Introduction
Endometrial carcinoma (EC) is one of three most
common types of malignant tumors of the female reproductive tract
worldwide, accounting for 20–30% of the total number of female
malignant tumors of the genital tract (1). Over the past few years, the incidence
of EC has been increasing. In 2014, there were 52,630 new EC cases
and 8,590 EC-related deaths, while in 2017, an estimated number of
61,380 would be diagnosed with EC and more than one-sixth of these
would perhaps succumb to the disease (2,3).
Currently, radical surgery is one of the most effective treatments
for early-stage EC; however, 40% of cases receiving surgery still
have a risk of recurrence and metastasis (4). Previous studies have indicated that
the main cause of the high mortality rate among patients with EC is
recurrence and metastasis (5,6).
Epithelial-mesenchymal transition (EMT) is closely associated with
tumor invasion and metastasis; EMT promotes the disengagement of
epithelial cells from cell-cell adhesion and cell polarity, thus
promoting cell migration and invasion, as well as the development
of an aggressive phenotype (7–9).
Previous research has revealed the molecular mechanisms of common
EMT in EC, including the downregulation of the E-cadherin level,
and other molecular alterations consistent with the mesenchymal
phenotype (10). In addition, it
has been indicated that EMT affects EC through estrogen signaling
and microRNAs (miRNAs or miRs) (11).
G protein-coupled estrogen receptor (GPER or GPR30)
is a member of the 7-transmembrane spanning G protein-coupled
receptor (GPCR) superfamily, structurally unrelated to the nuclear
estrogen receptors (nERs) (12).
Currently, high levels of GPER have been detected and it exists at
least 4 types of human tumor cells, including breast cancer
(13), thyroid cancer (14), ovarian cancer (15) and EC (16) cells. Moreover, increasing evidence
has indicated that GPER is significantly associated with tumor
progression, migration, infiltration and prognosis (17,18).
A previous study demonstrated that the abnormal expression of GPER
was mainly found in advanced tumors and that GPER was associated
with a poor survival of patients with EC; in that study, only 65.2%
of patients with EC with GPER overexpression survived, whereas a
100% survival rate was noted in patients with EC with normal GPER
levels (19). These findings
indicated GPER may be a promising biomarker for the progression of
EC. Moreover, GPER has also been reported to be associated with the
activation of several signaling pathways; for example, GPER binding
to estradiol can rapidly activate the phosphatidylinositol
3-kinase/protein kinase B (PI3K/AKT) pathway through non-genomic
effects, promoting cell proliferation (20,21).
miRNAs are a class of non-coding short RNAs (21–25
bp in length), which can modulate the expression of a large number
of genes by binding to the 3′-UTR of their transcripts (22). In 2014, by comparative analysis of
endometrioid EC (EEC) tissue, normal endometrium (NE) tissue and
blood cells of the same patient, miR-195 was demonstrated as one of
the key miRNAs of EC pathogenesis (23). In this study, we investigated the
effects of miR-195 on the EMT process in EC.
Materials and methods
Cells and cell culture
Human endometrial cancer cell lines (AN3-CA and
Hec1A) were obtained from the American Type Culture Collection
(ATCC). The cells was curtured with Eagle's minimum essential
medium (EMEM, #30-2003, ATCC) with 10% (v/v) fetal bovine serum
(FBS, #30-2020, ATCC), and grown at 37°C under 5% CO2
atmosphere.
Cell transfection
miR-195 mimics (5′-UAGCAGCACAGAAAUAUUGGC-3′) and
negative control (5′-UUCUCCGAACGUGUCACGUTT-3′) were synthesized by
GenePharma. The AN3-CA or Hec1A cells were embedded in 6-well
plates (5×104 cells/well) containing serum-free medium,
and the cells were then cultured for 3 h at 37°C. miR-195 mimics or
Mock vectors (50 nM) were added to 250 µl EMEM medium, supplemented
with Lipofectamine 2000 reagent (Invitrogen), keeping the mixture
at 37°C in 5% CO2 for 6 h. The transfected cells were
harvested and cultured with new medium for 24 h at 37°C. After 24
h, transfected cells were collected using EDTA (0.25%)-Trypsin
(Sigma-Aldrich; Merck KGaA), for the assessment of the transfection
efficiencies.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay (Dojindo Laboratories) was used for
the analysis of cell viability. In brief, the AN3-CA or Hec1A cells
were embedded in 6-well plates at a cell density of
1×105 per well and cultured in a 5% CO2
humidified atmosphere at 37°C. The cells were harvested at 3 time
points (12, 24 and 48 h), and the harvested cells were then
cultured with 10% CCK-8 reagent, respectively. Following incubation
for 1 h at 37°C, the absorbance at 450 nm was quantified using a
Microplate Reader (Multiskan FC, Thermo Fisher Scientific).
Wound healing assay
The AN3-CA or Hec1A cells were embedded in 6-cm
culture dishes and cultured at 37°C in 5% CO2 humidified
atmosphere until the cells reached 90% confluence. A sterile
pipette tip was used to create a cell-free line. At 24 h after the
scratch was made, the condition of wound healing was captured using
a Nikon ECLIPSE Ts2 light microscope (Nikon Corporation), and the
migration rates were calculated by comparing the width of the
wound.
Transwell assay
The invasive ability of the AN3-CA and Hec1A cells
was assessed by Transwell chamber assay. Briefly, 5×105
AN3-CA or Hec1A cells or transfected cells were seeded into the
8-µm pore size of 6-well Matrigel invasion chambers (BD
Biosciences). The top chamber was loaded with 200 µl serum-free
EMEM, while the bottom chamber was loaded with 600 µl EMEM medium
containing 20% FBS. The chamber was maintained at 37°C in a 5%
CO2 humidified incubator for 24 h, and the cells on the
upper surface of the membrane were removed using a cotton swab, and
the cells that had invaded into the bottom chamber were fixed with
4% paraformaldehyde for 15 min, followed by staining with 0.1%
crystal violet solution for 20 min at 37°C. Five random field views
were selected for counting.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
miRNAs from each experimental group were isolated
using the miRcute miRNA Isolation kit (Tiangen), and then reverse
transcribed using the miScript II RT kit (Qiagen GmbH) according to
the instructions of the manufacturer. All samples were reacted at
37°C for 60 min, heated at 95°C for 5 min, finally held at 4°C. The
miRNA levels were analyzed using the miScript SYBR-Green PCR kit
(Qiagen GmbH). The 20 µl volume of qPCR reaction consisted of 2 µl
cDNA, 1X QuantiTect SYBR-Green PCR Master Mix (Qiagen GmbH) and 0.5
mM of each primer. The relative miR-195 levels were normalized to
U6. RNAs from AN3-CA or Hec1A cells were extracted using TRIzol
reagent (Invitrogen). A total of 2 µl total RNAs were used for cDNA
generation using the Prime Script RT reagent kit (Takara). The
reaction protocol was as follows: 5 min at 65°C, then 6 min at 30°C
and 60 min at 50°C. SYBR-Green detection was used for the
assessment of the relative mRNA levels. The ABI 7500 real-time PCR
system was used to carry out the miRNA and mRNA level analysis,
using the following reaction parameters: Pre-degeneration at 95°C
for 15 min, followed by 40 cycles at 95°C for 5 sec, and annealing
at 60°C for 30 sec. Fold changes were calculated using the relative
quantification (2−ΔΔCq) method (24) and normalized against GAPDH. All
primers used are listed in Table
I.
 | Table I.Sequences of primers used for
RT-qPCR. |
Table I.
