Introduction
Mastitis, a common disease in dairy herds, causes
economic loss worldwide (1). A
survey in 2007 showed that clinical mastitis results in an economic
cost of 444 US dollars per average case in the USA, including
direct costs of 128 dollars and indirect costs of 316 dollars
(2). Previous research has
confirmed that Escherichia coli is responsible for mastitis
(3).
Lipopolysaccharide (LPS), also called endotoxin, is
the main element of the outer membrane of Gram-negative bacteria,
including Escherichia coli, and is a valuable tool for
inducing inflammation, such as mastitis (4,5). LPS
can stimulate Toll-like receptor 4 (TLR4), a member of the
Toll-like receptor (TLR) family, which is known as one of the
significant pathogen-recognition receptors (PRRs) (6). TLR4 signalling pathways then activate
the Toll/interleukin-1 (IL-1) receptor (TIR) signalling pathway
(7,8). Subsequently, myeloid differentiation
primary response 88 (MyD88), a universal adaptor protein, responses
to TIR first and activates IL-1 receptor-associated kinase 1
(IRAK1) and TNF receptor-associated factor 6 (TRAF6) (9). Then, phosphorylated IKKβ initiates
the degradation of IκBα, which associates with a nuclear
localization sequence to prevent the nuclear transfer of p65
(10). Then, NF-κB subunit p65
separates from IκB and is phosphorylated as pp65. Furthermore, it
displaces into the nucleus and exhibits its DNA-binding activity,
where NF-κB can trigger the release of various pro-inflammatory
cytokines, such as interleukin (IL)-1β, IL-6 and tumor necrosis
factor (TNF)α (11–13).
Many Chinese traditional herbs have attracted wide
attention over the past decade due to their biological function
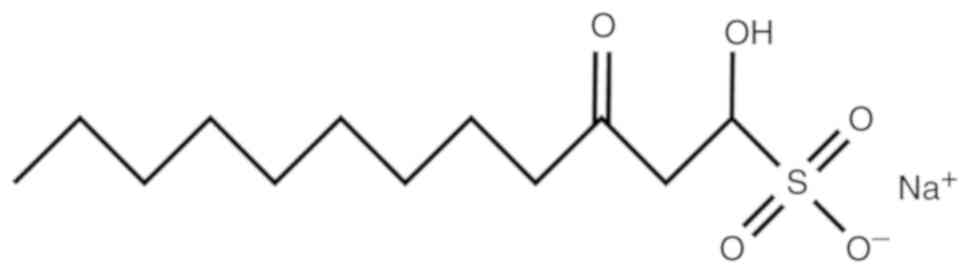
(14). Sodium houttuyfonate (SH)
(Fig. 1) is one of the main
compounds in the volatile oil of Houttuynia cordata Thunb.,
which has been used as an anti-pyretic and detoxicated herbal
medicine for the therapy of infections for an extensive period of
time (15,16). The effects of SH on diabetic
nephropathy and glomerulonephritis have been reported in
experimental animals (17,18). Moreover, previous research suggests
that SH inhibits LPS-induced inflammatory responses in bovine
mammary epithelial cells (bMECs) by depressing the NF-κB signalling
pathway (19). The authors
investigated the levels of IL-1β, IL-6 and TNF-α by real-time
quantitative PCR (RT-qPCR) and the expression of IκBα, NF-κB p65
and TLR4 by western blotting. All of the results showed that SH may
be a potential agent for the therapy of mastitis.
Therefore, in order to verify whether SH causes the
same effect in vivo, mouse models of LPS-induced mastitis
were utilized in the present study. The mouse mastitis model is
commonly used for the study of bovine intramammary infections as it
closely mimics the inflammatory responses observed in natural
mastitis (5,20,21).
In addition, we also assayed the function of SH using a mouse
mammary epithelial cell line.
Materials and methods
Reagents
Sodium houttuyfonate (SH) was purchased from
Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China; purity
≥98%). LPS (Escherichia coli 055:B5) was provided by
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). All of the
antibodies used in this experiment were provided by Cell Signaling
Technology (Beverly, MA, USA), including β-Actin (D6A8) Rabbit mAb
(8457), NF-κB p65 (D14E12) XP® Rabbit mAb (8242),
Phospho-NF-κB p65 (Ser468) Antibody (3039), IκBα (44D4) Rabbit mAb
(4812) and Anti-rabbit IgG, HRP-linked Antibody (7074). Other
chemicals were of reagent grade.
Animals
Twenty lactating female BALB/c mice (6-week old,
20–25 g) were purchased from the Wuhan Institute of Biological
Products Co., Ltd. (Wuhan, China). Three mice were raised per cage
with standard laboratory chow and ad libitum feeding. All of
the mice were housed in the facilities at a temperature of 25±2°C
with 50±2% humidity and a 12-h light/dark cycle for 1 week to adapt
to the environment before the experiment. All of the animal
experiments were performed according to the guidelines for the
Laboratory Animal Research Center of Hubei Province and approved by
the Ethics Committee on Animal Research of Huazhong Agricultural
University (Wuhan, China).
Animal modelling and grouping
The mice were randomly assigned into three groups of
six, namely, the negative control group (NC), the LPS group, and
the LPS + SH (50 mg/kg, gastric perfusion) group. Mice were
anaesthetized with ethyl ether using an anaesthesia machine. Mice
in the NC group were given PBS, while mice in all other groups were
infused with LPS using a 100-µl syringe into both the L4 (on the
left) and R4 (on the right) abdominal mammary glands after the
teats were disinfected with 75% alcohol (22). SH (at doses of 50 mg/kg, dissolved
in DMSO and PBS) was administered 1 h before LPS injection by
gastric perfusion in the LPS+SH group, and the mice in the control
group were administered equal volumes of PBS. At 24 h after LPS
injection, mice were sacrificed using cervical dislocation and the
mammary tissues were harvested, one part of the sample was fixed
into 4% paraformaldehyde, and the other part was stored at
−80°C.
Histopathologic analysis
At 24 h after fixation in 4% paraformaldehyde,
mammary tissues were embedded in paraffin and then the sections
(4-µm) were stained with hematoxylin and eosin (H&E). Finally,
pathological changes in the tissues were observed under a light
microscope (Olympus Corporation, Tokyo, Japan) at ×400
magnification.
