Introduction
Chronic obstructive pulmonary disease (COPD) and
obstructive sleep apnea (OSA) are the most prevalent chronic
respiratory disorders in the world (1). COPD is progressive and most commonly
arises from cigarette smoking and exposure to pollutants. It is
characterized by persistent respiratory symptoms and nonreversible
air-flow limitation (2).
Obstructive sleep apnea (OSA) is defined by repeated episodes of
upper airway occlusion, which results in brief periods of breathing
cessation (apnea) or a marked reduction in flow (hypopnea) during
sleep (3). David Flenley (4) was the first to coin the term ‘overlap
syndrome’ (OS) meaning the coexistence of COPD with OSA. Overlap
syndrome has an increased mortality compared with either COPD or
OSA alone. Marin et al (5)
found a decreased survival rate among patients with OS compared
with either COPD or OSA alone. Data are emerging that show worse
deleterious cardiac changes in individuals with OS compared to
those with COPD alone (6,7). Although much research still needs to
be conducted, more and more researchers are vigilant about OS.
Bone marrow-derived mesenchymal stem cells (BMSCs)
are pluripotent stem cells that can differentiate into osteoblasts,
chondrocytes or muscle cells and even into nerve cells or
hepatocytes (8). BMSCs have a good
paracrine function, enabling them to secrete a variety of
substances, such as neurotrophic factors, cytokines and chemokines
to regulate the local microenvironment of damage, reduce
inflammatory response, and promote the repair of damaged tissues.
MSCs have potential therapeutic roles in chronic lung diseases
(9). MSCs have the ability to
regulate the immune response to tissue injury, and MSCs also
promote repair in vivo and have been suggested as an
attractive therapeutic candidate for various types of lung
diseases, including chronic obstructive pulmonary disease (COPD),
among others (10). Studies
involving animal models have already demonstrated the effect of
MSCs on tissue regeneration after elastase-induced emphysema,
asbestos or endotoxin-induced lung injury (11,12).
OSA can induce oxidative stress, inflammation and endothelial
dysfunction, along with certain clinical consequences such as
cardiovascular diseases and neurocognitive alterations (13–15).
MSCs play an important role in the physiological response to OSA
(16). MSCs also have potential
therapeutic effects on COPD (17,18).
Emphysema is an important pathological
characteristic of COPD, characterized by a loss of alveolar walls
and permanently enlarged cavities of the terminal bronchioles
(19). Oxidative stress plays an
important role in the pathogenesis of COPD (20–23).
The increased apoptosis in alveolar cells of the COPD model and
MSCs can inhibit the apoptosis of alveolar cells (24,25).
Although it has been reported that MSCs play a protective and
reparative role in COPD and OSA, the data currently available on
the potential role of BMSCs in overlap syndrome (OS) are still
scarce. Moreover, the possible mechanisms of MSCs in OS have not
been reported.
In this study, rats were used to generate the OS
model by daily exposure to cigarette smoke and intermittent
hypoxia. To investigate the effects and possible mechanisms of
BMSCs on the OS model, BMSCs were intravenously injected into OS
model rats. We found that BMSCs were able to alleviate lung injury
in OS rats. Moreover, BMSCs inhibited oxidative stress and the
apoptosis of endotheliocytes. The experiment validated the
antioxidative stress and antiapoptotic effect of BMSCs in an OS
model.
Materials and methods
Isolation and culture of BMSCs
The extraction and characterization methods of BMSCs
in rats were performed as described previously (26). A total of 3 one-week-old
Sprague-Dawley (SD) rats (Vital River) were sacrificed by cervical
vertebra luxation. The femur and tibia of rats were removed under
sterile conditions. Bone marrow cells of rats were collected from
the femurs and tibias with Dulbecco's modified Eagle's medium
(DMEM; HyClone; GE Healthcare). The DMEM containing bone marrow
cells was added to a Percoll gradient (Pharmacia Biotech; GE
Healthcare) and centrifuged at 270 × g for 30 min. The mononuclear
cells were harvested and added to DMEM containing 10% fetal bovine
serum (FBS; Gibco/Thermo Fisher Scientific, Inc.). The cells were
cultured at 37°C in a 5% CO2 incubator. After 24 h,
nonadherent cells were removed, and the medium was replaced every
3–4 days. MSCs from the third passage were used in the subsequent
experiments. BMSCs were cultured and passaged with DMEM/F12 medium
(HyClone; GE Healthcare) containing 10% FBS, 100 U/ml penicillin
and 0.1 mg/ml streptomycin.
Identification of molecular markers on
the surface of the BMSCs
Third-generation cells were digested with trypsin
(HyClone/GE Healthcare), washed with PBS l-2 times, and then
centrifuged to collect the cells. A total of 1×l06 cells
were taken, and 350 µl of 1X PBS was added. After mixing, flow
tubes were added at 50 µl/tube. Next, 2 µl of a
fluorescence-labeled monoclonal antibody CD19-Fluor 488 (cat.
#NBP2-24965AF488, Novus Biologicals, USA), CD45-FITC (cat.
#85-11-0461-80, eBioscience), CD29-FITC (cat. #85-11-0291-82,
eBioscience), CD90-FITC (cat. #85-11-0900-85, eBioscience) or
isotype control FITC (cat. #85-11-4031-81, eBioscience) was added.
Next, 48 ml PBS was added to each tube and incubated at room
temperature in the dark for 30 min. Cell surface markers were
detected by flow cytometry (Sysmex Partec GmbH).
