Introduction
Several studies have indicated that short telomere
length is associated with aging-related diseases, including
cardiovascular diseases (CADs), stroke, cancer, arthritis,
osteoporosis, cataracts, diabetes type 2, hypertension, mental
diseases, chronic obstructive pulmonary disease (COPD) and dementia
(1). Telomere shortening can be
affected by environmental factors, including physical activity,
body mass index (BMI), hormone replacement therapy, smoking,
chronic inflammation, oxidative stress, dietary antioxidants and
vitamins (2–5). For instance, DNA-damage caused by
various environmental factors triggers a DNA-damage response at
telomeres that protects them from instability and shortening
(6,7). Moreover, Vakonaki et al
demonstrated an association between telomere length and drug abuse,
which leads to premature biological aging (8). Telomere length has been proposed to
be a biomarker of somatic cell aging, while the rate of increase of
short telomeres has been linked to longevity in mammals (9). Indeed, when the length of the
telomeres shortens below a threshold limit, cell growth is
restricted and cells undergo cellular senescence or apoptosis
(10). In a recent study, it was
found that the administration of nutraceutical supplements may be
linked to sustaining the telomere length in healthy adults
(11). To determine the rate of
telomere shortening and increase in the percentage of short
telomeres with aging, we generated ‘BIOTEL version 2.4’ that was
validated using data from Telomere Length Database Project (TLDP)
(12), and allows the easy
production of graphs and track telomere shortening in response to
stimuli.
The shortening of telomeres can be reversed by the
enzyme telomerase, which is active in high-proliferating cells,
such as in male germ cells, activated lymphocytes, stem cells and
cancer cells (13,14). It consists of two domains, namely a
reverse transcriptase catalytic subunit (TERT) and an associated
telomerase RNA component (TERC) (15). However, the majority of adult human
somatic cells are telomerase-deficient and their proliferation
contributes to progressive telomere shortening with age, ultimately
leading to aging and death(16). In addition, telomerase-related
gene mutations result in the development of certain diseases, such
as Dyskeratosis Congenita (DKC) that is the first disease to be
associated with mutations in human telomerase gene (17). Telomerase mutations have also been
detected in aplastic anemia, Hoyeraal-Hreidarsson syndrome and
idiopathic pulmonary fibrosis, while numerous epidemiological
studies have demonstrated that telomerase activity is associated
with pregnancy complications (18,19)
and mental disorders (20). Thus,
based on all the above, telomerase activators may be potent agents
in anti-aging and in the treatment of telomerase-dependent
diseases. It has been further demonstrated that telomerase
activators enhance the efficiency of the DNA repair process and
protect cells from stress and DNA-damaging conditions (21). Telomerase activation has been
achieved through natural molecules, synthetic molecules and genetic
manipulation and intervention (22). Several extracts from the
Astragalus membranaceus root have been studied as possible
telomerase activators (22–26).
Such an extract is TA-65, a natural product telomerase activator
marketed since 2008, that has been found to lengthen telomeres in
humans (23). A previous in
vitro study on human CD4 and CD8 T-cells suggested that
cycloastragenol (CAG), a triterpenoid saponin compound obtained
from Astragaloside IV hydrolysis that is the main compound in
Astragalus, increased telomerase activity and reduced the
effects of aging (24). Product B,
a herb nutraceutical that contains ‘telomere support’ compounds and
antioxidants, has also been suggested to be a potent telomerase
activator, although no long-term test data are currently
available.
The aim of the present study was to test supplements
and natural extracts for their capacity to enhance telomerase
activity in human peripheral blood mononuclear cells (PBMCs). We
demonstrate that Centella asiatica extract formulation
(08AGTLF) can lead to significantly higher telomerase activation
compared to untreated cells, as well as TA-65 and other supplements
containing Astragalus extract and CAG. This is the first
study, at least to our knowledge, to demonstrate that a natural
formulation, such as Centella asiatica extract formulation
(08AGTLF) can lead to such high telomerase activity relative to
control cells.
Materials and methods
Formulations
Centella asiatica extract formulation
(08AGTLF) which consisted of >95% high-purity triterpenes was
obtained from ApexBio Company. Oleanolic acid (OA) was obtained
from Sigma-Aldrich and maslinic acid (MA) was obtained from
Extrasynthese. Nutrient 1 and Nutrient 2 (contents shown below)
were obtained from Lumis Research S.A. Nutrient 3 and Nutrient 4
(contents shown below) were obtained from Natural Doctor S.A. Each
compound was dissolved in ethanol to achieve various concentrations
to be tested in the cell cultures.
Cell isolation and telomerase activity
measurements
The protocol of this study was approved by the
Ethics Committee for Patients and Biological Material of the
University of Crete with reference no. 63/22.03.2019. All
procedures performed involving human participants were carried out
under the ethical standards of the 1964 Helsinki declaration and
its later amendments, or comparable ethical standards. The study
was performed using samples prepared from healthy donors that
volunteered to participate in the study. The samples were
anonymized and personal data were managed according to the EU
General Data Protection Regulation (GDPR).
