Introduction
Among the life-threatening cancer types, lung cancer
is the most common cause of mortality worldwide. The principal
clinical therapeutic regimens for non-small cell lung cancer
(NSCLC) include surgery, radiotherapy and chemotherapy. Although a
number of antitumor drugs have been approved and used for lung
cancer therapy, the cancer-associated mortality rate remains high.
This may primarily be attributed to late diagnosis and low response
to chemotherapy (1,2). Therefore, at present, the 5-year
survival rate of patients with lung cancer worldwide is ~15%
(3–5). Lung cancer includes two principal
subtypes, small cell lung cancer and NSCLC, the later accounts for
~85% of all cases and the outcomes for NSCLC remain unsatisfactory
(6). Therefore, there is a
necessity to develop compounds against lung cancer, particularly
the NSCLC subtype.
Natural products have been regarded as important
sources for the development of novel and potent antitumor drugs
during the past decade. Bergapten (5-methoxypsoralen), a coumarine
derivative, has been demonstrated to exhibit anti-proliferative
activity against a number of malignant carcinoma cells, including
breast cancer cells (7). Through
photo-activation using UVA irradiation, it was determined that
psoralen, structurally associated with coumarine, may be used to
treat proliferative skin disorders; a human breast cancer model
that overexpressed the erb-b2 receptor tyrosine kinase 2 oncogene
was treated with psoralen to inhibit ErbB2 signaling (8). Panno et al (7) observed that bergapten inhibits the
proliferation of MCF-7 cells and tamoxifen-resistant MCF7-TR1 cells
by inducing the transforming growth factor-β/mothers against
decapentaplegic homolog 4-associated degradation of estrogen
receptor α (7). In addition, De
Amicis et al (9)
demonstrated that bergapten induces the phosphatase and tensin
homolog (PTEN)-mediated autophagic cascade, including increased
expression of PTEN, Beclin-1 and class III phosphatidylinositol
3-kinase, and microtubule-associated proteins 1A/1B light chain 3B
conversion in MCF-7 and ZR-75 cells. These findings demonstrated
that bergapten possesses potential anticancer activity by
triggering different signaling pathways. However, the effects of
bergapten on NSCLC cells require further examination.
Apoptosis, programmed cell death, is a common target
of a number of treatment strategies and serves a crucial role in
cancer treatment. Morphological alterations in cells undergoing
apoptosis include cell shrinkage, membrane blebbing, organelle
integrity loss, chromatin condensation and DNA fragmentation
(10). Apoptosis may be
categorized into two pathways: The extrinsic and
mitochondria-mediated pathways (11). The two apoptotic pathways are
associated with the activation of caspases, a family of cysteine
proteases that mediate efficient and non-inflammatory cell
destruction (12). A recent study
demonstrated that bergapten, isolated from Ruta angustifolia
L. Pers, has differential toxicity on A549 and MRC-5 cells
(3). Therefore, the present study
examined the anticancer effects of bergapten on NSCLC cells and the
associated signaling cascade.
Materials and methods
Reagents and antibodies
MTT, 2-propanol, dimethyl sulfoxide (DMSO),
deoxycholic acid, dithiothreitol, EDTA, bergapten (cat. no. 69664),
glycerol, Igepal CA-630, phenylmethylsulphonyl fluoride (PMSF),
NaCl, SDS, sodium phosphate, Tris-HCl, Tween-20, propidium iodide
(PI), RNase A, Triton X-100 and trypsin/EDTA were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Primary antibodies
against human apoptosis regulator Bcl-2-associated X protein (Bax,
cat. no. 2772), B cell lymphoma-2 (Bcl-2, cat. no. 2872, caspase-3
(cat. no. 9662), cyclin D1 (cat. no. 2978), cyclin-dependent kinase
4 (CDK4, cat. no. 9662), cyclin-dependent kinase inhibitor 1
(p21Cip1, cat. no. 2947), cyclin-dependent kinase
inhibitor 1B (p27Kip1, cat. no. 2552), cellular tumor
antigen p53 (p53, cat. no. 9282) and GAPDH (cat. no. 2118), and
horseradish peroxidase-conjugated secondary antibodies (cat. no.
