Introduction
Diabetes mellitus (DM) is a complex metabolic
disease, characterized by high blood glucose levels and
dysfunctional lipid metabolism. DM is further classified as type 1
(TIDM) and type 2 (T2DM), caused by absolute and relative insulin
insufficiency, respectively. In T1DM, inadequate mass of functional
pancreatic β cells causes insulin deficiency (1). A variety of traditional treatments
for diabetes, including insulin therapy, have limited ability to
simulate insulin secretion and are frequently associated with
severe hypoglycemic episodes (2,3). To
overcome the limitations of traditional treatment, researchers have
focused on replenishing the loss of insulin-producing cells and
expansion of existing pancreatic β cells through regeneration
(2).
The streptozotocin (STZ)-treated neonatal model is
useful for investigating β-cell regeneration (4). It provides a better understanding of
DM pathophysiology by exhibiting hyperglycemia, altered glucose
tolerance, and reduced insulin secretion after neonatal injection
of STZ (5,6).
Betatrophin, also known as lipasin (7), angiopoietin-like 8 (ANGPTL8)
(8), refeeding induced in fat and
liver (RIFL) (9), or
hepatocellular carcinoma-associated gene TD26, is a protein in the
ANGPTL family. Originally derived from liver and adipose tissues,
it regulates triglyceride (TG) clearance by inhibiting lipoprotein
lipase (LPL) in plasma (10).
Betatrophin is known to induce β-cell proliferation
and to improve glucose tolerance in insulin resistance states
(11). Jiao et al (12) found that elevated hepatic
betatrophin expression increased the proliferation of β cells
transplanted from mice. However, this finding is contentious. Cox
et al reported that β-cell replication was not obviously
altered after ANGPTL8 overexpression in B6.129 mice (13). Since the initial discovery relied
on cDNA overexpression rather than direct treatment using the
recombinant protein, we tested its direct effect on diabetes and β
cells using a neonatal diabetes model.
Furthermore, numerous clinical studies have
demonstrated that betatrophin levels are significantly increased in
individuals with obesity, who are pregnant (14), with T1DM (15), with newly identified type 2
diabetes mellitus (nT2DM), T2DM, and diabetic retinopathy (16,17).
These facts suggest that betatrophin may be involved in
compensatory mechanisms of enhanced insulin levels. In the present
study, we investigated the short- and long-term effects of
betatrophin in a neonatal STZ-induced diabetes mellitus model and
the underlying mechanisms.
Materials and methods
Reagents
Recombinant human betatrophin was purchased from
Creative Biomart (Shirley, NY, USA), and STZ was purchased from
Wako Pure Chemicals (Sigma-Aldrich; Merck KGaA).
Animals (rat model of diabetes)
Wistar rats (275.4±10.1 g per rat, n=5) at 16–18
days of pregnancy, were obtained from Qingdao Institute for Food
and Drug Control. They were kept in specific pathogen-free
conditions in filtered cages and were fed standard chow diets and
water in a 12-h light/dark cycle and housed in standard housing
conditions (22–24°C and 55±20% humidity). Each litter of pups was
randomly divided into four groups (STZ group n=13, STZ+betatrophin
group n=11, Untreated group n=13, Untreated+betatrophin group
n=18). For neonates within 10 h of birth, rats were injected
intraperitoneally (i.p.) with a single dose of STZ (100 mg/kg,
freshly dissolved in 0.1 M citrate buffer, pH 4.5), or citrate
buffer alone. Male animals were used for further study only if
their random glucose levels were higher than 11.1 mM on day 3
measured using a glucometer (B. Braun, Meilsungen, Germany).
Betatrophin (300 mg/kg, freshly dissolved in normal saline) or
normal saline alone was administered to the animals by i.p.
injection for 6 days (days 1–6). In the long term study, STZ and
STZ+betatrophin groups were injected with a single dose of STZ (100
mg/kg) within 10 h of birth. Betatrophin (300 mg/kg) was
administered to the STZ+betatrophin group by i.p. injection for 6
days (day 1–6). At least five rats in each group were maintained
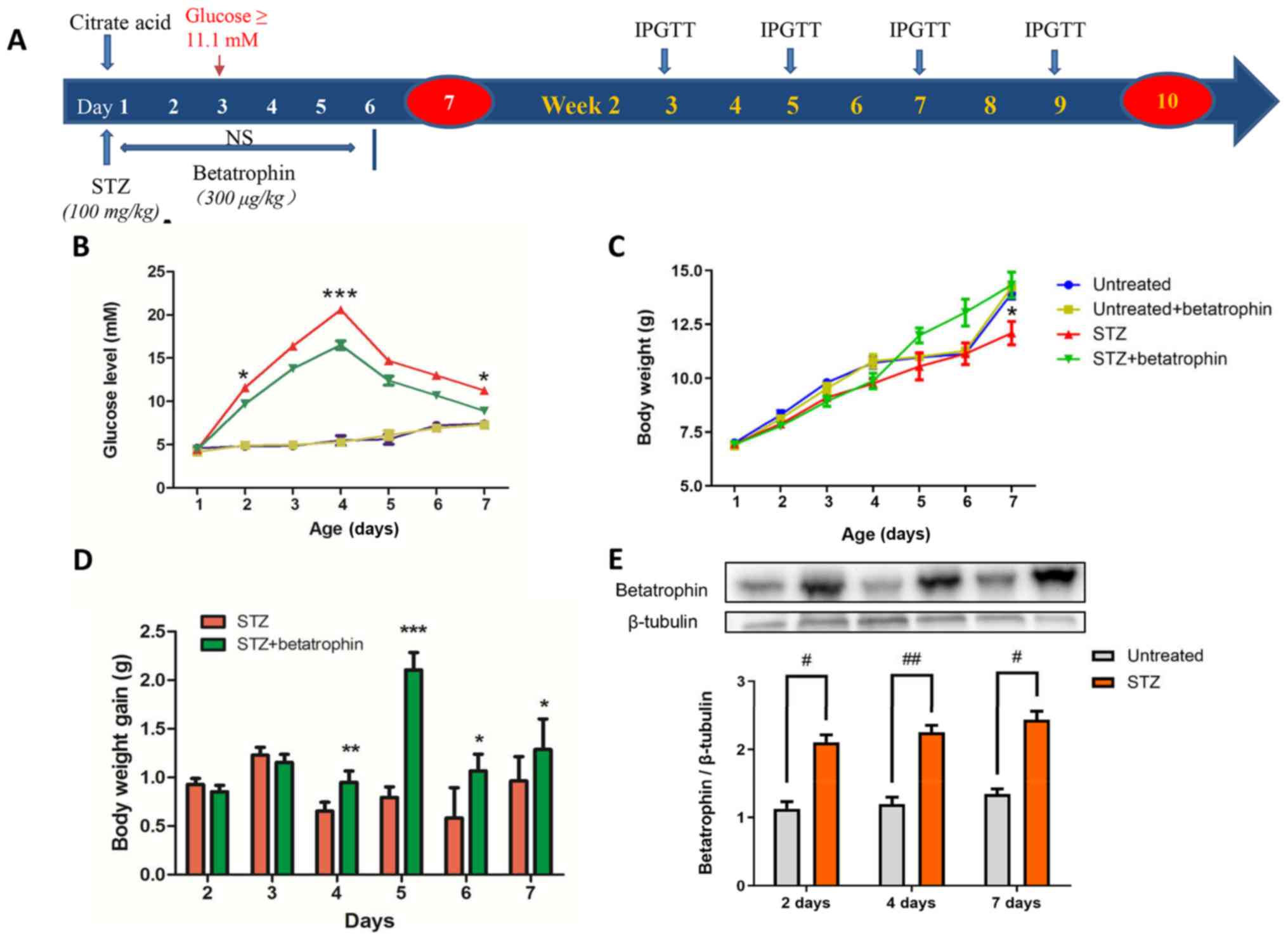
until the 10th week. The design of the study is presented in
Fig. 1A.