PBMCs where isolated from the blood samples by
Ficoll-Hypaque gradient centrifugation. The cells were grown in
DMEM (Biochrom AG; F0455) supplemented with 10% fetal bovine serum
(FBS; 10500-064, heat-inactivated; Invitrogen; Thermo Fisher
Scientific), glutamine (4 mM; Biosera XCT1715), gentamycin
(15710–049; Invitrogen; Thermo Fisher Scientific) and
penicillin/streptomycin (100 U/ml; Biosera LMA4118). Prior to the
addition of the test agents, the cells were cultured in serum-free
medium for 24 h at 37°C and 5% CO2. The PBMCs were then
treated with the compounds at various concentrations, for 24–72 h.
PBMCs samples were collected at 24–72 h following treatment, washed
in PBS buffer and stored at −80°C. Telomerase activity was measured
using a commercial telomerase PCR-ELISA (Sigma-Aldrich), based on
the telomeric repeat amplification protocol, as previously
described (27–30). All treatments for each condition
were performed in triplicates.
Contents of Nutrients 1, 2, 3 and
4
Nutrient 1 (My Shape) contained the following: Alpha
lipoic acid, cinnamon) bark dry extract 1/4 (Cinnamomum
zeylanycum N.), magnesium citrate, L-glutamine, L-carnitine
tartrate, potassium citrate, ascorbic acid, magnesium ascorbate,
green tea (Camellia sinensis K.) leaves dry extract titrated
to 95% polyphenols, natural vitamin E acetate 50%, enzimix
(amylase, protease, glucose amilase, lipase, cellulase, lactase,
pectinase), niacin, bitamin B1, vitamin K2 Mena Q7 0.2%, selenium
methionine, vitamin B2, β-carotene, vitamin B5, choline bitartrate,
inositol, para-aminobenzoic acid (PABA), vitamin B6, vitamin B12
1%, chromium picolinate, vitamin D3 2.5%, biotin, folic acid,
anti-caking agent (cellulose, mono- and diglycerides of fatty acid,
magnesium stearate, silica dioxide).
Nutrient 2 (My Health) contained the following: Mix
Vitamin (ascorbic acid, vitamin E acetate 50% natural, niacin,
vitamin B1, vitamin K2 Mena Q7 0.2%, vitamin B6, β-carotene,
vitamin B12 1%), anti-caking agents (microcrystalline cellulose,
mono- and diglycerides of fatty acids, magnesium stearate, silica
dioxide).
Nutrient 3 (Vit. D3&K2 Cofactors, 1 capsule)
contained the following: 2,000 OH25D3, 100 µg
vitamin K2 (MK7), 56 mg elemental magnesium as magnesium
bisglycinate.
Nutrient 4 (REYOUTH, 1 capsule) contained the
following: Vitamin C (50 mg), Magnesium (58 mg), CAG (16 mg) and
amino-acids mix containing L-glutamine, L-lysine, L-proline,
L-glycine, L-arginine, L-leucine, L-histidine, L-isoleucine,
L-valine, L-methionine, L-tyrosine, glutamic acid, L-phenylalanine,
L-serine, L-threonine, L-alanine, L-citrulline, L-taurine,
L-tryptophan, aspartic acid, Vitamin E (12 mg), calcium, Vitamin
B3, Broccoli dry extract, dry fruit and vegetable extract, blend of
digestive enzymes (Enzymix), potassium, Vitamin B1, Zinc, Vitamin
B6, manganese, phosphorus, B-carotene, Vitamin B5, inositol,
Vitamin K2, Vitamin B2, PABA, Vitamin D3, biotin, chromium, copper,
selenium, molybdenum, Vitamin B12.
TA-65 (1 capsule) contained the following:
Astragalus membranaceus moench extract (TA-65®MD, 8
mg).
Statistical analysis
The mean (xm), standard deviation (SD) and the
estimated approximated 95% confidence interval (95% CI) (xm±1.96
SD/√n (where n -n=3- was the number of replications) of the
absorbance values were applied. All experiments were performed in
triplicates and the mean values were used for the data presentation
of differences of telomerase activity triggered by each formulation
vs. the control, expressed as P-values resulting from one-way ANOVA
followed by Dunnett's post-hoc test for pairwise comparisons with
untreated cells. All statistical analyses were performed in IBM
SPSS Statistics 24.0 and diagrams were created using Excel 365 for
Windows (Microsoft Corp.) and a value of P<0.05 was considered
to indicate a statistically significant difference.
Results
A summary of the compounds used and the calculated
concentrations (µg/ml) in vitro is presented in Table I. Fig.
