Introduction
Mesenchymal stem cells (MSCs), which are derived
from the mesoderm and have the self-renewal capacity and
multi-directional differentiation potential of adult stem cells,
can be differentiated into cartilage, bone, skeletal muscle and
other cell types under certain conditions (1–4).
MSCs can be isolated from a number of diverse sources and exhibit
multi-directional differentiation in vitro. Recent studies
have demonstrated that MSCs repair damaged tissue (5,6).
MSCs can not only function as seed cells of engineered myocardial
tissue, bone and cartilage constructs, and important carrier cells
in gene therapy, they can also repair damaged endometrium and
inhibit graft vs. host responses (7). Stem cells have wide application
prospects in the repair of damaged tissue (8–10)
and may be used as a potential treatment for patients who suffer
from infertility caused by intrauterine adhesions (11,12).
MSC therapy is an effective treatment for myocardial necrosis of
myocardial infarction, osteoporosis, bone cysts, lupus nephritis,
diabetes, liver cirrhosis, liver failure, spinal cord injury and
Parkinson's disease (13,14).
MSCs can be derived from a wide range of tissues,
including the bone marrow, umbilical cord, placenta, amniotic fluid
and dental pulp tissue. Stem cells from different sources have
different molecular and growth characteristics; therefore, the
mechanisms and effects of treatment may be different (15,16).
In addition, the method of MSC culture differs in different
laboratories, including the tissue adherent method (14) and the enzyme digestion method
(11); therefore, the extent of
cell amplification and cell quality is different, as well as
clinical trial application solutions. Currently, there is no
consensus on the markers that identify or distinguish MSCs derived
from different tissues; an internationally recognized standard for
MSC culture has not been reached (17). The aim of the present study was to
compare the biological characteristics of MSCs derived from the
umbilical cord (UC-MSCs) MSCs derived from the decidua parietalis
(DP-MSCs), such as proliferation, immunophenotype and
differentiation potential under the same conditions. The results of
the present study may provide novel evidence for the selection of
seed cells in regenerative medicine.
Materials and methods
Sample collection and group
allocation
All procedures in the present study were approved by
the Ethical Committee of The First People's Hospital of Foshan and
written informed consent was obtained from all donors. Foetal
umbilical cord and decidua parietalis samples were obtained from 12
patients who underwent routine caesarean delivery for dystocia
between January and April 2016 at the Department of Obstetrics, The
First People's Hospital of Foshan. Samples were collected under
sterile conditions and transported to the laboratory in Dulbecco's
modified Eagle's medium (DMEM)/F-12 (Gibco; Thermo Fisher
Scientific, Inc.) in an icebox. Donors were negative for hepatitis
virus markers, syphilis and HIV.
Isolation and culture of UC-MSCs
Tissue samples comprising ~5 cm of the umbilical
cord were washed three times with PBS. Amniotic membrane and blood
vessels surrounding the umbilical cord were removed. The Wharton's
jelly from the umbilical cord was cleaned by PBS and mechanically
fragmented into 1 mm3 sections with ophthalmic scissors.
Sections of Wharton's jelly were then suspended in DMEM containing
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 1% penicillin and 1% streptomycin (Gibco; Thermo Fisher
Scientific, Inc.), and were incubated at 37°C in a humidified
atmosphere containing 5% CO2. The medium was replaced
every 3 days, and tissue block cells were passaged upon reaching
80% confluence. The morphology of UC-MSCs was observed under an
inverted optical microscope (Olympus Corporation). For further
study, UC-MSCs were resuspended in DMEM/F12 at the required density
and subjected to various assays.
Isolation and culture of DP-MSCs
Complete placenta tissue samples were washed three
times with sterile PBS. The coarse surface of the decidua
parietalis tissues close to the maternal side were scraped using
surgical instruments and soaked in PBS. A total of 2 g decidua
parietalis tissue was transferred to a 50-ml centrifuge tube and
mechanically fragmented into 1-mm3 sections with
ophthalmic scissors. Subsequently, tissue fragments were digested
with 0.1% (m/v) collagenase I (Sigma-Aldrich; Merck KGaA) for 1.5 h
at 37°C. Primary cells were obtained by filtering through cell
strainers (pore size, 70 µm), resuspended in DMEM/F12 supplemented
with 10% FBS, and seeded in 25 cm2 flasks at a density
of 1×105/ml. Cells were cultured in an incubator at 37°C
in a humidified atmosphere containing 5% CO2 and
passaged upon reaching 80% confluence. The medium was replaced
every 3 days and the morphology was observed under an inverted
optical microscope (Olympus Corporation). For further study,
DP-MSCs were resuspended in DMEM at the required density and
subjected to various assays.
