Introduction
Gastric carcinoma, a major cause of
cancer-associated mortality worldwide, remains a concern for
clinicians and the scientific community (1). The majority of patients with gastric
carcinoma are diagnosed at advanced stages and have poor outcomes,
with susceptibility to invasion and metastasis (2,3).
Unfortunately, the development of techniques for the treatment and
diagnosis of gastric carcinoma in previous years have only made a
modest contribution towards improving the prognosis of gastric
carcinoma, in particular for patients with advanced-stage disease
(4–6). Therefore, the exploration of novel
approaches for the early diagnosis and management of gastric
carcinoma is urgently required.
The matrix metalloproteinases (MMPs) family was
demonstrated to be overexpressed in multiple types of cancer and to
serve essential roles in the development and invasion of cancer
(7–9). Accumulating evidence has indicated
that several MMPs regulate the migration and invasion of cancer
cells by promoting epithelial-mesenchymal transition (EMT) of
cancer cells, including MMP-3, MMP-8 and MMP-14 (10–12).
Membrane-type 1 matrix metalloproteinase (MT1-MMP) was demonstrated
to be expressed at abnormally high levels in cancerous tissues from
patients with gastric cancer (13). A previous study demonstrated that
the overexpression of MT1-MMP was associated with poor prognoses of
patients with gastric carcinoma, including increased tumor
invasion, metastasis, advanced Tumor-Node-Metastasis stages and
worse overall survival (14).
Inhibition of MT1-MMP expression may inhibit cell migration,
invasion, proliferation and angiogenesis (13,15).
However, the specific mechanisms of MT1-MMP in gastric carcinoma
remain unclear.
In the present study, the expression of MT1-MMP was
determined in gastric carcinoma clinical samples. In addition,
MT1-MMP expression was suppressed using a short hairpin RNA (shRNA)
technique followed by proliferation and invasion assays. Using
these analyses, the present study aimed to provide useful new
information concerning the underlying mechanisms of gastric
carcinoma.
Materials and methods
Tissue samples
Clinic tissue samples were obtained from 15 patients
who underwent gastric carcinoma resection at the First Affiliated
Hospital of Suzhou University (Suzhou, China). Concomitantly,
normal gastric tissue samples were collected to serve as controls,
which were sourced from a site distant the cancerous lesion (≥5 cm)
and blindly confirmed by two experienced pathologists. Among the 15
patients, 5 were females and 10 were males. The age of patients
ranged from 49–81, and the average age was 61.267 years old.
According to the Goseki classification (16), a total of 5 tumors were classed as
moderately differentiated adenocarcinomas, 2 were moderately-poorly
differentiated, and 8 were poorly differentiated. The present study
was approved by the Research Ethics Committee of the First
Affiliated Hospital of Suzhou University and was performed
according to ethical standards of the Declaration of Helsinki. All
participants provided written consent for their clinical
information to be used for scientific research.
Immunohistochemistry and
hematoxylin-eosin staining (H&E)
Immunohistochemical staining and H&E staining
were performed on 4% paraformaldehyde-fixed (for 24 h at room
temperature), paraffin-embedded tissue sections. For
immunohistochemistry, the sections were blocked with 10% goat serum
(OriGene Technologies, Inc., Beijing, China) at 37°C for 1 h.
MT1-MMP expression was detected with anti-MT1-MMP antibody (cat.
no. ab51074; Abcam, Cambridge, UK; dilution, 1:100), followed by
horseradish peroxidase-conjugated goat anti-rabbit IgG (H+L) (cat.
no. 111-035-045; Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA, USA) antibody at a dilution of 1:400. Assessment of
immunohistochemical staining was performed as described previously
by Pang et al (17) and Di
Martino et al (18). For
H&E staining, sections were stained with hematoxylin solution
(0.2%) for 4 min, followed by eosin solution (0.5%) for 90 sec at
room temperature.
Cell culture
The human gastric cancer AGS cell line and normal
gastric epithelial GES-1 cell line were purchased from The Cell
Bank of Type Culture Collection of Chinese Academy of Science
(Shanghai, China). GES-1 cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 1% streptomycin and 1% penicillin (Gibco; Thermo
Fisher Scientific, Inc.). AGS cells were cultured in Dulbecco's
modified Eagles medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS, 1% streptomycin and 1% penicillin.
