Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a rare
but severe disease characterized by hyperactivation of T
lymphocytes and macrophages, and a potentially life-threatening
cytokine storm (1,2). Although there have been improvements
in the diagnosis and treatment of HLH, the mortality rate remains
high, with a 5-year overall survival of 54% reported by the
Histiocyte Society in 2011 (3).
Therefore, more effective tools are required for early HLH
diagnosis so that treatment can be initiated in a timely manner and
patient outcomes improved.
The pathogenesis of HLH is complex (4,5).
Infection is one of the most common triggers, but symptoms caused
by HLH are difficult to differentiate from those associated with
severe infection (6). Serum
expression levels of various cytokines including soluble
interleukin-2 receptor (7) and
plasma ferritin (8) have been used
to distinguish HLH from other syndromes. However, as the expression
levels of these cytokines can increase slowly over the course of
pathogenesis, they are not useful for evaluating the acute phase of
HLH (6). Interferon (IFN)-γ is a
potential biomarker for HLH pathogenesis, as serum concentrations
increase rapidly at disease onset and decrease upon treatment
(9). It has previously been
demonstrated in mouse models that neutralizing anti-IFN-γ antibody
(Ab) may be an effective therapy for HLH (10,11).
However, only a few studies have investigated anti-cytokine
antibodies produced by patients with HLH (12,13).
Biochip technology has the advantages of possessing
high throughput, sensitive and specific, and has broad biological
applications, for example, in genomic library construction or
oligonucleotide and protein detection. Biochip surfaces are
typically pretreated with different chemicals to produce a
self-assembled monolayer with varying sensitivity and capacity for
binding biomolecules (14–16). Polyamidoamine (PAMAM) dendrimer has
been used as a chemical linker that enhances signals in
fluorophore-based (17) or
electrical (18) biosensors. PAMAM
modification of a gold substrate was speculated to enhance the
sensitivity of biochips for protein detection. Although a variety
of assays have been used to detect IFN-γ in serum (19,20),
biochips are highly selective and require only small amounts of
sample, which is useful in clinical situations where blood sample
volume is very limited or where repeated sampling can put patients
at risk.
The present study developed a strategy for
PAMAM-based chemical modification on a gold biochip, and evaluated
the performance of the modified biochip in the detection of IFN-γ
protein and anti-IFN-γ Ab in serum samples from patients with
HLH.
Materials and methods
Study population
Serum samples (3 ml) from patients with HLH (n=77;
42 male patients and 35 female patients; age, 23.2±23.9 years) were
collected from Anhui Medical University (Hefei, China) between
November 2012 and September 2014. HLH diagnosis was based on the
guidelines of the Histiocyte Society (4). Serum samples from four healthy
individuals (two men and two women; age, 28.5±12.1 years) also
collected between November 2012 and September 2014 were used as
controls. Written informed consent was obtained from all study
participants, and the study protocol was approved by the
Institutional Ethics Committee of Anhui Medical University. All
procedures were performed in accordance with the ethical standards
of the institutional and/or national research committees and with
the 1964 Helsinki declaration and its later amendments.
Reagents and equipment
All chemicals were of reagent grade and used as
received unless otherwise indicated. PBS (0.01 M, pH 7.4), 1,
4-phenylene diisothiocyanate (PDITC), PAMAM G4.0, dimethylformamide
(DMF), acetone and bovine serum albumin (BSA) were purchased from
Sigma-Aldrich (Merck KGaA). Absolute ethanol was from Sangon
Biotech Co., Ltd. Rabbit anti-human IFN-γ polyclonal (p)Ab (cat.
no. ab9657), mouse anti-human IFN-γ monoclonal (m)Ab (cat. no.
ab9658) and recombinant human IFN-γ protein (cat. no. ab119140)
were from Abcam. Cy3-conjugated anti-human IgG (cat. no. D110142)
and Cy3-conjugated anti-mouse IgG (cat. no. D110088) were from
Sangon Biotech Co., Ltd.