Myeloperoxidase (MPO) activity
analysis
The levels of neutrophils and monocytes in mammary
tissue were assessed using an MPO kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). Mammary tissues (50 mg)
were homogenized with reaction buffer (950 µl, w/v 1:19), and then
the MPO activity was assayed according to the protocol of the
kit.
Cell culture and treatment
The cells were cultured in 90% RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with
10% foetal bovine serum (FBS) at 37°C with 5% CO2 for
incubation. The cells were divided into four groups: DMSO control
(DMSO) group, LPS group, and SH treatment (30 and 60 µg/ml) groups.
All of cells in the DMSO control group and the SH treatment groups
were pretreated with DMSO and SH (30 and 60 µg/ml) for 1 h,
respectively. Subsequently, the LPS group and the SH treatment
groups were stimulated by LPS (1 µg/ml) for 1 h. The DMSO group was
used as a negative control as the drug was diluted with DMSO.
Cell Counting Kit-8 (CCK-8)
analysis
Cell proliferation status was assayed by a CCK-8
kit. The cells were seeded in 96-well plates at a density of 2,000
cells per well with 100 µl culture medium. After cultivation for 24
h, SH was added to each well to the final concentrations (30 and 60
µg/ml SH, n=6). Subsequently, the cells were cultured for another
24 h. Then, 10 µl of CCK-8 solution was added to the medium, and
the culture was incubated for 1 h at 37°C with 5% CO2.
The optical density (OD) values were read at 450 nm by a microplate
reader (Bio-Rad Instruments, Hercules, CA, USA) (23).
ELISA analysis
The effects of SH on the levels of cytokines in cell
supernatants were examined. IL-1β, IL-6 and TNF-α levels were
measured with ELISA kits (Shanghai Hengyuan Biological Technology
Co., Ltd, Shanghai, China) according to the manufacturer's
instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) from
tissues and cells (1×106 cells/well) according to the
manufacturer's instructions. The concentration and purity of the
total RNA was measured using Q5000 (Quawell Technology Inc., San
Jose, CA, USA) at a 260/280 nm ratio. Reverse transcription was
performed immediately after measurement. qPCR was implemented using
the LightCycler® 480 SYBR® Green I Master
(Roche Diagnostics, Basel, Switzerland) and a PCR system
(LightCycler® 96; Roche Diagnostics). The sequences of
primers used in the study were designed with Primer Premier 5.0
(Premier, Canada), and the primers are shown in Table I. The relative quantitative assay
was performed using the 2−ΔΔCq comparative method
normalized by GAPDH (24).
 | Table I.Primers used for qPCR. |
Table I.
Primers used for qPCR.
| Gene name | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| IL-1β |
GCAGCAGCACATCAACAAGA | 121 |
|
|
GTTCATCTCGGAGCCTGTAGT |
|
| IL-6 |
TTCCATCCAGTTGCCTTCTTG | 134 |
|
|
CATTTCCACGATTTCCCAGAGA |
|
| TNF-α |
ACTGGCAGAAGAGGCACTC | 116 |
|
|
GGCTACAGGCTTGTCACTC |
|
| GAPDH |
CAATGTGTCCGTCGTGGATCT | 124 |
|
|
GTCCTCAGTGTAGCCCAAGATG |
|
Western blot analysis
Protein in tissues and cells were isolated by RIPA
buffer (Biosharp, China) with 1 mM phenylmethylsulfonyl fluoride
(PMSF) and phosphorylation of protease inhibitor cocktail. The
concentrations of protein were tested using a BCA protein assay kit
(Thermo Scientific). Total proteins (40 µg) were denatured by
boiling for 10 min and were then separated by 10% SDS-PAGE. After
transfer to polyvinylidene fluoride (PVDF) membranes and blocking
in 5% non-fat dry milk for 2 h at room temperature, the proteins
were incubated overnight (12 h) with primary antibodies (1:1,000
dilution) at 4°C. Then, the membranes were washed in TBST 3 times
(10 min each time) and incubated with HRP-conjugated secondary
antibody (1:4,000 dilution) for 1 h at room temperature. After
washing as described above, immunoblot signals were measured with
an enhanced chemiluminescence detection system (ImageQuant LAS 4000
mini; GE Healthcare, Chicago, IL, USA). Densitometry assays were
completed using Image Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA).
Statistical analysis
Statistical assays were performed using GraphPad
Prism 7 software (GraphPad Software, Inc., La Jolla, CA, USA). All
values are presented as the mean ± SEM. The significant differences
between groups were analysed using one-way ANOVA with the Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference. P-values are indicated as follows in the
figures and legends: #P<0.05 vs. the control group;
*P<0.05 vs. the LPS group; **P<0.01 vs. the LPS group.