Animals were sacrificed on days 2, 4, and 7 after
birth by decapitation. Adult rats were sacrificed at 70 days after
birth by bleeding following anesthesia with an i.p. injection of
sodium pentobarbital (50 mg/kg). Blood samples were immediately
collected and centrifuged at 20,000 × g for 2 min at 4°C and then
stored at −20°C until assayed. The animal experimental protocol was
approved by the Ethics Committee of the Medical Department of
Qingdao University (Qingdao, China). All experiments were conducted
in accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals.
Measurement of body weight, blood
glucose and plasma insulin levels
Blood glucose levels were measured with a glucometer
from the foot pad. Body weights were measured with an electric
balance (PL1501-S; Mettler Toledo, China) each day of the study.
Plasma insulin levels of the 4- and 7-day-old pups were measured
using an enzyme-linked immunosorbent assay kit (EIaab Science Co.,
Wuhan, China), following the manufacturer's protocol.
Glucose tolerance test (IPGTT)
Rats were fasted for 12 h prior to the
intraperitoneal glucose tolerance test (IPGTT). Glucose levels were
measured from the tail vein at 0, 30, 60 and 120 min, after an i.p.
injection of glucose (2 g/kg), using a One Touch Basic glucose
meter.
Western blot analysis
In the pancreas and liver tissues of 2-, 4- and
7-day-old neonates, western blot analysis of insulin promoter
factor, duodenal homeobox gene-1 (PDX-1) and anti-apoptosis factor
B-cell lymphoma-2 (Bcl-2) was carried out as previously described
(18). Primary antibodies included
rabbit anti-PDX-1 diluted at 1:4,000 (#2437, Cell Signaling
Technology), rabbit anti-Bcl-2 diluted at 1:4,000 (AB1722,
Millipore), rabbit anti-betatrophin diluted at 1:4,000 (ab180915,
Abcam), rabbit anti-Bax diluted at 1:1,000 (#2772T, Cell Signaling
Technology) and rabbit anti-β-tubulin diluted at 1:2,000 (#2128,
Cell Signaling Technology). The intensity of bands was analyzed
using Quantity One Software (Bio-Rad). Peroxidase-conjugated
secondary antibody was diluted at 1:8,000 (ZB2301, ZSGB-BIO,
Beijing, China). The protein concentration was measured using
Quantity One Software (Bio-Rad).
Immunohistochemistry of the
pancreas
The pancreas of each pup was rapidly harvested,
weighed and fixed in 4% paraformaldehyde for 24 h.
Immunohistochemistry and immunofluorescence staining analysis was
performed as described previously (19). The tissues were subsequently
dehydrated in graded concentrations of ethanol, cleared in xylene,
embedded in paraffin and sectioned into 5-µm tissue sections.
Adjacent sections at a fixed interval throughout the block were
immunostained for insulin using a technique adapted from the
peroxidase indirect labeling method. The sections were incubated
overnight with primary antibodies against insulin (ZSGB-BIO) after
1 h blocking with 1% bovine serum albumin. They were then incubated
with biotinylated secondary antibody (PV6000; ZSGB-BIO) for 3 h.
Insulin positive staining was visualized with hematoxylin and eosin
and mounted in 3,3′diaminobenzidine (DAB). Histological images were
captured using high-power light microscopy (DP72; Olympus, Tokyo,
Japan). Ten non-consecutive sections of different series per block
were immunostained for insulin; at least six random islets
(magnification, ×400) from each section were counted for
insulin-positive areas. The β-cell area ratio was quantified as the
area of insulin-positive cells divided by the total tissue area
using Image J software (National Institutes of Health, Bethesda,
MD, USA).
Immunofluorescence staining
The paraffin-embedded sections were deparaffinized
and rehydrated using xylene, ethanol and PBS. Antigen retrieval was
performed using 10 mM citrate buffer (pH 6.0) in a microwave oven
for two treatments of 10 min each. Sections were blocked in 10%
normal donkey serum, 1.0% bovine serum albumin (BSA) for 2 h to
prevent nonspecific binding. Incubation with the primary antibody
mix was performed at 4°C overnight in a wet chamber followed by
incubation with the mixture of fluorophore-conjugated secondary
antibody for 2 h in a wet chamber protected from light. The primary
antibodies used were anti-insulin (dilution 1:1,000, E11D7; Merck
Millipore) and anti-Ki67 (dilution 1:1,000, #2382594; Merck
Millipore). Corresponding secondary antibodies were
Rhodamine-conjugated Affinipure goat anti-mouse, and
Fluorescein-conjugated Affinipure goat anti-rabbit made by
ZSGB-BIO. Slides were mounted with coverslips with a drop of 50%
glycerol. Fluorescence images were captured with a laser confocal
microscope (FV500; Olympus). Ten non-consecutive sections of
different series per block were immunostained for insulin; at least
five random islets from each section were measured to determine the
proportion of insulin/Ki67 co-localized cells of the total β cells
which was counted using ImageJ software.
Statistical analysis
Data are expressed as mean ± SEM and analyzed using
ANOVA. The LSD (least significance difference) test was used as the
post-hoc test, with the Student's t-test and the Fisher's exact
test, with statistical significance set at P<0.05.
Results
Betatrophin treatment decreases
STZ-promoted hyperglycemia in neonatal rats
In order to establish neonatal diabetes by
specifically destroying β cells, STZ at 100 mg/kg was injected into
newborn pups 10 h after birth, as previously described (20). As shown in Fig. 1B, compared to the untreated pups,
STZ treatment caused a steady elevation in blood glucose level from
5 to 22 mM over 4 days. Glucose levels then gradually declined from
4 to 7 days to 13 mM, suggesting a partial recovery from diabetes.
The expression of endogenous betatrophin in the liver doubled from
day 2 in the STZ group compared to levels in untreated pups,
suggesting possible involvement of betatrophin in diabetes
(Fig. 1E).
Betatrophin+STZ treatments also caused substantial
increases in glucose levels, however, to a significantly lesser
extent especially on days 2, 4 and 7 and only peaked at 16 mM
(Fig. 1B). Body weight was
significantly different between the STZ and STZ+betatrophin groups
on day 7 (12.81±0.37 g, n=9 vs. 13.82±0.25 g, n=11). There was a
tendency of the STZ+betatrophin rats to grow faster from the 4th to
the 7th day of age (Fig. 1C). STZ
treatment caused a slight delay in weight gain (Fig. 1D) and a decrease in pancreatic
weight, especially on day 7, both of which were prevented by
betatrophin treatment (Fig. 1 and
Table I).
 | Table I.Effects of STZ and betatrophin
treatment on 4- and 7-day-old neonatal rats. |
Table I.