Biochips were generously supplied by the Interactiva
Division of Thermo-Hybaid. The dimensions of the biochip were
previously described (21). Glass
slides (75×25 mm) were coated with gold film (0.1 µm) and a layer
of Teflon (50 µm) was added that divided the gold surface into an
8×24 matrix spot layout; the diameter of each spot was 1.5 mm. The
chip was scanned using a LuxScan 10K-A microarray scanner
(CapitalBio Technology Inc.). Atomic force microscopy (AFM) was
performed using a Dimension atomic force microscope (Digital
Instruments, Inc.) or an Innova microscope (Veeco Instruments
Inc.). Attenuated total reflectance Fourier transform infrared
spectrometry (ATR-FTIR) was performed using a Nicolet 8700
instrument (Thermo Fisher Scientific, Inc.).
Chemical modification and
characterization of the gold biochip
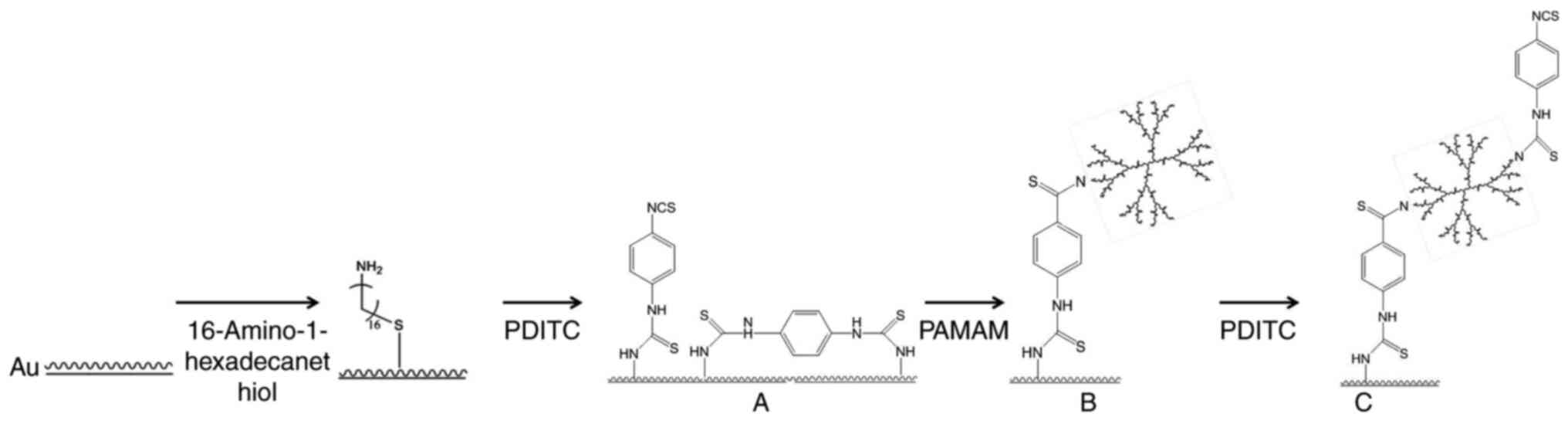
A workflow for chemical modification and assay
evaluation in detection of IFN-γ in serum from patients with HLH
with the biochip is presented in Figs.
1 and 2. The gold slides were
cleaned in a 5:1:1 (v/v/v)
H2O:H2O2:NH3•H2O
solution at 82°C for 5 min, then rinsed with sterile water followed
by anhydrous ethanol and dried under a flow of nitrogen (5 l/min).
PDITC was dissolved in DMF solution to a final concentration of 10
mM. PAMAM G4.0 was dissolved in PBS to a final concentration of
0.01 mM. The cleaned slides were incubated in PDITC solution for 2
h in the dark with gentle shaking; they were then washed 3 times
for 3 min each with DMF, followed by ethanol and dried under a
nitrogen flow as aforementioned. The PDITC-modified slides were
incubated in PAMAM solution (0.01 mM) for 8 h in the dark with
vigorous shaking. The slides were washed 3 times for 3 min each in
ethanol followed by PBS-Tween (PBST; 0.1% v/v Tween-20) and dried
under a nitrogen flow. For PAMAM activation by PDITC, the slides
were incubated in PDITC solution for 2 h in the dark with shaking,
washed with 3 times for 3 min each in DMF solution followed by
absolute ethanol and dried under a nitrogen flow. All the steps
were performed at room temperature (RT; 24°C). The ready-to-use
biochips were stored at 4°C until further use.