Results
Effects of SH on pathological changes
in the mouse mammary gland
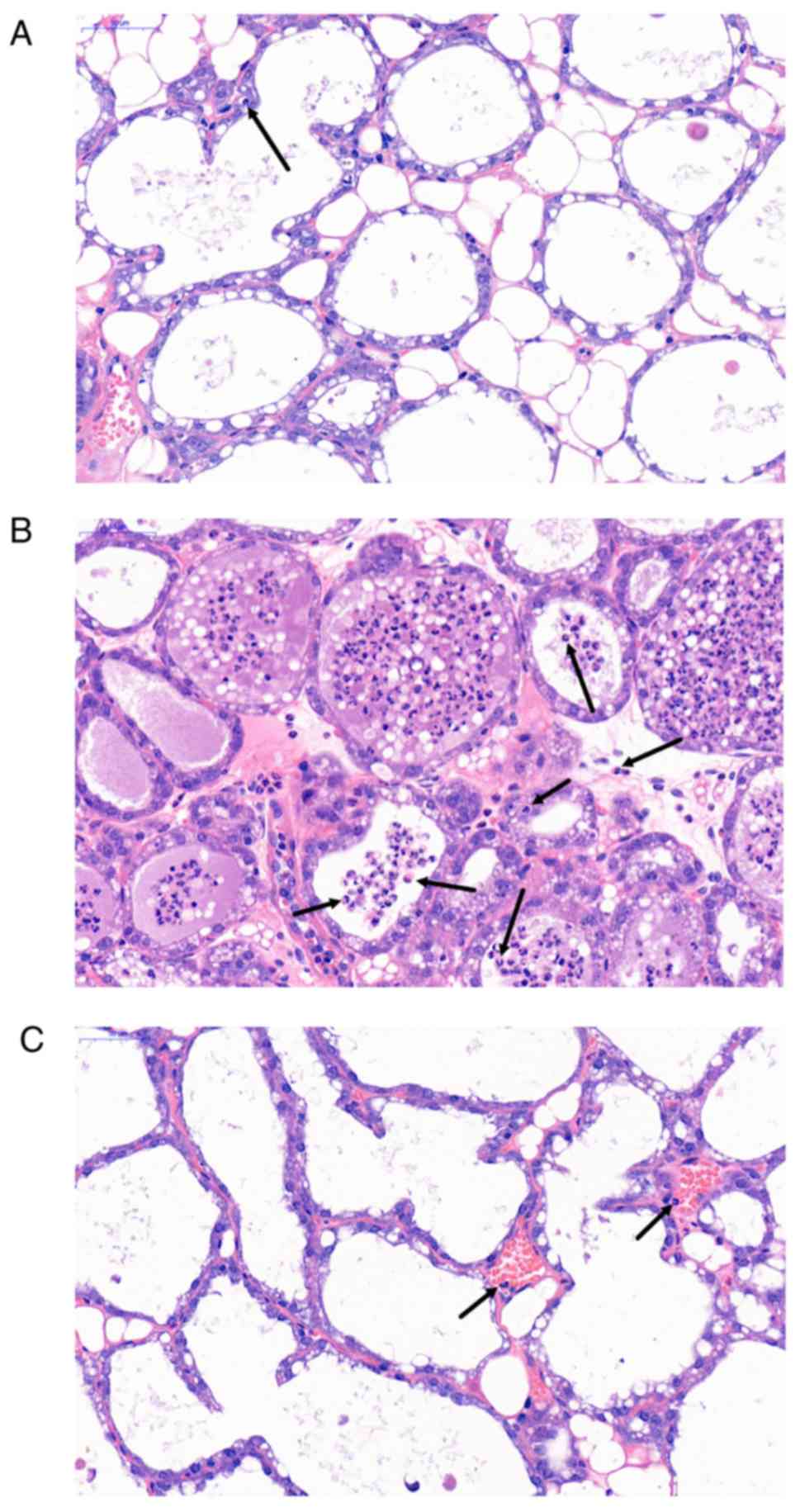
The histopathological results are shown in Fig. 2. Compared with the NC group
(Fig. 2A), the LPS group (Fig. 2B) exhibited significant mammary
gland hyperaemia, mammary gland wall thickening and invasion of
inflammatory cells such as neutrophils (as indicated by black
arrows). However, SH (50 mg/kg; Fig.
2C) treatment obviously ameliorated these morphological changes
in the mouse mammary gland tissues.
Effects of SH on MPO activity and the
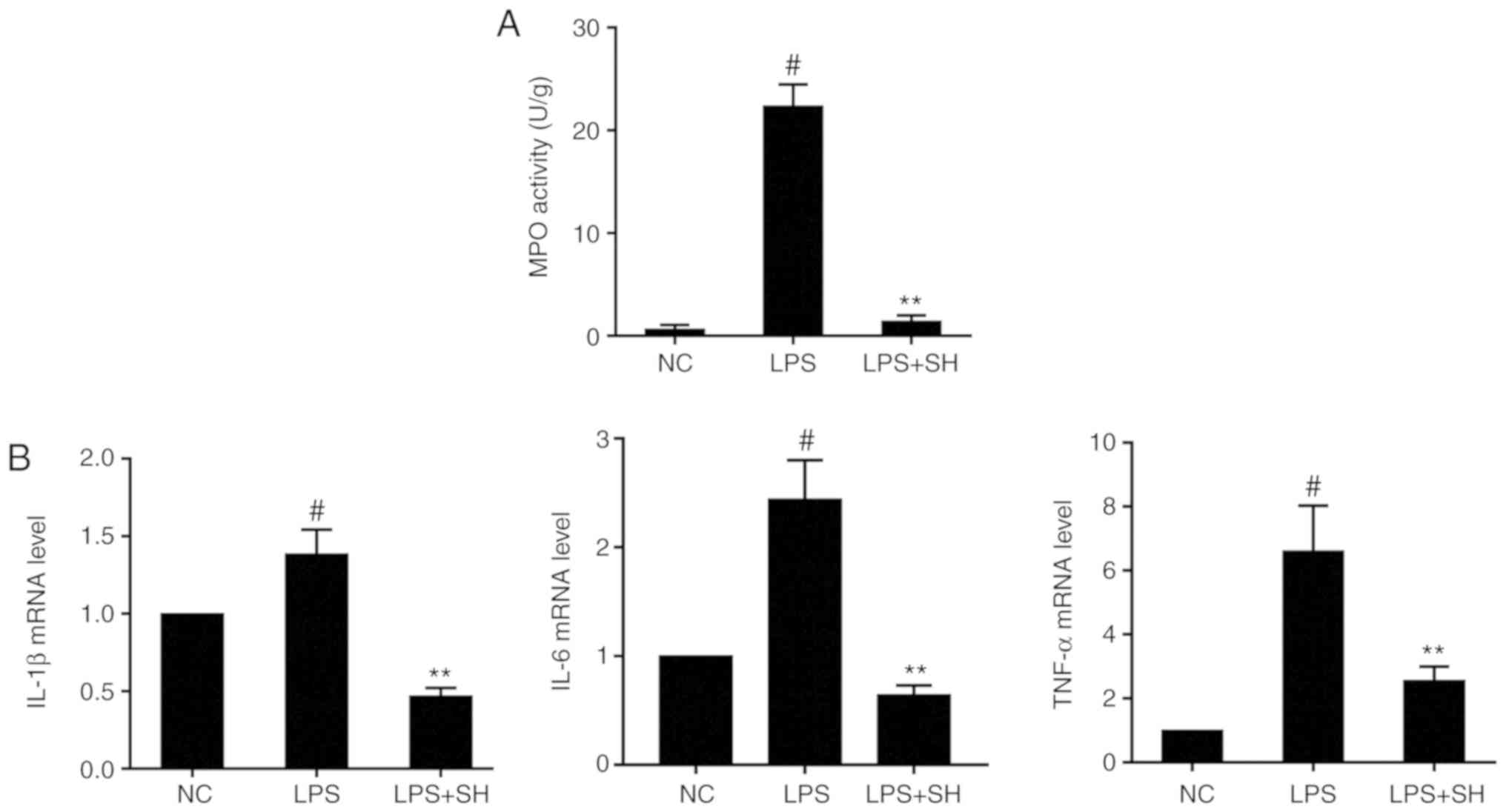
expression of inflammatory cytokines in vivo
MPO is most abundantly expressed in neutrophil
granulocytes and can immediately reflect the levels of inflammation
(25). The MPO assay showed that
MPO activity in the LPS group was significantly increased when
compared with that in the negative control (NC) group (P<0.05;
Fig. 3A), while SH treatment
decreased MPO activity relative to that in the LPS group, similar
to the histopathology results. A previous study showed that LPS can
markedly elevate the expression levels of inflammatory cytokines
such as IL-1β, IL-6 and TNF-α (26). As shown in Fig. 3B, mRNA levels of cytokines in the
LPS group were significantly higher than those in the NC group. SH
also significantly downregulated the expression of these cytokines.