All cells were maintained in a CO2 incubator (Thermo
Fisher Scientific, Inc.) with 5% CO2 at 37°C.
Construction of shRNA vector and cell
transfection
A total of 4 shRNA sequences against MT1-MMP were
designed, synthesized and inserted (50 ng) into pLKO.1-puro vector
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) through Age I
(ACCGGT) and Eco RI (GAATTC) restriction enzyme cutting sites. The
sequences of the 4 oligonucleotides are summarized in Table I. A scrambled shRNA negative
control (NC) sequence (shRNA-NC; Sangon Biotech Co., Ltd.,
Shanghai, China) was generated through complementary pairs of
primers: shNC- forward,
5′-CCGGGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTGGTACC-3′
and shNC-reverse,
3′-CAAGAGGCTTGCACAGTGCAGTTCTCTAATGCACTGTGCAAGCCTCTTAAAAAACCATGGTTAA-5′
and used as the negative control. Different shMT1-MMP (3 µg) and
negative control shRNA vectors (3 µg) were transduced into AGS
cells by lentivirus. Briefly, the recombinant plasmids were
transfected into 293T cells by lentiviruses using a Lipofectamine
2000 transfection kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Then, 293T cells were cultured in DMEM (Sigma-Aldrich; Merck KGaA)
with 10% FBS for 24 h. Following replication, the viruses were
harvested for the infection of the AGS cells. Subsequent
experiments were then performed after 48 h transfection.
 | Table I.Sequences of four short hairpin RNAs
(shRNA) |
Table I.
Sequences of four short hairpin RNAs
(shRNA)
| Oligonucleotides | Primer sequences |
|---|
| shRNA-1 |
5′-ACCGGTGGGTCTCAAATGGCAACATAATTCAAGAGATTATGTTGCCATTTGAGACCCTTTTTTGAATTC−3′ |
| shRNA-2 |
5′-ACCGGTGGGAGATGTTTGTCTTCAAGGTTCAAGAGACCTTGAAGACAAACATCTCCCTTTTTTGAATTC−3′ |
| shRNA-3 |
5′-ACCGGTGCGGGTGAGGAATAACCAAGTTTCAAGAGAACTTGGTTATTCCTCACCCGCTTTTTTGAATTC−3′ |
| shRNA-4 |
5′-ACCGGTGGAAACAAGTACTACCGTTTCTTCAAGAGAGAAACGGTAGTACTTGTTTCCTTTTTTGAATTC−3′ |
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was isolated from GES-1 and AGS cells
using the total RNA extraction reagent RNAiso Plus (Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocol. cDNA was generated from RNA using
PrimeScript RT Master Mix (Takara Biotechnology Co., Ltd.). RT-qPCR
was performed with SYBR Premix EX Taq (Takara Biotechnology Co.,
Ltd.) as previously described (13). Briefly, reactions were performed
with the following components: 5 µl 2X SYBR Premix EX Taq, 3.4 µl
cDNA and 10 µM primers, in a final volume of 10 µl. β-actin was
used as the control. The PCR thermocycler conditions were as
follows: 50°C for 3 min and 95°C for 3 min, followed by 30 cycles
of 95°C for 10 sec and 60°C for 30 sec, and finally 72°C for 5 min.
The relative quantities of mRNAs were estimated using the
2−ΔΔCq method (19).
The gene primers used are summarized in Table II.
 | Table II.Sequences of primers used in the
reverse transcription quantitative polymerase chain reaction
assay. |
Table II.
Sequences of primers used in the
reverse transcription quantitative polymerase chain reaction
assay.
| Genes | Primer
sequences |
|---|
| MT1-MMP | F:
5′-GGCTACAGCAATATGGCTACC-3′ |
|
| R:
5′-GATGGCCGCTGAGAGTGAC-3′ |
| Vimentin | F:
5′-GGACCAGCTAACCAACGACA-3′ |
|
| R:
5′-AAGGTCAAGACGTGCCAGAG-3′ |
| E-cadherin | F:
5′-CTTTGACGCCGAGAGCTACA-3′ |
|
| R:
5′-TCGACCGGTGCAATCTTCAA-3′ |
| β-actin | F:
5′-TGACAACTTTGGTATCGTGGAAGG-3′ |
|
| R:
5′-AGGCAGGGATGATGTTCTGGAGAG-3′ |
Western blot analysis
GES-1 and AGS cells were lysed with
radioimmunoprecipitation assay lysis buffer III (Sangon Biotech
Co., Ltd., Shanghai, China), and concentration was quantified using
a bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). A total of 20 µg protein was loaded in each lane
of a 10% SDS-PAGE gel and separated by electrophoresis. Then, the
proteins were transferred onto a polyvinylidene fluoride (PVDF)
membrane followed by blocking with 5% skim milk for 1 h at room
temperature. Following washing with PBS + 0.1% Tween-20 (PBST)
buffer for 5 min, PVDF membranes were incubated with primary
antibodies against MT1-MMP (dilution, 1:200; cat. no. ab51074;
Abcam) and β-actin (dilution, 1:1,000; cat. no. 4967; Cell
Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight.