Determination of growth curves and
population doubling times
The second and fifth generation of UC-MSCs and
DP-MSCs were digested by trypsin and cultured in 24-well plates
(1×104 cells/ml) at 37°C in a humidified atmosphere
containing 5% CO2. Randomly selected wells (n=3) were
digested with trypsin and counted daily using a Countess automatic
cell counter (Invitrogen; Thermo Fisher Scientific, Inc.). Each
well was counted three times, and the daily average was calculated
for 7 days. The population doubling time during the logarithmic
growth phase was calculated using the Patterson formula. Cell
population doubling time (Td) was calculated using the following
equation: Td=t × lg2/(lgNt-lgNo), where T indicates incubation
time; No refers to cell number after inoculation and Nt refers to
cell number at T hour culture.
Colony formation assay
The second and fifth generations of UC-MSCs and
DP-MSCs were resuspended as single-cell suspensions following
trypsin digestion and added in triplicate to 60-mm Petri dishes
(100 cells/dish). Cells were cultured for 7 days, fixed with 4%
paraformaldehyde for 5 min and stained with 0.1% crystal violet for
15 min at room temperature. After three washes with
triple-distilled water, colonies that consisted of >30 nucleated
cells were counted under an inverted optical microscope (Olympus
Corporation) and were washed with ddH2O. The number of
colonies was determined using a camera, and the colony formation
rate was calculated using the following formula: Colony formation
rate = colony number/cell number ×100%.
Immunophenotype analysis
Flow cytometric analysis was performed using an FC
500 flow cytometry instrument (Beckman Coulter, Inc.). Cell surface
markers CD34, CD45, CD73, CD90 and CD105, which are considered as
standard by the International Society For Cellular Therapy, were
used to identify the MSC phenotype (18). Briefly, 2×105 cells from
the fifth generation of UC-MSCs and DP-MSCs were stained for 10 min
with FITC -conjugated or phycoerythrin (PE)-conjugated monoclonal
antibodies at 37°C. The following monoclonal antibodies were used:
Mouse FITC anti-CD34 antibody (cat. no. 555821; 1:200); mouse FITC
anti-CD45 antibody (cat. no. 555482; 1:200); mouse PE anti-CD73
antibody (cat. no. 550257; 1:200); mouse PE anti-CD90 antibody
(cat. no. 561970; 1:200); and mouse FITC anti-CD105 antibody (cat.
no. 561443; 1:200) (all from BD Biosciences).
Adipogenic differentiation
UC-MSCs and DP-MSCs at passage 5 were seeded in
6-well plates (2×103 cells/ml, 3 ml). Adipogenic medium
A (Cyanogen, Inc.) was added when cells reached 90% confluence and
was replaced by adipogenic medium B (Cyanogen, Inc.) after 3 days.
At 24 h, adipogenic medium B was replaced with adipogenic medium A,
and this cycle was repeated three times. The adipogenic induction
was then maintained by incubation in adipogenic medium A. Oil red-O
staining (Sigma-Aldrich; Merck KGaA) was performed for 15 min at
37°C following a 14 day-induction and cells were observed under an
inverted optical microscope (Olympus Corporation).
Osteogenic differentiation
UC-MSCs and DP-MSCs at passage 5 were resuspended at
a density of 5×103 cells/ml (3 ml) and seeded in 6-well
plates. Osteogenic medium (Cyanogen, Inc.) was added when cells
reached 60% confluence and replaced every 3 days. After 14 days,
the culture medium was removed, and cells were fixed with 4%
paraformaldehyde for 15 min at room temperature. Fixed MSCs were
then stained with alizarin red for 15 min at 37°C (Sigma-Aldrich;
Merck KGaA) and observed under an inverted optical microscope
(Olympus Corporation).