GFP-labeled BMSCs
Before infection, the eGFP-lentivirus (GM100202-2,
Shanghai Genomeditech Co., Ltd.) was diluted in complete medium
according to the manufacturer's instructions. The old medium was
removed, and the virus described above and polybrene were added to
the cells for infection and bleeding. After 48 h of infection, the
medium containing virus was replaced with fresh medium for further
culture. After 48 h, cells were injected intravenously into rats.
Four weeks after the last injection, the BMSC location was observed
under a confocal fluorescence microscope (Leica Microsystems
GmbH).
Establishment of the OS model by
cigarette smoke and intermittent hypoxia
The OS model was established by the cigarette smoke
(27) and intermittent hypoxia
exposure method. A total of 24 SD rats (female, 150–200 g,
6-week-old) were raised under controlled temperature (19–23°C) and
humidity (40–60%), and maintained on a 12-h light/dark cycle
lights. Food and water were provided ad libitum. All animal
experiments were approved by the Institutional Animal Care and Use
Committee of Kunming Medical University. Rats in the OS group were
exposed to cigarette smoke (15 cigarettes once, 2 times daily) and
intermittent hypoxia (30 times per h, 8 h per day) exposure for 8
weeks.
Grouping and BMSC transplantation
Female rats were randomly divided into three groups
(n=8): Control, OS and OS+BMSCs. Rats in the control group were
treated with false smoke and air exposure. From 24 h after
cigarette smoke and intermittent hypoxia exposure, the rats in the
OS group were injected intravenously with PBS (50 µl) and those in
the OS+BMSC group were injected intravenously with 50 µl of MSCs
(total number of cells: 2×106/rat) via the tail vein (1
time/week on the 7th day of each week, total 4 times). Three of the
OS model rats were injected intravenously with the GFP-labeled
BMSCs to investigate the location and differentiation of the
transplanted BMSCs, and five of the OS model rats were injected
intravenously with unlabeled BMSCs for examination with the TUNEL
assay. All rats were used to perform H&E staining and
immunocytochemistry assays.
Lung morphologic analysis,
quantification of emphysema and lung injury score
On week 4 of the last BMSCs injection, all rats were
euthanized with an overdose of anesthetic (3% sodium pentobarbital
180 mg/kg, i.p.). Lung tissues were fixed in 4% formalin, embedded
in paraffin, cut into 4-µm-thick sections, and stained with
hematoxylin and eosin (H&E). Histological assessment of the
sections was determined using the mean alveolar number (MAN) and
the mean linear intercept (MLI) method as previously described
(28,29) by Image-Pro Plus 6.0 software (Media
Cybernetics, Inc.). Three visual fields were observed in each
slice, in which no trachea or large blood vessels were observed.
The ‘+’ symbol was located in the middle of each field of each
section; the number of alveolar intervals (NS) that passed the ‘+’
symbol was determined, the total length (L) of the ‘+’ symbol was
measured (mm), the area of the field was determined (S), and the
number of alveola (Na) in each field was counted (MLI=L/NS and
MAN=Na/S). The pathological scores were detected as a reference
(30). Medium size (300–1100 µm)
membranous bronchi were selected to observe the following
indicators: i) epithelium abscission, erosion and ulcer formation;
ii) hyperplasia and hypertrophy of epithelial goblet cells; iii)
mucosal epithelial ciliary lodging; iv) inflammatory cell
infiltration and exudation of the airway wall; v) the formation of
lymphatic nodules in the vessel wall; vi) stenosis of the small
airway lumen; vii) a proliferation disorder of respiratory smooth
muscle; viii) connective tissue hyperplasia of the airway wall; ix)
squamous metaplasia of mucosal cells; x) wall congestion and edema;
and xi) pigmentation on the wall. Each item was scored 0, 1 for
mild, 2 for moderate, or 3 for severe. Three membranous bronchioles
were randomly selected from the pathological sections of each
rat.
Determination of SOD and MDA
levels
The contents of SOD (xanthine oxidase assay method)
and MDA (thiobarbituric acid assay method) were measured using
respective kits purchased from Nanjing Jiancheng Bio-Engineering
Institute Co., Ltd. (according to the manufacturer's
instructions).
TUNEL assay
The apoptosis of alveolar septal cells was also
detected using a TUNEL assay kit (cat. no. 11684817910, Roche
Diagnostics). Tissue samples were fixed in 4% formaldehyde for 24 h
and embedded in paraffin. Next, 4-µm-thick paraffin sections were
adhered to slides. Sections were deparaffinized by heating the
slides for 30 min at 60°C (or 10 min at 70°C), followed by two
5-min incubations in a xylene bath at room temperature. The tissue
samples were rehydrated by transferring the slides through a graded
ethanol series: 2×3 min 90% ethanol, 1×3 min 80% ethanol, 1×3 min
70% ethanol, and 1×3 min double-distilled water (ddH2O).
Excess water was carefully blotted away, and 20 mg/ml proteinase K
solution was pipetted to cover the sections. The mixture was then
incubated for 15 min at room temperature. Following proteinase K
treatment, the slides were washed 3×5 min with ddH2O.
Then, the sections were covered with 50 µl of TUNEL reaction buffer
for 60 min at 37°C. The slides were washed with PBS and air dried.
The tissue sections were examined by confocal microscopy
(magnification, ×400).