Chemical characterization of the PDITC-activated
PAMAM-modified biochip was performed by AFM and ATR-FTIR. Scan
rates ranged from 1–5 Hz, and scan size was set according to a 5-µm
engagement of the cantilever. The instrument was operated in
tapping mode to obtain height images that were processed using
Nanoscope VII software (Version 6.13; Veeco Instruments, Inc.). The
images were flattened to remove scan lines, and the height scale
was set to 20 nm. Feedback control was modulated in real time as
images were being generated. Integral and proportional gains were
set between 2.0 and 0.5. ATR-FTIR spectra were obtained in the
674–3,999 cm−1 spectral range over 128 scans at an
atomic resolution of 8 cm−1.
Comparative analysis of immobilization
efficiency on PDITC, PAMAM and PDITC-activated PAMAM biochips
Serial two-fold dilutions of human IgG were prepared
to test the protein-binding efficiency of the chemically modified
biochips. IgG solutions with concentrations ranging between 0.001
and 10 µg/ml were immobilized on PDITC, PAMAM and PDITC-activated
PAMAM biochips for 2 h at RT, then washed three times with PBST and
dried under a nitrogen flow. The biochips were incubated with 2.5
µg/ml Cy3-conjugated anti-human IgG at RT for 30 min in the dark.
After washing with PBS-0.1% Tween-20 buffer and drying with a
nitrogen flow, the fluorescence intensity of each spot on the
biochips was scanned and measured by the microarray scanner and
semi-quantified using ImageJ software (Version 1.52t; National
Institutes of Health).
Quantification of immobilized
anti-IFN-γ pAb and detected anti-IFN-γ mAb on biochips
To optimize the concentration of anti-human IFN-γ
pAb (capture Ab) on the biochips, serial two-fold dilutions of
rabbit anti-human IFN-γ pAb in PBST-BSA buffer [0.1% Tween-20
(v/v), 0.1% BSA (wt/v), pH 7.4] with various concentrations ranging
between 0.000006 and 25 µg/ml were immobilized onto the biochips at
RT for 2 h. The biochips were washed three times with PBST, dried
under a nitrogen flow, then incubated with 10 µg/ml recombinant
human IFN-γ protein and 10 µg/ml mouse anti-human IFN-γ mAb
(detection Ab) at RT for 1 h, followed by 2.5 µg/ml of
Cy3-conjugated anti-mouse IgG at RT for 30 min in the dark. After
washing and drying as aforementioned, the fluorescence intensity of
each spot on the biochips was semi-quantified using ImageJ
software.
To determine the optimal reaction concentration of
detection Ab, serial two-fold dilutions of mouse anti-human IFN-γ
mAb were prepared in PBST-BSA buffer (pH 7.4) with concentrations
ranging between 0.000006 and 25 µg/ml. The reaction steps were the
same as those used for the aforementioned capture Ab. Briefly, 6.25
µg/ml rabbit anti-human IFN-γ pAb was immobilized on the chemically
modified biochips at RT for 2 h; subsequently, 10 µg/ml recombinant
human IFN-γ protein was applied to each spot at RT for 1 h,
followed by incubation of the biochips with serial five-fold
diluted mouse anti-human IFN-γ mAb solution at RT for 1 h. Finally,
the biochips were incubated with 2.5 µg/ml Cy3-conjugated
anti-mouse IgG Ab at RT for 30 min in the dark. After washing and
drying as aforementioned, the fluorescence intensity of each spot
on the biochip was semi-quantified using ImageJ software.