Sequences of primers used for
RT-qPCR.
| Gene name | Primer
sequences |
|---|
| miR-195 | Forward:
5′-GGGGAGCCAAAAGGGTCATCATCT-3′ |
|
| Reverse:
5′-GAGGGGCCATCCACAGTCTTCT-3′ |
| TIMP-2 | Forward:
5′-CCAAAGCAGTGAGCGAGAA-3′ |
|
| Reverse:
5′-CATCCAGAGGCACTCATCC-3′ |
| MMP-2 | Forward:
5′-GGAGGCACGATTGGTCTG-3′ |
|
| Reverse:
5′-TTGGTTTCCGCATGGTCT-3′ |
| MMP-9 | Forward:
5′-TTGACAGCGACAAGAAGTGG-3′ |
|
| Reverse:
5′-GGCACAGTAGTGGCCGTAG-3′ |
| GPER | Forward:
5′-TCACGGGCCACATTGTCAACCTC-3′ |
|
| Reverse:
5′-GCTGAACCTCACATCTGACTGCTC-3′ |
| U6 | Forward:
5′-CTCGCTTCGGCAGCACA-3′ |
|
| Reverse:
5′-AACGCTTCACGAATTTGCGT-3′ |
| GAPDH | Forward:
5′-ACCACAGTCCATGAAATCAC-3′ |
|
| Reverse:
5′-AGGTTTCTCCAGGCGGCATG-3′ |
Western blot analysis
Subconfluent AN3-CA or Hec1A cells were suspended in
RIPA buffer (Beyotime). The concentration of the cell lysates was
quantified by BCA protein assay (Pierce). Proteins (15 µg) were
separated on sodium dodecyl sulfate-polyacrylamide gels and
electrophoretically transferred onto polyvinylidene fluoride (PVDF)
membranes (Pall Inc.). After being blocked with 5% non-fat milk
overnight at 4°C, the membranes were incubated with several types
of primary antibodies at 4°C overnight. All antibodies were
obtained from Abcam, including anti-GAPDH (rabbit, 1:1,000, cat.
no. ab8245, 37 kDa), anti-tissue inhibitor of metalloproteinase 2
(TIMP-2, rabbit, 1:1,000, cat. no. ab180630, 24 kDa), anti-matrix
metalloproteinase (MMP)-2, (rabbit, 1:1,000, cat. no. ab37150, 72
kDa), anti-MMP-9 (rabbit, 1:1,000, cat. no. ab73734, 95 kDa), PI3K
(mouse, 1:2,000, cat. no. ab140307, 127 kDa), phospho-PI3K (p-PI3K,
rabbit, 1:1,000, cat. no. ab138364, 85 kDa), AKT (rabbit, 1:1,000,
cat. no. ab18785, 55 kDa), p-AKT (rabbit, 1:1,000, cat. no.
ab38449, 56 kDa) and GPER (rabbit, 1:1,000, cat. no. ab137479, 42
kDa). After washing with PBS, secondary antibodies, such as goat
anti-mouse IgG, HRP-linked antibody (1:2,000, cat. no. ab205719,
Abcam) and goat anti-rabbit IgG H&L (HRP) (1:2,000, cat. no.
ab205718) (both from Abcam) were incubated with the membranes at
room temperature for 2 h. The membranes were stained with enhanced
chemiluminescence solution (Pierce), visualized using a laser
densitometer GeneGnome XRQ and analyzed using GeneSys image
acquisition software version 3.0 (SynGene).
Luciferase reporter assay
GPER was a potential target of miR-195 based on the
prediction algorithm of TargetScan (http://www.targetscan.org/vert_72/). Dual-luciferase
reporter assay system (E1910; Promega) was performed to further
verify this predicted target. In brief, miR-195 was inserted into
the GV272 plasmid (GV272-miR-195; Shanghai Genepharma Co., Ltd.),
and the 3′-UTR of GPER containing miR-195-binding site was inserted
into the GV268 plasmid (GV268-GPER-3′UTR; Shanghai Genepharma Co.,
Ltd.). The QuikChangeH Site-Directed Mutagenesis kit (Agilent
Technologies) was used to create a mutant GPER 3′-UTR containing a
mutant sequence in the miR-195 binding site, and the mutant was
also cloned into GV268 (GV268-GPER-3′-UTR mut). GV272-miR-195 was
co-transfected with GV268-GPER-3′-UTR or GV268-GPER-3′-UTR mut into
293T cells (#CRL-3216, ATCC) by Lipofectamine 2000, and the control
was transfected with GV268-GPER-3′-UTR alone. The cells were lysed
and separated under a 5 min centrifugation at 15,000 × g at 4°C.
The prepared dual luciferase reporter mixture was added into the
supernatant of lysed cells, and the relative Firefly and
Renilla luciferase activity was then immediately detected
using a Sinergy 2 luminometer (Biotek Instruments).
Statistical analysis
All tests were carried out using SPSS 15.0 software
(SPSS Inc.). The values are reported as the means ± SEM. One-way
analysis of variance (ANOVA) followed by a post-hoc Tukey's test
was used for comparisons between various groups. Differences were
considered statistically significant at P<0.05.