Immunofluorescence
Fixed tissues were dehydrated with various
concentrations of xylene and ethanol (50% ethanol for 4 h; 75%
ethanol for 4 h; 85% ethanol for 3 h; 95% ethanol for 2 h; 100%
ethanol for 1 h; 100% ethanol for 1 h; 1:1 ethanol-xylene for 1 h;
xylene for 1 h; and xylene for 30 min at room temperature) and
embedded in paraffin. Sections (4-µm thickness) were cut from a
paraffin block. Sections were dewaxed with various concentrations
of xylene and ethanol (xylene for 10 min; xylene for 5 min; 100%
ethanol for 5 min; 95% ethanol for 2 min; 80% ethanol for 2 min;
and 70% ethanol for 2 min). Antigen retrieval was performed on the
sections with 0.01 M citric acid buffer (pH 6.0) at 100°C and 80
kPa pressure. Sections were blocked by incubation with 5% v/v
normal goat serum (cat. no. SL038, Beijing Solarbio Science &
Technology Co., Ltd.) in PBS for 15 min at room temperature.
Sections were incubated with an anti-CD34 antibody (dilution 1:100,
cat. no. A10796; ABclonal Biotech Co., Ltd.) overnight at 4°C and
with a CoraLite594-conjugated secondary antibody (dilution 1:100;
cat. no. SA00013-4; ProteinTech Group, Inc.) for 2 h at room
temperature. Nuclei were stained with 10 µg/ml DAPI in the dark for
5 min at room temperature. Sections were observed using a
fluorescence microscope (magnification, ×200). Three representative
fields of stained cells were analyzed using Image-Pro Plus software
(version 6.0; Media Cybernetics, Inc.) to obtain the mean optical
density (OD), which represents the staining strength per positive
pixel.
Immunocytochemistry
Sections were incubated with an anti-CD31 antibody
(dilution 1:100, A3181; ABclonal) or an anti-VWF antibody (dilution
1:100, A1335; ABclonal) overnight at 4°C followed by incubation
with goat anti-rabbit IgG (H+L) horseradish peroxidase
(HRP)-conjugated secondary antibody (dilution 1:200, AS014,
ABclonal) for 2 h at room temperature. The reactions were
visualized using a 3,3′-diaminobenzidine visualization kit (Fuzhou
Maixin Biotech Co., Ltd.). Sections were counterstained with
hematoxylin to visualize nuclei, stained with hematoxylin for 5–10
min at room temperature, and dehydrated. Sections were examined
under a light microscope at a magnification of ×200. Brown staining
indicated immunoreactive positive cells, and blue staining
indicated the nuclei.
Statistical analysis
All values are expressed as the mean ± SD.
Comparisons were assessed with one-way analysis of variance (ANOVA)
(Tukey's post hoc test) by using GraphPad Prism software version
5.0a (GraphPad, USA). All experiments were conducted at least three
times. P<0.05 was considered statistically significant.
Results
Identification of BMSCs
As shown in Fig.
1A, BMSCs exhibited shapes similar to stars and spindles, with
round nuclei in the center, cytoplasmic hypertrophy and a clear
membrane. Flow cytometry detection showed that the third-generation
BMSCs expressed high levels of CD29 (99.9%) and CD90 (99.9%), and
low levels of CD19 (1.0%) and CD45 (3.1%) (Fig. 1B). These results indicate that rat
BMSCs were successfully collected.
Location and differentiation of BMSCs
transplanted in the lung tissue
The OS model was established by exposure to
cigarette smoke and intermittent hypoxia. The model profile for
each day is shown in Fig. 2A. At
the beginning of the smoke exposure, the rats were agitated,
irritable and pulsating, followed by less movement, eye closing,
wheezing, salivating and intermittent cough. After 2~3 weeks, rats
showed fur with yellowish color which easily fell off, a dispirited
demeanor, a decline in appetite, an emaciated bodily form, and a
decrease in activity ability. To evaluate the effect of BMSCs, we
used an OS model with intravenous injection of BMSCs (Fig. 2B). GFP-labeled BMSCs showed green
fluorescence, and green fluorescent cells (BMSCs) were observed in
the alveolar walls of lung tissues in the OS+BMSC group (Fig. 2C). Immunofluorescence detection
found that some cells had both green and red fluorescent
(CD34-positive) cells (Fig. 2C).
There are three important cell types in alveolar tissues: Alveolar
type I epithelial cells, alveolar type II epithelial cells and
endotheliocytes. These three types of cells are important for
maintaining the integrity of alveolar tissue, and the loss of one
of these cell types directly affects the integrity of the alveolar
tissue. CD34 is a marker of endotheliocytes; thus, we believe that
some of the transplanted BMSCs had differentiated into
endotheliocytes of the alveolar walls. Our results suggest that
BMSCs can migrate into alveolar walls and differentiate into the
endotheliocytes of alveolar walls.
BMSC transplantation reduces
emphysematous changes in the OS rat model
H&E staining was performed to detect emphysema
and pathological scores in the lung tissues of rats. We quantified
the enlargement of the alveolar spaces and alveolar septum fracture
by MLI, MAN and the pathological scores in the lung tissue by the
lung injury score. The alveolar structure was intact in the control
group (Fig. 3A and D). In the OS
group, there were emphysematous changes, the alveolar size was
different, the alveolar number was decreased, the alveolar space
was enlarged, and the alveolar septum was fractured (Fig. 3B). Moreover, in the OS group, a
small number of bronchial epithelial cells were shed, and the
bronchial epithelial cells exhibited edema and degeneration; the
bronchial tubes were irregular in shape and narrow in the lumen;
the alveolar walls were thickened; the alveoli fused into a large
cystic cavity; and inflammatory cell infiltration in bronchial and
lung tissue was observed in the OS group (Fig. 3E). In the OS+BMSC group, the
inflammatory cell infiltration and emphysematous changes were
significantly reduced after BMSC transplantation compared with
before BMSC transplantation (Fig. 3C
and F).