7076 and 7074) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Cell culture and treatment with
bergapten
The human NSCLC cell lines A549 and NCI-H460 and
non-tumorigenic lung fibroblast MRC-5 were obtained from The
American Type Culture Collection (Manassas, VA, USA) and maintained
in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% v/v fetal
bovine serum (FBS), 1% nonessential amino acids, 1% L-glutamine
(both Gibco; Thermo Fisher Scientific, Inc.) and 100 µg/ml
penicillin/streptomycin (Sigma-Aldrich; Merck KGaA) at 37°C in a
humidified atmosphere with 5% CO2, as previously
described (13). Cells were seeded
in a 6-well culture plate at an initial density of 2×105
cells/ml and cultured until they reached ~80% confluence. For
treatment with bergapten, cells were starved for 16 h in serum-free
medium, and subsequently treated with different concentrations (10,
20, 30, 40 and 50 µM for cell viability assay and 10, 30, and 50 µM
for the other analyses) of bergapten in DMEM for 24 or 48 h (cell
viability assay) and 24 h (flow cytometry and western blot
analysis). Following the treatments, the treated cells were washed
with PBS (25 mM sodium phosphate; 150 mM NaCl; pH 7.2), and
subsequently collected by centrifugation (800 × g; 5 min; 25°C) for
subsequent analyses.
Cell viability assay
Cell viability was determined by an MTT assay as
previously described (14). Cells
were seeded at a density of 4×104 cells/well in a
24-well plate and cultured for 24 h. Subsequently, the cells were
treated with bergapten at various concentrations (10, 20, 30, 40
and 50 µM) for 24 h. Each treatment was performed in triplicate for
statistical analysis. Following the treatments, the medium was
removed and the cells were washed with PBS. The washed cells were
incubated with MTT solution (5 mg/ml) for 4 h. Subsequent to
removing the supernatant, 2-propanol was added to solubilize the
formazan for determination of absorbance at 563 nm. The percentage
of viable cells was estimated by comparing with untreated
cells.
Flow cytometric analysis
Cells were synchronized at the G0 phase by serum
starvation for 24 h, and subsequently incubated with fresh serum
containing medium to allow cell-cycle progression. Following serial
treatments, cells were collected, fixed with 1 ml ice-cold 70%
ethanol and incubated at −20°C for 24 h, and centrifuged at 380 × g
for 5 min at room temperature to spin down the cells. The cell
pellets were treated with l ml cold staining solution containing 20
µg/ml PI, 20 µg/ml RNase A and 1% Triton X-100, incubated for 15
min in dark at room temperature, and subsequently analyzed using a
flow cytometer (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA). CellQuest software (version 2.0; BD Biosciences) was used to
determine cell cycle distribution (15). Representative data were acquired
from three independent experiments.
Protein extraction
Cellular proteins were extracted as previously
described (16). Cells were
digested using trypsin/EDTA, and subsequently homogenized in
ice-cold lysis buffer [50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1%
(v/v) Igepal CA-630, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v)
SDS, 1 mM dithiothreitol, 0.1 mM EDTA and 1 mM PMSF]. Following
sonication at a frequency of 20 kHz at 4°C for 30 min, the
homogenate was centrifuged at 14,000 × g at 4°C for 10 min, and the
supernatant was subsequently transferred to a 1.5 ml-Eppendorf tube
and stored at −70°C until subsequent analysis. The protein
concentration was quantified using the bicinchoninic acid (BCA)
method (BCA Protein Assay kit; Pierce; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol.
Western blot analysis
Cellular proteins (20 µg for each lane) were
electrophoresed using 12.5% SDS-PAGE, and subsequently transferred
onto a nitrocellulose membrane, as previously described (16). Following blocking with 5% nonfat
milk at 25°C for 1 h, the membrane was incubated with
1:1,000-diluted primary antibodies at 25°C for 2 h, washed with PBS
containing 0.5% Tween-20, and subsequently incubated with
1:2,000-diluted peroxidase-conjugated secondary antibody at 25°C
for 1 h. GAPDH was used as the loading control. Following the final
wash, the signal was developed with enhanced chemiluminescence (EMD
Millipore, Billerica, MA, USA), and the relative density was
quantified using the ImageQuant LAS-3000 image analysis system
equipped with Multi Gauge software version 3.0 (Fujifilm, Tokyo,
Japan).