1 depicts the mean values of the telomerase activity (expressed
in absorbance units, A450nm-A690nm) of cells
treated with the formulations and compounds (08AGTLF, TA-65,
Nutrient 4, OA and MA) in comparison with the ethanol-only treated
cells, hereafter referred as untreated cells. Importantly, all the
compounds tested were not toxic for the cells, as they only led to
small amount of apoptosis or necrosis (13–15%), similar with the
untreated cells (13%; data not shown). 08AGTLF exhibited the
highest telomerase activity, 1.35 absorbance units (95% CI,
1.154–1.546) at the concentration of 0.02 µg/ml and 1.18 (95% CI,
1.088–1.278) at the concentration of 0.2 µg/ml, while it decreased
at the concentration of 2 µg/ml. The difference in telomerase
activity reached the levels of 8.8-fold increase relative to the
untreated cells at the concentration of 0.02 µg/ml. Importantly,
the differences in telomerase activity of the treated cells
compared to the untreated ones were statistically significant with
P-values <0.001 and <0.0001 for the 0.02 and 0.2 µg/ml
concentrations, respectively.
 | Table I.Concentrations in µg/ml of all the
formulations and compounds used for measuring telomerase activity
in PBMCs. |
Table I.
Concentrations in µg/ml of all the
formulations and compounds used for measuring telomerase activity
in PBMCs.
| Nutrients | Concentration
(µg/ml) |
|---|
| Nutrient 1 | 20, 120, 600 |
| Nutrient 2 | 10, 60, 330 |
| Nutrient 3 | 4, 20, 100 |
| Nutrient 4 | 12.8, 25, 51 |
| 08AGTLF | 0.02, 0.2, 2 |
| TA-65 | 0.16, 0.32,
0.64 |
| OA | 1, 5, 10 |
| MA | 1, 5, 10 |
Telomerase activity levels increased with all the 3
concentrations used for Nutrient 4 compared to the untreated cells
(up to 4.3-fold increase) with the highest activation at the
concentration of 12.8 µg/ml (absorbance 0.38; 95% CI, 0.311–0.456)
and followed a slightly decreasing pattern at the concentrations of
25 and 51 µg/ml (95% CI, 0.321–0.426 and 0.320–0.414,
respectively). In addition, the difference in telomerase activity
relative to the untreated cells were all statistically significant,
with P<0.01 for the concentration of 12.8 µg/ml and P<0.001
for the concentrations of 25 and 52 µg/ml (Fig. 1).
Treatment with TA-65 also exhibited telomerase
activation compared to the untreated cells (approximately 2-fold
increase). The highest values were acquired at the concentration of
0.16 and 0.32 µg/ml (95% CI, 0.197–0.210 and 0.190–0.203,
respectively), while there was also a small activation at the
concentration of 0.64 µg/ml (95% CI, 0.155–0.182). Importantly,
differences in telomerase activity compared to the untreated cells
were statistically significant in all the 3 concentrations
(P<0.001; Fig 1).
OA and MA triggered higher levels of telomerase
activation at the concentrations of 1 and 10 µg/ml, respectively.
Telomerase activation was significantly higher, approximately
6-fold, at 1 µg/ml of OA treatment (95% CI, 0.391–0.549) and
approximately 2-fold higher at 10 µg/ml of MA treatment (95% CI,
0.197–0.210), compared to the untreated cells. The increase in
telomerase activity was statistically significant for the
treatments with 1 and 5 µg/ml for OA with P<0.001 and P<0.01,
respectively, and 10 µg/ml for MA with P<0.001.
Fig. 2 depicts
telomerase activity measured in absorbance units in the cells
treated with Nutrient 1, Nutrient 2 and Nutrient 3 compared with
the untreated cells. Telomerase activity triggered by Nutrient 1
did not differ significantly compared to the untreated cells at any
of the concentrations used (20, 120 and 600 µg/ml) and the P-values
for the difference in telomerase activity at the same
concentrations compared to the untreated were higher than 0.05.
Nutrient 2 triggered a 1.5-fold increase in telomerase activity
(95% CI, 0.119–0.154) that was statistically significant at the
concentration of 330 µg/ml (P<0.05), but not at the other 2
concentrations used (10 and 60 µg/ml) that remained at levels
similar with the untreated cells with P-values higher than 0.05.
Finally, Nutrient 3 exerted a greater effect on telomerase activity
(95% CI, 0.157–0.203) at the concentration of 100 µg/ml relative to
the untreated that was statistically significant (P<0.01), but
not at the concentrations of 4 and 20 µg/ml which had values
similar to the untreated cells (P>0.05).