These results suggested that SH may inhibit LPS-induced mastitis in
mice.
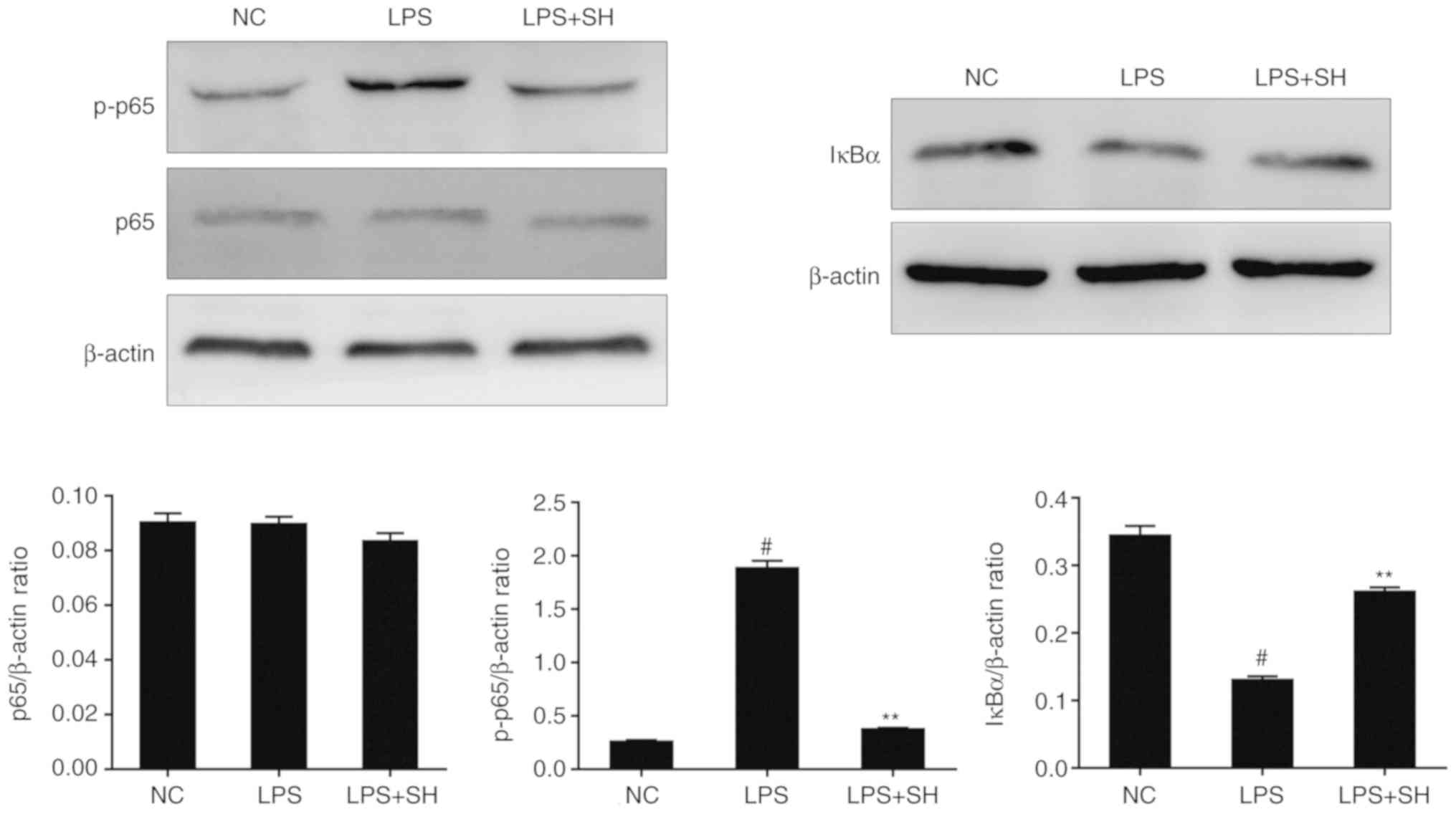
Effects of SH on the NF-κB pathway in
vivo
Phosphorylation of p65 plays a significant role in
the activation of the NF-κB pathway (27). Phosphorylation and rapid
degradation of IκBα is the sign of NF-κB-IκB complex changes
(28). In this study, to clarify
the function of SH on the NF-κB pathway, we detected the expression
of IκBα and p65 protein in tissues by western blotting. As shown in
Fig. 4, the phosphorylation of p65
protein was upregulated in the LPS group, while the levels of IκBα
were downregulated. However, SH treatment suppressed the
LPS-induced phosphorylation of p65 proteins and the degradation of
IκBα, thus inhibiting the NF-κB pathway. These results demonstrated
that SH ameliorates LPS-induced mastitis in mouse models through
the NF-κB pathway.