Effects of STZ and betatrophin
treatment on 4- and 7-day-old neonatal rats.
| Age (days) | Treatment | Number | Body weight
(g) | Pancreas weight
(mg) |
|---|
| 4 | Untreated | 13 | 10.72±0.25 | 23.3±0.002 |
|
|
Untreated+betatrophin | 11 | 10.79±0.31 | 25.4±0.6 |
|
| STZ | 13 | 10.01±0.15 | 22.95±0.002 |
|
|
STZ+betatrophin | 18 | 9.88±0.336 | 24.89±0.001 |
| 7 | Untreated | 7 | 14.71±0.3 | 35.2±1.3 |
|
|
Untreated+betatrophin | 6 | 14.5±0.2 | 36.2±1.0 |
|
| STZ | 9 | 12.81±0.37 | 24.2±1.0 |
|
|
STZ+betatrophin | 11 |
13.82±0.25a |
38.4±4.1b |
Betatrophin treatment reverses the
depletion of plasma insulin and the mortality caused by STZ
With partial destruction of β cells, plasma insulin
levels in neonatal rats were significantly lower by approximately
30% at 4 and 7 days after STZ injection, an effect that was
completely reversed by the treatment of recombinant betatrophin
(Fig. 2A and B). Treatment with
betatrophin alone in normal neonates did not significantly affect
insulin levels.
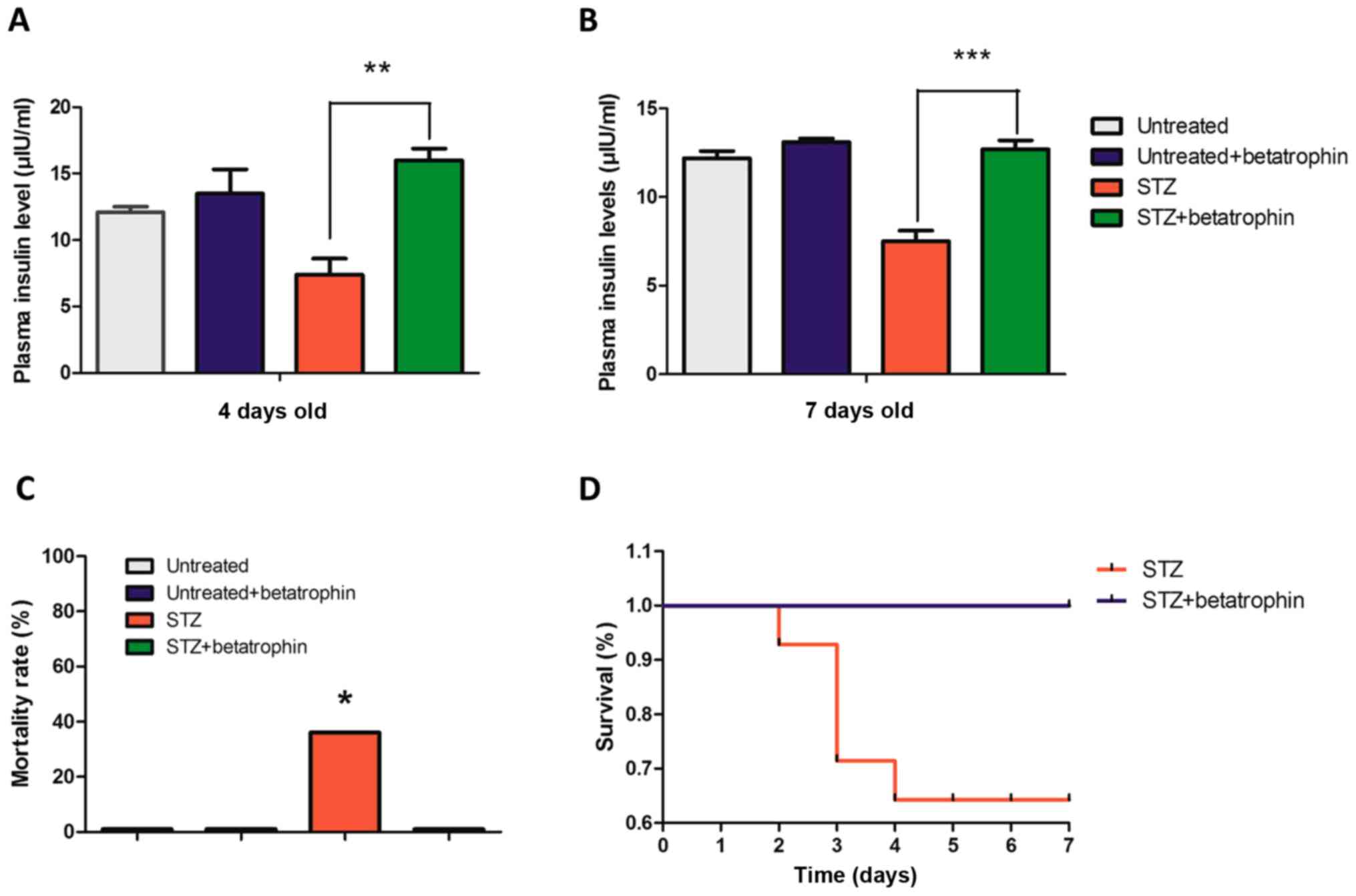 | Figure 2.Betatrophin treatment reverses the
depletion of plasma insulin and the mortality caused by STZ. Plasma
insulin levels (µIU/ml) of (A) 4- and (B) 7-day-old neonates in the
untreated (n=6, 7), Untreated+betatrophin (n=5, 6), STZ (n=4, 9)
and STZ+betatrophin groups (n=7, 11). (C) The mortality rate of the
STZ-induced diabetic rat neonates with (STZ+betatrophin group) and
without (STZ group) betatrophin treatment was calculated at day 7
after birth. A Fisher exact test with Freeman-Halton extension was
used to determine significance for mortality, P=0.00037681;
significance was determined at P<0.05. (D) Changes in the
survival rate. Bars represent mean ± SEM *P<0.05, **P<0.01,
***P<0.001, STZ rats vs. STZ+betatrophin rats. STZ,
streptozotocin. |
STZ injection caused very severe hyperglycemia. The
affected pups became pale and inactive, and 36% of them in fact
died within 2–4 days (Fig. 2C and
D). This high mortality rate was once again completely
prevented by co-treatment of betatrophin, supporting the notion
that early administration of betatrophin protected the newborns
from diabetes and mortality.