Determination of the limit of
detection (LOD) for IFN-γ
The LOD of IFN-γ protein by the PDITC-activated
PAMAM-modified biochip was determined. Serial five-fold dilutions
of IFN-γ protein in PBST-BSA buffer (pH 7.4) were prepared with
concentrations ranging between 0.00001 and 100 µg/ml. Rabbit
anti-human IFN-γ pAb (6.25 µg/ml) was immobilized on the biochip at
RT for 2 h. Subsequently, the biochip was incubated with the serial
two-fold diluted IFN-γ protein solution at RT for 1 h, along with
3.12 µg/ml mouse anti-human IFN-γ mAb at RT for 1 h, and 2.5 µg/ml
Cy3-conjugated anti-mouse IgG Ab at RT for 30 min in the dark.
After washing and drying as aforementioned, the fluorescence
intensity of each reaction spot on the biochip was semi-quantified
using ImageJ software.
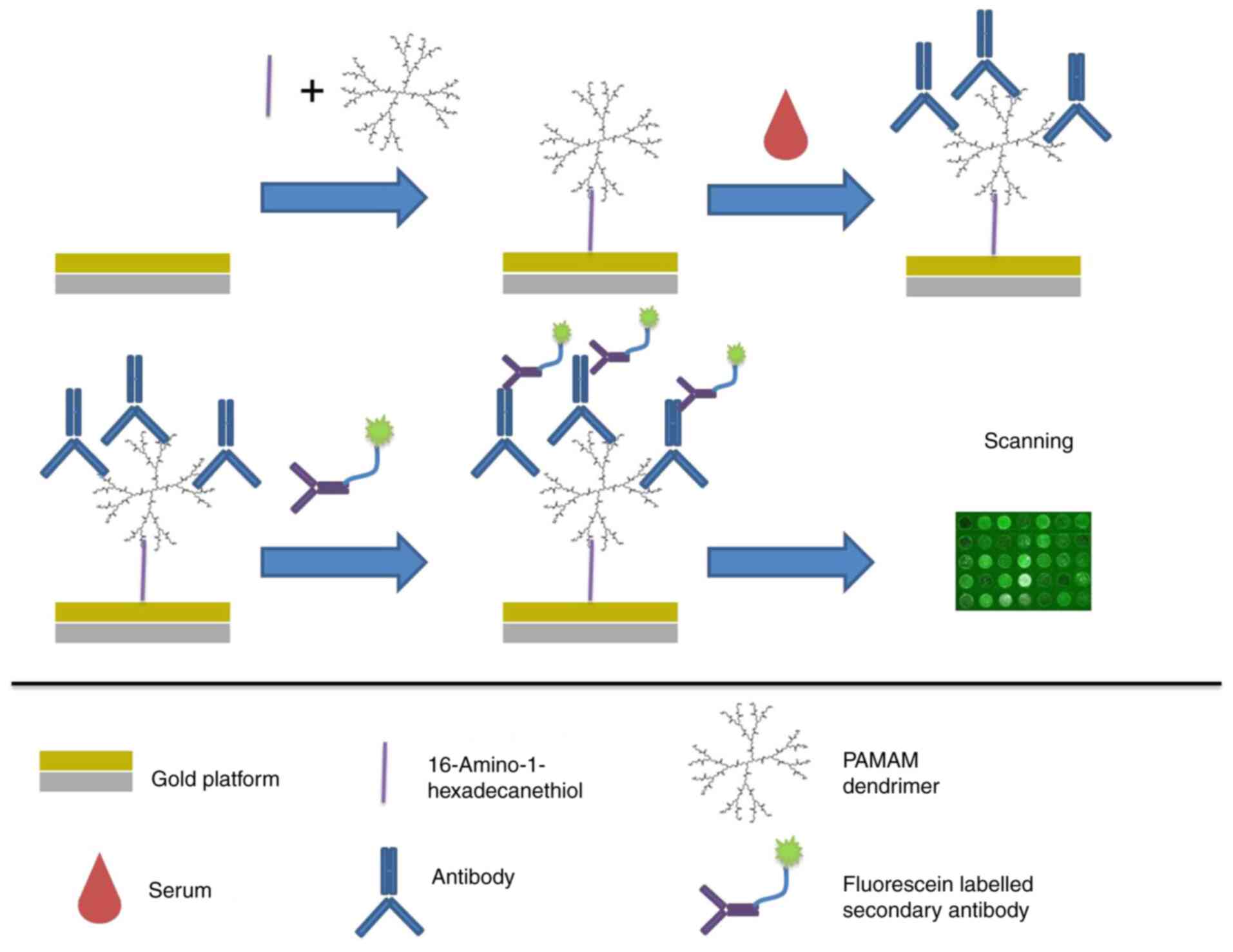
On-chip assay for serum IFN-γ and
anti-human IFN-γ Ab detection in patients with HLH
Ready-to-use probe biochips were produced by
immobilization of 6.25 µg/ml anti-human IFN-γ pAb at RT for 2 h. A
total of 77 serum samples from patients with HLH and four samples
from healthy subjects (negative control) were diluted 1:40 with
0.01 mM PBST-BSA (pH 7.4). The biochip was incubated with the
samples at RT for 1 h, followed by 3.12 µg/ml mouse anti-human
IFN-γ mAb at RT for 1 h and 2.5 µg/ml Cy3-conjugated anti-mouse IgG
at RT for 30 min in the dark. After washing and drying as
aforementioned, the fluorescence intensity of each spot on the
biochip was semi-quantified using ImageJ software. PBST-BSA buffer
was used as the blank control.
Immunological blocking assays of IFN-γ
and anti-human IFN-γ Ab
Before blocking assay of serum samples from patients