Detection of secreted factors
The double antibody sandwich method was used
according to the ELISA kit manufacturer's protocol (ExCell Biology)
to detect the content of basic fibroblast growth factor (bFGF; cat.
no. EH022-48), epidermal growth factor (EGF; cat. no. EH016-96),
keratinocyte growth factor (KGF; cat. no. EH122-96), stem cell
factor (SCF; cat. no. EH231-48), transforming growth factor-β
(TGF-β; cat. no. EH010-96) and vascular endothelial growth factor
(VEGF; cat. no. EH015-96) in culture supernatants of UC-MSCs and of
DP-MSCs. Supernatants were obtained from MSCs cultured for 3 days
and were centrifuged at 1,000 × g for 10 min. Briefly, 200 µl
standard substance and samples were added to coated wells and
incubated for 2 h at room temperature. After three washes, 200 µl
enzyme-labelled antibody was added to the wells and incubated for 1
h at room temperature. After a further three washes, 200 µl
substrate was added to the wells and incubated for 20 min at room
temperature. Stop buffer was added (50 µl/well) to terminate the
reaction. The absorbance value at 450 nm was measured by automatic
microplate reader within 30 min. The values of three replicates
were averaged for each sample. CurveExpert 1.4 software (Hyams
Development) was used to fit the curves and select the most
appropriate equation to calculate the amounts of cytokines.
Statistical analysis
SPSS 17.0 software (SPSS, Inc.) was used for
statistical analysis. Values are expressed as the means ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance with the least-significant difference post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Isolated culture of UC-MSCs and
DP-MSCs
The morphology of the two types of MSCs was compared
and the results revealed that the umbilical cord and the placental
wall decidua parietalis produced MSCs, which were spindle
fibroblast-like cells with spiral growth (Fig. 1). The cell bodies of DP-MSCs were
elongated compared with UC-MSCs.
 | Figure 1.Morphological observations of UC-MSCs
and DP-MSCs in different generations. (A-C) Morphology of UC-MSCs
in the first, second and fourth generation, respectively. (D-F)
Morphology of DP-MSCs in the first, second and fourth generation,
respectively. Scale bar, 200 µm. DP-MSCs, mesenchymal stem cells
derived from the decidua parietalis; P1, first generation; P2,
second generation; P4, fourth generation; UC-MSCs, mesenchymal stem
cells derived from the umbilical cord. |
UC-MSCs have a higher proliferative
rate and shorter cell doubling time compared with DP-MSCs
The proliferative rate of MSCs was used to assess
cell growth. The growth curves of the two types of MSCs exhibited a
similar pattern. During the same time period, the number of UC-MSCs
in the second and fifth generation was higher compared with the
number of DP-MSCs (Fig. 2A). The
cell doubling time in the second generation was 17.73±0.51 h for
the UC-MSC group and 21.93±0.72 h for the DP-MSC group, and the
difference between the two groups was significant (P<0.05). The
cell doubling time in the fifth generation was 14.50±0.81 h for the
UC-MSC group and 19.63±1.1 h for the DP-MSC group, and the
difference between the two groups was significant (P<0.05)
(Fig. 2B). The cell doubling time
of MSCs was evaluated after culturing the two groups (Fig. 2B).
UC-MSCs exhibit higher colony forming
efficiency compared with DP-MSCs
Colony forming efficiency of the two groups of MSCs
was evaluated following culture (Fig.
2C). The colony forming efficiency of the UC-MSCs was
significantly higher (16.00±1.41%) compared with that of DP-MSCs
(13.60±1.50%) in the second generation (P<0.05); the colony
forming efficiency was 12.80±0.75% in the UC-MSC group and
8.60±1.85% in the DP-MSC group in the fifth generation, and the
difference between the two groups was significant (P<0.05)
(Fig. 2C).