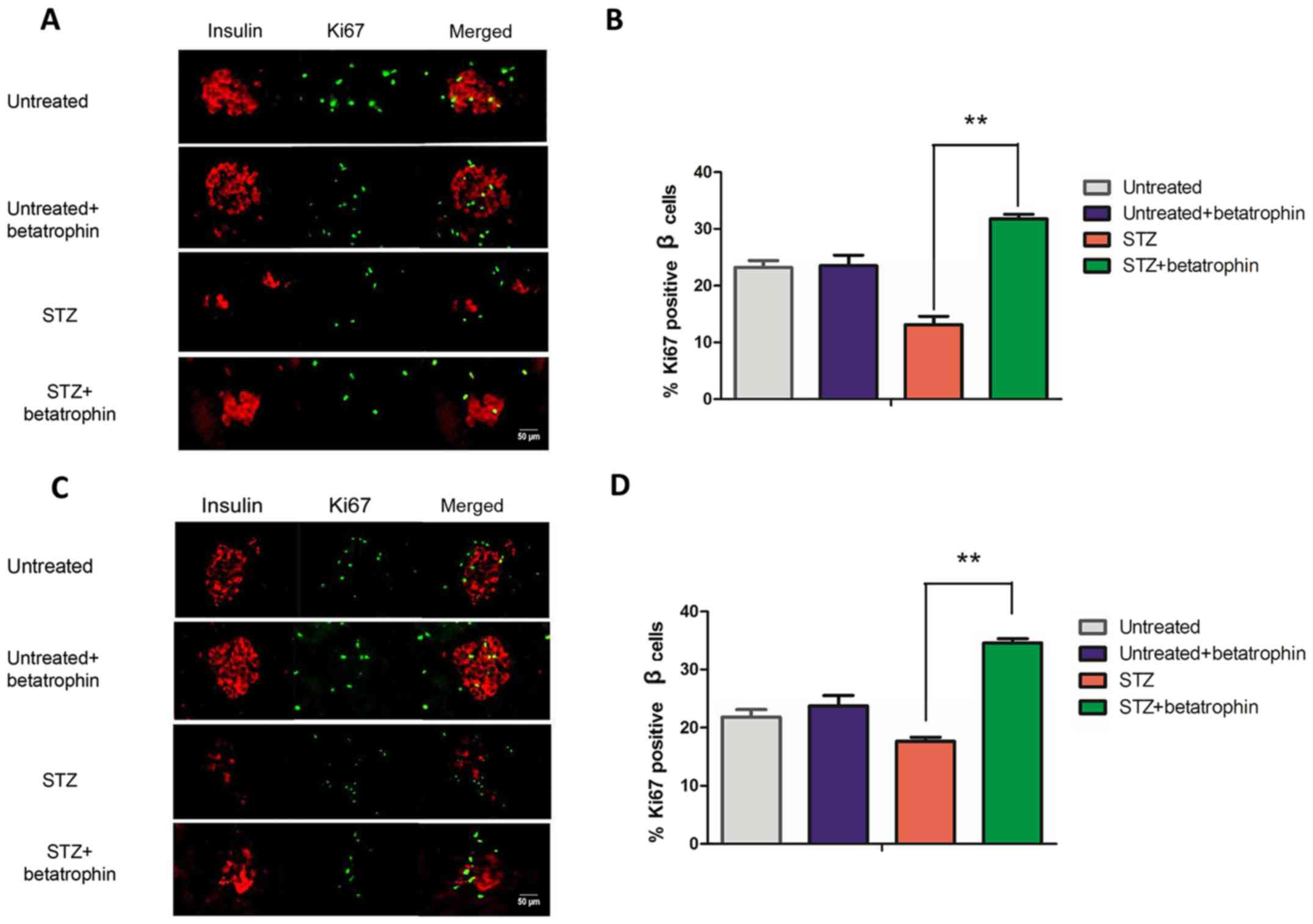
Betatrophin treatment promotes β-cell
proliferation in STZ-treated rats
Betatrophin may protect neonatal pups by preventing
β-cell death caused by STZ, or by promoting β-cell proliferation
thereby replacing the lost cells. In order to determine whether the
increase in plasma insulin level after betatrophin administration
in the STZ+betatrophin rats was due to enhancement in β-cell
replication, we performed immunofluorescence on pancreatic sections
from 4- and 7-day-old pups. Cell proliferation was labeled using
nuclear protein Ki67 and colocalized to β cells using insulin
(Fig. 3) The ratios of
Ki67-positive β cells were calculated from representative images,
and the results illustrated using bar graphs. It was found that, in
4-day-old pups, 23% of the β cells underwent proliferation
normally; STZ treatment caused a 50% reduction to 12%, while
betatrophin+STZ treatment resulted in higher than normal ratios of
β-cell proliferation to 32% (Fig. 3A
and B, 4th vs. 3rd bar). The rate of β-cell proliferation was
greater than restored, consistent with the restored insulin level.
A similar result was obtained at 7 days, while a 20% decrease in
β-cell proliferation by STZ treatment was more than fully
compensated to 35% of the normal ratio (Fig. 3C and D, 4th vs. 3rd bar).
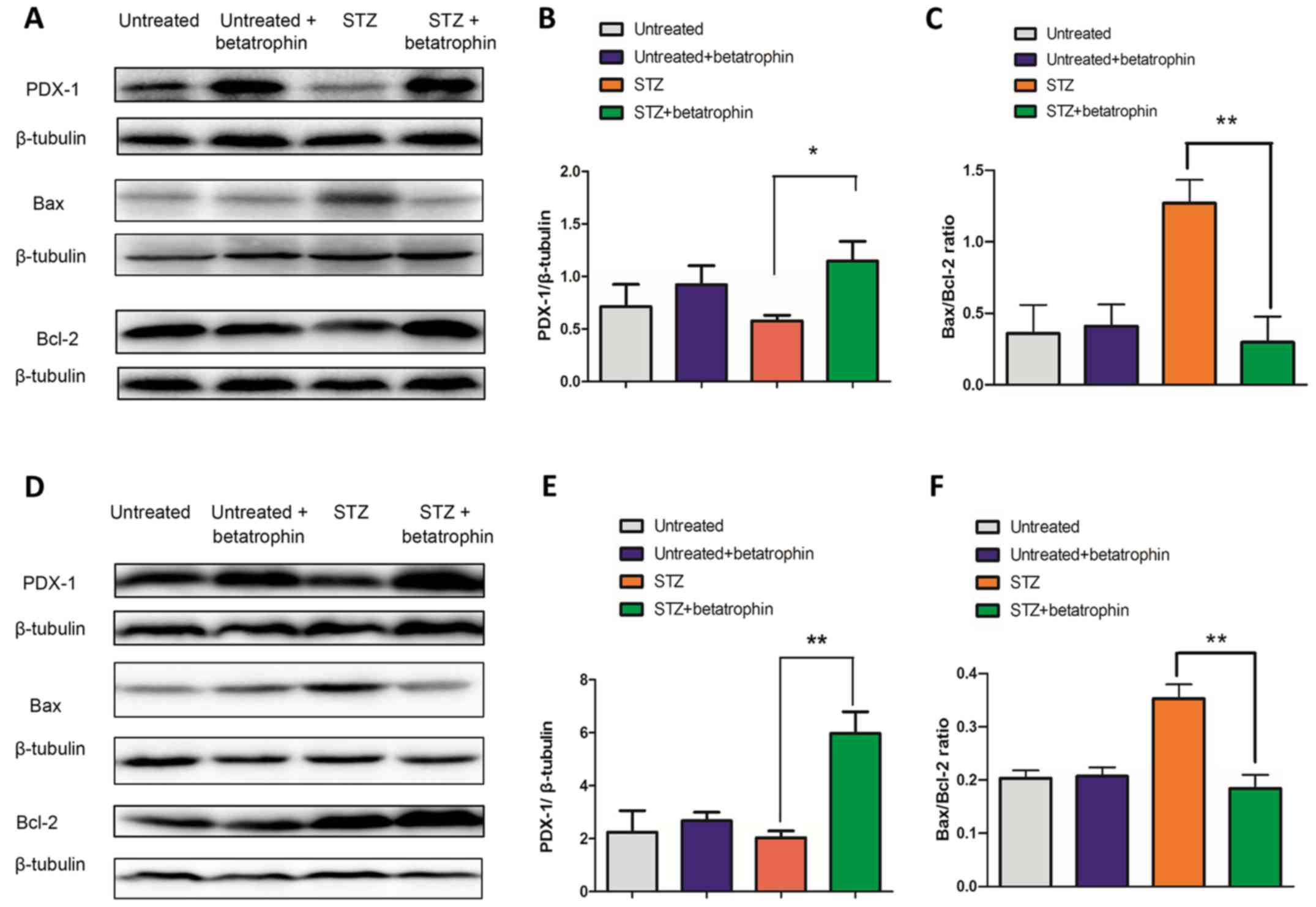
Betatrophin treatment increases PDX-1
and reduces the Bax/Bcl-2 ratio in STZ-treated neonatal
pancreas
To identify the mechanism governing the restoration
of β-cell proliferation and insulin production, we examined
possible changes in expression of PDX-1, a key transcriptional
factor involved in pancreatic development and β-cell function, as
well as that of pro-apoptosis protein Bax and anti-apoptotic
protein Bcl-2. Western blot analysis and densitometry showed that
PDX-1 levels were only slightly decreased by STZ treatment at
either 4 or 7 days (Fig. 4A, B, D and
E, 3rd vs. 1st bars); betatrophin+STZ treatments significantly
increased PDX-1 levels to 1.3 and 3-fold above STZ-treated levels,
respectively (4th vs. 3rd bars). Betatrophin treatment itself had
no effect on normal PDX-1 expression in young rats (2nd vs. 1st
bars).