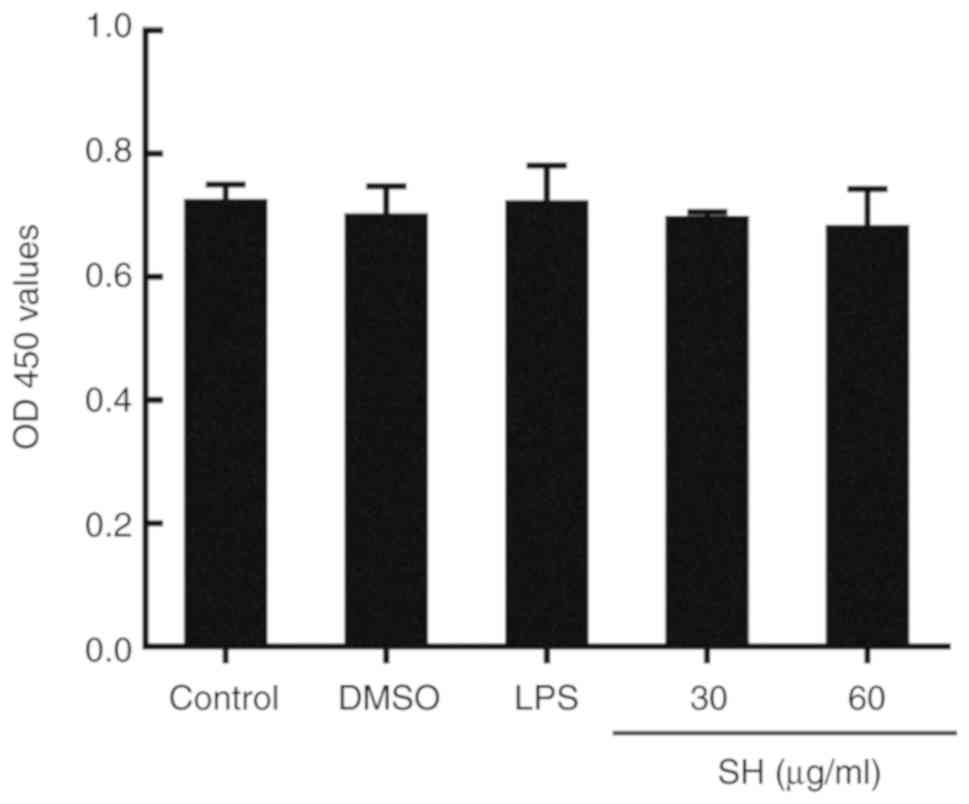
Effects of SH on cell viability
Cell viability was measured using CCK-8 assay in
this study. The results as shown in Fig. 5 demonstrated that SH (30 and 60
µg/ml) and DMSO (used as solvent) had no cytotoxicity on the mouse
mammary epithelial cell line.
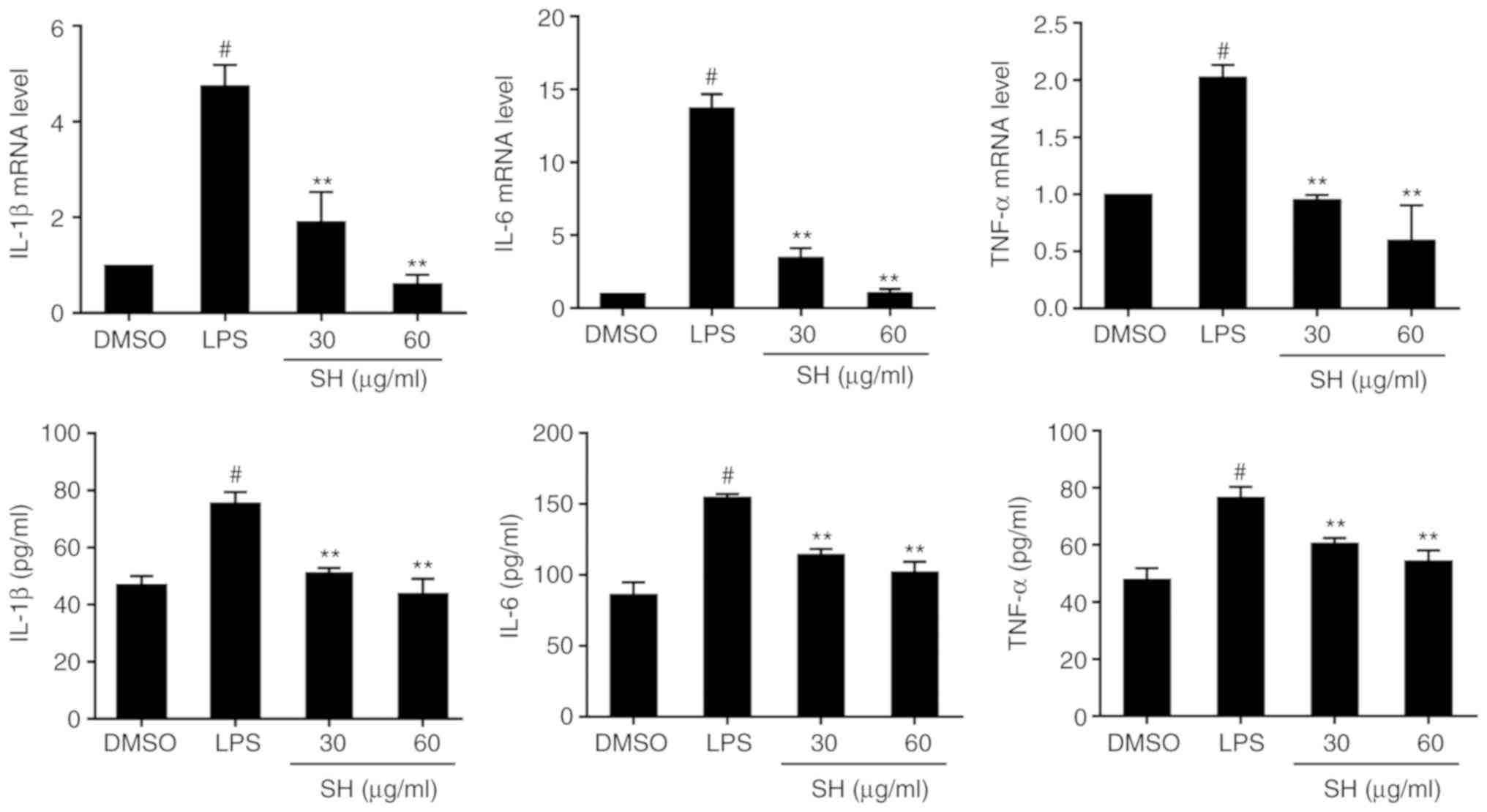
Effects of SH on the levels of
inflammatory cytokines in vitro
To investigate the function of SH on cytokine
levels, IL-1β, IL-6 and TNF-α production was detected by qPCR and
ELISA. According to the results shown in Fig. 6, it was found that LPS stimulation
promoted higher expression of cytokines than DMSO. Additionally, SH
significantly decreased the expression of these cytokines.