Stem cell marker expression on UC- and
DP-MSCs
MSCs from the UC and DP highly expressed the
characteristic cell surface markers of MSCs CD73, CD90 and CD105,
but did not express CD45 and CD34, which are characteristic cell
surface markers of haematopoietic stem cells. The positive
expression rate for CD73, CD90 and CD105 in UC-MSCs and DP-MSCs was
>95%, and the positive expression rate for CD45 and CD34 was
<1% (Table I; Fig. 3).
 | Table I.Stem cell marker expression on
MSCs. |
Table I.
Stem cell marker expression on
MSCs.
|
| Expression (%) |
|---|
|
|
|
|---|
| Surface marker | UC-MSCs (n=5) | DP-MSCs (n=5) |
|---|
| CD34 | 0.22±0.08 | 0.77±0.26 |
| CD45 | 0.17±0.10 | 0.34±0.12 |
| CD73 | 99.12±0.17 | 95.50±0.83 |
| CD90 | 98.83±0.86 | 98.63±1.04 |
| CD105 | 98.42±0.84 | 98.33±0.91 |
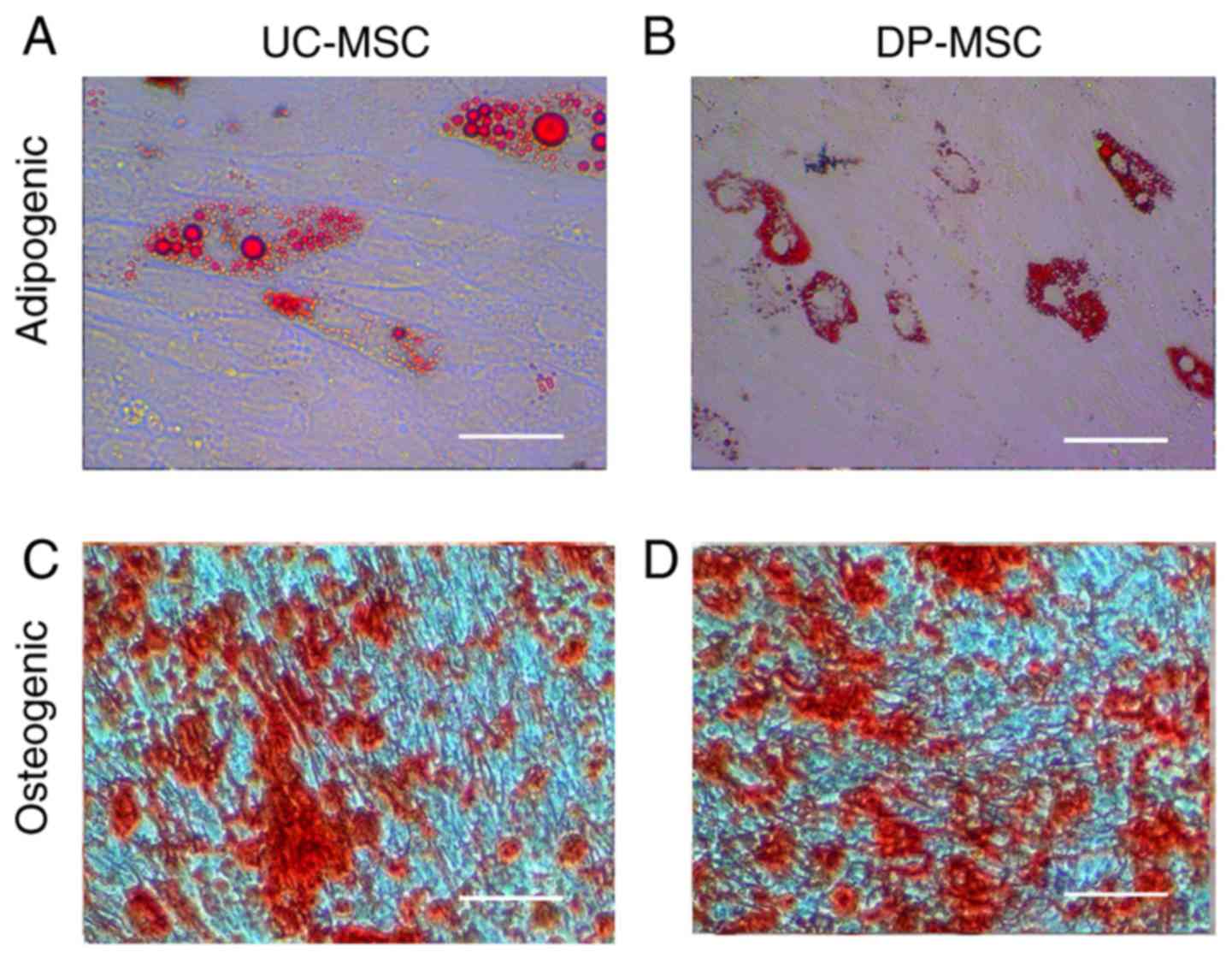
Differentiation of UC-MSCs and
DP-MSCs
After induction with adipogenic medium, UC-MSCs and
DP-MSCs gradually changed from fibroblast-like cells to flattened
cells, and lipid droplets accumulated within them. The adipogenic
differentiated MSCs were visualized by staining with Oil red-O on
day 15; cellular staining was positive and the multiple lipid
vacuoles in differentiated cells were stained red. After incubation
with osteogenic medium for 15 days, MSCs exhibited obvious
morphological alterations. Tightly packed colonies forming
nodule-like structures were observed and deposition of calcium in
these cells was observed by staining with alizarin red (Fig. 4).