Then, membranes were incubated for 2 h at room temperature with
horseradish peroxidase-conjugated goat anti-rabbit IgG (H+L) (cat.
no. ab0101; ProteinTech Group, Inc., Chicago, IL, USA; dilution,
1:1,000) secondary antibodies. Subsequent to washing 3 times with
PBST, proteins were detected by chemiluminescence (ECL; EMD
Millipore, Billerica, MA, USA), and the expression was quantified
by Image Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA).
Proliferation analysis
A Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology, Shanghai, China) was used to evaluate the
proliferation ability and viability of AGS cells. The transfected
AGS cells were resuspended, and 100 µl AGS cells were seeded in
96-well plates (2×103 cells/well). AGS cells were
cultivated for 24, 48, 72 and 96 h, then 10 µl CCK-8 solution was
added at each time point and cultivated for 2 h at 37°C. The
optical density values were evaluated at 450 nm by microplate
reader (Epoch; BioTek Instruments, Inc., Winooski, VT, USA). All
experiments were performed in quintuplicate.
Transwell analysis
Matrigel® (Corning Incorporated, Corning,
NY, USA) was diluted 1:6 by serum-free culture media, added to the
upper Transwell chamber and incubated for 1 h at 37°C prior to use.
The transfected AGS cells were cultivated for 12 h and then the
culture media was refreshed. After 48 h incubation, the cells were
resuspended in serum-free media, counted and seeded into the upper
chamber with 100 µl cell suspension for incubation. The bottom
chamber was loaded with 500 µl DMEM with 20% FBS. After 24 h
incubation at 37°C, media for AGS cells in the upper chamber was
removed and washed twice by PBS. The cells were fixed in 4%
formaldehyde at room temperature for 15 min, washed and stained by
0.01% crystal violet at room temperature for 20 min. Cells on the
upper surface were removed by cotton swabs and the invasive cells
were counted under an inverted microscope (IX73; Olympus
Corporation, Tokyo, Japan) at ×200 magnification.
Statistical analysis
SPSS statistical software 19.0 (IBM Corp., Armonk,
NY, USA) was used to analyze the data. Measurement data were
presented as mean ± standard deviation. An unpaired Student's
t-test was used to analyze the statistical significance between two
groups. One- and two-way analysis of variance were used to compare
data between three or more groups, followed by Bonferroni's
post-hoc test. Enumeration data were presented as percentage, and
analyzed by Chi-square test. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
conducted in triplicate.
Results
H&E analysis
The H&E analysis results of tissue sections from
15 patients are summarized in Table
III. In accordance with the clinical diagnosis of gastric
carcinoma, all patients exhibited different levels of tumor
differentiation and invasion. In the present study, the results of
the H&E staining from a representative patient is presented in
Fig. 1.
 | Table III.Hematoxylin and eosin analysis of
tissue sections from 15 patients |
Table III.
Hematoxylin and eosin analysis of
tissue sections from 15 patients
| Clinicopathological
features | Number |
|---|
| Sample type |
|
|
Normal | 15 |
| Gastric
carcinoma | 15 |
| Sex |
|
|
Male | 10 |
|
Female | 5 |
| Age, years |
|
|
Range | 49-81 |
|
Mean | 61.267 |
| Tumor invasion
depth |
|
| Serosal
layer | 6 |
|
Muscular layer | 1 |
|
Superficial muscular
layer | 1 |
| Deep
muscular layer | 2 |
| Tunica
muscularis mucosae | 2 |
| Tela
submucosa | 3 |
| Tumor
differentiation |
|
|
Moderate | 5 |
|
Moderate-poor | 2 |
|
Poor | 8 |
Immunohistochemical analysis of
MT1-MMP expression
Immunohistochemical analysis was performed to detect
MT1-MMP expression. The immunohistochemical analysis results
revealed that MT1-MMP expression was observed in all patients with
gastric carcinoma (Table IV).