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Statistical significance analysis
was determined by one-way analysis of variance followed by
Dunnett's test for multiple comparisons with the control using SPSS
(version 17.0; SPSS Inc. Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Bergapten reduces the cell viability
of NSCLC cell lines A549 and NCI-H460
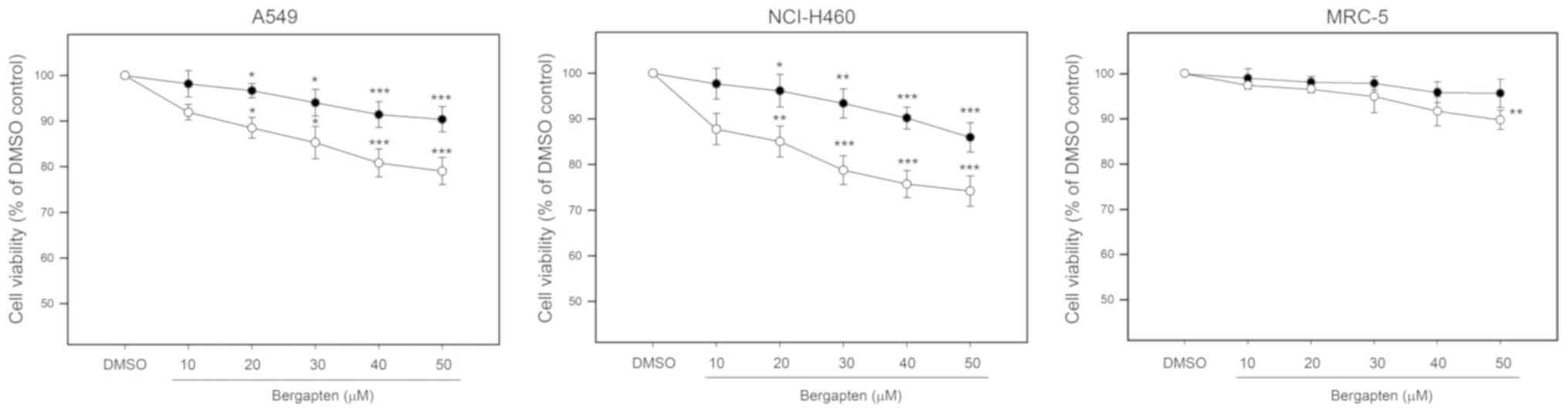
The cytotoxic effects of bergapten on human NSCLC
cell lines A549 and NCI-H460 were investigated by an MTT assay. As
presented in Fig. 1, it was
observed that the 24 h-treatments with bergapten dose-dependently
and significantly decreased the viability of A549 and NCI-H460
cells to 90.2±3.4% and 87.3±3.9%, respectively, compared with the
DMSO controls (50 µM; P<0.005). Whereas, treatments with the
low-dose of bergapten (10 µM) did not significantly affect the
viability of A549 and NCI-H460 cells (P=0.172 and 0.214,
respectively). In addition, it was demonstrated that the 48
h-treatments with bergapten further decreased the viability of A549
and NCI-H460 cells to 79.1±2.8% and 74.5±3.1%, respectively,
compared with the DMSO controls (50 µM; P<0.005). The
cytotoxicity of bergapten on human non-cancer lung fibroblast MRC-5
was additionally evaluated, and the findings demonstrated that the
24-h treatments with bergapten did not significantly affect the
viability of MRC-5; however, the 48-h treatment with bergapten at a
high dose (50 µM) significantly decreased the viability of MRC-5
cells to 89.8±1.8%, compared with the control (P=0.008).
Collectively, these findings demonstrated that bergapten exerted
significant cytotoxic effects on the NSCLC cell lines A549 and
NCI-H460; however, negligibly affected the viability of the
non-cancerous MRC-5 cells.
