplasmic reticulum. The two pathways
lead to the activation of caspases. Apoptosis is important in the
pathophysiology of different diseases (24). Inadequate, excessive or limited
apoptosis may all contribute to the pathogenesis of disease
(25,26). Perhaps the current challenge in
investigating lncRNAs is the knowledge embedded in pathways
regarding how various lncRNAs interact with their target genes. The
results of the present study demonstrated that the biological
processes of the dysregulated lncRNA may contribute to the
apoptotic pathway. The PI3K-Akt pathway regulates several cellular
processes, including cell apoptosis (27), ERS (28) and autophagy (29). Apoptosis is also involved in acute
ischemic hypoxia (30). Whether
lncRNA regulates acute ischemic hypoxia through the apoptotic
pathway remains to be fully elucidated and further investigations
are required in order to elucidate this. Of note, although the
remaining lncRNAs were not selected for further analysis, this does
not mean that they have no biological relevance. One possible
explanation that may account for this is that lncRNAs may utilize
different mechanisms to function, depending on their location.
In conclusion, the present study is the first, to
the best of our knowledge, to present comprehensive data regarding
the differential profile of lncRNAs in acute ischemic hypoxia. The
findings suggest that numerous lncRNAs are involved in AMI and
provide adequate resources for the future functional investigation
of lncRNAs. There is no doubt that examining the role and mechanism
of lncRNAs in the pathophysiology of acute ischemic hypoxia will
provide further knowledge regarding AMI.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81570209, 81873540 and
81800283).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LQ, JZ and HL designed and supervised the study. HL,
YT, and JX performed the experiments. ZC acquired the data. MF, AY,
QZ and HZ analyzed the data. ZC and HL wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li YF, Wang H, Fan Y, Shi HJ, Wang QM,
Chen BR, Khurwolah MR, Long QQ, Wang SB, Wang ZM and Wang LS:
Epigallocatechin-3-gallate inhibits matrix metalloproteinase-9 and
monocyte chemotactic protein-1 expression through the 67-κDa
laminin receptor and the TLR4/MAPK/NF-κB signalling pathway in
lipopolysaccharide-induced macrophages. Cell Physiol Biochem.
43:926–936. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lyngbakken MN, Skranes JB, De Lemos JA,
Nygård S, Dalen H, Hveem K, Røsjø H and Omland T: Impact of smoking
on circulating cardiac troponin I concentrations and cardiovascular
events in the general population: The HUNT study (Nord-Trøndelag
Health Study). Circulation. 134:1962–1972. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mccarroll CS, He W, Foote K, Bradley A,
Mcglynn K, Vidler F, Nixon C, Nather K, Fattah C, Riddell AH, et
al: Runx1 deficiency protects against adverse cardiac remodeling
following myocardial infarction. Circulation. 137:57–70. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maejima Y, Kyoi S, Zhai P, Liu T, Li H,
Ivessa A, Sciarretta S, Del Re DP, Zablocki DK, Hsu CP, et al: Mst1
inhibits autophagy by promoting the interaction between Beclin1 and
Bcl-2. Nat Med. 19:1478–1488. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simon MC, Liu L, Barnhart BC and Young RM:
Hypoxia-induced signaling in the cardiovascular system. Annu Rev
Physiol. 70:51–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cui X, You L, Li Y, Zhu L, Zhang F, Xie K,
Cao Y, Ji C and Guo X: A transcribed ultraconserved noncoding RNA,
uc.417, serves as a negative regulator of brown adipose tissue
thermogenesis. FASEB J. 30:4301–4312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Dai C, Jia G, Xu S, Fu Z, Xu J, Li
Q, Ruan H and Xu P: Microarray analysis reveals differentially
expressed lncRNAs in benign epithelial ovarian cysts and normal
ovaries. Oncol Rep. 38:799–808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song G, Shen Y, Ruan Z, Li X, Chen Y, Yuan
W, Ding X, Zhu L and Qian L: lncRNA-uc.167 influences cell
proliferation, apoptosis and differentiation of P19 cells by
regulating Mef2c. Gene. 590:97–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang P, Xu J, Wang Y and Cao X: An
interferon-independent lncRNA promotes viral replication by
modulating cellular metabolism. Science. 358:1051–1055. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Joung J, Engreitz JM, Konermann S,
Abudayyeh OO, Verdine VK, Aguet F, Gootenberg JS, Sanjana NE,
Wright JB, Fulco CP, et al: Genome-scale activation screen
identifies a lncRNA locus regulating a gene neighbourhood. Nature.