At 4 and 7 days, betatrophin treatment significantly
reduced Bax protein expression, while increased Bcl-2 protein level
in the STZ-treated rats (Fig. 4A and
D). Thus, betatrophin caused inhibitory effect on the ratio of
Bax/Bcl-2 (P<0.05, Fig. 4C and
F, 4th vs. 3rd bars). No difference in the Bax/Bcl-2 ratio was
observed between the untreated and untreated+betatrophin group
(P>0.05, Fig. 4C and 4F, 1st vs. 2nd bars). The changes in
PDX-1 and Bcl-2 levels indicate potential stimulation by the
treatment of betatrophin and potential involvement in restoring
β-cell mass, function and/or rescuing from diabetes.
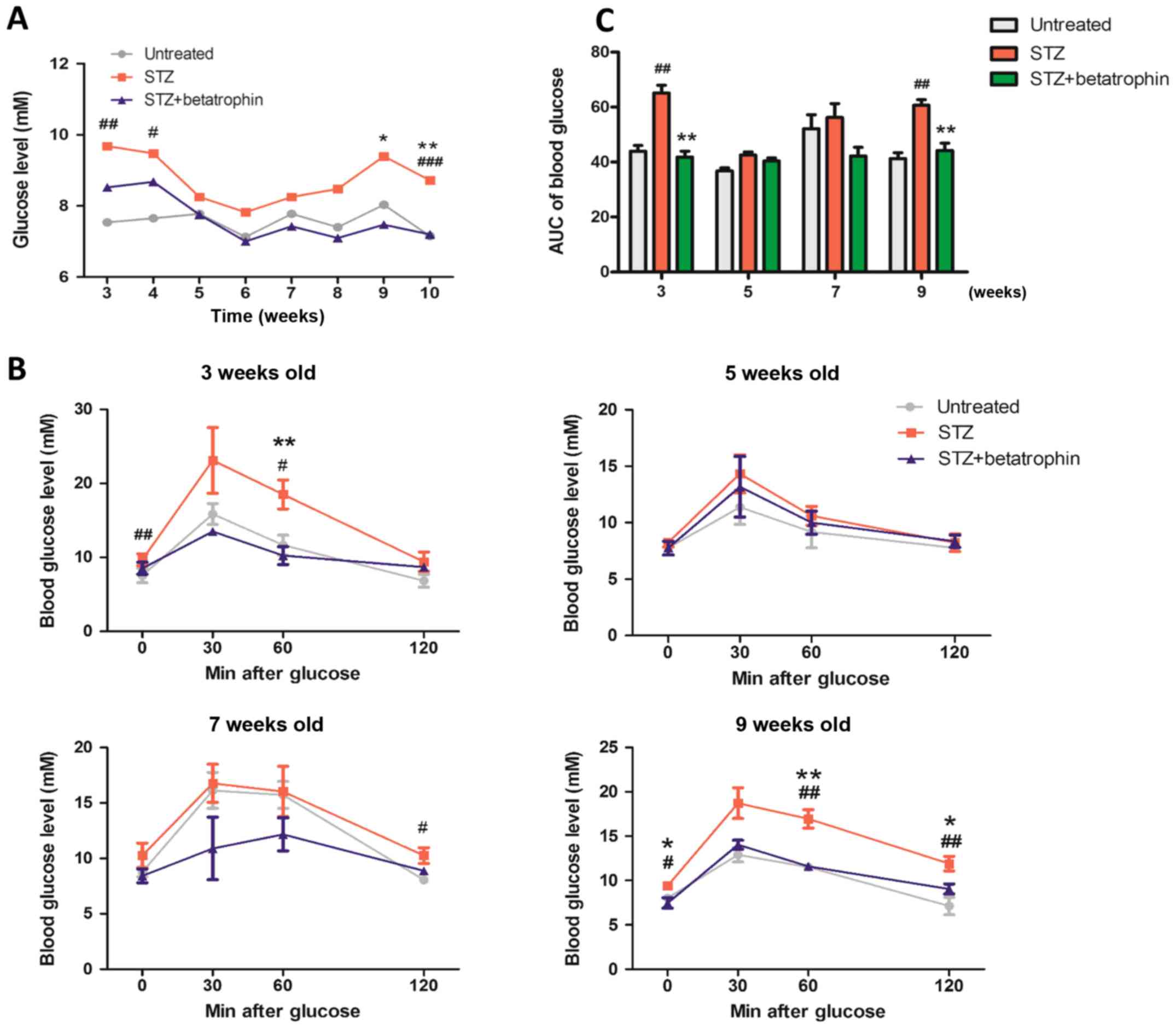
Long-term effects on blood glucose,
insulin levels, glucose tolerance and β-cell mass after neonatal
treatments of STZ and betatrophin
To study the recovery from acute STZ and betatrophin
treatments in the newborn period and in addition to studying the
possible long-term influence of betatrophin administration, we
followed a group of pups until they reached adulthood at 10 weeks.
Fig. 5A illustrates the changes in
overnight fasting blood glucose levels. There was hyperglycemia at
3, 4, 9 and 10 weeks with transient normalization to
close-to-normal over 5–8 weeks. Betatrophin-treated mice displayed
largely normalized glycemia from STZ, close to that of the
untreated rats.