The increase in telomerase activity triggered in
PBMCs treated with the natural activators can be also expressed as
telomerase activation relative to the positive control that is
usually telomerase activity in a cancer cell line. In this study,
the positive control was HeLa extract telomerase activity that
corresponded to an absorbance value of 7.8. It is common to measure
telomerase activation in a cell line relative to a cancer cell line
telomerase activity that reaches very high levels, in order to show
the potency of an activator (according to the telomerase PCR-ELISA
kit instructions). According to our results, following treatment
with 08AGTLF, telomerase activity reached the 17.3% of the positive
control, while after treatment with Nutrient 4 and TA-65 it reached
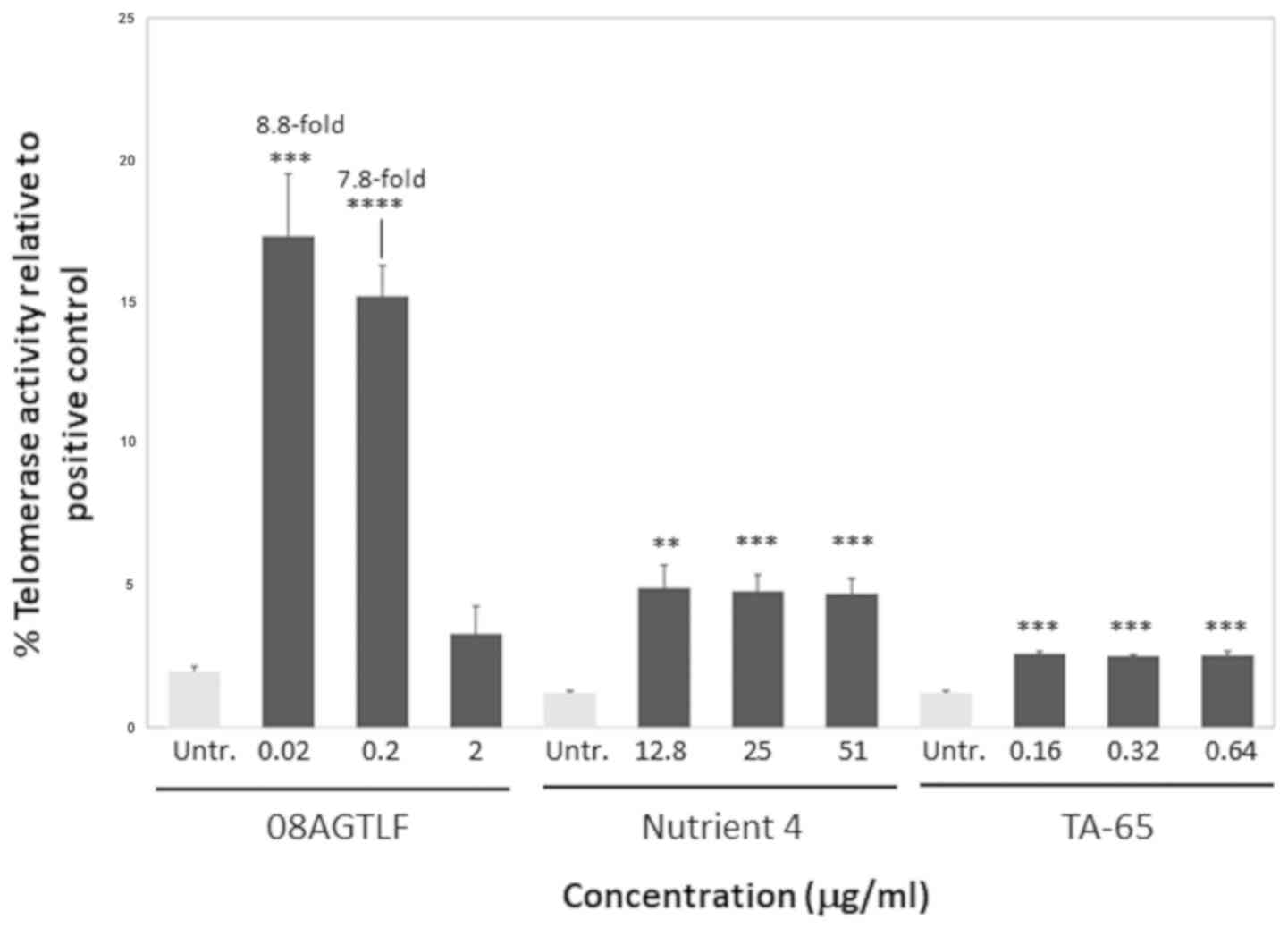
the 5.5 and 2.6% of the positive control, respectively (Fig. 3).
Discussion
Telomerase activators are important for anti-aging
and telomerase-dependent disease treatments, since telomere
shortening has been associated with cellular aging and
telomerase-related gene mutations with several diseases (31). In the current study, we
characterized the effects of 08AGTLF, TA-65, MA, OA, and Nutrients
1, 2, 3 and 4 for their ability to induce telomerase activity in
PBMCs. The active constituents of 08AGTLF, TA-65, OA and MA include
pentacyclic triterpene derivatives. Herein, we demonstrate that
08AGTLF, Nutrient 4, TA-65, OA and MA trigger different levels of
telomerase activation with the most potent of the compounds being
the formulation containing 08AGTL at 0.02 µg/ml concentration
(1.35).
We demonstrated that 08AGTL formulation containing
Centella asiatica extract was able to trigger an almost
9-fold increase in telomerase activity compared to the untreated
cells, much higher than the rest of the compounds used in this
study, suggesting that it could be a novel strong natural
telomerase activator with important anti-aging effects. Centella
asiatica is a widely used Ayurvedic medicine and traditional
Chinese medicine, which has been shown to be effective in improving
cognitive ability, increasing antioxidant response, as well as
treating wound healing disturbances (32–34).