Bergapten induces
G0/G1 phase arrest of A549 and NCI-H460
cells
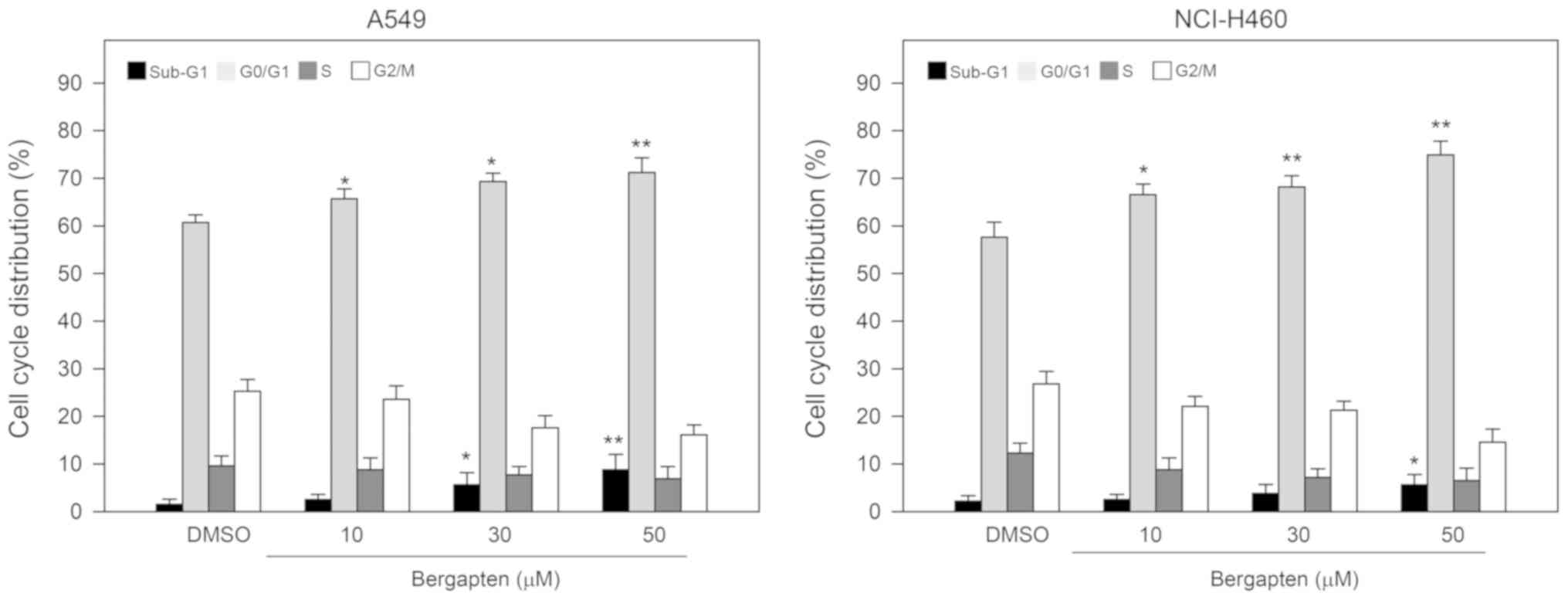
The effects of bergapten on cell cycle distribution
in A549 and NCI-H460 cells were additionally examined. By flow
cytometry, it was identified that treatment with bergapten (10, 30
and 50 µM) dose-dependently increased the percentage of cells in
G0/G1 phase between 61.8±2.1% and 72.1±3.2%
in A549 cells and between 57.2±2.8% and 76.4±3.3% in NCI-H460
cells, and the increase in the percentage of cells in the
G0/G1 phase in response to the treatments
with bergapten was significant (P<0.05; Fig. 2). Simultaneously, the percentage of
cells in sub-G1 phase additionally increased to 9.1±2.6% in A549
cells and 5.8±2.2% in NCI-H460 cells in the presence of bergapten
at a concentration of 50 µM compared with the DMSO control
(P<0.05; Fig. 2). The results
suggested that bergapten induced the apoptosis of A549 and NCI-H460
cells.
Bergapten downregulates the expression
of cyclin D1 and CDK4, and upregulates the expression of p53,
p21Cip1 and p27Kip1 in A549 and NCI-H460
cells
Based on the observation that bergapten induced
G0/G1 phase arrest, it was examined whether
the treatments with bergapten regulated the expression levels of
cyclins and CDKs associated with G0/G1
accumulation (17). The expression
levels of cyclin D1 and CDK4 were determined by western blot
analysis and quantified by densitometric analysis. It was observed
that the 24 h treatments with bergapten (10, 30 and 50 µM)
dose-dependently decreased the expression levels of cyclin D1 and
CDK4 (Fig. 3A). The treatment with
50 µM bergapten decreased the expression levels of cyclin D1 and
CDK4 to 21 and 33%, respectively, compared with the DMSO controls
(Fig. 3A). p53 and its downstream
proteins p21Cip1 and p27Kip1 are
well-documented negative cell cycle regulators that contribute to
cell cycle arrest (17).