Intraperitoneal glucose tolerance was assessed at 3,
5, 7 and 9 weeks (Fig. 5B). On
both occasions, STZ-treated rats exhibited glucose intolerance as
opposed to untreated animals. Those treated with betatrophin
exhibited completely normalized responses, indistinguishable from
those of the untreated rats, but significantly lower than those of
STZ alone treated rats (Fig. 5C
using AUC). These results suggest that STZ treatment resulted in a
long-term glucose intolerance that was corrected by treatment with
betatrophin during the neonatal period. The effect was
persistent.
The animals were then sacrificed at 10 weeks of age.
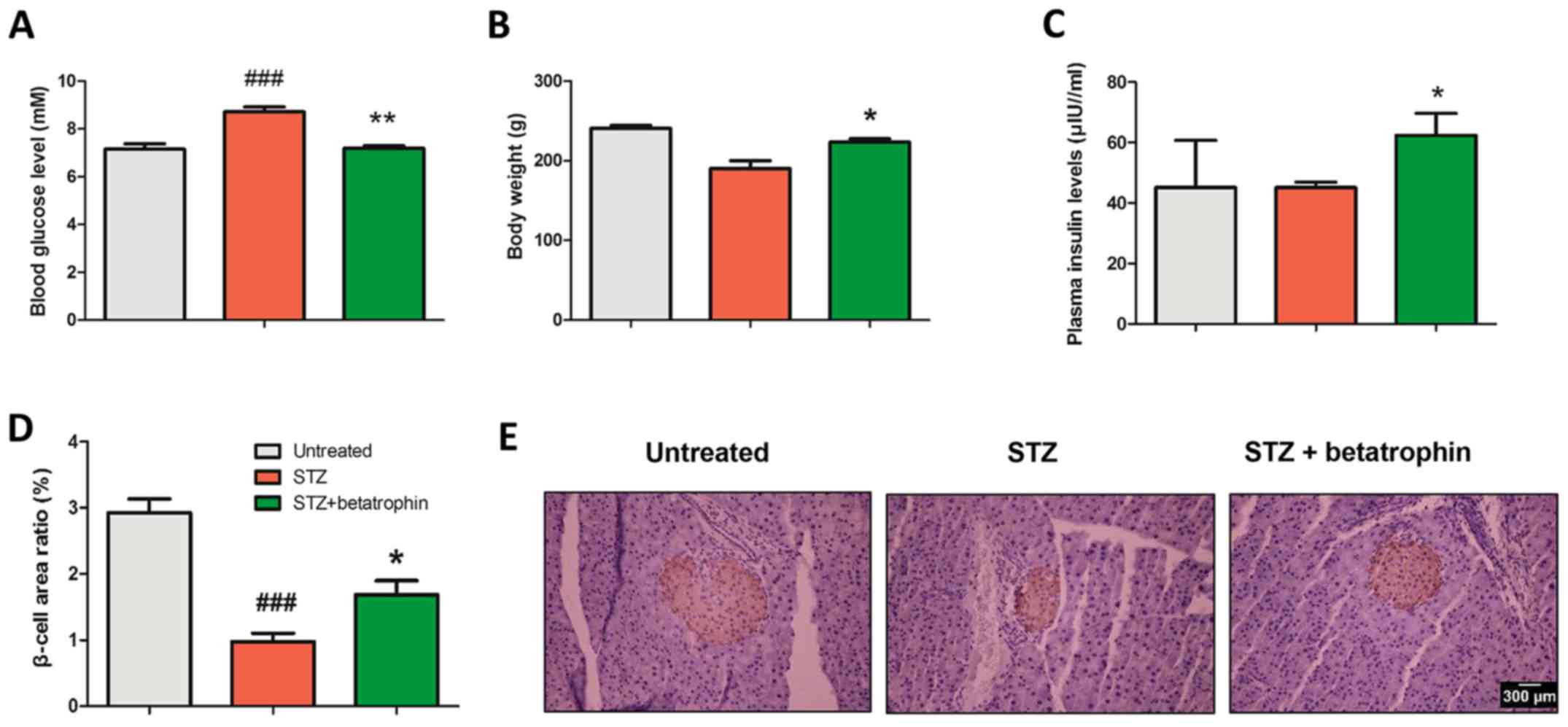
As shown in Fig. 6A and B, STZ
rats with glucose intolerance and hyperglycemia had significantly
lower body weights (−15%) and elevated blood glucose levels (+15%).
Early treatment with betatrophin significantly improved body weight
to 95% of the normal as well as normalizing blood glucose levels.
Betatrophin treatment significantly increased serum insulin levels
by 15% compared to the STZ group (Fig.
6C).
Finally, we assessed the islet morphology using
immunohistochemistry (Fig. 6E). On
average, STZ-treated rats exhibited smaller islets that were
stained using insulin antibody. Treatment of betatrophin in
addition to STZ caused significant normalization (Fig. 6D). STZ rats exhibited only 1/3 of
the β-cell area of the untreated rats, that was increased to 55% by
treatment with betatrophin. These results suggest that neonatal STZ
administration causes reduced insulin production in the pancreas
and reduced hyperglycemia from newborn to 10 weeks of age.
Betatrophin treatment may prevent this deterioration via
maintenance of insulin-positive cells and insulin production.
Discussion
The neonatal STZ-induced diabetes mellitus model is
valuable to study the regeneration of β cells. Streptozotocin (STZ)
administration with 100 mg/kg body weight in newborn rats causes
severe destruction in β cells, followed by spontaneous remission.
Several studies have reported that this partial recovery of β cells
may be modulated by exogenous factors (20,21).
In the present study, we investigated whether recombinant
betatrophin has a potentially protective effect on neonatal
STZ-induced diabetic rats. Our results showed that administration
of recombinant betatrophin during the neonatal period alleviated
hyperglycemia by promoting β-cell proliferation, and exhibited
anti-diabetic properties in adults in a neonatal STZ-induced
diabetic rat model.
We found that endogenous betatrophin levels in the
liver were higher in STZ rats than that in the untreated rats
(Fig. 1E), consistent with the
observation that serum betatrophin levels were increased in
long-term T1DM patients (15).
These observations suggest that betatrophin may participate in a
compensatory mechanism. Further investigation of the effect of
betatrophin in T1DM, it was found that betatrophin ameliorated
hyperglycemia in neonatal STZ-induce diabetic rats (Fig. 1), in accordance with previous
reports showing that betatrophin improved hyperglycemia (22,23).
It was also found that STZ-induced diabetic rats gained
significantly less weight in the first week than did the untreated
rats (Fig. 1C), while betatrophin
improved the body weight gain of diabetic rats from days 4 to 7
(Fig. 1D), suggesting an
anti-diabetic effect of betatrophin in the increasing severity of
the diabetic state. In Fig. 1B,
the STZ treated rats exhibited insulin deficient acute diabetes
mellitus 3–5 days after birth. The hyperglycemia observed in the
neonates following STZ is only transient which is followed by
spontaneous remission (24). Both
high rates of replication and neogenesis are known to contribute to
the increase in β-cell mass in the neonatal pancreas during the
first week (25). Thus, a possible
explanation for the decline in glucose level could be the
compensatory proliferation of exciting β cells.