Detection of cytokines secreted by
UC-MSCs and DP-MSCs
MSC-secreted cytokines were assessed using ELISA.
The results demonstrated that the bFGF content in cell supernatants
of UC-MSCs was significantly higher compared with that in DP-MSCs
(P<0.05; Fig. 5). The KGF, VFGF
and SCF contents in cell supernatants of UC-MSCs were significantly
lower compared with those of DP-MSCs (P<0.05). However, the
differences in EGF and TGF-β expression between UC-MSC and DP-MSC
supernatants were not significant (Fig. 5).
 | Figure 5.Comparison of bFGF, EGF, KGF, SCF,
TGF-β and VEGF levels in UC-MSCs and DP-MSCs. Secretion of bFGF in
UC-MSCs was higher compared with that in DP-MSCs. Secretion levels
of KGF, SCF and VEGF in UC-MSCs were lower compared with those in
DP-MSCs. No significant differences were observed between EGF and
TGF-β secretion in UC-MSCs and DP-MSCs. *P<0.05. bFGF, basic
fibroblast growth factor; DP-MSCs, mesenchymal stem cells derived
from the decidua parietalis; EGF, epidermal growth factor; KGF,
keratinocyte growth factor; SCF, stem cell factor; TGF-β,
transforming growth factor-β; UC-MSCs, mesenchymal stem cells
derived from the umbilical cord; VEGF, vascular endothelial growth
factor. |
Discussion
MSCs are important sources of stem cells for
regenerative medicine due to their high self-renewal, proliferation
and differentiation potential (19). MSCs are pluripotent stem cells
derived from mesodermal interstitial tissue of early development
(18), which are used for tissue
repair and regeneration during individual ontogenesis and
autoimmune diseases (20,21). Due to their high proliferation
rates and multifunctional differentiation ability, MSCs can
differentiate into endometrial cells, osteoblasts, chondrocytes and
cardiomyocytes under certain conditions (22–25).
MSCs have been successfully isolated from a number of adult tissues
(26), such as adipose tissue
(27), bone marrow (28) and umbilical tissue (29). However, MSCs from various tissue
sources may have different biological characteristics. In the
present study, the characteristics of UC-MSCs and DP-MSCs were
analysed. The results revealed that the MSCs from the two sources
demonstrated some biological distinctions with regards to growth
characteristics and cytokine secretion, which may provide profound
understanding of their biological functions.
In the present study, established methods (30) were used to isolate and culture MSCs
from the umbilical cord and the placenta decidua parietalis tissue.