Therefore, the effects of bergapten on the
p53/p21Cip1/p27Kip1 axis were subsequently
examined. It was identified that bergapten increased the expression
of p53, in addition to p21Cip1 and p27Kip1
(Fig. 3B). Collectively, the
present results demonstrated that bergapten downregulated the
expression levels of cyclin D1 and CDK4, and upregulated the
expression levels of p53/p21Cip1/p27Kip1 in
A549 and NCI-H460 cells.
 | Figure 3.Bergapten downregulates cyclin D1 and
CDK4, and increased the expression levels of p53,
p21Cip1 and p27Kip1 in non-small cell lung
cancer A549 and NCI-H460 cells. (A) Cells were exposed to serial
concentrations of Bergapten (10, 30 and 50 µM) for 24 h, and
subsequently western blot analysis was performed using specific
antibodies against cyclin D1 and CDK4. (B) Cells were exposed to
serial concentrations of Bergapten (10, 30 and 50 µM) for 24 h, and
subsequently western blot analysis was performed using specific
antibodies against p53, p21Cip1 and p27Kip1.
GAPDH was used as the internal control. Approximate molecular
weights for the immunodetected signals are indicated. CDK4,
cyclin-dependent kinase 4; p53, cellular tumor antigen p53; DMSO,
dimethyl sulfoxide; p21Cip1, cyclin-dependent kinase
inhibitor 1; p27Kip1, cyclin-dependent kinase inhibitor
1B. The quantitative data were acquired from three independent
experiments. *P<0.05, **P<0.01 vs. respective DMSO
control. |
Bergapten decreases the expression
level of Bcl-2, and increases the expression level of Bax and
cleavage of caspase-3 in A549 and NCI-H460 cells
As bergapten induced significant sub-G1 phase
accumulation, apoptosis-associated components were subsequently
investigated. By western blot analysis, the expression levels of
anti-apoptotic Bcl-2 and pro-apoptotic Bax, and the cleavage of
effector caspase-3 were determined. As presented in Fig. 4, treatments with bergapten
decreased the Bcl-2 expression level; however, increased the Bax
expression level and induced caspase-3 cleavage/activation in A549
and NCI-H460 cell lines. These observations suggested that
bergapten decreased the anti-apoptotic signal and enhanced the
apoptotic signals in A549 and NCI-H460 cells.
Discussion
In the present study, the anticancer effects of
bergapten on the malignant human NSCLC cell lines A549 and NCI-H460
were examined and it was demonstrated that bergapten was able to
inhibit the viability of the two cell types, which may be
attributed to the induction of cell cycle arrest and apoptosis. It
was previously identified that bergapten may inhibit the growth of
a number of carcinoma cell types, including bladder transitional
cell carcinoma T-24 (18),
mucoepidermoid carcinoma cell MEC-1 (19) and hepatocellular carcinoma cell J5
(20). Similarly, the present
results demonstrated that bergapten is able to suppress the
viability of NSCLC cells, suggesting that bergapten has potential
use in NSCLC treatment. However, although the in vitro
findings indicated that bergapten has potential anticancer activity
on NSCLC cells, further investigation is still needed to elucidate
the in vivo anticancer effects of bergapten. p53 is a
well-studied tumor suppressor protein in humans. It regulates cell
fate in response to DNA damage, including cell cycle arrest,
apoptosis and cellular senescence (21). Obstruction of p53 functions through
mutations or deletions of p53 and p53-associated regulators has
been permanently and widely discovered in human tumors (22). Recently, accumulating evidence
demonstrated that p53 additionally serves an important role in
regulating tumor metastasis and invasion in lung cancer (23,24).
As a result, activation or gain-of-function of p53 is a potential
anticancer treatment for numerous types of cancer. In the present
study, A549 and NCI-H460 cell lines were used, which are derived
from NSCLCs that express detectable p53 mRNA at expression levels
comparable to normal lung tissue and exhibit no gross structural
DNA abnormalities (25).
Accordingly, the present findings demonstrated that bergapten
increased the expression levels of p53 and downstream
p21Cip1 and p27Kip1, suggesting that
bergapten may exert its anticancer activity by inducing activation
of the p53 cascade.