In particular, Gray et al investigated the effect of
Centella asiatica on cognitive ability, as well as
mitochondrial and antioxidant response pathways in healthy mice. It
was shown that treatment with Centella asiatica enhanced
cognitive ability in mice and led to higher expression of
mitochondrial and antioxidant genes in the brain and liver, which
could contribute to cognitive improvement (33). Moreover, it has been suggested that
Centella asiatica can heal wounds due to the specific plant
chemicals that it contains, known as triterpenoid saponins.
Somboonwong et al reported the wound healing activities of
sequential hexane, ethyl acetate, methanol and water extract of
Centella asiatica in incision and partial-thickness burn
wound models in rats. It was found that all extracts of Centella
asiatica facilitated the wound healing process in both
incisions and burn wounds due to the formulation inhibiting
bacterial growth, fueling the growth of new skin cells and
increasing skin ‘tensile strength’ and resilience (34).
Formulations that included Astragalus extract with
different compositions (e.g. Nutrient 4 and TA-65) also exhibited
statistically significant effect on telomerase activity, but much
lower compared with 08AGTLF, reaching a 4.3-fold increase for
Nutrient 4 and 2-fold increase for TA-65 relative to the untreated
cells. In agreement with our results, Molgora et al
demonstrated that TA-65 containing CAG, an algycone of
Astragaloside IV, increased telomerase activity significantly 1.3
to 3.3 folds relative to controls in human T-cells cultures.
Similarly, it has been shown that CAG activates telomerase both
in vitro and in vivo (22). In particular, it has been shown
that CAG activates telomerase and lengthens telomeres in a
telomerase-dependent manner in vitro and decreases the
percentage of critically short telomeres and DNA damage in the cell
(35).
Notably, Nutrient 4, a mixture of nutrients that
contains CAG of Astragalus extract exhibited a higher effect on
telomerase activity compared to TA-65 (4.3-fold increase),
suggesting the synergistic effect of Astragalus extract with the
other nutrients contained in Nutrient 4. In a recent study by Bruno
et al the authors examined the effects of a multivitamin
supplement on telomere length and they suggested that telomerase
activation mediated by this supplement resulted in higher telomere
length (36).
Furthermore, we demonstrated that MA and OA were
also potent activators of telomerase in specific concentrations,
leading to a 5.9-fold and 2-fold increase in telomerase activity
relative to the untreated, respectively. MA is a bioactive
pentacyclic triterpenoid and has been associated with a low
incidence of inflammation-related diseases (37). Fukumitsu et al demonstrated
that MA, which was extracted from olive fruit, exerted an
anti-inflammatory effect in humans. This study, that included
middle-aged and elderly volunteers with mild knee joint pain,
demonstrated that MA at the concentration of 50 mg/day improved
joint pain by promoting weight loss (37), while in another study, MA had a
positive effect on the resistance to oxidative stress in animals
(38). Moreover, Nur and Al-Jasabi
determined the significant antioxidant properties of MA extracted
from Plumeria rubra leaves, by performing quantitative and
qualitative biochemical analysis (39). Similarly, OA is a pentacyclic
triterpenoid widely found in plants, including fruits and
vegetables that has been suggested to have a variety of
pharmacological activities (40).
However, very little is known about its effects on anti-aging.
Zhang et al investigated whether OA has an effect on
longevity in vivo in Caenorhabditis elegans and they
showed that indeed OA could extend the lifespan by increasing
resistance to stress and reducing the intracellular reactive oxygen
species in wild-type worms (41).
Another study evaluating the anti-wrinkle effects of OA, showed
that not only it was innocuous to human skin fibroblasts, but could
also significantly decrease the expression of both matrix
metalloproteinase (MMP)-1 and MMP-2, and increase that of collagen
type I alpha 1 chain (COL1A1), thus promoting collagen synthesis
(42).
There are numerous studies that have associated
nutraceutical supplementation with telomerase activity, telomere
length and oxidative stress and it should be noted that natural
products containing more than one antioxidant are more effective
than the administration of a single one, suggesting a synergistic
effect among these compounds (43,44).
In this study, we tested Nutrient 1 and Nutrient 2, which consist
of a mix of vitamins and antioxidants and found that these
supplements trigger a slight increase in telomerase activity. In
agreement with this, Balcerczyk et al examined the effect of
a diet supplement on parameters related to redox homeostasis and
aging, and found that telomerase activity in PBMCs from healthy
women, increased by >25% (45).
Surprisingly, Nutrient 3, which contains vitamin D, led to a
significantly higher increase in telomerase activity (around 2-fold
increase), when compared to the untreated cells. This finding is in
accordance with the fact that vitamin D supplementation
significantly increased PBMC telomerase activity in overweight
African Americans, as shown by Zhu et al, suggesting that
vitamin D may improve telomere maintenance, as well as prevent cell
senescence and obesity-induced acceleration of cellular aging
(46).
In conclusion, according to our in vitro
model, an increase in telomerase activity between 2 to 9 folds
compared with the untreated cells was observed with our tested
molecules. Importantly the 08AGTL formulation containing
Centella asiatica extract was the most potent activator
among other commercially available supplements causing an almost
9-fold increase in telomerase activity at 0.02 µg/ml. Moreover, the
potency of 08AGTL in increasing telomerase activity was evident
when translated in telomerase activation relative to the positive
control, since it reached the 17.3% of the telomerase activity of
the positive control, significantly higher percentage than the rest
of the compounds tested (Fig. 3).