 | Figure 3.The therapeutic effects of BMSCs on
OS model rats. Hematoxylin and eosin (H&E) staining of lung
tissue sections in the control group (A and D), OS group (B and E)
and OS+BMSC group (C and F). Scale bar, 50 µm. (A) Alveolar
structure was intact. (B) Alveolar size was different, the alveolar
number was decreased, the alveolar space was enlarged and the
alveolar septum was fractured. (C) Less enlarged alveolar space and
less alveolar walls were thickened. (D) Alveolar structure was
intact, and no inflammatory cell infiltration was observed. (E) A
small number of bronchial epithelial cells were shed, and the
bronchial epithelial cells exhibited edema and degeneration; the
bronchial tubes were irregular in shape and narrow in the lumen;
alveolar walls were thickened; the alveoli fused into a large
cystic cavity; and much inflammatory cell infiltration. (F)
Decreased inflammatory cell infiltration. Histological assessment
of the sections was determined using the MLI (G), MAN (H) and
pathological scores (I). A small number of bronchial epithelial
cells are shed in the field, as shown by the black arrow. More
bronchial epithelial cells showed edema and degeneration, cell
swelling, loose cytoplasm and light staining, as shown by red
arrows. Thickening of the alveolar walls is noted in the field, as
shown by yellow arrows. The alveoli fused into a large cystic
cavity as shown by green arrows. Inflammatory cells infiltrate the
alveolar walls as shown by a blue arrow. ***P<0.001 vs. control
group; ##P<0.01 and ###P<0.001 vs. OS
group. BMSCs, bone marrow-derived mesenchymal stem cells; OS,
overlap syndrome; MLI, mean linear intercept; MAN, mean alveolar
number. |
The MLI and pathological scores were significantly
increased, and the MAN was significantly decreased in the OS group
compared with the control group. Compared with the OS group, the
MLI and pathological scores were significantly reduced and the MAN
was promoted in the OS+BMSC group (Fig. 3G-I). BMSC transplantation
alleviated lung injury in the OS rats.
BMSC transplantation has an
antioxidant effect on OS rats
We also investigated the functions of BMSCs on
oxidative stress in OS rats. As shown in Table I, there was an increase in the MDA
content and a decrease in SOD activity in serum and lung tissues in
the OS group, while BMSCs attenuated the elevated MDA content and
activation of SOD.
 | Table I.SOD activity and MDA content in the
different rat groups in the OS model (mean ± SD). |
Table I.
SOD activity and MDA content in the
different rat groups in the OS model (mean ± SD).
|
| Serum | Lung tissues |
|---|
|
|
|
|
|---|
| Rat groups | SOD (U/ml) | MDA (nmol/ml) | SOD (U/ml) | MDA (nmol/ml) |
|---|
| Control | 102.13±5.12 |
6.07±2.12 | 139.64±9.12 | 5.73±63 |
| OS |
26.43±1.33a |
12.32±1.72a |
43.12±1.87a |
10.43±2.11a |
| OS+BMSC |
93.84±2.08b |
7.23±0.75b |
129.32±1.87b |
5.98±1.2b |
BMSC transplantation reduces
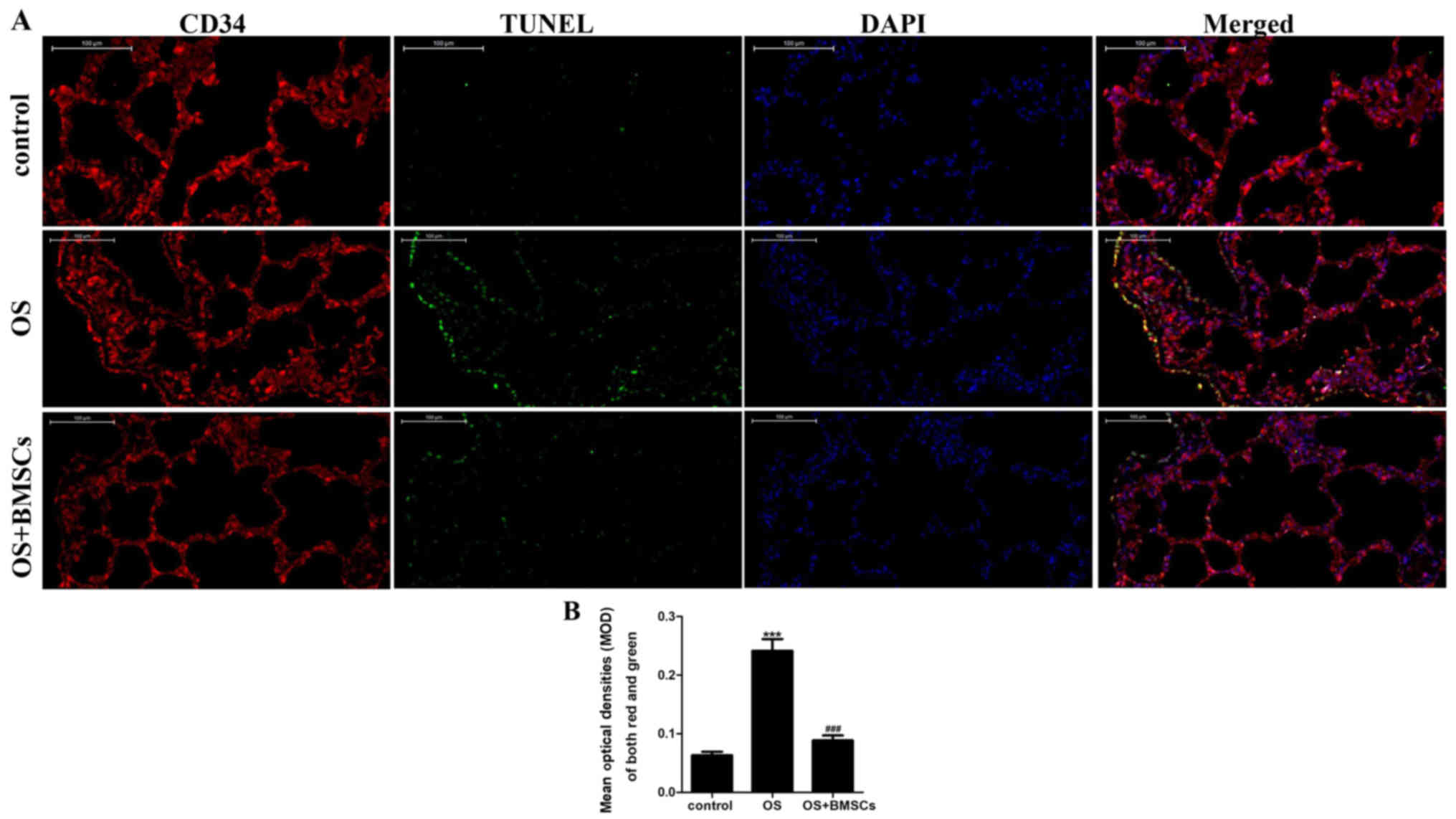
endotheliocyte apoptosis in the alveolar walls of OS rats
The apoptotic cells under fluorescence microscopy
displayed green fluorescence, while the endotheliocytes under
fluorescence microscopy fluoresced red. The co-expression of red
and green fluorescence indicated apoptotic endotheliocytes. As
shown in Fig. 4A, few apoptotic
cells were observed in the alveolar wall of the control group, and
apoptosis was more obvious in the OS group than it was in the
control group. BMSC transplantation significantly reduced