Notably, the expression of MT1-MMP in gastric carcinoma tissues was
prominently overexpressed compared with non-cancerous adjacent
tissues (Fig. 2 and Table IV; P<0.01). Concomitantly,
MT1-MMP was predominantly localized in the cytoplasm of the tumor
cells in gastric carcinoma (Fig.
2A).
 | Table IV.Expression of MT1-MMP proteins in
gastric carcinoma tissues and non-cancerous adjacent tissues. |
Table IV.
Expression of MT1-MMP proteins in
gastric carcinoma tissues and non-cancerous adjacent tissues.
|
|
| MT1-MMP expression
(%) |
|---|
|
|
|
|
|---|
| Groups | Total cases | − | + | ++ | +++ | P-value |
|---|
| Gastric
carcinoma | 15 | 0 (0.00) | 3
(20.00) | 8 (53.33) | 4
(26.67) | <0.01 |
| Control | 15 | 0 (0.00) | 10 (66.67) | 5 (33.33) | 0 (0.00) | – |
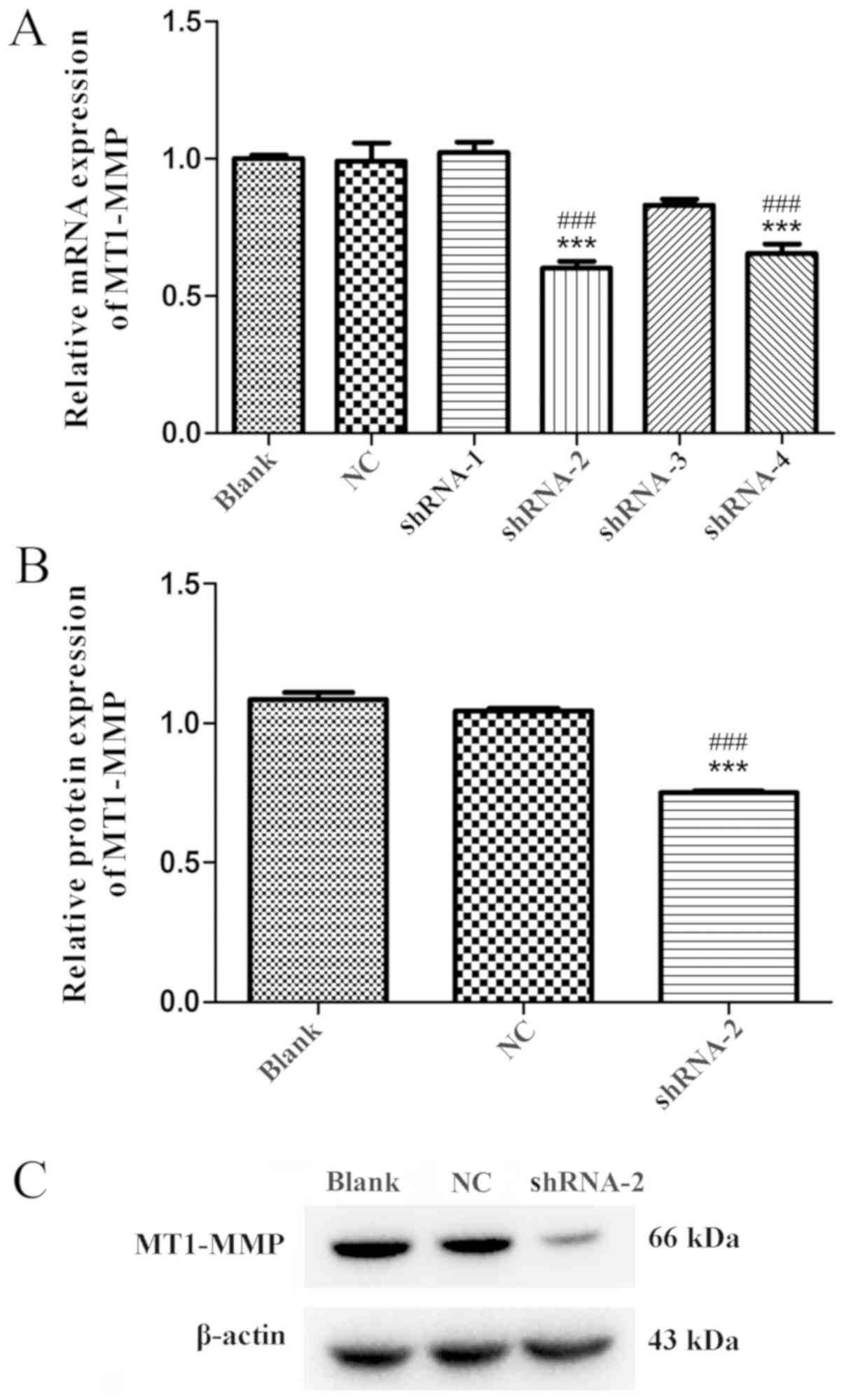
Verification and selection of
shRNA
A total of 4 interfering vectors targeting MT1-MMP
were constructed and transfected into AGS cells. To select the most
effective shRNA sequence, the mRNA expression level of MT1-MMP in
transfected AGS cells was measured by RT-qPCR. ShRNA-2 exhibited
the most significant level of interference (0.601±0.026; Fig. 3A; P<0.001). The western blot
analysis results also indicated that the effect of shRNA-2
(0.750±0.004) on the expression level of MP1-MMP was significant
(Fig. 3B and C; P<0.001).
According to these data, shRNA-2 was used to transfect AGS cells
and to evaluate the effect of MT1-MMP.
Expression of MT1-MMP in GES-1 and AGS
cells
The expression level of MT1-MMP in GES-1 cells and
AGS cells was evaluated. Compared with the GES-1 cells, the gastric
adenocarcinoma-derived AGS cells exhibited significantly increased
expression levels of MT1-MMP at the mRNA (Fig. 4A; P<0.001) and protein levels
(Fig. 4B and C; P<0.001).
Inhibition of AGS cells
proliferation
A CCK-8 assay was performed to explore the role of
MT1-MMP in the proliferation of AGS cells. The results of the CCK-8
assay revealed that inhibiting MT1-MMP by shRNA-2 significantly
decreased the proliferation rate of AGS cells compared to the
blank-transfected cells at 72 h (P<0.001) and 96 h (P<0.01;