We measured plasma insulin levels on days 4 and 7
(Fig. 2A and B), and found an
increase in insulin levels after betatrophin treatment in the
diabetic rats, consistent with the observed glucose changes
(Fig. 1B). We also found an
interesting phenomenon to the effect that neonates became pale and
inactive after STZ administration. Betatrophin improved the
survival rate from 64 to 100% on day 7, suggesting that early
betatrophin treatment may protect neonates from death induced by
acute hyperglycemia and liver damage. Zhang et al reported
that betatrophin is a stress response protein that increases
expression of early response transcription factor (Egr1) (26). Other studies reported marked
elevation of betatrophin levels in cord blood and placental tissues
(27,28). Taken together, these data suggest
that betatrophin may play a key role in growth and development
during the fetal and perinatal period.
Both high rates of replication and neogenesis
contribute to the increase in β-cell mass in the neonatal pancreas
during the first week of life (24). Betatrophin displayed dramatic
effects on proliferation and expansion of pancreatic β cells
(29). In the present study,
betatrophin did not trigger the proliferation of β cells in normal
neonates, but increased the proliferating β-cell number.
Nevertheless, this function of betatrophin on β-cell replication
has been disputed in subsequent studies (13,30),
that used ANGPTL8-deficient and ANGPTL8-overexpressing mice. This
phenomenon can be explained as betatrophin promotes β-cell
proliferation only under circumstances of β-cell deficiency.
Another possible reason is that there are several interconnected
compensatory mechanisms that regulate cell homeostasis in mice. We
therefore hypothesized that betatrophin treatment would promote
β-cell proliferation in STZ-induced diabetic neonates with insulin
deficiency. In support of our hypothesis, we found increased
Ki67-positive cells, representing an elevated rate of cell
proliferation in response to betatrophin treatment at two different
ages (Fig. 3B and D). This result
agrees with previous findings showing that betatrophin promotes
β-cell proliferation and expansion (12,22,23).
We found that betatrophin upregulated the expression
of PDX-1 and decreased the Bax/Bcl-2 ratio in the pancreas in
neonatal STZ-induced diabetic rats. PDX-1 is a necessary
transcription factor for pancreatic development and β-cell
differentiation. β cells with reduced PDX-1 expression have an
increased rate of apoptosis (31).
Bcl-2 is an important anti-apoptotic protein, and Bax is an
pro-apoptotic protein (32). In
the present study, the upregulated pancreatic expression of PDX-1
and the decreased Bax/Bcl-2 ratio suggest that betatrophin may
expand the β-cell mass by enhancing activation of anti-apoptotic
mechanisms and promoting differentiation of stem cells into β cells
as well as promoting β-cell proliferation. It was reported that
betatrophin regulates inflammation via the NF-κB pathway (33–35).
Inflammation is one of the factors leading to apoptosis. We
assessed a pro-apoptosis marker with western blot analysis. The
ratio of Bax/Bcl-2 indicates that inflammation may exist in the
early stage, eventually leading to β-cell apoptosis. Betatrophin
may reduce inflammation and eventually improve the apoptosis rate
of β cells.
This study was the first to determine that
betatrophin preserves islet cell mass and prevents diabetes in
adult rats in a neonatal STZ-diabetic rat model. Early
administration of betatrophin showed anti-diabetic potential by
reducing plasma glucose, elevating insulin levels, and improving
body weight and glucose tolerance test (IPGTT) in adult rats
(Fig. 5). It is likely that STZ
has no long-acting effects on pancreatic islets. Therefore, at
weeks 5 and 7, the result of the IPGTT was normal in the STZ group.
Moreover, there was a higher demand for β-cell mass to maintain
normal glucose tolerance. The regeneration and proliferation of β
cells in the STZ group might account for the recovery of impaired
glucose tolerance. Although plasma insulin levels in the STZ group
were equal to that of the untreated group, blood glucose levels of
the STZ rats was higher than those of untreated rats (Fig. 6A and C). It is possible that newly
regenerated islets in the STZ group are able to release similar
amounts of insulin. However, the insulin responsiveness may have
been impaired due to earlier diabetic damage. There may be other
unaccounted compensatory changes behind this discovery.
Immunohistochemistry revealed that betatrophin increased pancreatic
islet area (Fig. 6D). There were
few large islets in the STZ-treated rats (Fig. 6E). These results strongly favor the
conclusion that betatrophin prevents development of diabetes in
adult rats by stimulating β-cell proliferation in the neonatal
STZ-induced diabetes model. Betatrophin as an adipokine, played a
beneficial role in β-cell proliferation and function. We previously
reported that betatrophin (ANGPTL8) is closely linked to T2DM and
insulin resistance (17). In the
present study, we confirmed that betatrophin improved glucose
tolerance and promoted β-cell proliferation. Moreover, our group
also found that betatrophin decreased the FFA level (36). Thus, betatrophin may be a promising
and attractive target for the treatment of obesity and T2DM.
In summary, this study firstly illuminated that
administration of recombinant betatrophin during the neonatal
period promoted β-cell proliferation, and exhibited anti-diabetic
properties in the adult rat in a neonatal STZ-induced diabetic rat
model.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (no. 31872791 to JD), Natural Science
Foundation of Shandong Province of China (no. ZR2019MC046 to JD),
and Natural Science and Engineering Research Council of Canada
(NSERC) to JLL (RGPIN-2017-05246).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ and YJY designed and performed the experiments,
and researched the data. DZ wrote the manuscript. JHY, RW, CSZ,
LXW, YL and LMS contributed to discussion of the experiments and
analysis of the data and results. JD and JLL directed the project,
contributed to the discussion and analysis of the results, and
reviewed and edited the manuscript. JD as the corresponding author
had full access to all the data in the study and had final
responsibility for the decision to submit for publication. FSX
interpreted the results and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Ethics Committee of
the Medical Department of Qingdao University (Qingdao, China). All
experiments were conducted in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shan R, Sarkar S and Martin SS: Digital
health technology and mobile devices for the management of diabetes
mellitus: State of the art. Diabetologia. 62:877–887. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H, Bender A, Wang P, Karakose E,
Inabnet WB, Libutti SK, Arnold A, Lambertini L, Stang M, Chen H, et
al: Insights into beta cell regeneration for diabetes via
integration of molecular landscapes in human insulinomas. Nat
Commun. 8:7672017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu LT, Yang MQ, Liu JL, Alfred MO, Li X,
Zhang XQ, Zhang J, Wu MY, Wang M and Luo C: Recombinant Reg3α
protein protects against experimental acute pancreatitis in mice.
Mol Cell Endocrinol. 422:150–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kataoka M, Kawamuro Y, Shiraki N, Miki R,
Sakano D, Yoshida T, Yasukawa T, Kume K and Kume S: Recovery from
diabetes in neonatal mice after a low-dose streptozotocin
treatment. Biochem Biophys Res Commun. 430:1103–1108. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Howarth FC and Qureshi MA:
Characterisation of ventricular myocyte shortening after
administration of streptozotocin (STZ) to neonatal rats. Arch
Physiol Biochem. 109:200–205. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takada J, Machado MA, Peres SB, Brito LC,
Borges-Silva CN, Costa CE, Fonseca-Alaniz MH, Andreotti S and Lima
FB: Neonatal streptozotocin-induced diabetes mellitus: A model of
insulin resistance associated with loss of adipose mass.