endotheliocyte apoptosis in the alveolar walls of OS rats.
Quantitative analysis showed that the apoptosis index of
endotheliocytes in the OS+BMSC group was significantly lower than
that in the OS group (Fig. 4B).
These results suggest that BMSCs can reduce endotheliocyte
apoptosis in the alveolar walls of OS rats.
BMSC transplantation promotes the
expression of CD31 and VWF in the alveolar walls of OS rats
CD31 and VWF are markers of endotheliocytes. The
levels of CD31 and VWF were detected by an immunohistochemical
assay. As shown in Fig. 5A and C,
the cytoplasm of alveolar wall cells in the lung tissue sections of
the transplanted rats was stained brown. A small amount of brown
was observed in the alveolar wall of the OS group compared with the
control group. The quantitative results (Fig. 5B and D) of the immunohistochemical
assay are consistent with Fig. 5A and
C. BMSC transplantation significantly increased the brown
staining in the alveolar walls of OS rats. These results indicate
that BMSCs suppressed the apoptosis of endotheliocytes in the
alveolar walls of OS rats.
Discussion
Several studies have confirmed that individuals with
chronic obstructive pulmonary disease (COPD) and obstructive sleep
apnea (OSA) have more profound nocturnal oxygen desaturation and
sleep disturbances compared with those with either disease alone
(31). Marin et al
(5) found decreased survival among
patients with overlap syndrome (OS) compared to those with either
COPD or OSA alone. Bone marrow-derived mesenchymal stem cells
(BMSCs) are stem cells that are found in connective tissues
throughout the whole body; they are easy to harvest, easy to
culture, quick to proliferate and weakly immunogenic (32–34).
Previous studies have shown that BMSCs can migrate into damaged
lung tissue (35,36). Moreover, BMSCs not only
differentiate into lung epithelial cells in damaged lung tissue
(37) but also secrete various
growth factors and cytokines in response to lung injury (38). Some studies have focused on the
effect of BMSCs on COPD alone or OSA alone, but few studies have
focused on the OS model.
One of the characteristics of OSA is intermittent
hypoxemia, and smoking is the main cause of COPD development. Thus,
we constructed the OS rat model with exposure to cigarette smoke
and intermittent hypoxia, yet the intermittent hypoxia model cannot
simulate upper airway obstruction. At present, there are no
relevant studies on spontaneous and natural OS animal models, and
there is no accepted evaluation standard for the OS model.
Theoretically, the establishment of the model can be judged based
on the evaluation criteria of OSA and COPD animal models, and the
main pathological characteristics of the two can determine the
success of the model. Building an ideal OS animal model requires
more exploration and practice. Compared with the control group, the
OS rats were in poor condition, with acidosis, hypoxemia and carbon
dioxide retention, and right ventricular hypertrophy (data not
shown). In this study, we first examined the effects of BMSC
transplantation in the OS model induced by cigarette smoke and
intermittent hypoxia and its potential mechanisms in vitro.
Our results revealed that BMSC transplantation relieved emphysema
and lung injury in the OS model by inhibiting oxidative stress and
apoptosis of endotheliocytes in the alveolar walls. We created an
OS model induced by cigarette smoke and intermittent hypoxia.