Results
Overexpression of miR-195 exerts an
inhibitory effect on AN3-CA and Hec1A cell viability
To examine the effects of miR-195 on EC development,
a miR-195 overexpression AN3-CA and Hec1A cell model was
constructed and the cell viability was detected using the CCK-8
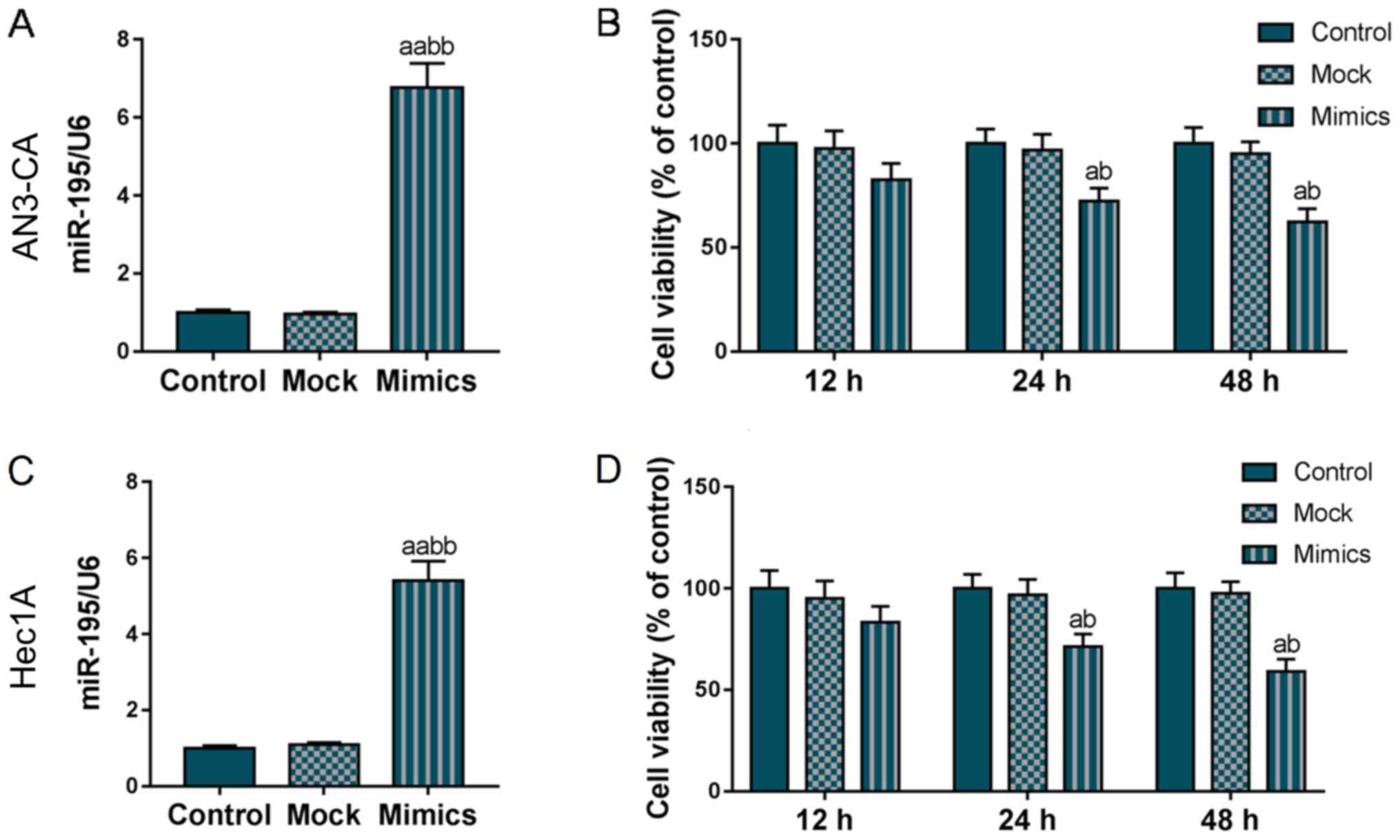
kit. As shown in Fig. 1A and C,
miR-195 was overexpressed in the Mimics group, indicating that the
miR-195 overexpression vector was stably expressed in the AN3-CA
and Hec1A cells. The results of CCK-8 kit assay revealed that the
viability of both the AN3-CA and Hec1A cells gradually decreased
with the increasing incubation time and from the 24-h time point,
the overexpression of miR-195 significantly reduced the viability
of the AN3-CA and Hec1A cells (P<0.05, Fig. 1B and D). These findings suggested