548:343–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang K, Liu F, Zhou LY, Long B, Yuan SM,
Wang Y, Liu CY, Sun T, Zhang XJ and Li PF: The long noncoding RNA
CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res.
114:1377–1388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang JZ, Chen M, Chen, Gao XC, Zhu S,
Huang H, Hu M, Zhu H and Yan GR: A peptide encoded by a putative
lncRNA HOXB-AS3 suppresses colon cancer growth. Mol Cell.
68:171–184.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamaguchi A, Taniguchi M, Hori O, Ogawa S,
Tojo N, Matsuoka N, Miyake S, Kasai K, Sugimoto H, Tamatani M, et
al: Peg3/Pw1 is involved in p53-mediated cell death pathway in
brain ischemia/hypoxia. J Biol Chem. 277:623–629. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, Liu K, Yao K, Reddy K, Zhang Y,
Fu Y, Yang G, Zykova TA, Shin SH, Li H, et al: HOI-02 induces
apoptosis and G2-M arrest in esophageal cancer mediated by ROS.
Cell Death Dis. 6:e19122015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Liu J, Zheng Y, Wang J, Wang Z, Gu
S, Tan J, Jing Q and Yang H: Uncoupling protein 3 mediates
H2O2 preconditioning-afforded
cardioprotection through the inhibition of MPTP opening. Cardiovasc
Res. 105:192–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu H, Jing X, Dong A, Bai B and Wang H:
Overexpression of TIMP3 protects against cardiac
ischemia/reperfusion injury by inhibiting myocardial apoptosis
through ROS/mapks pathway. Cell Physiol Biochem. 44:1011–1023.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y and Cao X: Long noncoding RNAs in
innate immunity. Cell Mol Immunol. 13:138–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hosono Y, Niknafs YS, Prensner JR, Iyer
MK, Dhanasekaran SM, Mehra R, Pitchiaya S, Tien J, Escara-Wilke J,
Poliakov A, et al: Oncogenic role of THOR, a conserved
cancer/testis long non-coding RNA. Cell. 171:1559–1572.e20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo S, Lu JY, Liu L, Yin Y, Chen C, Han X,
Wu B, Xu R, Liu W, Yan P, et al: Divergent lncRNAs regulate gene
expression and lineage differentiation in pluripotent cells. Cell
Stem Cell. 18:637–652. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Wu LJ, Gu M, Chen YM, Zhang QJ, Li
H, Cheng ZJ, Hu P, Han SP, Yu ZB and Qian LM: Peptidomic analysis
of amniotic fluid for identification of putative bioactive peptides
in ventricular septal defect. Cell Physiol Biochem. 38:1999–2014.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whelan RS, Kaplinskiy V and Kitsis RN:
Cell death in the pathogenesis of heart disease: Mechanisms and
significance. Annu Rev Physiol. 72:19–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagata S and Tanaka M: Programmed cell
death and the immune system. Nat Rev Immunol. 17:333–340. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hetz C and Saxena S: ER stress and the
unfolded protein response in neurodegeneration. Nat Rev Neurol.
13:477–491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ashkenazi A, Fairbrother WJ, Leverson JD
and Souers AJ: From basic apoptosis discoveries to advanced
selective BCL-2 family inhibitors. Nat Rev Drug Discov. 16:273–284.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ho L, Tan SY, Wee S, Wu Y, Tan SJ,
Ramakrishna NB, Chng SC, Nama S, Sczerbinska I, Chan YS, et al:
ELABELA Is an endogenous growth factor that sustains hESC
self-renewal via the PI3K/AKT pathway. Cell Stem Cell. 17:435–447.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng L, Xiao Q, Chen M, Margariti A,
Martin D, Ivetic A, Xu H, Mason J, Wang W, Cockerill G, et al:
Vascular endothelial cell growth-activated XBP1 splicing in
endothelial cells is crucial for angiogenesis. Circulation.
127:1712–1722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nikoletopoulou V, Sidiropoulou K, Kallergi
E, Dalezios Y and Tavernarakis N: Modulation of autophagy by BDNF
underlies synaptic plasticity. Cell Metab. 26:230–242.e5. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Flevaris P, Khan SS, Eren M, Schuldt AJ,
Shah SJ, Lee DC, Gupta S, Shapiro A, Burridge P, Ghosh AK, et al:
Plasminogen activator inhibitor type I controls
cardiomyocytetransforming growth factor-β and cardiac fibrosis.
Circulation. 136:664–679. 2017. View Article : Google Scholar : PubMed/NCBI
|