Alveolar wall rupture and emphysematous changes were observed in
the OS group. The MLI and pathological scores were higher and the
MAN was lower in the OS group than these parameters in the control
group. Moreover, the malondialdehyde (MDA) content was increased
and the superoxide dismutase (SOD) activity was decreased in the OS
group compared with the control group. These results suggest that
cigarette smoke and intermittent hypoxia could promote oxidative
stress, emphysema, alveolar congestion, alveolar wall edema and
inflammatory cell infiltration and increase the lung injury score.
The BMSC transplantation group exhibited significantly alleviated
lung injury compared with the OS group. SOD is an important free
radical scavenger in the body that can protect cell membranes from
oxidation and lipid peroxidation (39). Decreased SOD activity in blood and
lung tissues is one of the manifestations of smoking-induced COPD
damage. MDA is a product of lipid peroxidation induced by free
radical attack on biofilms. The MDA level can reflect the degree of
lipid peroxidation in the body (40), and its level in serum and lung can
indirectly reflect the degree of cell damage. The increased SOD
activity and decreased MDA content in the serum and lung of the
OS+BMSC group rats, suggest that BMSCs can enhance the activity of
free radical scavenging enzymes and enhance the antioxidant
capacity and reduce the damage caused by lipid peroxidation.
To investigate whether the transplanted BMSCs could
migrate into the lung in OS rats, we marked BMSCs with an eGFP
lentivirus before injection. We also detected endotheliocytes by
CD34 immunofluorescence. We observed green fluorescence in the
alveolar walls and its co-expression with CD34. This implied that
BMSCs can migrate to the injury lung tissues, but not only lung
tissues. We found that BMSCs also can migrate into the aorta (data
not shown). Moreover, decreased apoptosis of endotheliocytes in
alveolar walls was observed in the OS+BMSC group, and a high rate
of apoptosis was found in the OS group. These results suggest that
BMSCs can migrate into lung tissues and differentiate into
endotheliocytes in alveolar walls, and BMSC transplantation
markedly reduced the apoptosis of endotheliocytes in alveolar
walls, which may represent one mechanism for emphysema therapy.
Some studies have indicated that the apoptosis of alveolar type II
epithelial cells is directly associated with emphysema (41,42)
and that MSCs can differentiate into alveolar type I and II
epithelial cells (43–45). These studies indicate that MSCs can
differentiate into alveolar cells. In the present study,
transplanted BMSCs with CD34-positive expression differentiated
into alveolar wall endotheliocytes, and the apoptosis of
endotheliocytes in the alveolar walls is important for OS, which
may help to alleviate emphysema. We also examined the expression of
the endothelial cell markers CD31 and VWF by immunohistochemical
assay. The results suggested that BMSCs suppress the apoptosis of
endothelial cells, which agrees with previous TUNEL results. There
was a possibility that the release of growth factors and structural
support may be a determinant for the regenerative effects observed
following treatment with BMSCs. Since BMSCs have strong
proliferative properties, they are theoretically tumorigenic in
tissue or cell transplantation (46). Therefore, the numbers of BMSCs for
treatment should be strictly controlled, so that the excessive
accumulation of BMSCs do not cause negative effects. BMSCs can
migrate to damaged tissues, so they are present not only in the
lungs, but also in other tissues, such as the aorta and the heart.
This indicates that the OS model can lead not only to lung injury
but also to other organ injury. We will study the repair effect of
BMSCs on other organs in OS rats in further research. There is also
the question of whether the presence of undifferentiated BMSCs
causes damage to organs, since excessive injection increases the
risk of tumors. Therefore, the use of BMSCs must be strictly
controlled within a safe range, which requires more in-depth
research.
In summary, we demonstrated the therapeutic effect
of BMSC administration in OS rats induced by cigarette smoke and
intermittent hypoxia. BMSCs can suppress pulmonary emphysema and
oxidative stress in OS rats. Moreover, BMSCs were able to migrate
into the alveolar walls and differentiate into endotheliocytes in
the alveolar wall, inhibiting endotheliocyte apoptosis. This
experiment confirms that the inhibition of the progression of
emphysema by BMSCs in the OS model may be through the
differentiation of BMSCs into endotheliocytes consequently
suppressing endotheliocyte apoptosis and by their antioxidative
stress function. However, further elucidation of the relationship
between BMSCs and endotheliocyte proliferation and whether they can
promote endotheliocyte proliferation remain to be further studied.
Our study indicates that BMSCs are potent and may serve as a basis
for clinical trials with OS patients in the near future.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural
Science Foundation of China (no. 81560010) and the Science and
Technology Planning Project of Yunnan Province [no.
2017FE467(−100)].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC, ZH and ZJ conceived and designed the study. HB,
XP, JH, LH and XH performed the experiments. JD, KZ, LW, QW and XG
collected and processed the data. MC, ZH and QW wrote the
manuscript. MC, ZH, XG and ZJ reviewed and edited the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The animal experiments were approved by the Animals
Ethics Committee of Kunming Medical University and the Research
Medical Ethics Committee of Kunming Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McNicholas WT, Verbraecken J and Marin JM:
Sleep disorders in COPD: The forgotten dimension. Eur Respir Rev.