that miR-195 overexpression notably inhibited AN3-CA and Hec1A cell
viability.
Overexpression of miR-195 suppresses
the migration and invasion of AN3-CA and Hec1A cells
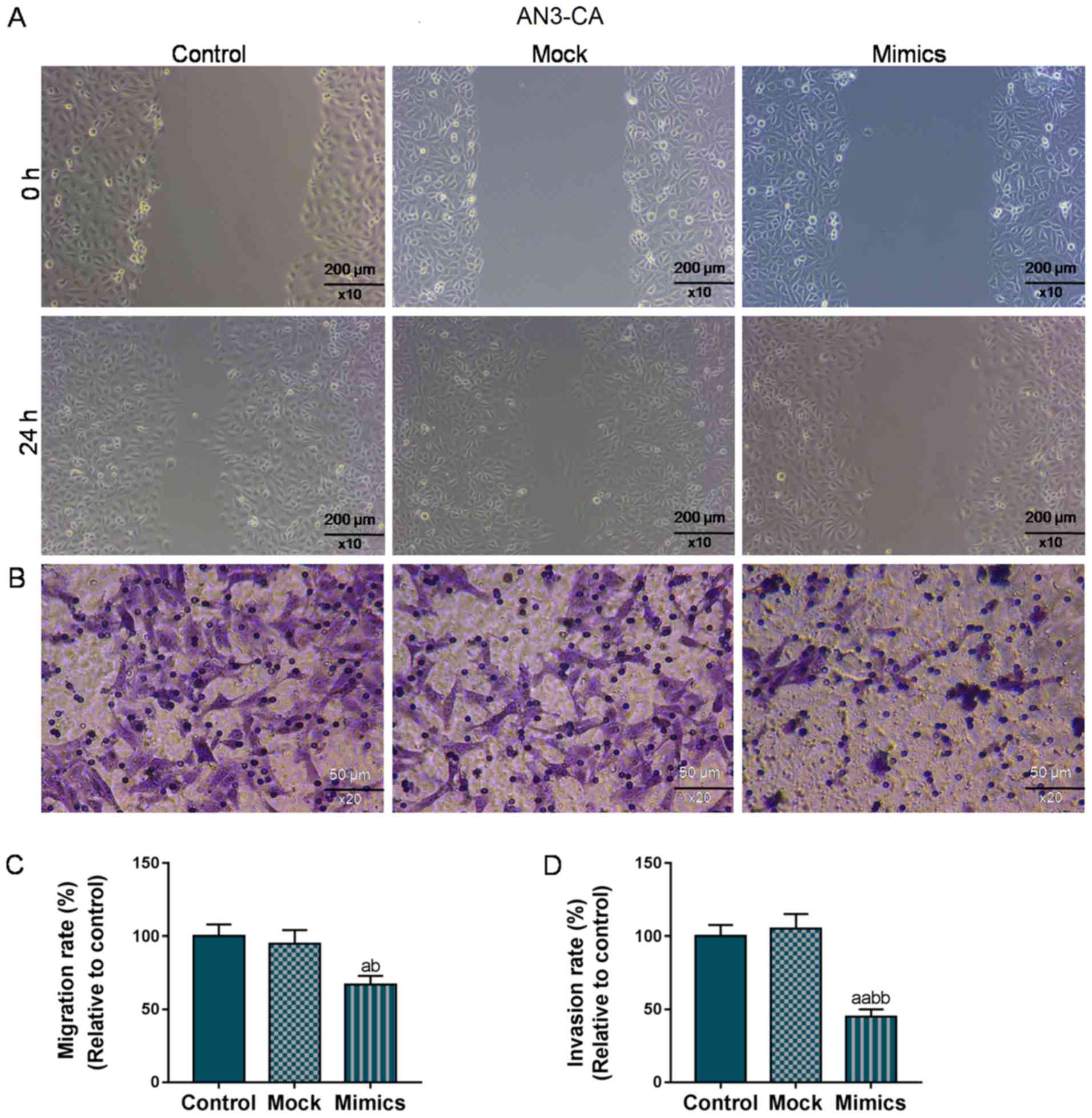
As shown in Fig. 2A and
C, the wound closure rate in the AN3-CA cells in the Mimics
group significantly decreased to 70% (from 100%), compared with the
Control and Mock groups (P<0.05). At the same time, the number
of invaded cells in the Mimics group was also markedly decreased
(50 vs. 100%, P<0.01; Fig. 2B and
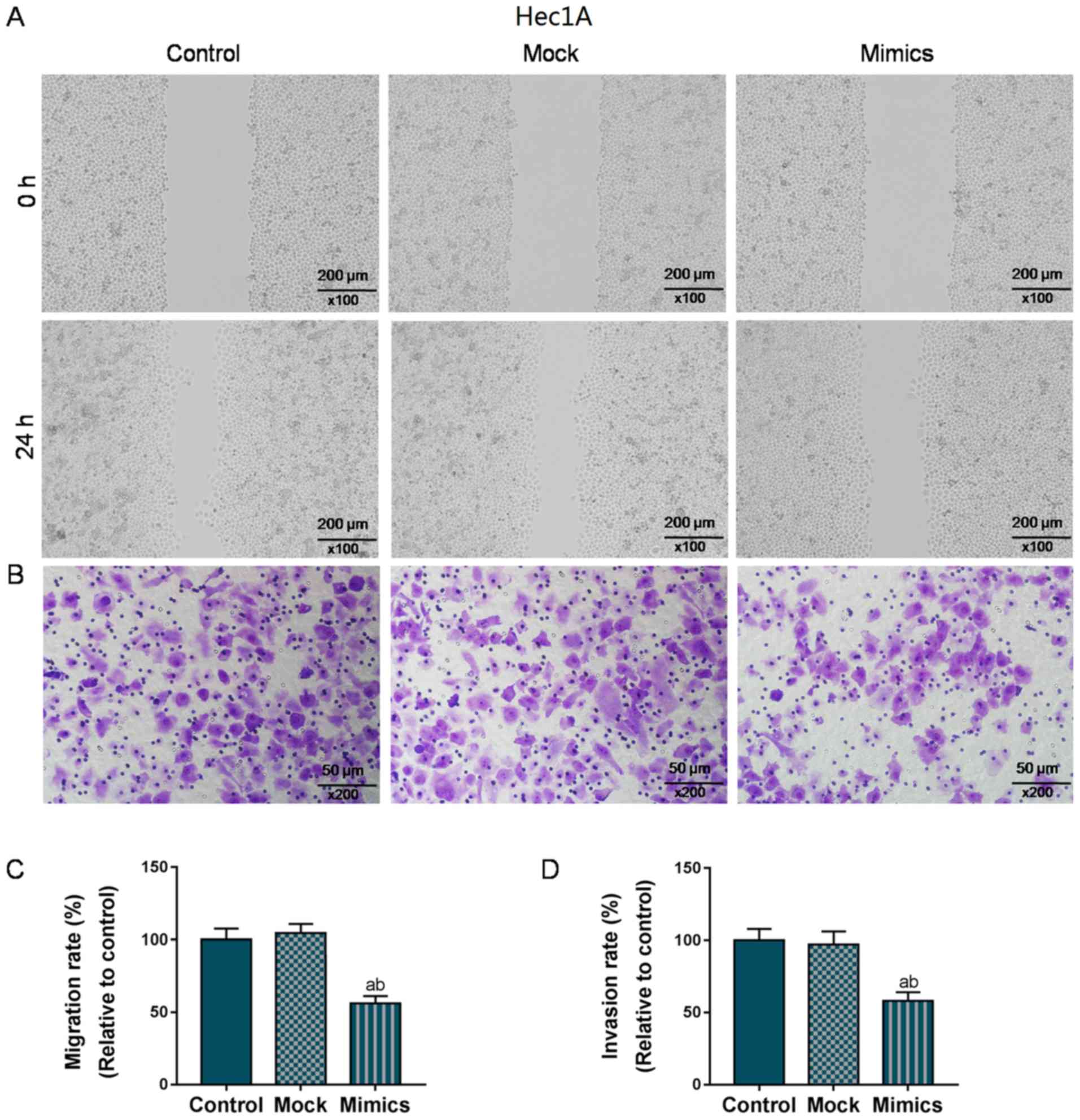
D). Similarly, in the Hec1A cells, the overexpression of
miR-195 significantly suppressed migration and invasion (P<0.05;
Fig. 3). Taken together, the
migratory and invasive capacities of the AN3-CA or Hec1A EC cells
were effectively suppressed in the cells in which miR-195 was
overexpressed.