Fig. 5). These results suggested
that MT1-MMP may promote the proliferation of gastric carcinoma
cell.
Inhibition of MT1-MMP resulted in
reduced invasion ability
To assess the effect of MT1-MMP on gastric carcinoma
cells, the invasion abilities of AGS cells were assessed using a
Transwell invasion assay following silencing of MT1-MMP. As
indicated in Fig. 6, the invasion
ability of AGS cells was significantly inhibited following
transfection with shRNA-2 (P<0.001). The results suggested that
inhibition of MT1-MMP may suppress the invasion of cancer
cells.
Suppression of genes associated with
invasion
The mRNA expression levels of EMT-associated genes,
including vimentin and epithelial cadherin (E-cadherin), were
examined by RT-qPCR. Following transfection of the AGS cells with
shRNA-2, the mRNA expression level of vimentin (0.396±0.009) was
significantly inhibited compared with the NC group (0.661±0.040;
Fig. 7A; P<0.001). Conversely,
the mRNA expression level of E-cadherin (0.774±0.038) was
significantly increased following the transfection of AGS cells
with shRNA-2 compared with the NC group (0.412±0.012; Fig. 7B) (P<0.001). In addition, it was
observed that the mRNA levels of vimentin and E-cadherin were
significantly decreased in the NC (vimentin and E-cadherin,
P<0.001) and shRNA-2 group (vimentin, P<0.001; E-cadherin,
P<0.01) compared with the blank group, which may due to a
general cell response to transfection reagent toxicity. These
results suggested that the suppression of MT1-MMP expression may
decrease the invasion ability of gastric carcinoma cells.
Discussion
Gastric carcinoma, the second most common cause of
tumor-associated mortality worldwide, contributes greatly to the
global disease burden (20).
Previously, with advances in surgical intervention and
chemotherapy, the overall survival rate has been greatly improved.
However, the 5-year mortality rate for advanced gastric carcinoma
remains as high as 30–50% (21).
Therefore, extensive investigations are required for elucidating
crucial molecules that participate in the pathogenesis of gastric
carcinoma.