Metabolism. 56:977–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang R: Lipasin, a novel
nutritionally-regulated liver-enriched factor that regulates serum
triglyceride levels. Biochem Biophys Res Commun. 424:786–792. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abu-Farha M, Abubaker J and Tuomilehto J:
ANGPTL8 (betatrophin) role in diabetes and metabolic diseases.
Diabetes Metab Res Rev. 332017.doi: 10.1002/dmrr.2919.
|
|
9
|
Ren G, Kim JY and Smas CM: Identification
of RIFL, a novel adipocyte-enriched insulin target gene with a role
in lipid metabolism. Am J Physiol Endocrinol Metab. 303:E334–E351.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang R and Abou-Samra AB: Emerging roles
of Lipasin as a critical lipid regulator. Biochem Biophys Res
Commun. 432:401–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raghow R: Betatrophin: A liver-derived
hormone for the pancreatic β-cell proliferation. World J Diabetes.
4:234–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiao Y, Le Lay J, Yu M, Naji A and
Kaestner KH: Elevated mouse hepatic betatrophin expression does not
increase human β-cell replication in the transplant setting.
Diabetes. 63:1283–1288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cox AR, Lam CJ, Bonnyman CW, Chavez J,
Rios JS and Kushner JA: Angiopoietin-like protein 8
(ANGPTL8)/betatrophin overexpression does not increase beta cell
proliferation in mice. Diabetologia. 58:1523–1531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trebotic LK, Klimek P, Thomas A, Fenzl A,
Leitner K, Springer S, Kiefer FW and Kautzky-Willer A: Circulating
betatrophin is strongly increased in pregnancy and gestational
diabetes mellitus. PLoS One. 10:e01367012015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Espes D, Lau J and Carlsson PO: Increased
circulating levels of betatrophin in individuals with long-standing
type 1 diabetes. Diabetologia. 57:50–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu Z, Berhane F, Fite A, Seyoum B,
Abou-Samra AB and Zhang R: Elevated circulating lipasin/betatrophin
in human type 2 diabetes and obesity. Sci Rep. 4:50132014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang YY, Zhang D, Jiang ZY, Lu XQ, Zheng
X, Yu YJ, Wang YG and Dong J: Positive association between
betatrophin and diabetic retinopathy risk in Type 2 diabetes
patients. Horm Metab Res. 48:169–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan JH, Chen X, Dong J, Zhang D, Song K,
Zhang Y, Wu GB, Hu XH, Jiang ZY and Chen P: Nesfatin-1 in the
lateral parabrachial nucleus inhibits food intake, modulates
excitability of glucosensing neurons, and enhances UCP1 expression
in brown adipose tissue. Front Physiol. 8:2352017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Q, Li B, Miao X, Ramgattie C, Gao ZH
and Liu JL: Reg2 Expression is required for pancreatic islet
compensation in response to aging and high-fat diet-induced
obesity. Endocrinology. 158:1634–1644. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Irako T, Akamizu T, Hosoda H, Iwakura H,
Ariyasu H, Tojo K, Tajima N and Kangawa K: Ghrelin prevents
development of diabetes at adult age in streptozotocin-treated
newborn rats. Diabetologia. 49:1264–1273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tourrel C, Bailbé D, Meile MJ, Kergoat M
and Portha B: Glucagon-like peptide-1 and exendin-4 stimulate
beta-cell neogenesis in streptozotocin-treated newborn rats
resulting in persistently improved glucose homeostasis at adult
age. Diabetes. 50:1562–1570. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Chen S, Huang P, Meng XL, Clayton
S, Shen JS and Grayburn PA: In vivo targeted delivery of ANGPTL8
gene for beta cell regeneration in rats. Diabetologia.
58:1036–1044. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun LL, Liu TJ, Li L, Tang W, Zou JJ, Chen
XF, Zheng JY, Jiang BG and Shi YQ: Transplantation of
betatrophin-expressing adipose-derived mesenchymal stem cells
induces β-cell proliferation in diabetic mice. Int J Mol Med.
39:936–948. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang RN, Bouwens L and Klöppel G:
Beta-cell proliferation in normal and streptozotocin-treated
newborn rats: Site, dynamics and capacity. Diabetologia.
37:1088–1096. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kassem SA, Ariel I, Thornton PS,
Scheimberg I and Glaser B: Beta-cell proliferation and apoptosis in
the developing normal human pancreas and in hyperinsulinism of
infancy. Diabetes. 49:1325–1333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Li S, Donelan W, Xie C, Wang H,
Wu Q, Purich DL, Reeves WH, Tang D and Yang LJ: Angiopoietin-like
protein 8 (betatrophin) is a stress-response protein that
down-regulates expression of adipocyte triglyceride lipase. Biochim
Biophys Acta. 1861:130–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martinez-Perez B, Ejarque M, Gutierrez C,
Nuñez-Roa C, Roche K, Vila-Bedmar R, Ballesteros M, Redondo-Angulo
I, Planavila A, Villarroya F, et al: Angiopoietin-like protein 8
(ANGPTL8) in pregnancy: A brown adipose tissue-derived endocrine
factor with a potential role in fetal growth. Transl Res. 178:1–12.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wawrusiewicz-Kurylonek N, Telejko B,
Kuzmicki M, Sobota A, Lipinska D, Pliszka J, Raczkowska B, Kuc P,
Urban R, Szamatowicz J, et al: Increased maternal and cord blood
betatrophin in gestational diabetes. PLoS One. 10:e01311712015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stewart AF: Betatrophin versus
bitter-trophin and the elephant in the room: Time for a new normal
in β-cell regeneration research. Diabetes. 63:1198–1199. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gusarova V, Alexa CA, Na E, Stevis PE, Xin
Y, Bonner-Weir S, Cohen JC, Hobbs HH, Murphy AJ, Yancopoulos GD and
Gromada J: ANGPTL8/betatrophin does not control pancreatic beta
cell expansion. Cell. 159:691–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spaeth JM, Gupte M, Perelis M, Yang YP,
Cyphert H, Guo S, Liu JH, Guo M, Bass J, Magnuson MA, et al:
Defining a novel role for the Pdx1 transcription factor in islet
β-cell maturation and proliferation during weaning. Diabetes.
66:2830–2839. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Zheng L and Huang K: A new way to
regulate inflammation: Selective autophagic degradation of
IKKY mediated by ANGPTL8. Cell Stress. 2:66–68. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo D, Chen X, Yang W, Ran W and Wen Z:
Angiopoietin-like 8 improves insulin resistance and attenuates
adipose tissue inflammation in diet-induced obese mice. Exp Clin
Endocrinol Diabetes. Sep 26–2018.doi: 10.1055/a-0725-7897 (Epub
ahead of print).
|
|
35
|
Zhang Y, Guo X, Yan W, Chen Y, Ke M, Cheng
C, Zhu X, Xue W, Zhou Q, Zheng L, et al: ANGPTL8 negatively
regulates NF-κB activation by facilitating selective autophagic
degradation of IKKY. Nat Commun. 8:21642017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang R, Yuan J, Zhang C, Wang L, Liu Y,
Song L, Zhong W, Chen X and Dong J: Neuropeptide Y-positive neurons
in the dorsomedial hypothalamus are involved in the anorexic effect
of Angptl8. Front Mol Neurosci. 11:4512018. View Article : Google Scholar : PubMed/NCBI
|