Overexpression of miR-195 inhibits the
expression of metastasis-associated genes and the activation of the
PI3K/AKT signaling pathway
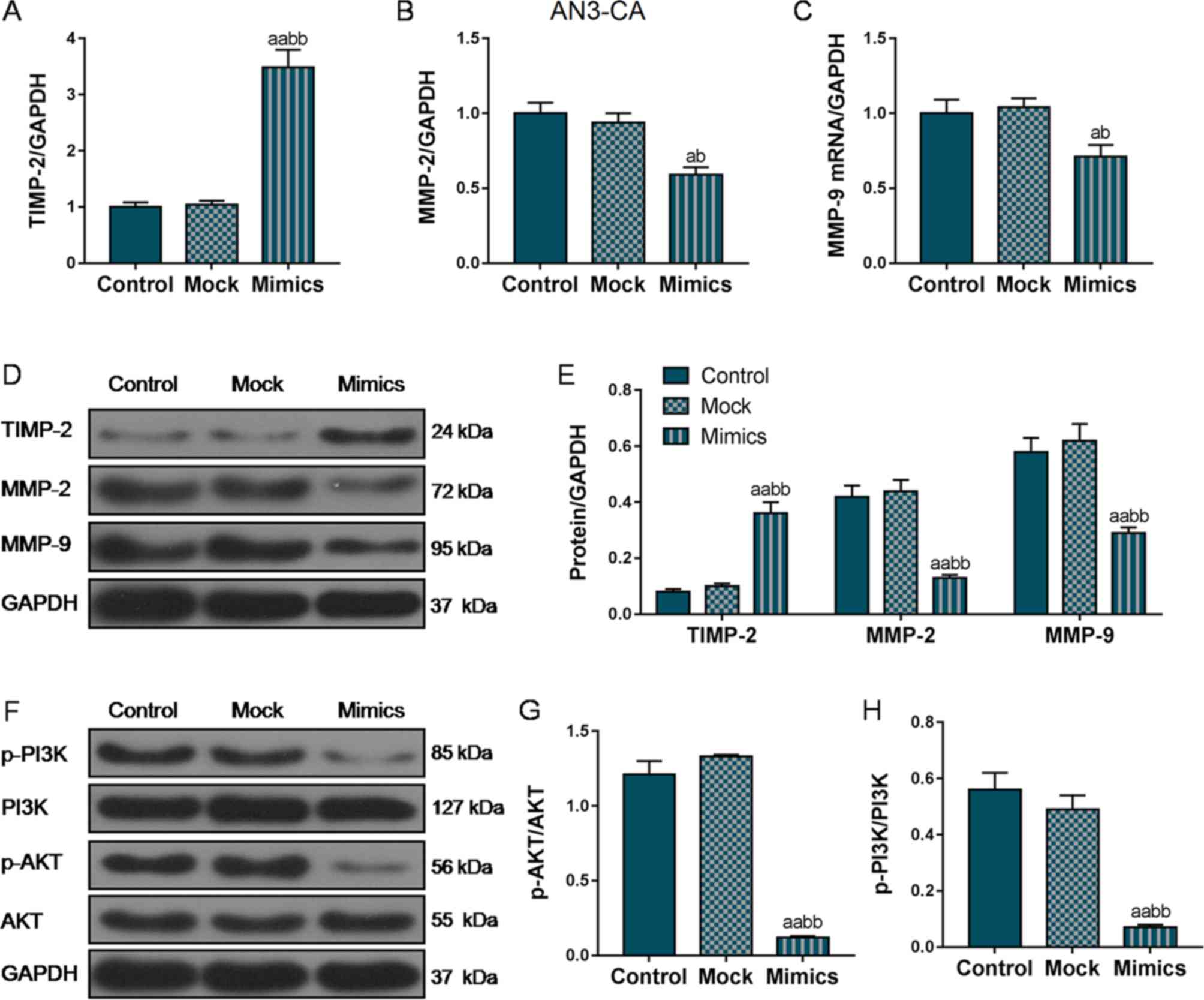
To further investigate the mechanisms through which
miR-195 overexpression suppressed the migratory and invasive
ability of the EC cells, RT-qPCR and western blot analysis were
performed to analyze the expression of several EMT-associated
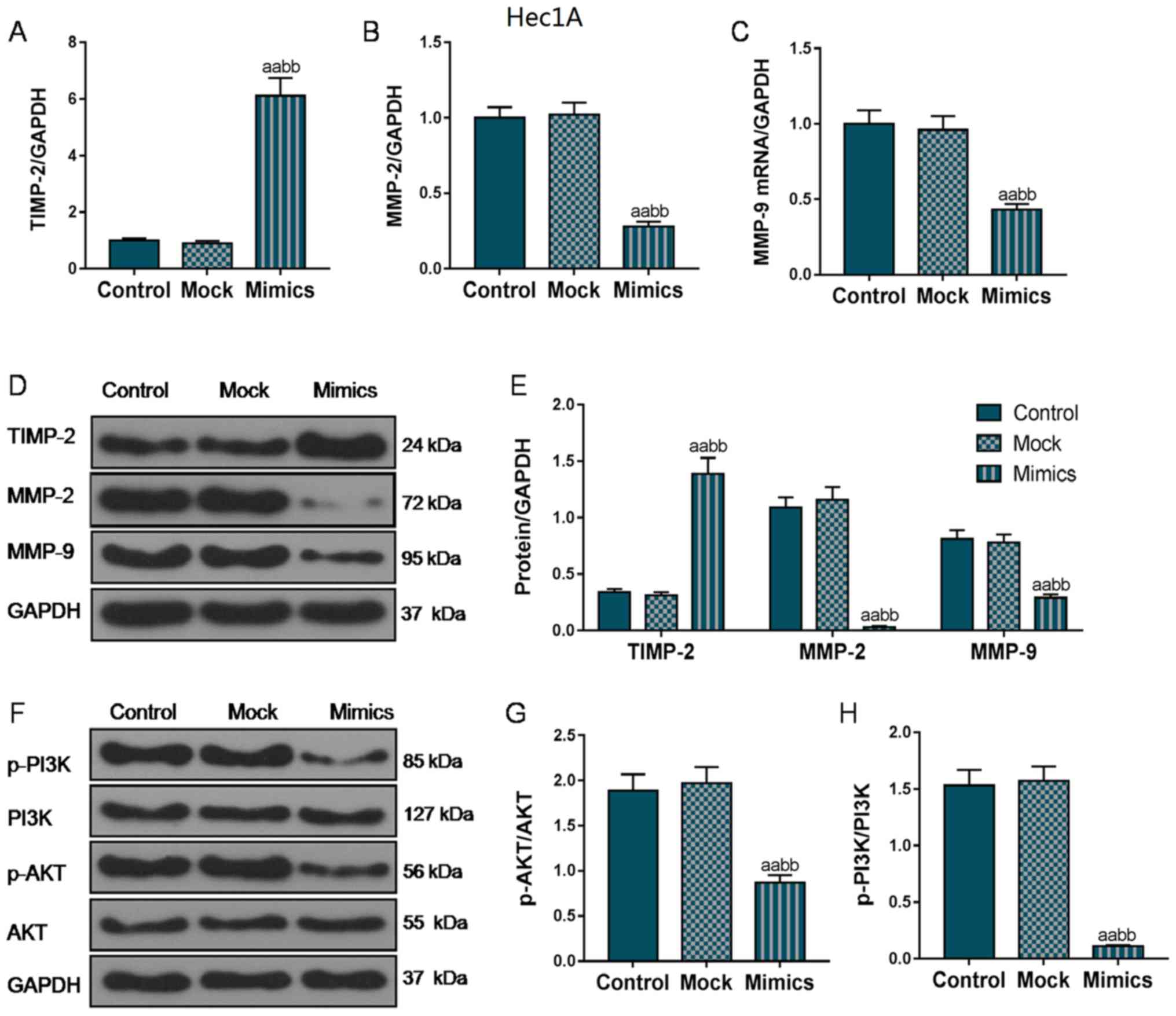
genes. As shown in Fig. 4A, D and
E, in the AN3-CA cells, TIMP2 mRNA and protein expression
markedly increased in the Mimics group compared with that in the
Control and Mock groups. In addition, the MMP-2 and MMP-9
expression levels were also markedly decreased in the Mimics group
compared with the Control and Mock groups (P<0.01; Fig. 4B-E). Furthermore, we also examined
the changes in the expression levels of MMPs and TIMP-2 in the
Hec1A cells, and the changes in the expression trends of these
EMT-related proteins in the Hec1A cells was basically consistent
with that in the AN3-CA cells (Fig.
5A-E), indicating that miR-195 overexpression suppressed the
invasion and metastasis of EC cells by inhibiting the expression of
these EMT-associated genes.