22:365–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rabe KF, Hurd S, Anzueto A, Barnes PJ,
Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R,
van Weel C, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 176:532–555. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alkhalil M, Schulman E and Getsy J:
Obstructive sleep apnea syndrome and asthma: What are the links? J
Clin Sleep Med. 5:71–78. 2009.PubMed/NCBI
|
|
4
|
Flenley DC: Sleep in chronic obstructive
lung disease. Clin Chest Med. 6:651–661. 1985.PubMed/NCBI
|
|
5
|
Marin JM, Soriano JB, Carrizo SJ, Boldova
A and Celli BR: Outcomes in patients with chronic obstructive
pulmonary disease and obstructive sleep apnea: The overlap
syndrome. Am J Respir Crit Care Med. 182:325–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neilan TG, Bakker JP, Sharma B, Owens RL,
Farhad H, Shah RV, Abbasi SA, Kohli P, Wilson J, DeMaria A, et al:
T1 measurements for detection of expansion of the myocardial
extracellular volume in chronic obstructive pulmonary disease. Can
J Cardiol. 30:1668–1675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma B, Neilan TG, Kwong RY, Mandry D,
Owens RL, McSharry D, Bakker JP and Malhotra A: Evaluation of right
ventricular remodeling using cardiac magnetic resonance imaging in
co-existent chronic obstructive pulmonary disease and obstructive
sleep apnea. COPD. 10:4–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Antoniou KM, Karagiannis K, Tsitoura E,
Bibaki E, Lasithiotaki I, Proklou A, Spandidos DA and Tzanakis N:
Clinical applications of mesenchymal stem cells in chronic lung
diseases. Biomed Rep. 8:314–318. 2018.PubMed/NCBI
|
|
10
|
Gupta N, Su X, Popov B, Lee JW, Serikov V
and Matthay MA: Intrapulmonary delivery of bone marrow-derived
mesenchymal stem cells improves survival and attenuates
endotoxin-induced acute lung injury in mice. J Immunol.
179:1855–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishizawa K, Kubo H, Yamada M, Kobayashi S,
Numasaki M, Ueda S, Suzuki T and Sasaki H: Bone marrow-derived
cells contribute to lung regeneration after elastase-induced
pulmonary emphysema. FEBS Lett. 556:249–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spees JL, Pociask DA, Sullivan DE, Whitney
MJ, Lasky JA, Prockop DJ and Brody AR: Engraftment of bone marrow
progenitor cells in a rat model of asbestos-induced pulmonary
fibrosis. Am J Respir Crit Care Med. 176:385–394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caples SM, Garcia-Touchard A and Somers
VK: Sleep-disordered breathing and cardiovascular risk. Sleep.
30:291–303. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Campana L, Eckert DJ, Patel SR and
Malhotra A: Pathophysiology & genetics of obstructive sleep
apnoea. Indian J Med Res. 131:176–187. 2010.PubMed/NCBI
|
|
15
|
Jelic S, Padeletti M, Kawut SM, Higgins C,
Canfield SM, Onat D, Colombo PC, Basner RC, Factor P and LeJemtel
TH: Inflammation, oxidative stress, and repair capacity of the
vascular endothelium in obstructive sleep apnea. Circulation.
117:2270–2278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carreras A, Almendros I and Farré R:
Potential role of bone marrow mesenchymal stem cells in obstructive
sleep apnea. Int J Stem Cells. 4:43–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JS, Kim HK, Kang EY, Cho R and Oh YM:
Potential therapeutic strategy in chronic obstructive pulmonary
disease using pioglitazone-augmented wharton's jelly-derived
mesenchymal stem cells. Tuberc Respir Dis (Seoul). 82:158–165.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu HM, Liu YT, Zhang J and Ma LJ: Bone
marrow mesenchymal stem cells ameliorate lung injury through
anti-inflammatory and antibacterial effect in COPD mice. J Huazhong
Univ Sci Technolog Med Sci. 37:496–504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miglino N, Roth M, Tamm M and Borger P:
Asthma and COPD-the C/EBP connection. Open Respir Med J. 6:1–13.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pryor WA and Stone K: Oxidants in
cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and
peroxynitrite. Ann N Y Acad Sci. 686:12–28. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Antus B, Harnasi G, Drozdovszky O and
Barta I: Monitoring oxidative stress during chronic obstructive
pulmonary disease exacerbations using malondialdehyde. Respirology.
19:74–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cristóvão C, Cristóvão L, Nogueira F and
Bicho M: Evaluation of the oxidant and antioxidant balance in the
pathogenesis of chronic obstructive pulmonary disease. Rev Port
Pneumol. 19:70–75. 2013.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Montaño M, Cisneros J, Ramírez-Venegas A,
Pedraza-Chaverri J, Mercado D, Ramos C and Sansores RH:
Malondialdehyde and superoxide dismutase correlate with FEV(1) in
patients with COPD associated with wood smoke exposure and tobacco
smoking. Inhal Toxicol. 22:868–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hodge S, Hodge G, Holmes M and Reynolds
PN: Increased airway epithelial and T-cell apoptosis in COPD
remains despite smoking cessation. Eur Respir J. 25:447–454. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhen G, Xue Z, Zhao J, Gu N, Tang Z, Xu Y
and Zhang Z: Mesenchymal stem cell transplantation increases
expression of vascular endothelial growth factor in papain-induced
emphysematous lungs and inhibits apoptosis of lung cells.
Cytotherapy. 12:605–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Y, Xu A, Xu Q, Zhao W, Li D, Fang X
and Ren Y: Bone marrow mesenchymal stem cell transplantation for
treatment of emphysemic rats. Int J Clin Exp Med. 7:968–972.