UC-MSCs and DP-MSCs exhibited similar cell growth characteristics,
surface markers, multipotent differentiation capabilities and
paracrine ability. UC-MSCs and DP-MSCs were similar morphologically
and had a long fusiform shape and spiral growth, being grown under
the same conditions. However, the doubling time of UC-MSCs was
shorter compared with that of DP-MSCs, and the colony formation
rate of UC-MSCs was higher compared with that of DP-MSCs at the
same generation, possibly owing to a stronger proliferative
capacity of UC-MSCs compared with that of DP-MSCs. Therefore,
UC-MSCs may be used as a source of stem cells for the treatment of
a number of diseases. Additionally, UC-MSCs and DP-MSCs had similar
cell surface markers. The data revealed that the positive rates of
CD73, CD90 and CD105 in each of the two MSCs groups surpassed 95%,
whereas those of CD34 and CD45 were <1%. Both UC-MSCs and
DP-MSCs had similar multilineage differentiation potential capacity
towards osteogenesis and adipogenesis. These results indicated the
feasibility of the isolation and culture of MSCs from umbilical
cord and placenta wall decidual tissue.
A number of cytokines and signalling factors are
secreted by MSCs, and MSCs from different species and sources
produce different factors (31).
MSC-secreted cytokines, such as bFGF, KGF, VEGF and SCF, serve
important roles in cell proliferation, differentiation, growth and
tissue repair (32–34). However, the characteristics of
proteins secreted by UC-MSCs and DP-MSCs have not been extensively
studied. Transplanted neural stem cells with high expression of
bFGF have been demonstrated to promote cell migration and
functional recovery following transient ischaemic stroke in rats
(35). The results of the present
study indicated that UC-MSCs and DP-MSCs secreted different
cytokines; UC-MSCs exhibited a higher expression level of bFGF, and
lower levels of KGF, VEGF and SCF compared with DP-MSCs, which
suggested that these two types of tissue have different capacities
for the secretion of signalling factors. These results may provide
a theoretical basis for the study of stem cell therapy.
In summary, the present study compared the
morphological and molecular characteristics of DC-MSCs and UC-MSCs.
The results demonstrated clear biological distinctions between
UC-MSCs and DP-MSCs, and revealed that UC-MSCs had a higher
proliferative rate and colony forming efficiency compared with
DP-MSCs. Therefore, UC-MSCs may have better application prospects.
However, only two different sources of MSCs and several biological
parameters were studied and compared in the present study.
Nevertheless, the data extend the characterization of MSCs derived
from different tissue types. As stem cell research develops, more
potential applications of stem cells are being discovered,
including injury repair (36). As
cell therapy products are used in the human body, it is necessary
to establish quality control and quality assurance systems, which
will ensure product safety, efficacy and stability. The present
study laid the foundation for the performance of clinical trials
focussing on stem cell therapy. More extensive studies are required
to facilitate clinical applications of MSCs.
Acknowledgements
Not applicable.
Funding
This research was supported by the Science and
Technology Programs of Foshan (grant no. 2017AB002801) and Medical
Research Foundation of Guangdong Province, China (grant no.
B2018034).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YTG and GW designed the study. DSL, YYZ, YPC and LJX
performed the experiments and analysed the data. YX and PFL
collected the samples. XLZ and YLF performed flow cytometry and
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures in the present study were approved by
the Ethical Committee of The First People's Hospital of Foshan and
written informed consent was obtained from all donors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MSCs
|
mesenchymal stem cells
|
|
KGF
|
keratinocyte growth factor
|
|
bFGF
|
basic fibroblast growth factor
|
|
DMEM/F12
|
Dulbecco's modified Eagle's
medium/F12
|
|
EGF
|
epidermal growth factor
|
|
SCF
|
stem cell factor
|
|
VEGF
|
vascular endothelial growth factor
|
|
TGF-β
|
transforming growth factor-β
|
References
|