The disruption of cell cycle progression in cancer
cells is considered an effective strategy to control tumor growth
(26). The transition between a
dormant quiescent stage (G0) to an active growing state is a
prerequisite for the majority of cells entering the cell cycle, and
it is a critical step for cancer cells (27). The progression of the cell cycle is
regulated by a number of negative regulators termed CDK inhibitors,
including p21Cip1 and p27Kip1 (27). p21Cip1 is a universal
cell cycle inhibitor that binds to cyclin-CDK complexes and
proliferating cell nuclear antigen, thereby inducing cell cycle
arrest at the G1 phase (28). In
addition, the upregulation of p21Cip1 and
p27Kip1 enhances the formation of complexes with G1-S
CDKs and cyclins, thereby, inhibiting their activities (29–31).
The results of the present study demonstrated that bergapten
upregulated the expression levels of p53, p21Cip1 and
p27Kip1; however, it downregulated the expression levels
of CDK4 and cyclin D1. Overall, the results suggested that the
bergapten-induced G1 phase arrest of NSCLC cells may be due to the
upregulation of p53/p21Cip1/p27Kip1 and the
consequent disruption of CDK4-cyclin D complexes.
A number of previous studies demonstrated that cell
cycle arrest and apoptosis may be directly associated (32–34).
For instance, the apoptotic cascade may be inhibited or induced via
cell cycle manipulation depending on the cellular circumstance
(35). In addition, the CDK
inhibitors of the Cip/Kip family have been suggested to be
indirectly involved in apoptosis. The upregulation of
p21Cip1 may be achieved via p53-dependent and
p53-independent pathways following stress (36), and the overexpression of
p21Cip1 may trigger apoptosis (37). The present results demonstrated
that treatment with bergapten increased the sub-G1 phase ratio in
A549 and NCI-H460 cells, suggesting that bergapten not only induced
G1 phase arrest; however, may additionally induce the apoptotic
cascade in NSCLC cells.
Combination chemotherapy is a promising and
effective treatment for cancer, which may maximize therapeutic
efficacy, reduce side effects and overcome drug resistance
(38). Previous clinical trials
have additionally suggested that treatment with platinum-based
combination chemotherapy may be considered as the first-line
therapy for patients with advanced NSCLC and is superior to the
single-agent treatments in terms of overall survival (39,40).
The present study demonstrated that bergapten significantly
decreases the viability of malignant human NSCLC cell lines A549
and NCI-H460, and induced G1 and sub-G1 phase accumulation, which
may be attributed to the upregulation of p53, p21Cip1
and p27Kip1. The present findings suggested that
bergapten exerts potential antitumor effects against NSCLC A549 and
NCI-H460 cells and may be used in combination with chemotherapy to
treat malignant human lung cancer.
Acknowledgements
The authors thank Dr Jing-Ting Tung (Doctor of
Pharmacy, Monterey Park Hospital AHMC, Monterey Park, CA, USA) for
her invaluable suggestions for the improvement of manuscript
writing.
Funding
The present study was supported by the Intercollege
grant from the Chung Shan Medical University (Taichung, Taiwan;
grant no. CSMU-CMMC-105-02), Chi Mei Medical Center (Tainan,
Taiwan, grant no. CMCSMU10503), and the China Medical University
Hospital (Taichung, Taiwan; grant no. DMR-106-033; 2016).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
S-RC and S-HK conceived and designed the
experiments, which were performed by C-SL, H-HL, and P-CS. S-RC,
C-SL and S-HK analyzed the data. S-RC and S-HK contributed
reagents, materials and analysis tools. S-HK wrote the paper. S-RC,
C-SL, and H-HL provided additional technical assistance and
contributed to interpretation of the data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heger Z, Polanska H, Krizkova S, Balvan J,
Raudenska M, Dostalova S, Moulick A, Masarik M and Adam V:
Co-delivery of VP-16 and Bcl-2-targeted antisense on PEG-grafted
oMWCNTs for synergistic in vitro anti-cancer effects in non-small
and small cell lung cancer. Colloids Surf B Biointerfaces.
150:131–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richardson JS, Sethi G, Lee GS and Malek
SN: Chalepin: Isolated from Ruta angustifolia L. Pers
induces mitochondrial mediated apoptosis in lung carcinoma cells.