MT1-MMP, also known as MMP14, belongs to the MMPs
family, which is correlated with invasion and metastasis of cancer
cell (22). At present, MT1-MMP
has been identified to be overexpressed in a variety of cancer
tissues, including colorectal and breast cancer (23,24).
A previous study demonstrated that MT1-MMP may promote breast tumor
growth and angiogenesis through increasing the expression of
vascular endothelial growth factor (VEGF) (25). Besides, the overexpression of
MT1-MMP resulted in increased migration ability of esophageal
squamous cell carcinomas (22).
Corresponding results were also identified in the present study. In
the present study, the tumor cell proliferation rate and invasion
abilities were decreased by shRNA targeting MT1-MMP.
A previous study revealed that MT1-MMP was
overexpressed in gastric carcinoma compared with that in adjacent
tissues (26). The results from
the present study also indicated that MT1-MMP was overexpressed in
gastric carcinoma tissues through immunohistochemistry analysis.
The overexpression of MT1-MMP was correlated with invasive lesions
(22). Therefore, high expression
levels of MT1-MMP may be associated with the invasion of gastric
carcinoma. Furthermore, compared with the non-cancer-derived GES-1
cells, MT1-MMP was overexpressed in the cancer-derived cell line
AGS cells. Subsequently, the present study screened an effective
shRNA vector (shRNA-2) targeting MT1-MMP. Following transfection of
the AGS cells with shRNA-2, the expression of MT1-MMP was markedly
suppressed at mRNA and protein levels. Additionally, it was
observed that inhibiting the expression of MT1-MMP was able to
significantly decrease the proliferation rate and invasion ability
of AGS cells. MT1-MMP is a critical protease that participates in
the progress of cancer cell proliferation, migration and invasion
(27). Tomari et al
(28) revealed that the growth,
invasion and metastasis of tumors was promoted by increasing
MT1-MMP expression in tumor cells. Concomitantly, Pahwa et
al (29) demonstrated that
MT1-MMP was a crucial player in the growth and progression of
melanoma. Therefore, these results indicated that MT1-MMP may
promote gastric carcinoma cells growth and metastasis during the
development of cancer.
In addition, the expression level of EMT-associated
genes was examined, including vimentin and E-cadherin, to
investigate the underlying mechanism of MT1-MMP in the progression
of gastric carcinoma. Pang et al (22) suggested that MT1-MMP prompted
esophageal squamous cell carcinoma invasion and metastasis by
suppressing E-cadherin and subsequently inducing EMT. At present, a
number of studies have demonstrated that EMT was associated with
different types of tumors, including gastric, esophageal and
hepatocellular carcinoma (22,30,31).
Additionally, Sakamoto and Seiki (27) revealed that MT1-MMP was involved in
the EMT progress of tumor development, by increasing the expression
levels of hypoxia-inducible factors (32) and regulating the expression of
epithelial cell surface markers (22). The results of the present study
suggested that the vimentin mRNA level was markedly decreased and
the E-cadherin mRNA level was markedly increased following
silencing of MT1-MMP. Concomitantly, differences in vimentin and
E-cadherin expression between untreated AGS cells and empty
pLKO.1-puro vector-treated AGS cells were observed in the present
study, which may be due to a general cell response to transfection
reagent toxicity, and require additional investigation. Taken
together, we hypothesized that MT1-MMP was likely to mediate the
invasion process via EMT, reflected by the altered expression of
vimentin and E-cadherin. In summary, these results suggested that
MT1-MMP may contribute to gastric carcinoma cell proliferation and
invasion via regulating vimentin and E-cadherin expression. Future
studies will investigate the molecular mechanisms underlying the
effects of MT1-MMP1 on gastric cancer cell growth and invasion.
In conclusion, the present study confirmed that
MT1-MMP was overexpressed in gastric carcinoma cells compared with
non-cancerous adjacent tissues. Recombinant shRNA vectors targeting
MT1-MMP successfully inhibited MT1-MMP expression in gastric
carcinoma cells. In particular, silencing of MT1-MMP may inhibit
cell proliferation and invasion via regulating the expression of
EMT-associated genes, including vimentin and E-cadherin. In
conclusion, the present study revealed that MT1-MMP may promote the
proliferation and invasion of gastric carcinoma cells by regulating
vimentin and E-cadherin expression.
Acknowledgements
Not applicable.