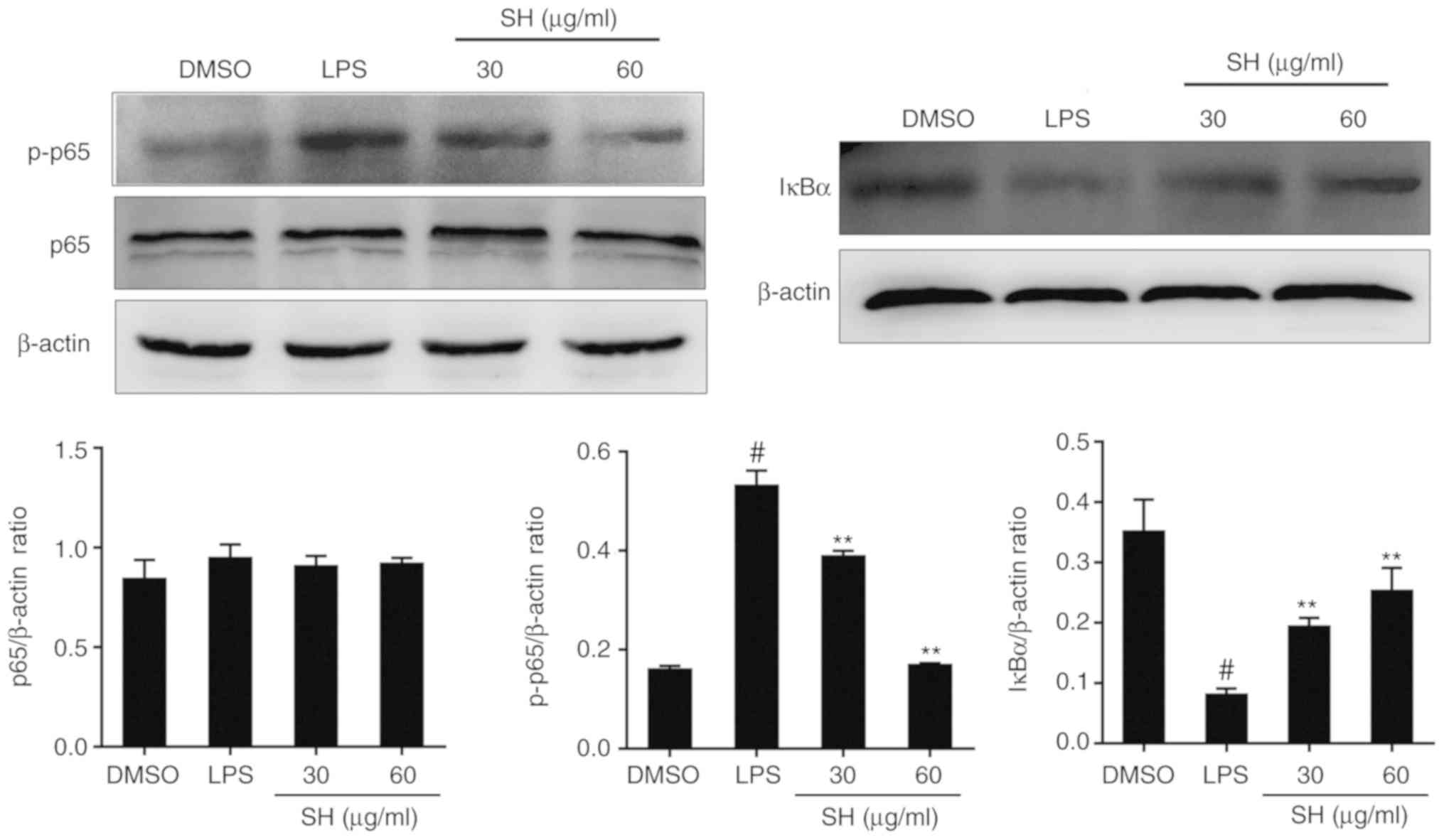
Effects of SH on the NF-κB pathway in
vitro
Related to the in vivo experiments, NF-κB
pathway protein expression in cells was also verified. As shown in
Fig. 7, the level of
phosphorylated (p)-p65 was suppressed dose-dependently in the SH
treatment groups, and the expression of IκBα was upregulated. In
conclusion, SH depressed the NF-κB pathway by inhibiting the
phosphorylation of p65 and the degradation of IκBα.
Discussion
Bovine mastitis leads to loss of milk production and
extra treatment costs (29,30).
Because mastitis is caused by various different pathogens, it is a
challenge for many different countries (31). E. coli infection was
suggested to be the most common cause of fatal mastitis (32). Lipopolysaccharide (LPS), a unique
structure on the walls of Gram-negative bacteria, including E.
coli, interacts with LPS-binding protein (LBP) and CD14 and
further promotes the activation of TLR4 (33). TLR4 accelerates inflammatory gene
expression via the NF-κB pathway (34). Sodium houttuyfonate (SH), first
extracted from Houttuynia cordata Thunb. by Kosuge in 1952,
is considered an anti-inflammatory medication for suppressing many
bacteria, such as E. coli (35,36).
Although the effects of SH on mastitis in bovine mammary epithelial
cells have been elucidated (19),
few studies have focused on the anti-inflammatory function of SH in
animals. In the present study, we first investigated whether SH
treatment ameliorates LPS-induced mastitis in mice
Histopathological examination of mammary glands
showed that the changes in SH treatment groups were not as obvious
as in the LPS group. The results indicate that SH has a protective
effect on LPS-induced mastitis. In addition, MPO analysis and
inflammatory cytokines assays also confirmed the protective effect
of SH.
Some inflammatory cytokines, such as IL-1β, IL-6 and
TNF-α, may cause tissue damage. As primary cytokines, IL-1β and
TNF-α induce the secretion of IL-6, which plays an important role
in the induction of acute disease (37). In the present study, the expression
of IL-1β, IL-6 and TNF-α was substantially enhanced in LPS-induced
mastitis. However, SH reduced the levels of these cytokines in
vivo and in vitro. These results demonstrated that the
anti-inflammatory activity of SH might be due to decreasing levels
of inflammatory cytokines.