2014.PubMed/NCBI
|
|
27
|
Lee JH, Lee DS, Kim EK, Choe KH, Oh YM,
Shim TS, Kim SE, Lee YS and Lee SD: Simvastatin inhibits cigarette
smoking-induced emphysema and pulmonary hypertension in rat lungs.
Am J Respir Crit Care Med. 172:987–993. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thurlbeck WM: Measurement of pulmonary
emphysema. Am Rev Respir Dis. 95:752–764. 1967.PubMed/NCBI
|
|
29
|
Zhen G, Liu H, Gu N, Zhang H, Xu Y and
Zhang Z: Mesenchymal stem cells transplantation protects against
rat pulmonary emphysema. Front Biosci. 13:3415–3422. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vernooy JH, Dentener MA, van Suylen RJ,
Buurman WA and Wouters EF: Long-term intratracheal
lipopolysaccharide exposure in mice results in chronic lung
inflammation and persistent pathology. Am J Respir Cell Mol Biol.
26:152–159. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanders MH, Newman AB, Haggerty CL,
Redline S, Lebowitz M, Samet J, O'Connor GT, Punjabi NM and Shahar
E; Sleep Heart Health Study, : Sleep and sleep-disordered breathing
in adults with predominantly mild obstructive airway disease. Am J
Respir Crit Care Med. 167:7–14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kashiwakura Y, Katoh Y, Tamayose K,
Konishi H, Takaya N, Yuhara S, Yamada M, Sugimoto K and Daida H:
Isolation of bone marrow stromal cell-derived smooth muscle cells
by a human SM22alpha promoter: In vitro differentiation of putative
smooth muscle progenitor cells of bone marrow. Circulation.
107:2078–2081. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Devine SM, Cobbs C, Jennings M,
Bartholomew A and Hoffman R: Mesenchymal stem cells distribute to a
wide range of tissues following systemic infusion into nonhuman
primates. Blood. 101:2999–3001. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bos C, Delmas Y, Desmoulière A, Solanilla
A, Hauger O, Grosset C, Dubus I, Ivanovic Z, Rosenbaum J, Charbord
P, et al: In vivo MR imaging of intravascularly injected
magnetically labeled mesenchymal stem cells in rat kidney and
liver. Radiology. 233:781–789. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamada M, Kubo H, Kobayashi S, Ishizawa K,
Numasaki M, Ueda S, Suzuki T and Sasaki H: Bone marrow-derived
progenitor cells are important for lung repair after
lipopolysaccharide-induced lung injury. J Immunol. 172:1266–1272.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ortiz LA, Gambelli F, McBride C, Gaupp D,
Baddoo M, Kaminski N and Phinney DG: Mesenchymal stem cell
engraftment in lung is enhanced in response to bleomycin exposure
and ameliorates its fibrotic effects. Proc Natl Acad Sci USA.
100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kotton DN, Ma BY, Cardoso WV, Sanderson
EA, Summer RS, Williams MC and Fine A: Bone marrow-derived cells as
progenitors of lung alveolar epithelium. Development.
128:5181–5188. 2001.PubMed/NCBI
|
|
38
|
Weiss DJ, Kolls JK, Ortiz LA,
Panoskaltsis-Mortari A and Prockop DJ: Stem cells and cell
therapies in lung biology and lung diseases. Proc Am Thorac Soc.
5:637–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chandra Jagetia G, Rajanikant GK, Rao SK
and Shrinath Baliga M: Alteration in the glutathione, glutathione
peroxidase, superoxide dismutase and lipid peroxidation by ascorbic
acid in the skin of mice exposed to fractionated gamma radiation.
Clin Chim Acta. 332:111–121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang RQ, Li DY, Xu TD, Zhu SS, Pan HJ,
Fang F, Wu X and Sun H: Antioxidative effect of luteolin
pretreatment on simulated ischemia/reperfusion injury in
cardiomyocyte and perfused rat heart. Chin J Integr Med.
23:518–527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mauck RL, Yuan X and Tuan RS: Chondrogenic
differentiation and functional maturation of bovine mesenchymal
stem cells in long-term agarose culture. Osteoarthritis Cartilage.
14:179–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Snykers S, Vanhaecke T, Papeleu P, Luttun
A, Jiang Y, Vander Heyden Y, Verfaillie C and Rogiers V: Sequential
exposure to cytokines reflecting embryogenesis: The key for in
vitro differentiation of adult bone marrow stem cells into
functional hepatocyte-like cells. Toxicol Sci. 94:330–341;
discussion 235–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Grove JE, Lutzko C, Priller J, Henegariu
O, Theise ND, Kohn DB and Krause DS: Marrow-derived cells as
vehicles for delivery of gene therapy to pulmonary epithelium. Am J
Respir Cell Mol Biol. 27:645–651. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abe S, Lauby G, Boyer C, Rennard SI and
Sharp JG: Transplanted BM and BM side population cells contribute
progeny to the lung and liver in irradiated mice. Cytotherapy.
5:523–533. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rojas M, Xu J, Woods CR, Mora AL, Spears
W, Roman J and Brigham KL: Bone marrow-derived mesenchymal stem
cells in repair of the injured lung. Am J Respir Cell Mol Biol.
33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu CJ, Kuo FC, Hu HM, Chen CY, Huang YB,
Cheng KH, Yokoyama KK, Wu DC, Hsieh S and Kuo CH: 17β-Estradiol
inhibition of IL-6-Src and Cas and paxillin pathway suppresses
human mesenchymal stem cells-mediated gastric cancer cell motility.
Transl Res. 164:232–243. 2014. View Article : Google Scholar : PubMed/NCBI
|