Funding
The present study was supported by Medicine and
Health Science and Technology Plan Projects in Zhejiang Province
(grant nos. 2014KYA013 and 2014KYA016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ designed the study and performed the experiments.
GL collected the data and performed the experiments. BL contributed
to data analysis, data interpretation and discussion. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of the First Affiliated Hospital of Suzhou
University and was performed according to ethical standards of the
Declaration of Helsinki. All participants provided written consent
for their clinical information to be used for scientific
research.
Patient consent for publication
All participants provided written consent for their
clinical information to be used for scientific research.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ren JS, Masuyer E and Ferlay J:
Global estimates of cancer prevalence for 27 sites in the adult
population in 2008. Int J Cancer. 132:1133–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah MA and Kelsen DP: Gastric cancer: A
primer on the epidemiology and biology of the disease and an
overview of the medical management of advanced disease. J Nati
Compr Canc Netw. 8:437–447. 2010. View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Camargo MC, Kim WH, Chiaravalli AM, Kim
KM, Corvalan AH, Matsuo K, Yu J, Sung JJ, Herrera-Goepfert R,
Meneses-Gonzalez F, et al: Improved survival of gastric cancer with
tumour Epstein-Barr virus positivity: An international pooled
analysis. Gut. 63:236–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ueda Y, Fujishima H, Hirashita T,
Shiroshita H, Etoh T, Inomata M and Shiraishi N: Clinical impact of
small advanced gastric cancer (≤40 mm) in elderly patients: A
retrospective cohort study. Int J Surg. 45:131–137. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Isobe Y, Nashimoto A, Akazawa K, Oda I,
Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y and
Kaminishi M: Gastric cancer treatment in Japan: 2008 annual report
of the JGCA nationwide registry. Gastric Cancer. 14:301–316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng TC, Din ZH, Su JH, Wu YJ and Liu CI:
Sinulariolide suppresses cell migration and invasion by inhibiting
matrix metalloproteinase-2/-9 and urokinase through the
PI3K/AKT/mTOR signaling pathway in human bladder cancer cells.
Marine drugs. 15:E2382017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
D'Costa Z, Jones K, Azad A, van Stiphout
R, Lim SY, Gomes AL, Kinchesh P, Smart SC, Gillies McKenna W, Buffa
FM, et al: Gemcitabine-induced TIMP1 attenuates therapy response
and promotes tumor growth and liver metastasis in pancreatic
cancer. Cancer Res. 77:5952–5962. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Milovanovic J, Todorovic-Rakovic N and Abu
Rabi Z: The role of interleukin 8 and matrix metalloproteinases 2
and 9 in breast cancer treated with tamoxifen. J BUON. 22:628–637.
2017.PubMed/NCBI
|
|
10
|
Vos MC, Hollemans E, Ezendam N, Feijen H,
Boll D, Pijlman B, van der Putten H, Klinkhamer P, van Kuppevelt
TH, van der Wurff AA and Massuger LF: MMP-14 and CD44 in
epithelial-to-mesenchymal transition (EMT) in ovarian cancer. J
Ovarian Res. 9:532016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robichaud N, Rincon SVD, Huor B, Alain T,
Petruccelli LA, Hearnden J, Goncalves C, Grotegut S, Spruck CH,
Furic L, et al: Phosphorylation of eIF4E promotes EMT and
metastasis via translational control of SNAIL and MMP-3. Oncogene.
34:2032–2042. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin G, Luo M, Chen J, Dang Y, Chen G, Li
L, Zeng J, Lu Y and Yang J: Reciprocal activation between MMP-8 and
TGF-β1 stimulates EMT and malignant progression of hepatocellular
carcinoma. Cancer Lett. 374:85–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng L, Li D, Xiang X, Tong L, Qi M, Pu
J, Huang K and Tong Q: Methyl jasmonate abolishes the migration,
invasion and angiogenesis of gastric cancer cells through
down-regulation of matrix metalloproteinase 14. BMC Cancer.
13:742013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He L, Chu D, Li X, Zheng J, Liu S, Li J,
Zhao Q and Ji G: Matrix metalloproteinase-14 is a negative
prognostic marker for patients with gastric cancer. Dig Dis Sci.
58:1264–1270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueda J, Kajita M, Suenaga N, Fujii K and
Seiki M: Sequence-specific silencing of MT1-MMP expression
suppresses tumor cell migration and invasion: Importance of MT1-MMP
as a therapeutic target for invasive tumors. Oncogene.