Furthermore, we also measured the phosphorylation
levels of PI3K and AKT. As shown in Figs. 4F-H and 5F-H, the protein levels of p-PI3K and
p-AKT were markedly decreased in both the AN3-CA and Hec1A cells in
which miR-195 was overexpressed, compared with those in the Control
and Mock groups (P<0.01), indicating that the overexpression of
miR-195 exerted its functional effects partly through the
regulation of the PI3K/AKT signaling pathway.
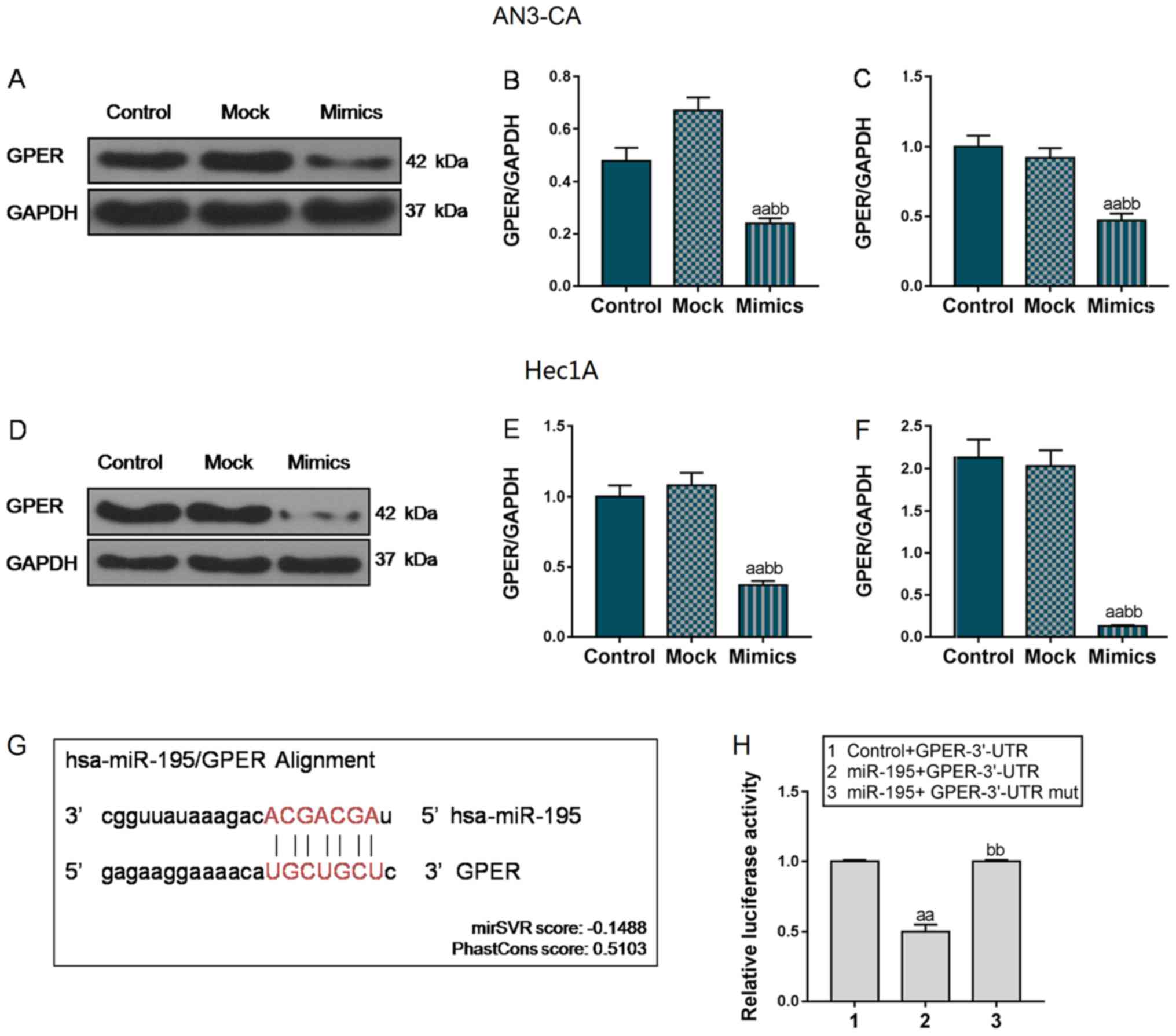
GPER is a predicted target of
miR-195
Following transfection with miR-195 overexpression
vector, GPER mRNA and protein expression was markedly decreased
reduced in the AN3-CA and Hec1A cells (P<0.01, Fig. 6A-F). According to the results, we
hypothesized that miR-195 could specifically target GPER. To
further verify the association between miR-195 and GPER, luciferase
plasmids were compared and transfected into 293T cells. Using
TargetScan prediction software, the GPER 3′-UTR was predicted to
contain 7 nucleotides (5′-UGCUGCU-′3) that were completely paired
with miR-195 (Fig. 6G). As shown
in Fig. 6H, in the Control +
GPER-3′-UTR group, the luciferase activity was significantly higher
than that in the 293T cells co-transfected with miR-195 and
GPER-3′-UTR plasmids. However, when miR-195 was co-transfected with
the GPER-3′-UTR mut plasmid, the luciferase activity was notably
enhanced, compared with that in the miR-195 + GPER-3′-UTR group
(P<0.01). Collectively, these data indicate that miR-195 can
directly regulate GPER expression.
Discussion
In the present study, we observed miR-195
overexpression exerted an inhibitory effect on the viability of
AN3-CA and Hec1A cells, and induced the upregulation of TIMP-2 and
the downregulation of MMP-2 and MMP-9 expression. miR-195
overexpression also suppressed the migratory and invasive abilities
of the AN3-CA and Hec1A cells. Moreover, the suppression of the
PI3K/AKT signaling pathway was found to contribute to the
functional effects of miR-195. It was also found that GPER was a
predicted target of miR-195. Hence, these findings suggest that the
suppressive effects of miR-195 overexpression on the EMT process of
EC cells are associated with the inhibition of PI3K/AKT signaling
and GPER expression.
It is known that the EMT process is an essential
step for the invasion and metastases formation of a tumor cell, as
during this process, activated MMPs participate in the degradation
of extracellular matrix elements, the reduction of cell adhesion
and the promotion of neoplastic cell migration (25). TIMPs have been reported to function
as natural inhibitors, of which TIMP-2 is the most effective
inhibitor of MMP-2 than other TIMP members (26). miR-195, a member of miR-15, has
been demonstrated to function as a tumor suppressor in a number of
types of cancer (27–29). In 2013, miR-195 was reported to
exert an inhibitory effect on the proliferation and development of
endometrial stromal cells by blocking fractalkine expression
(30). Moreover, Kong et al
found that the restoration of miR-195 expression by blocking
plasmacytoma variant translocation 1 (PVT1) not only inhibited the
upregulation of cell proliferation, migration and
invasion-associated proteins in both type I and II EC cell lines,
but also induced EC tumor regression in vivo (31), which was consistent with the
results of this study. It can thus be concluded that miR-195
overexpression can rebalance the expression of TIMP-2 and MMPs,
which may protect the extracellular matrix from matrixins, and
subsequently inhibit the migratory and invasive ability of AN3-CA
and Hec1A cells, and ultimately contribute to impeding the
progression of EMT in EC.