The aim of this study was to identify natural compounds that
significantly increase telomerase activation, and may lead to a
longer life expectancy and healthy aging. 08AGTLF, containing
Centella asiatica extract, seems to be such a natural
compound with a strong effect on telomerase activity that remains
to be validated with future research based on independent
randomized controlled studies investigating the underlying
mechanisms. Importantly, future intervention studies on humans are
warranted to examine its effect on telomere length, aging and human
health.
Acknowledgements
The study is part of the special part of the Ph.D.
thesis from the University of Medicine and Pharmacy and Craiova.
The authors would like to thank all the administrative, the
technical and the medical staff of Toxplus S.A., the Metabolomic
Medicine Health Clinic S.A., and the Laboratory of Toxicology for
their dedicated involvement in this study.
Funding
This study was funded by Metabolomic Medicine S.A.
and Toxplus S.A. and supported by the Special Research Account of
University of Crete (ELKE nos. 4602, 4920 and 3963).
Availability of data and materials
The datasets presented in this study are available
from the corresponding author upon reasonable request.
Authors' contributions
DT, PF, AT, DAS and DC conceived and designed the
study and wrote the manuscript. MT, PF, MPR and ES performed the
data processing and quality control assessment. AKA, AOD and MT
performed the statistical analysis and data interpretation. All
authors have reviewed and approved the manuscript before
submission.
Ethics approval and consent to
participate
The protocol of this study was approved by the
Ethics Committee for Patients and Biological Material of the
University of Crete with reference number 63/22.03.2019. Biological
Material and information of patients were obtained with written
informed consent according to the EU General Data Protection
Regulation (GDPR). All procedures performed in studies involving
human participants were under the ethical standards with the 1964
Helsinki declaration and its later amendments, or comparable
ethical standards.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
DT is a scientific advisor for Lumis Research S.A. and Natural
Doctor S.A. The remaining authors declare that they have no
competing interests. To avoid any bias in the collection of the
experimental data, the experiments were conducted by the Laboratory
of Toxicology of the Medical School of the University of Crete.
Lumis Research S.A. and Natural Doctor S.A. had no involvement in
the preparation of the manuscript, the results and the supervision
of the study.
Glossary
Abbreviations
Abbreviations:
|
CAD
|
cardiovascular disease
|
|
BMI
|
body mass index
|
|
TLDP
|
telomere length database project
|
|
COPD
|
chronic obstructive pulmonary
disease
|
|
TERT
|
transcriptase catalytic subunit
|
|
TERC
|
telomerase RNA component
|
|
DKC
|
Dyskeratosis congenita
|
|
08AGTLF
|
Centella asiatica extract
formulation
|
|
MA
|
maslinic acid
|
|
OA
|
oleanolic acid
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
CAG
|
cycloastragenol
|
References
|
1
|
Willeit P, Raschenberger J, Heydon EE,
Tsimikas S, Haun M, Mayr A, Weger S, Witztum JL, Butterworth AS,
Willeit J, et al: Leucocyte telomere length and risk of type 2
diabetes mellitus: New prospective cohort study and
literature-based meta-analysis. PLoS One. 9:e112483. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Armanios M: Telomeres and age-related
disease: How telomere biology informs clinical paradigms. J Clin
Invest. 123:996–1002. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pusceddu I, Herrmann M, Kirsch SH, Werner
C, Hübner U, Bodis M, Laufs U, Wagenpfeil S, Geisel J and Herrmann
W: Prospective study of telomere length and LINE-1 methylation in
peripheral blood cells: The role of B vitamins supplementation. Eur
J Nutr. 55:1863–1873. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pusceddu I, Herrmann M, Kirsch SH, Werner
C, Hübner U, Bodis M, Laufs U, Widmann T, Wagenpfeil S, Geisel J,
et al: One-carbon metabolites and telomere length in a prospective
and randomized study of B- and/or D-vitamin supplementation. Eur J
Nutr. 56:1887–1898. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richards JB, Valdes AM, Gardner JP,
Paximadas D, Kimura M, Nessa A, Lu X, Surdulescu GL, Swaminathan R,
Spector TD, et al: Higher serum vitamin D concentrations are
associated with longer leukocyte telomere length in women. Am J
Clin Nutr. 86:1420–1425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thanasoula M, Escandell JM, Martinez P,
Badie S, Muñoz P, Blasco MA and Tarsounas M: p53 prevents entry
into mitosis with uncapped telomeres. Curr Biol. 20:521–526. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thanasoula M, Escandell JM, Suwaki N and
Tarsounas M: ATM/ATR checkpoint activation downregulates CDC25C to
prevent mitotic entry with uncapped telomeres. EMBO J.