IκBα is associated with and tightly regulates the
NF-κB complex (38). IκBα has been
shown to have ankyrin repeats composing a 205-amino-acid internal
region (39). Mutations of these
ankyrin repeats can inhibit IκBα from interacting with NF-κB
(40). Under normal conditions,
IκBα preserves NF-κB in the cytoplasm by masking the nuclear
localization sequences (41). When
IKK initiates the sequence-specific phosphorylation and
ubiquitination of IκBα, the result is the rapid degradation of IκB
(42). Thus the present study
detected the level of IκBα. In the LPS group, the expression of
IκBα was obviously downregulated in vivo and in
vitro, which can lead to the release of NF-κB p65 in the LPS
group. However, SH treatment recovered the level of IκBα, which
inhibits the activation of the NF-κB pathway.
In addition, the phosphorylation and nuclear
translocation of p65 is generally regarded as a marker of
initiation of the NF-κB pathway (27). With the degradation of IκB, NF-κB
p65 is released from IκB molecules (43), and p65 is then free to translocate
to the nucleus and bind to target genes to promote the expression
of pro-inflammatory cytokines (44). In our study, we detected the
expression of p65 and phosphorylated (p)-p65 protein using western
blot analysis. Significantly, the expression of p-p65 protein was
upregulated in the LPS group. In the in vivo study, SH had a
marked effect on NF-κB pathway inhibition. In the in vitro
study, SH reduced the p-p65 protein.
Based on the above experimental results, we can
hypothesize that SH exhibits an anti-inflammatory function in
LPS-induced mouse mastitis. Furthermore, the mechanisms underlying
the effects of SH may be due to the inhibition of the NF-κB
pathway, but the direct targets of SH and accurate molecular
mechanisms warrant further investigation. All of these results
suggest that SH is a potential therapeutic strategy for bovine
mastitis.
Acknowledgements
Not applicable.
Funding
This study was sponsored by the National Natural
Science Foundation of China (no. 31772816).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
GD conceived and designed the research. PL and CY
acquired data, analysed and interpreted data and performed
statistical analysis. PL was responsible for manuscript writing and
contributed to animal experiment with CY. CY and GZ contributed to
most of the experimental designs and operations. SL contributed to
interpretation of the data, manuscript review and editing. TZ and
SG performed qPCR. KJ and HW gave technical guidance on western
blotting and contributed to analysis of its results. TZ, SG, KJ and
HW revised the manuscript for important intellectual content. CQ
and MG contributed to data analysis. All authors read and approved
the final manuscript and agree to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All of the animal experiments were performed
according to the guidelines for the Laboratory Animal Research
Center of Hubei Province and approved by the Ethics Committee on
Animal Research of Huazhong Agricultural University (Wuhan, Hubei,
China).
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SH
|
sodium houttuyfonate
|
|
bMECs
|
bovine mammary epithelial cells
|
|
CCK-8
|
Cell Counting Kit-8
|
|
LPS
|
lipopolysaccharide
|
References
|
1
|
Le M, aréchal C, Thiéry R, Vautor E and Le
Loir Y: Mastitis impact on technological properties of milk and
quality of milk products-a review. Dairy Sci Technol. 91:247–282.
2011. View Article : Google Scholar
|
|
2
|
Rollin E, Dhuyvetter KC and Overton MW:
The cost of clinical mastitis in the first 30 days of lactation: An
economic modeling tool. Prev Vet Med. 122:257–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halasa T, Nielen M, Huirne RBM and
Hogeveen H: Stochastic bio-economic model of bovine intramammary
infection. Livestock Sci. 124:295–305. 2009. View Article : Google Scholar
|
|
4
|
Kauf AC, Vinyard BT and Bannerman DD:
Effect of intramammary infusion of bacterial lipopolysaccharide on
experimentally induced Staphylococcus aureus intramammary
infection. Res Vet Sci. 82:39–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JW, Paape MJ, Elsasser TH and Zhao X:
Elevated Milk soluble CD14 in bovine mammary glands challenged with
Escherichia coli lipopolysaccharide. J Dairy Sci.
86:2382–2389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeuke S, Ulmer AJ, Kusumoto S, Katus HA
and Heine H: TLR4-mediated inflammatory activation of human
coronary artery endothelial cells by LPS. Cardiovasc Res.
56:126–134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeuchi O, Kaufmann A, Grote K, Kawai T,
Hoshino K, Morr M, Mühlradt PF and Akira S: Cutting edge:
Preferentially the R-stereoisomer of the mycoplasmal lipopeptide
macrophage-activating lipopeptide-2 activates immune cells through
a toll-like receptor 2- and MyD88-dependent signaling pathway. J
Immunol. 164:554–557. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burns K, Martinon F, Esslinger C, Pahl H,
Schneider P, Bodmer JL, Di Marco F, French L and Tschopp J: MyD88,
an adapter protein involved in interleukin-1 signaling. J Biol
Chem. 273:12203–12209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arancibia SA, Beltrán CJ, Aguirre IM,
Silva P, Peralta AL, Malinarich F and Hermoso MA: Toll-like
receptors are key participants in innate immune responses. Biol
Res. 40:97–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beg AA, Ruben SM, Scheinman RI, Haskill S,
Rosen CA and Baldwin AS: I kappa B interacts with the nuclear
localization sequences of the subunits of NF-kappa B: A mechanism
for cytoplasmic retention. Genes Dev. 6:1899–1913. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruifeng G, Yunhe F, Zhengkai W, Ershun Z,
Yimeng L, Minjun Y, Xiaojing S, Zhengtao Y and Naisheng Z:
Chlorogenic acid attenuates lipopolysaccharide-induced mice
mastitis by suppressing TLR4-mediated NF-κB signaling pathway. Eur
J Pharmacol. 729:54–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Triantafilou M and Triantafilou K: The
dynamics of LPS recognition: Complex orchestration of multiple
receptors. J Endotoxin Res. 11:5–11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Wang Y, Xiao C, Wei Z, Wang J,
Yang Z and Fu Y: Resveratrol inhibits LPS-induced mice mastitis
through attenuating the MAPK and NF-κB signaling pathway. Microb
Pathog. 107:462–467. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Al-Sheraji SH, Ismail A, Manap MY, Mustafa
S, Yusof RM and Hassan FA: Prebiotics as functional foods: A
review. J Functional Foods. 5:1542–1553. 2013. View Article : Google Scholar
|
|
15
|
Wang D, Noda Y, Zhou Y, Nitta A, Nabeshima
T and Yu Q: Effects of sodium houttuyfonate on phosphorylation of
CaMK II CREB and ERK 1/2 and expression of c-Fos in macrophages.