Furthermore, we also observed the significant
downregulation of p-PI3K and p-AKT in the cells transfected with
the miR-195 mimics vector. The PI3K/AKT pathway has been reported
to participate in the metastasis of numerous cancer types (32,33).
Kong et al revealed that both acidic fibroblast growth
factor receptor (FGFR1) and basic fibroblast growth factor (FGF2)
were effective targets of miR-195, and their inhibition by miR-195
suppressed the activities of the PI3K/AKT and MAPK/ERK pathways,
ultimately resulting in the inhibition of malignancy in EC
(31). In this study, miR-195 also
exerted significantly inhibitory effects on the phosphorylation of
PI3K and AKT, which indicated that the involvement of the PI3K/AKT
signaling pathway may be required for the anticancer effects of
miR-195 on EC development.
Previous studies have suggested that EC can be
separated into two types (34);
type I EC represents mostly low-grade endometrioid tumors driven by
estrogen, and type II EC is categorized as estrogen non-dependent
due to a lack of ER expression (35). However, Petrie et al
revealed that Hec50 cells, a typical type II EC cell line, did not
express the classical nERs, but GPER (17), indicating type II EC also strongly
depended on estrogen in vivo that was attributed to GPER
(17). In 2016, GPER was reported
to promote ovarian cancer cell migration and invasion by
upregulating the protein abundancy and functional activities of
MMP-2 and MMP-9 (33). More
importantly, it was also reported that GPER was involved in the
regulation of several signaling pathways, including the MEK/ERK
mitogen-activated protein kinase (MAPK) (36) and PI3K/AKT pathways (37). A previous study indicated that the
activation of GPER notably enhanced the expression levels of MMPs
and promoted cell migration and invasion in renal cell carcinoma
(RCC) by regulating MAPK and PI3K/AKT; however, only the inhibitor
of the PI3K/AKT pathway could effectively counteract the effects of
GPER by downregulating the expression of MMPs and inhibiting the
migratory and invasion ability of RCC cells (38,39).
Therefore, miR-195 binds to the 3′-UTR of GPER and markedly reduces
GPER protein levels and subsequently inhibits the positive effects
of GPER on PI3K/AKT activation, leading to a significantly
reduction in the levels of p-PI3K and p-AKT. Taken together, the
results of this study indicate that the suppressive effects of
miR-195 on EC cell migration and invasion are closely associated
with the PI3K/AKT signaling pathway and GPER expression.
There are some limitations to this study. For
example, although we have demonstrated miR-195 is able to directly
target GPER, whether the negative correlation of miR-195 with GPER
also applies to clinical EC samples remains to be investigated.
This study found that the inhibitory effects of miR-195 on EC
progression by targeting GPER involved the regulation of PI3K/AKT
signaling; however, the detailed underlying mechanisms remain
unclear. In addition, our data suggested that miR-195
overexpression notably suppressed the migration and invasion of EC
cells through GPER; however, whether the knockdown or
overexpression of GPER could cancel or enhance the suppressive
effects of miR-195 on EC cells warrants further investigation.
In additions, some other limitations are that, for
instance, although we have explored the association of miR-195 and
the EMT process through the expression levels of MMPs and TIMPs,
the effects of miR-195 on the expression levels of key EMT-related
markers, including E-cadherin, Vimentin, FN and Slug need to be
investigated.
In conclusion, this study found that the
overexpression of miR-195 significantly suppressed cell migration
and invasion, which may subsequently impede the EMT progress of EC
cells, and that the PI3K/AKT signaling pathway was involved in this
process. In addition, GPER was identified as a target of miR-195.
Based on the essential role of GPER in type II EC cell development,
we hypothesized that it also played an important role in the
suppressive effects of miR-195 on EC progression; however, the
underlying molecular mechanisms remain to be further elucidated.
This study suggests that miR-195 may prove to be a promising
therapeutic target for the treatment of EC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JD and WW made substantial contributions to the
conception and design of the study, and were also involved in the
drafting of the article or critically revising it for important
intellectual content. GY was involved in data acquisition, data
analysis and interpretation. XM was involved in all experiments.
All authors have read and approved the final version of the
manuscript. All authors agree to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang G, Cheng Y, Zhang Q, Li X, Zhou J,
Wang J and Wei L: ATX-LPA axis facilitates estrogen-induced
endometrial cancer cell proliferation via MAPK/ERK signaling
pathway. Mol Med Rep. 17:4245–4252. 2018.PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ward KK, Shah NR, Saenz CC, McHale MT,
Alvarez EA and Plaxe SC: Cardiovascular disease is the leading
cause of death among endometrial cancer patients. Gynecol Oncol.
126:176–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elit L and Hirte H: Novel strategies for
systemic treatment of endometrial cancer. Expert Opin Investig
Drugs. 9:2831–2853. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong P, Kaneuchi M, Konno Y, Watari H,
Sudo S and Sakuragi N: Emerging therapeutic biomarkers in
endometrial cancer. Biomed Res Int. 2013:1303622013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gong B, Yue Y, Wang R, Zhang Y, Jin Q and
Zhou X: Overexpression of microRNA-194 suppresses the
epithelial-mesenchymal transition in targeting stem cell
transcription factor Sox3 in endometrial carcinoma stem cells.
Tumour Biol. 39:10104283177062172017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oyanadel C, Holmes C, Pardo E, Retamal C,
Shaughnessy R, Smith P, Cortés P, Bravo-Zehnder M, Metz C,
Feuerhake T, et al: Galectin-8 induces partial
epithelial-mesenchymal transition with invasive tumorigenic
capabilities involving a FAK/EGFR/proteasome pathway in Madin-Darby
canine kidney cells. Mol Biol Cell. 29:557–574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng X, Kong DH, Li N, Zong ZH, Liu BQ, Du
ZX, Guan Y, Cao L and Wang HQ: Knockdown of BAG3 induces
epithelial-mesenchymal transition in thyroid cancer cells through
ZEB1 activation. Cell Death Dis. 5:e10922014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitra A, Mishra L and Li S: EMT, CTCs and
CSCs in tumor relapse and drug-resistance. Oncotarget.