with HLH on biochips, four samples from the patients with HLH that
were previously tested to be positive were randomly chosen. These
serum samples were incubated in vials at RT for 1 h with anti-human
IFN-γ mAb and recombinant human IFN-γ protein, respectively, at
ten-fold diluted concentrations ranging between 0.01 and 10 µg/ml
The same samples without pretreatment and four serum samples from
healthy individuals were used as positive and negative controls,
respectively. Anti-human IFN-γ pAb (6.25 µg/ml) was immobilized
onto the biochip at RT for 1 h. Subsequently, the biochip was
incubated with the pretreated or untreated serum samples and 10
µg/ml mouse anti-human IFN-γ mAb at RT for 1 h, followed by 2.5
µg/ml Cy3-conjugated anti-mouse IgG at RT for 30 min in the dark.
The fluorescence intensity of each spot was semi-quantified using
ImageJ software.
Statistical analysis
SPSS software (v19.0; IBM Corp.) was used for
statistical analysis. All numerical data were presented as the mean
± SD. Correlations between variables were calculated using
Pearson's correlation analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characterization of activated
PAMAM-modified biochip
The efficiency for biocompatibility of anti-human
IgG immobilization was visually superior for the PDITC-activated
PAMAM biochip compared with the PDITC and PAMAM biochips (Fig. S1). Activated PAMAM was crosslinked
between biochip substrate and probe proteins. A chemical
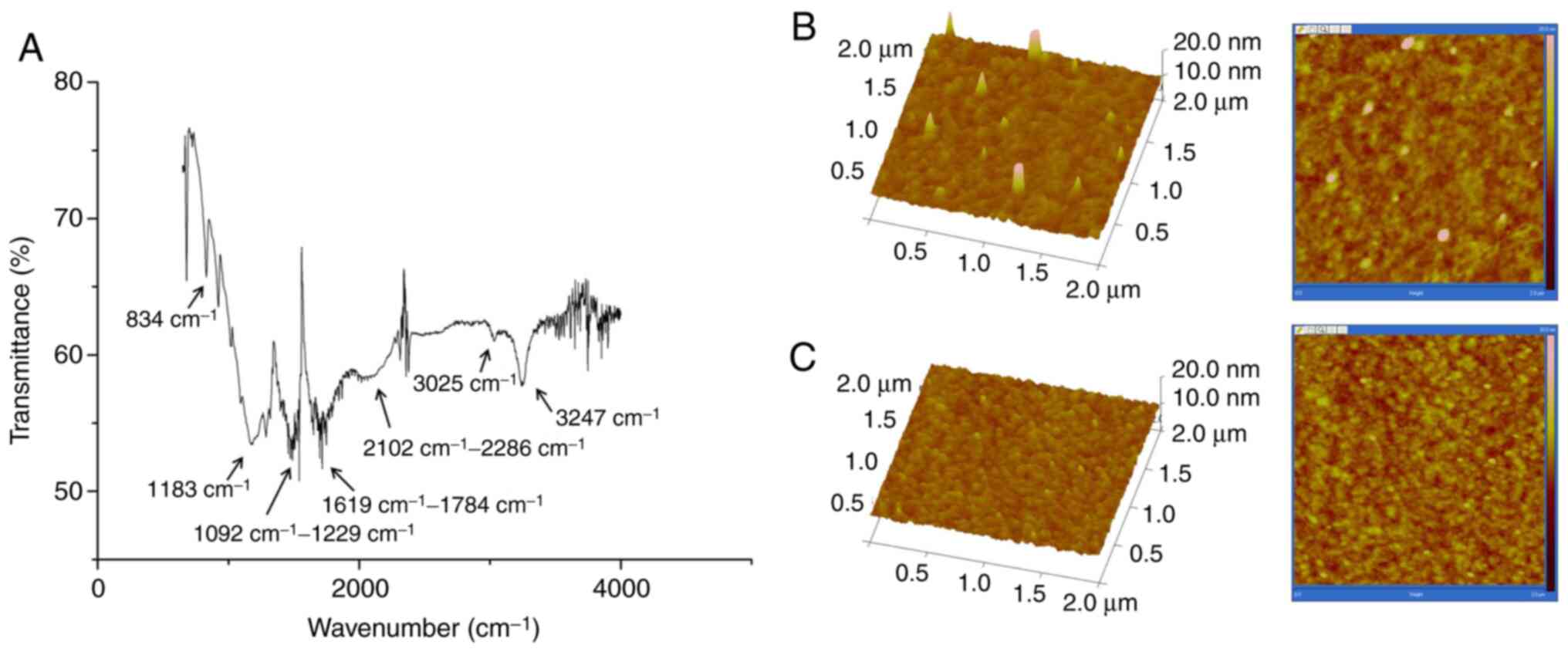
characterization of PAMAM was performed via ATR-FTIR. The major
absorbance of functional groups in PAMAM were 834 cm−1
(C-H out-of-plane bending vibration of the benzene ring), 1,183