31:3398–3410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vakonaki E, Tzatzarakis M, Tsiminikaki K,
Nathena D, Fragkiadaki P, Kalliantasi K, Kanaki K, Vaki G, Plaitis
S, Tsoukalas D, et al: Effect of chronic and heavy drug abuse on
biological aging. World Acad Sci J. 1:67–73. 2019.
|
|
9
|
Vera E, Bernardes de Jesus B, Foronda M,
Flores JM and Blasco MA: The rate of increase of short telomeres
predicts longevity in mammals. Cell Rep. 2:732–737. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shay JW and Wright WE: Hallmarks of
telomeres in ageing research. J Pathol. 211:114–123. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsoukalas D, Fragkiadaki P, Docea AO,
Alegakis AK, Sarandi E, Vakonaki E, Salataj E, Kouvidi E, Nikitovic
D, Kovatsi L, et al: Association of nutraceutical supplements with
longer telomere length. Int J Mol Med. 44:218–226. 2019.PubMed/NCBI
|
|
12
|
Tsatsakis A, Tsoukalas D, Fragkiadaki P,
Vakonaki E, Tzatzarakis M, Sarandi E, Nikitovic D, Tsilimidos G and
Alegakis AK: Developing BIOTEL: A semi-automated spreadsheet for
estimating telomere length and biological age. Front Genet.
10:842019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blackburn EH, Chan S, Chang J, Fulton TB,
Krauskopf A, McEachern M, Prescott J, Roy J, Smith C and Wang H:
Molecular manifestations and molecular determinants of telomere
capping. Cold Spring Harb Symp Quant Biol. 65:253–263. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beyne-Rauzy O, Prade-Houdellier N, Demur
C, Recher C, Ayel J, Laurent G and Mansat-De Mas V: Tumor necrosis
factor-alpha inhibits hTERT gene expression in human myeloid normal
and leukemic cells. Blood. 106:3200–3205. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Starkweather AR, Alhaeeri AA, Montpetit A,
Brumelle J, Filler K, Montpetit M, Mohanraj L, Lyon DE and
Jackson-Cook CK: An integrative review of factors associated with
telomere length and implications for biobehavioral research. Nurs
Res. 63:36–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tárkányi I and Aradi J: Pharmacological
intervention strategies for affecting telomerase activity: Future
prospects to treat cancer and degenerative disease. Biochimie.
90:156–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fragkiadaki P, Tsoukalas D, Fragkiadoulaki
I, Psycharakis C, Nikitovic D and Spandidos D: and Tsatsakis A:
Telomerase activity in pregnancy complications (Review). Mol Med
Rep. 14:16–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vasilopoulos E, Fragkiadaki P, Kalliora C,
Fragou D, Docea AO, Vakonaki E, Tsoukalas D, Calina D, Buga AM,
Georgiadis G, et al: The association of female and male infertility
with telomere length (Review). Int J Mol Med. 44:375–389.
2019.PubMed/NCBI
|
|
20
|
Vakonaki E, Tsiminikaki K, Plaitis S,
Fragkiadaki P, Tsoukalas D, Katsikantami I, Vaki G, Tzatzarakis MN,
Spandidos DA and Tsatsakis AM: Common mental disorders and
association with telomere length. Biomed Rep. 8:111–116.
2018.PubMed/NCBI
|
|
21
|
Westin ER, Aykin-Burns N, Buckingham EM,
Spitz DR, Goldman FD and Klingelhutz AJ: The p53/p21(WAF/CIP)
pathway mediates oxidative stress and senescence in dyskeratosis
congenita cells with telomerase insufficiency. Antioxid Redox
Signal. 14:985–997. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu Y, Zhou L, Yang Y and Liu Y:
Cycloastragenol: An exciting novel candidate for age-associated
diseases. Exp Ther Med. 16:2175–2182. 2018.Review. PubMed/NCBI
|
|
23
|
Bernardes de Jesus B, Schneeberger K, Vera
E, Tejera A, Harley CB and Blasco MA: The telomerase activator
TA-65 elongates short telomeres and increases health span of
adult/old mice without increasing cancer incidence. Aging Cell.
10:604–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fauce SR, Jamieson BD, Chin AC, Mitsuyasu
RT, Parish ST, Ng HL, Kitchen CM, Yang OO, Harley CB and Effros RB:
Telomerase-based pharmacologic enhancement of antiviral function of
human CD8+ T lymphocytes. J Immunol. 181:7400–7406.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harley CB, Liu W, Blasco M, Vera E,
Andrews WH, Briggs LA and Raffaele JM: A natural product telomerase
activator as part of a health maintenance program. Rejuvenation
Res. 14:45–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mutly AG: Telomerase inhibitors and
activators: Pharmaceutical importance. Enzyme Inhibitors and
Activators. Chapter 5. 125–138. 2017.
|
|
27
|
Ozcagli E, Kara M, Kotil T, Fragkiadaki P,
Tzatzarakis MN, Tsitsimpikou C, Stivaktakis PD, Tsoukalas D,
Spandidos DA, Tsatsakis AM, et al: Stanozolol administration
combined with exercise leads to decreased telomerase activity
possibly associated with liver aging. Int J Mol Med. 42:405–413.