6:10697–10711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huszar M, Pfeifer M, Schirmer U, Kiefel H,
Konecny GE, Ben-Arie A, Edler L, Munch M, Muller-Holzner E,
Jerabek-Klestil S, et al: Up-regulation of L1CAM is linked to loss
of hormone receptors and E-cadherin in aggressive subtypes of
endometrial carcinomas. J Pathol. 220:551–561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kent CN and Guttilla Reed IK: Regulation
of epithelial-mesenchymal transition in endometrial cancer:
Connecting PI3K, estrogen signaling, and microRNAs. Clin Transl
Oncol. 18:1056–1061. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prossnitz ER and Barton M: The
G-protein-coupled estrogen receptor GPER in health and disease. Nat
Rev Endocrinol. 7:715–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Molina L, Figueroa CD, Bhoola KD and
Ehrenfeld P: GPER-1/GPR30 a novel estrogen receptor sited in the
cell membrane: Therapeutic coupling to breast cancer. Expert Opin
Ther Targets. 21:755–766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu P, Liao LY, Zhao TT, Mo XM, Chen GG
and Liu ZM: GPER/ERK&AKT/NF-κB pathway is involved in
cadmium-induced proliferation, invasion and migration of
GPER-positive thyroid cancer cells. Mol Cell Endocrinol. 442:68–80.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan Y, Jiang X, Zhao Y, Wen H and Liu G:
Role of GPER on proliferation, migration and invasion in
ligand-independent manner in human ovarian cancer cell line SKOV3.
Cell Biochem Funct. 33:552–559. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Li Y, Lan L, Liu R, Wu Y, Qu Q
and Wen K: Tamoxifen has a proliferative effect in endometrial
carcinoma mediated via the GPER/EGFR/ERK/cyclin D1 pathway: A
retrospective study and an in vitro study. Mol Cell Endocrinol.
437:51–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petrie WK, Dennis MK, Hu C, Dai D,
Arterburn JB, Smith HO, Hathaway HJ and Prossnitz ER: G
protein-coupled estrogen receptor-selective ligands modulate
endometrial tumor growth. Obstet Gynecol Int. 2013:4727202013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He YY, Cai B, Yang YX, Liu XL and Wan XP:
Estrogenic G protein-coupled receptor 30 signaling is involved in
regulation of endometrial carcinoma by promoting proliferation,
invasion potential, and interleukin-6 secretion via the MEK/ERK
mitogen-activated protein kinase pathway. Cancer Sci.
100:1051–1061. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith HO, Leslie KK, Singh M, Qualls CR,
Revankar CM, Joste NE and Prossnitz ER: GPR30: A novel indicator of
poor survival for endometrial carcinoma. Am J Obstet Gynecol.
196:386.e1–e9.e11. 2007. View Article : Google Scholar
|
|
20
|
Fan DX, Yang XH, Li YN and Guo L:
17β-estradiol on the expression of G-protein coupled estrogen
receptor (GPER/GPR30) mitophagy, and the PI3K/Akt signaling pathway
in ATDC5 chondrocytes in vitro. Med Sci Monit. 24:1936–1947. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Zhao Y and Guo L: 17β-estradiol
protects INS-1 insulinoma cells from mitophagy via G
protein-coupled estrogen receptors and the PI3K/Akt signaling
pathway. Int J Mol Med. 41:2839–2846. 2018.PubMed/NCBI
|
|
22
|
Bhaskaran M and Mohan M: MicroRNAs:
History, biogenesis, and their evolving role in animal development
and disease. Vet Pathol. 51:759–774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsukamoto O, Miura K, Mishima H, Abe S,
Kaneuchi M, Higashijima A, Miura S, Kinoshita A, Yoshiura K and
Masuzaki H: Identification of endometrioid endometrial
carcinoma-associated microRNAs in tissue and plasma. Gynecol Oncol.
132:715–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drzewiecka-Jędrzejczyk M, Wlazeł R,
Terlecka M and Jabłoński S: Serum metalloproteinase-2 and tissue
inhibitor of metalloproteinase-2 in lung carcinoma patients. J
Thorac Dis. 9:5306–5313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators
intumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang T, Shan G and Zeng F: Knockdown of
DGCR5 enhances the radiosensitivity of human laryngeal carcinoma
cells via inducing miR-195. J Cell Physiol. 234:12918–12925. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu W, Liang X, Li X, Zhang Y, Sun Z, Liu Y
and Wang J: MicroRNA-195: A review of its role in cancers. Onco
Targets Ther. 11:7109–7123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J and Li W: Long noncoding RNA CYTOR
sponges miR-195 to modulate proliferation, migration, invasion and
radiosensitivity in nonsmall cell lung cancer cells. Biosci Rep.
38(pii): BSR201815992018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Chen H, Fu Y, Ai A, Xue S, Lyu Q
and Kuang Y: MiR-195 inhibits proliferation and growth and induces
apoptosis of endometrial stromal cells by targeting FKN. Int J Clin
Exp Pathol. 6:2824–2834. 2013.PubMed/NCBI
|
|
31
|
Kong F, Ma J, Yang H, Yang D, Wang C and
Ma X: Long non-coding RNA PVT1 promotes malignancy in human
endometrial carcinoma cells through negative regulation of
miR-195-5p. Biochim Biophys Acta Mol Cell Res. Jul 19–2018.doi:
10.1016/j.bbamcr.2018.07.008 (Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Segarra J, Balenci L, Drenth T, Maina F
and Lamballe F: Combined signaling through ERK, PI3K/AKT, and
RAC1/p38 is required for met-triggered cortical neuron migration. J
Biol Chem. 281:4771–4778. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian PC, Wang HL, Chen GH, Luo Q, Chen Z,
Wang Y and Liu YF: 2,2′,4,4′-Tetrabromodiphenyl ether promotes
human neuroblastoma SH-SY5Y cells migration via the GPER/PI3K/Akt
signal pathway. Hum Exp Toxicol. 35:124–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ulrich LS: Endometrial cancer, types,
prognosis, female hormones and antihormones. Climacteric.
14:418–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Geletina NS, Kobelev VS, Babayants EV,
Feng L, Pustylnyak VO and Gulyaeva LF: PTEN negative correlates
with miR-181a in tumour tissues of non-obese endometrial cancer
patients. Gene. 655:20–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yin H, Zhu Q, Liu M, Tu G, Li Q, Yuan J,
Wen S and Yang G: GPER promotes tamoxifen-resistance in ER+ breast
cancer cells by reduced Bim proteins through MAPK/Erk-TRIM2
signaling axis. Int J Oncol. 51:1191–1198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Birnbaumer L and Teng CT: Regulation
of ERRalpha gene expression by estrogen receptor agonists and
antagonists in SKBR3 breast cancer cells: Differential molecular
mechanisms mediated by g protein-coupled receptor GPR30/GPER-1. Mol
Endocrinol. 24:969–980. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He YY, Du GQ, Cai B, Yan Q, Zhou L, Chen
XY, Lu W, Yang YX and Wan XP: Estrogenic transmembrane receptor of
GPR30 mediates invasion and carcinogenesis by endometrial cancer
cell line RL95-2. J Cancer Res Clin Oncol. 138:775–783. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guan BZ, Yan RL, Huang JW, Li FL, Zhong
YX, Chen Y, Liu FN, Hu B, Huang SB and Yin LH: Activation of G
protein coupled estrogen receptor (GPER) promotes the migration of
renal cell carcinoma via the PI3K/AKT/MMP-9 signals. Cell Adh Migr.
12:109–117. 2018. View Article : Google Scholar : PubMed/NCBI
|