1
|
Kristjansson B and Honsawek S: Mesenchymal
stem cells for cartilage regeneration in osteoarthritis. World J
Orthop. 8:674–680. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bao R, Xu P, Wang Y and Wang J: Bone
marrow derived mesenchymal stem cells transplantation rescues
premature ovarian insufficiency induced by chemotherapy. Gynecol
Endocrinol. 34:1–7. 2017.PubMed/NCBI
|
|
3
|
Sibov TT, Severino P, Marti LC, Pavon LF,
Oliveira DM, Tobo PR, Campos AH, Paes AT, Amaro E Jr, F Gamarra L
and Moreira-Filho CA: Mesenchymal stem cells from umbilical cord
blood: Parameters for isolation, characterization and adipogenic
differentiation. Cytotechnology. 64:511–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huntsman HD, Zachwieja N, Zou K, Ripchik
P, Valero MC, De Lisio M and Boppart MD: Mesenchymal stem cells
contribute to vascular growth in skeletal muscle in response to
eccentric exercise. Am J Physiol Heart Circ Physiol. 304:H72–H81.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mahmoud EE, Kamei N, Kamei G, Nakasa T,
Shimizu R, Harada Y, Adachi N, Misk NA and Ochi M: Role of
mesenchymal stem cells densities when injected as suspension in
joints with osteochondral defects. Cartilage. 10:61–69. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sabry D, Mostafa A, Marzouk S, Ibrahim W,
Ali HHM, Hassan A and Shamaa A: Neupogen and mesenchymal stem cells
are the novel therapeutic agents in regeneration of induced
endometrial fibrosis in experimental rats. Biosci Rep. 37(pii):
BSR201707942017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Ju B, Pan C and Zhang Y, Sun L,
Zhang B and Zhang Y: Application of bone marrow-derived mesenchymal
stem cells in the treatment of intrauterine adhesions in rats. Cell
Physiol Biochem. 39:1553–1560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao GP, Choi Y, Vojnits K, Xue H, Aroom
K, Meng F, Pan HY, Hetz RA, Corkins CJ, Hughes TG, et al: Tissue
engineering to repair diaphragmatic defect in a rat model. Stem
Cells Int. 2017:17645232017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Spigna G, Iannone M, Ladogana P,
Salzano S, Ventre M, Covelli B, De Marinis E and Postiglione L:
Human cardiac multipotent adult stem cells in 3D matrix: New
approach of tissue engineering in cardiac regeneration
post-infarction. J Biol Regul Homeost Agents. 31:911–921.
2017.PubMed/NCBI
|
|
10
|
Fu Y, Karbaat L, Wu L, Leijten J, Both SK
and Karperien M: Trophic effects of mesenchymal stem cells in
tissue regeneration. Tissue Eng Part B Rev. 23:515–528. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alawadhi F, Du H, Cakmak H and Taylor HS:
Bone marrow-derived stem cell (BMDSC) transplantation improves
fertility in a murine model of asherman's syndrome. PLoS One.
9:e966622014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azizi R, Aghebati-Maleki L, Nouri M,
Marofi F, Negargar S and Yousefi M: Stem cell therapy in asherman
syndrome and thin endometrium: Stem cell-based therapy. Biomed
Pharmacother. 102:333–343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Biancone L, Bruno S, Deregibus MC, Tetta C
and Camussi G: Therapeutic potential of mesenchymal stem
cell-derived microvesicles. Nephrol Dial Transplant. 27:3037–3042.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joerger-Messerli MS, Marx C, Oppliger B,
Mueller M, Surbek DV and Schoeberlein A: Mesenchymal stem cells
from wharton's jelly and amniotic fluid. Best Pract Res Clin Obstet
Gynaecol. 31:30–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim IJ and Phan TT: Epithelial and
mesenchymal stem cells from the umbilical cord lining membrane.
Cell Transplant. 23:497–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu LN, Lin N, Xu BN, Li JB and Chen SQ:
Effect of human umbilical cord mesenchymal stem cells on
endometriotic cell proliferation and apoptosis. Genet Mol Res.
14:16553–16561. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harris DT: Umbilical cord tissue
mesenchymal stem cells: Characterization and clinical applications.
Curr Stem Cell Res Ther. 8:394–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoon DS, Choi Y and Lee JW: Cellular
localization of NRF2 determines the self-renewal and osteogenic
differentiation potential of human MSCs via the P53-SIRT1 axis.