2018.PubMed/NCBI
|
|
28
|
Kara M, Ozcagli E, Fragkiadaki P, Kotil T,
Stivaktakis PD, Spandidos DA, Tsatsakis AM and Alpertunga B:
Determination of DNA damage and telomerase activity in
stanozolol-treated rats. Exp Ther Med. 13:614–618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zafiropoulos A, Tsarouhas K, Tsitsimpikou
C, Fragkiadaki P, Germanakis I, Tsardi M, Maravgakis G,
Goutzourelas N, Vasilaki F, Kouretas D, et al: Cardiotoxicity in
rabbits after a low-level exposure to diazinon, propoxur, and
chlorpyrifos. Hum Exp Toxicol. 33:1241–1252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsitsimpikou C, Tzatzarakis M, Fragkiadaki
P, Kovatsi L, Stivaktakis P, Kalogeraki A, Kouretas D and Tsatsakis
AM: Histopathological lesions, oxidative stress and genotoxic
effects in liver and kidneys following long term exposure of
rabbits to diazinon and propoxur. Toxicology. 307:109–114. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Holohan B, Wright WE and Shay JW: Cell
biology of disease: Telomeropathies: An emerging spectrum disorder.
J Cell Biol. 205:289–299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brinkhaus B, Lindner M, Schuppan D and
Hahn EG: Chemical, pharmacological and clinical profile of the East
Asian medical plant Centella asiatica. Phytomedicine.
7:427–448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gray NE, Harris CJ, Quinn JF and
Soumyanath A: Centella asiatica modulates antioxidant and
mitochondrial pathways and improves cognitive function in mice. J
Ethnopharmacol. 180:78–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Somboonwong J, Kankaisre M, Tantisira B
and Tantisira MH: Wound healing activities of different extracts of
Centella asiatica in incision and burn wound models: an
experimental animal study. BMC Complement Altern Med. Jul
20–2012.(Epub ahead of print). doi: 10.1186/1472-6882-12-103.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Molgora B, Bateman R, Sweeney G, Finger D,
Dimler T, Effros RB and Valenzuela HF: Functional assessment of
pharmacological telomerase activators in human T cells. Cells.
2:57–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bruno EJ, Simpson GD and Martin RL:
Extending telomere length with a multivitamin: A pilot study. J
Health Educ Res Dev. 5:2382017. View Article : Google Scholar
|
|
37
|
Fukumitsu S, Villareal MO, Aida K, Hino A,
Hori N, Isoda H and Naito Y: Maslinic acid in olive fruit
alleviates mild knee joint pain and improves quality of life by
promoting weight loss in the elderly. J Clin Biochem Nutr.
59:220–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Montilla MP, Agil A, Navarro MC, Jiménez
MI, García-Granados A, Parra A and Cabo MM: Antioxidant activity of
maslinic acid, a triterpene derivative obtained from Olea
europaea. Planta Med. 69:472–474. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nur NM and Al-Jasabi SM: Antioxidant
properties of maslinic acid extracted from Plumeria Rubra
leaves. IJCRR. 8:20178–20183. 2017.
|
|
40
|
Ayeleso TB, Matumba MG and Mukwevho E:
Oleanolic acid and its derivatives: Biological activities and
therapeutic potential in chronic diseases. Molecules. 22:19152017.
View Article : Google Scholar :
|
|
41
|
Zhang J, Lu L and Zhou L: Oleanolic acid
activates daf-16 to increase lifespan in Caenorhabditis
elegans. Biochem Biophys Res Commun. 468:843–849. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hong YD, Yoo DS, Nam MH, Kim HC, Park SJ,
Shin SS, Cheon JW and Park YH: Excellent anti-aging effects of
ursolic acid and oleanolic acid present in Ligustrum
lucidum. J Soc Cosmet Sci Korea. 38:181–187. 2012.
|
|
43
|
Bai H, Liu R, Chen HL, Zhang W, Wang X,
Zhang XD, Li WL and Hai CX: Enhanced antioxidant effect of caffeic
acid phenethyl ester and Trolox in combination against radiation
induced-oxidative stress. Chem Biol Interact. 207:7–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stefanska B, Salamé P, Bednarek A and
Fabianowska-Majewska K: Comparative effects of retinoic acid,
vitamin D and resveratrol alone and in combination with adenosine
analogues on methylation and expression of phosphatase and tensin
homologue tumour suppressor gene in breast cancer cells. Br J Nutr.
107:781–790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Balcerczyk A, Gajewska A,
Macierzyńska-Piotrowska E, Pawelczyk T, Bartosz G and Szemraj J:
Enhanced antioxidant capacity and anti-ageing biomarkers after diet
micronutrient supplementation. Molecules. 19:14794–14808. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu H, Guo D, Li K, Pedersen-White J,
Stallmann-Jorgensen IS, Huang Y, Parikh S, Liu K and Dong Y:
Increased telomerase activity and vitamin D supplementation in
overweight African Americans. Int J Obes. 36:805–809. 2012.
View Article : Google Scholar
|