BMC Complement Altern Med. 16:3892016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tricker EM, Xu C, Uddin S, Capelletti M,
Ercan D, Ogino A, Pratilas CA, Rosen N, Gray NS, Wong KK and Jänne
PA: Combined EGFR/MEK inhibition prevents the emergence of
resistance in EGFR-mutant lung cancer. Cancer Discov. 5:960–971.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Janne PA, Yang JC, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Travis WD: Pathology of lung cancer. Clin
Chest Med. 32:669–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Panno ML, Giordano F, Rizza P, Pellegrino
M, Zito D, Giordano C, Mauro L, Catalano S, Aquila S, Sisci D, et
al: Bergapten induces ER depletion in breast cancer cells through
SMAD4-mediated ubiquitination. Breast Cancer Res Treat.
136:443–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia W, Gooden D, Liu L, Zhao S, Soderblom
EJ, Toone EJ, Beyer WF Jr, Walder H and Spector NL: Photo-activated
psoralen binds the ErbB2 catalytic kinase domain, blocking ErbB2
signaling and triggering tumor cell apoptosis. PLoS One.
9:e889832014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Amicis F, Aquila S, Morelli C, Guido C,
Santoro M, Perrotta I, Mauro L, Giordano F, Nigro A, Andò S and
Panno ML: Bergapten drives autophagy through the up-regulation of
PTEN expression in breast cancer cells. Mol Cancer. 14:1302015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kroemer G, El-Deiry WS, Golstein P, Peter
ME, Vaux D, Vandenabeele P, Zhivotovsky B, Blagosklonny MV, Malorni
W and Knight RA: Classification of cell death: Recommendations of
the Nomenclature Committee on Cell Death. Cell Death Differ. 12
Suppl 2:S1463–S1467. 2005. View Article : Google Scholar
|
|
11
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta 1807. 735–745. 2011.
|
|
12
|
Li J and Yuan J: Caspases in apoptosis and
beyond. Oncogene. 27:6194–6206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin CH, Lin HH, Kuo CY and Kao SH:
Aeroallergen Der p 2 promotes motility of human non-small cell lung
cancer cells via toll-like receptor-mediated up-regulation of
urokinase-type plasminogen activator and integrin/focal adhesion
kinase signaling. Oncotarget. 8:11316–11328. 2017.PubMed/NCBI
|
|
14
|
Hansen MB, Nielsen SE and Berg K:
Re-examination and further development of a precise and rapid dye
method for measuring cell growth/cell kill. J Immunol Methods.
119:203–210. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang WC, Tsai JJ, Kuo CY, Chen HM and Kao
SH: Non-proteolytic house dust mite allergen, Der p 2, upregulated
expression of tight junction molecule claudin-2 associated with
Akt/GSK-3β/β-catenin signaling pathway. J Cell Biochem.
112:1544–1551. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin CH, Hong YC and Kao SH: Aeroallergen
Der p 2 induces apoptosis of bronchial epithelial BEAS-2B cells via
activation of both intrinsic and extrinsic pathway. Cell Biosci.
5:712015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu JD, Wang YJ, Chen CH, Yu CF, Chen LC,
Lin JK, Liang YC, Lin SY and Ho YS: Molecular mechanisms of G0/G1
cell-cycle arrest and apoptosis induced by terfenadine in human
cancer cells. Mol Carcinog. 37:39–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keane TE, Petros JA, Velimirovich B, Yue
KT and Graham SD Jr: Methoxypsoralen phototherapy of transitional
cell carcinoma. Urology. 44:842–846. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu JZ, Situ ZQ, Wang W, Chen JY and Liu B:
Antitumor activity of psoralen on mucoepidermoid carcinoma cell
line MEC-1. Chin Med J (Engl). 105:913–917. 1992.PubMed/NCBI
|
|
20
|
Lee YM, Wu TH, Chen SF and Chung JG:
Effect of 5-methoxypsoralen (5-MOP) on cell apoptosis and cell
cycle in human hepatocellular carcinoma cell line. Toxicol In
Vitro. 17:279–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun SY, Yue P, Wu GS, El-Deiry WS, Shroot
B, Hong WK and Lotan R: Implication of p53 in growth arrest and
apoptosis induced by the synthetic retinoid CD437 in human lung
cancer cells. Cancer Res. 59:2829–2833. 1999.PubMed/NCBI
|
|
22
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang D, Yue L, Yao R, Zhou L, Yang Y, Lu L
and Gao W: P53 prevent tumor invasion and metastasis by
down-regulating IDO in lung cancer. Oncotarget. 8:54548–54557.