Cell Death Dis. 7:e20932016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Furtado MB, Nim HT, Gould JA, Costa MW,
Rosenthal NA and Boyd SE: Microarray profiling to analyse adult
cardiac fibroblast identity. Genom Data. 2:345–350. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uccelli A and Prockop DJ: Why should
mesenchymal stem cells (MSCs) cure autoimmune diseases? Curr Opin
Immunol. 22:768–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sahoo AK, Das JK and Nayak S: Isolation,
culture, characterization, and osteogenic differentiation of canine
endometrial mesenchymal stem cell. Vet World. 10:1533–1541. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zanetta M, Quirici N, Demarosi F, Tanzi
MC, Rimondini L and Farè S: Ability of polyurethane foams to
support cell proliferation and the differentiation of mscs into
osteoblasts. Acta Biomater. 5:1126–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sekine W, Haraguchi Y, Shimizu T, Yamato
M, Umezawa A and Okano T: Chondrocyte differentiation of human
endometrial gland-derived mscs in layered cell sheets.
ScientificWorldJournal. 2013:3591092013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yannarelli G, Tsoporis JN, Desjardins JF,
Wang XH, Pourdjabbar A, Viswanathan S, Parker TG and Keating A:
Donor mesenchymal stromal cells (MSCs) undergo variable cardiac
reprogramming in vivo and predominantly co-express cardiac and
stromal determinants after experimental acute myocardial
infarction. Stem Cell Rev. 10:304–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prockop DJ: Repair of tissues by adult
stem/progenitor cells (MSCs): Controversies, myths, and changing
paradigms. Mol Ther. 17:939–946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim YJ, Kim HJ and Im GI: PTHrP promotes
chondrogenesis and suppresses hypertrophy from both bone
marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res
Commun. 373:104–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prockop DJ, Kota DJ, Bazhanov N and Reger
RL: Evolving paradigms for repair of tissues by adult
stem/progenitor cells (MSCs). J Cell Mol Med. 14:2190–2199. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miranda JP, Filipe E, Fernandes AS,
Almeida JM, Martins JP, De la Fuente A, Abal M, Barcia RN, Cruz P,
Cruz H, et al: The human umbilical cord tissue-derived MSC
population UCX(®) promotes Early motogenic effects on
keratinocytes and fibroblasts and G-CSF-mediated mobilization of
BM-MSCs when transplanted in vivo. Cell Transplant. 24:865–877.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blanco JF, Graciani IF, Sanchez-Guijo FM,
Muntión S, Hernandez-Campo P, Santamaria C, Carrancio S, Barbado
MV, Cruz G, Gutierrez-Cosío S, et al: Isolation and
characterization of mesenchymal stromal cells from human
degenerated nucleus pulposus: Comparison with bone marrow
mesenchymal stromal cells from the same subjects. Spine (Phila Pa
1976). 35:2259–2265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Majumdar MK, Thiede MA, Haynesworth SE,
Bruder SP and Gerson S: Human marrow-derived mesenchymal stem cells
(MSCs) express hematopoietic cytokines and support long-term
hematopoiesis when differentiated toward stromal and osteogenic
lineages. J Hematother Stem Cell Res. 9:841–848. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marquez MP, Alencastro F, Madrigal A,
Jimenez JL, Blanco G, Gureghian A, Keagy L, Lee C, Liu R, Tan L, et
al: The role of cellular proliferation in adipogenic
differentiation of human adipose tissue-derived mesenchymal stem
cells. Stem Cells Dev. 26:1578–1595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carrington LM and Boulton M: Hepatocyte
growth factor and keratinocyte growth factor regulation of
epithelial and stromal corneal wound healing. J Cataract Refract
Surg. 31:412–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chi Y, Jin Y, He Z and Yu T: Detection of
cytokines in supernatant from hematopoietic stem/progenitor cells
co-cultured with mesenchymal stem cells and endothelial progenitor
cells. Cell Tissue Bank. 15:397–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang JJ, Zhu JJ, Hu YB, Xiang GH, Deng
LC, Wu FZ, Wei XJ, Wang YH, Sun LY, Lou XQ, et al: Transplantation
of bFGF-expressing neural stem cells promotes cell migration and
functional recovery in rat brain after transient ischemic stroke.
Oncotarget. 8:102067–102077. 2017.PubMed/NCBI
|
|
36
|
Wang S, Qu X and Zhao RC: Clinical
applications of mesenchymal stem cells. J Hematol Oncol. 5:192012.
View Article : Google Scholar : PubMed/NCBI
|