22:8716–8722. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berlth F, Bollschweiler E, Drebber U,
Hoelscher AH and Moenig S: Pathohistological classification systems
in gastric cancer: Diagnostic relevance and prognostic value. World
J Gastroenterol. 20:5679–5684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pang L, Li Q, Wei C, Zou H, Li S, Cao W,
He J, Zhou Y, Ju X, Lan J, et al: TGF-β1/Smad signaling pathway
regulates epithelial-to-mesenchymal transition in esophageal
squamous cell carcinoma: in vitro and clinical analyses of cell
lines and nomadic Kazakh patients from northwest Xinjiang, China.
PloS One. 9:e1123002014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Martino E, Wild CP, Rotimi O, Darnton
JS, Olliver RJ and Hardie LJ: IGFBP-3 and IGFBP-10 (CYR61)
up-regulation during the development of Barrett's oesophagus and
associated oesophageal adenocarcinoma: potential biomarkers of
disease risk. Biomarkers. 11:547–561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clini. 65:87–108. 2015. View Article : Google Scholar
|
|
21
|
Hamashima C, Shabana M, Okada K, Okamoto M
and Osaki Y: Mortality reduction from gastric cancer by endoscopic
and radiographic screening. Cancer Sci. 106:1744–1749. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pang L, Li Q, Li S, He J, Cao W, Lan J,
Sun B, Zou H, Wang C, Liu R, et al: Membrane type 1-matrix
metalloproteinase induces epithelial-to-mesenchymal transition in
esophageal squamous cell carcinoma: Observations from clinical and
in vitro analyses. Sci Rep. 6:221792016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang WG, Davies G, Martin TA, Parr C,
Watkins G, Mason MD and Mansel RE: Expression of membrane type-1
matrix metalloproteinase, MT1-MMP in human breast cancer and its
impact on invasiveness of breast cancer cells. Int J Mol Med.
17:583–590. 2006.PubMed/NCBI
|
|
24
|
Shields MA, Dangi-Garimella S, Krantz SB,
Bentrem DJ and Munshi HG: Pancreatic cancer cells respond to type I
collagen by inducing snail expression to promote membrane type 1
matrix metalloproteinase-dependent collagen invasion. J Biol Chem.
286:10495–10504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sounni NE, Devy L, Hajitou A, Frankenne F,
Munaut C, Gilles C, Deroanne C, Thompson EW, Foidart JM and Noel A:
MT1-MMP expression promotes tumor growth and angiogenesis through
an up-regulation of vascular endothelial growth factor expression.
FASEB J. 16:555–564. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen B, Zheng MQ, Xu XY, Mo FG, Zhang T
and Feng JF: Expression of MT1-MMP and RECK protein in human
gastric carcinoma. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za
Zhi. 25:364–367. 2011.(In Chinese). PubMed/NCBI
|
|
27
|
Sakamoto T and Seiki M: Integrated
functions of membrane-type 1 matrix metalloproteinase in regulating
cancer malignancy: Beyond a proteinase. Cancer Sci. 108:1095–1100.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomari T, Koshikawa N, Uematsu T, Shinkawa
T, Hoshino D, Egawa N, Isobe T and Seiki M: High throughput
analysis of proteins associating with a proinvasive MT1-MMP in
human malignant melanoma A375 cells. Cancer Sci. 100:1284–1290.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pahwa S, Stawikowski MJ and Fields GB:
Monitoring and inhibiting MT1-MMP during cancer initiation and
progression. Cancers. 6:416–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ouyang S, Zhu G, Ouyang L, Luo Y, Zhou R,
Pan C, Bin J, Liao Y and Liao W: Bapx1 mediates transforming growth
factor-β- induced epithelial-mesenchymal transition and promotes a
malignancy phenotype of gastric cancer cells. Biochem Biophys Res
Commun. 486:285–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji C, Liu H, Yin Q, Li H and Gao H: miR-93
enhances hepatocellular carcinoma invasion and metastasis by EMT
via targeting PDCD4. Biotechnol Lett. 1-9:2017.
|
|
32
|
Sakamoto T and Seiki M: A membrane
protease regulates energy production in macrophages by activating
hypoxia-inducible factor-1 via a non-proteolytic mechanism. J Biol
Chem. 285:29951–29964. 2010. View Article : Google Scholar : PubMed/NCBI
|