Int Immunopharmacol. 4:1083–1088. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Z, Tan B, Zhang H, Guo Y, Tu Y, Qiu F
and Yang A: Effects of sodium houttuyfonate on pulmonary
inflammation in COPD model rats. Inflammation. 40:2109–2117. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lan S, Hai YQ, Huang Q, Sinica A and
Shengyang: The effect of Houttuyninum on cellar immunoiogic
function in splenectomy animals. Chin Pharmacol Bulletin. 17:51–54.
2001.
|
|
18
|
Pan P, Wang YJ, Han L, Liu X, Zhao M and
Yuan YF: Effects of sodium houttuyfonate on expression of NF-κB and
MCP-1 in membranous glomerulonephritis. J Ethnopharmacol.
131:203–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Hu X, Peng S, Zhang N and Fu Y:
Sodium houttuyfonate inhibits LPS-induced inflammatory response via
suppressing TLR4/NF-κB signaling pathway in bovine mammary
epithelial cells. Microb Pathog. 107:12–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng J, Watson AD and Kerr DE:
Genome-wide expression analysis of lipopolysaccharide-induced
mastitis in a mouse model. Infect Immun. 74:1907–1915. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Notebaert S and Meyer E: Mouse models to
study the pathogenesis and control of bovine mastitis. A review.
Vet Q. 28:2–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li D, Zhang N, Cao Y, Zhang W, Su G, Sun
Y, Liu Z, Li F, Liang D, Liu B, et al: Emodin ameliorates
lipopolysaccharide-induced mastitis in mice by inhibiting
activation of NF-κB and MAPKs signal pathways. Eur J Pharmacol.
705:79–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Shen J, Wu WK, Yu X, Liang J, Qiu G
and Liu J: Leptin induces cyclin D1 expression and proliferation of
human nucleus pulposus cells via JAK/STAT, PI3K/Akt and MEK/ERK
pathways. PLoS One. 7:e531762012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klebanoff SJ: Myeloperoxidase: Friend and
foe. J Leukoc Biol. 77:598–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vels L, Røntved CM, Bjerring M and
Ingvartsen KL: Cytokine and acute phase protein gene expression in
repeated liver biopsies of dairy cows with a
lipopolysaccharide-induced mastitis. J Dairy Sci. 92:922–934. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Neumann M, Grieshammer T, Chuvpilo S,
Kneitz B, Lohoff M, Schimpl A, Franza BR Jr and Serfling E:
RelA/p65 is a molecular target for the immunosuppressive action of
protein kinase A. EMBO J. 14:1991–2004. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hiscott J, Marois J, Garoufalis J,
D'Addario M, Roulston A, Kwan I, Pepin N, Lacoste J, Nguyen H,
Bensi G, et al: Characterization of a functional NF-kappa B site in
the human interleukin 1 beta promoter: Evidence for a positive
autoregulatory loop. Mol Cell Biol. 13:6231–6240. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miller GY, Bartlett PC, Lance SE, Anderson
J and Heider LE: Costs of clinical mastitis and mastitis prevention
in dairy herds. J Am Vet Med Assoc. 202:1230–1336. 1993.PubMed/NCBI
|
|
30
|
Wilson DJ, González RN, Hertl J, Schulte
HF, Bennett GJ, Schukken YH and Gröhn YT: Effect of clinical
mastitis on the lactation curve: A mixed model estimation using
daily milk weights. J Dairy Sci. 87:2073–2084. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gröhn YT, Wilson DJ, González RN, Hertl
JA, Schulte H, Bennett G and Schukken YH: Effect of
pathogen-specific clinical mastitis on milk yield in dairy cows. J
Dairy Sci. 87:3358–3374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Menzies FD, Bryson DG, Mccallion T and
Matthews DI: A study of mortality among suckler and dairy cows in
Northern ireland in 1992. Vet Rec. 137:531–536. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Burvenich C, Van MV, Mehrzad J,
Diez-Fraile A and Duchateau L: Severity of E. coli mastitis is
mainly determined by cow factors. Vet Res. 34:521–564. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Akira S: Toll-like receptors and innate
immunity. Adv Immunol. 388:621–625. 2009.
|
|
35
|
Ye X, Li X, Yuan L, Ge L, Zhang B and Zhou
S: Interaction of houttuyfonate homologues with the cell membrane
of gram-positive and gram-negative bacteria. Colloids Surf A
Physicochemical Eng Aspects. 301:412–418. 2007. View Article : Google Scholar
|
|
36
|
Kosuge T: Structure of an antimicrobial
substance isolated from Houttuynia cordata thunb. J
Pharmaceutical Soc Japan. 72:1227–1231. 1952. View Article : Google Scholar
|
|
37
|
Xing Z, Gauldie J, Cox G, Baumann H,
Jordana M, Lei XF and Achong MK: IL-6 is an antiinflammatory
cytokine required for controlling local or systemic acute
inflammatory responses. J Clin Invest. 101:311–320. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huxford T, Huang DB, Malek S and Ghosh G:
The crystal structure of the IkappaBalpha/NF-kappaB complex reveals
mechanisms of NF-kappaB Inactivation. Cell. 95:759–770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baldwin AS Jr: THE NF-kappaB and I kappa B
proteins: New discoveries and insights. Ann Rev Immunol.
14:649–683. 1996. View Article : Google Scholar
|
|
40
|
Siebenlist U, Franzoso G and Brown K:
Structure, regulation and function of NF-kappa B. Ann Rev Cell
Biol. 10:405–455. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chae SW: Function and activation of
NF-kappa B in immune system. J Cell Biol. 33:307–318. 2005.
|
|
42
|
Scherer DC, Brockman JA, Chen Z, Maniatis
T and Ballard DW: Signal-induced degradation of I kappa B alpha
requires site-specific ubiquitination. Proc Natl Acad Sci USA.
92:11259–11263. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mercurio F and Manning AM: Multiple
signals converging on NF-kappaB. Curr Opin Cell Biol. 11:226–232.
1999. View Article : Google Scholar : PubMed/NCBI
|