2017.PubMed/NCBI
|
|
24
|
Alaee M, Nool K and Pasdar M: Plakoglobin
restores tumor suppressor activity of p53R175H mutant by
sequestering the oncogenic potential of β-catenin. Cancer Sci.
109:1876–1888. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu W, Cheng F, Yan W, Li X, Yao X, Song W,
Liu M, Shen X, Jiang H, Chen J, et al: Selective targeting
p53WT lung cancer cells harboring homozygous p53 Arg72
by an inhibitor of CypA. Oncogene. 36:4719–4731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Janssen A and Medema RH: Mitosis as an
anti-cancer target. Oncogene. 30:2799–2809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abukhdeir AM and Park BH: p21 and p27:
Roles in carcinogenesis and drug resistance. Expert Rev Mol Med.
10:e192008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Valesky EM, Hrgovic I, Doll M, Wang XF,
Pinter A, Kleemann J, Kaufmann R, Kippenberger S and Meissner M:
Dimethylfumarate effectively inhibits lymphangiogenesis via p21
induction and G1 cell cycle arrest. Exp Dermatol. 25:200–205. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Wang H, Li M, Agrawal S, Chen X
and Zhang R: MDM2 is a negative regulator of p21WAF1/CIP1,
independent of p53. J Biol Chem. 279:16000–16006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hiyama H, Iavarone A, LaBaer J and Reeves
SA: Regulated ectopic expression of cyclin D1 induces
transcriptional activation of the cdk inhibitor p21 gene without
altering cell cycle progression. Oncogene. 14:2533–2542. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
LaBaer J, Garrett MD, Stevenson LF,
Slingerland JM, Sandhu C, Chou HS, Fattaey A and Harlow E: New
functional activities for the p21 family of CDK inhibitors. Genes
Dev. 11:847–862. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee HL, Lin CS, Kao SH and Chou MC: Gallic
acid induces G1 phase arrest and apoptosis of triple-negative
breast cancer cell MDA-MB-231 via p38 mitogen-activated protein
kinase/p21/p27 axis. Anticancer Drugs. 28:1150–1156. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen SY, Lin CH, Lin JT, Cheng YF, Chen HM
and Kao SH: Adenine causes cell cycle arrest and autophagy of
chronic myelogenous leukemia K562 cells via AMP-activated protein
kinase signaling. Oncol Lett. 14:5575–5580. 2017.PubMed/NCBI
|
|
34
|
Huang WS, Kuo HY, Kuo HC, Hsieh MC, Huang
CY, Lee KC, Lee KF, Shen CH, Tung SY and Teng CC: CIL-102-induced
cell cycle arrest and apoptosis in colorectal cancer cells via
upregulation of p21 and GADD45. PLoS One. 12:e01689892017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Evan GI, Brown L, Whyte M and Harrington
E: Apoptosis and the cell cycle. Curr Opin Cell Biol. 7:825–834.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gartel AL and Tyner AL: The role of the
cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer
Ther. 1:639–649. 2002.PubMed/NCBI
|
|
37
|
Kang KH, Kim WH and Choi KH: p21 promotes
ceramide-induced apoptosis and antagonizes the antideath effect of
Bcl-2 in human hepatocarcinoma cells. Exp Cell Res. 253:403–412.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Greco F and Vicent MJ: Combination
therapy: Opportunities and challenges for polymer-drug conjugates
as anticancer nanomedicines. Adv Drug Deliv Rev. 61:1203–1213.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morth C and Valachis A: Single-agent
versus combination chemotherapy as first-line treatment for
patients with advanced non-small cell lung cancer and performance
status 2: A literature-based meta-analysis of randomized studies.
Lung Cancer. 84:209–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Masters GA, Temin S, Azzoli CG, Giaccone
G, Baker S Jr, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller
JH, et al: Systemic therapy for stage IV non-small-cell lung
cancer: American society of clinical oncology clinical practice
guideline update. J Clin Oncol. 33:3488–3515. 2015. View Article : Google Scholar : PubMed/NCBI
|