cm−1 (C-N stretching vibration), 1,092-1,229
cm−1 (C=S stretching vibration of the thiourea group),
1,619-1,784 cm−1 (C-C framework vibration of the benzene
ring and N-H stretching vibration of the secondary amino group),
2,102-2,286 cm−1 (broad peak corresponding to -N=C=S
moiety vibration), 3,025 cm−1 (C-C stretching vibration
of the hexadecane group and C-H stretching vibration of the benzene
ring) and 3,247 cm−1 (N-H stretching vibration of the
primary amino group) (Fig. 3A).
Images of 3-dimensional AFM of a PDITC-activated PAMAM-modified
biochip and a cleaned biochip are shown in Fig. 3B and C. The peak height was higher
on the PAMAM-modified gold surface than on the non-modified
surface, which indicated a strong anchoring of the protein. Height
measurements determined by AFM reflected the binding of chemical
components as well as their interaction with proteins; the peak
height was >20 nm on the PAMAM-modified surface compared with
0–3 nm on the cleaned surface, which indicated that the functional
groups were covalently bound.
Optimization of capture and detection
Ab concentrations
The concentrations of anti-human IFN-γ pAb and
anti-human IFN-γ mAb immobilized on the PDITC-activated
PAMAM-modified biochip were optimized. Fluorescence signal
intensity increased with anti-human IFN-γ pAb concentration, but
reached a plateau at 6.25 µg/ml, which indicated saturation of the
biochip surface with anti-IFN-γ pAb. Thus, the optimal
concentration for immobilization of captured Ab was determined as
6.25 µg/ml (Fig. S2). Similarly,
the optimal concentration for the detection Ab was determined as
3.12 µg/ml (Fig. S3).
LOD of IFN-γ protein by
PDITC-activated PAMAM-modified biochip
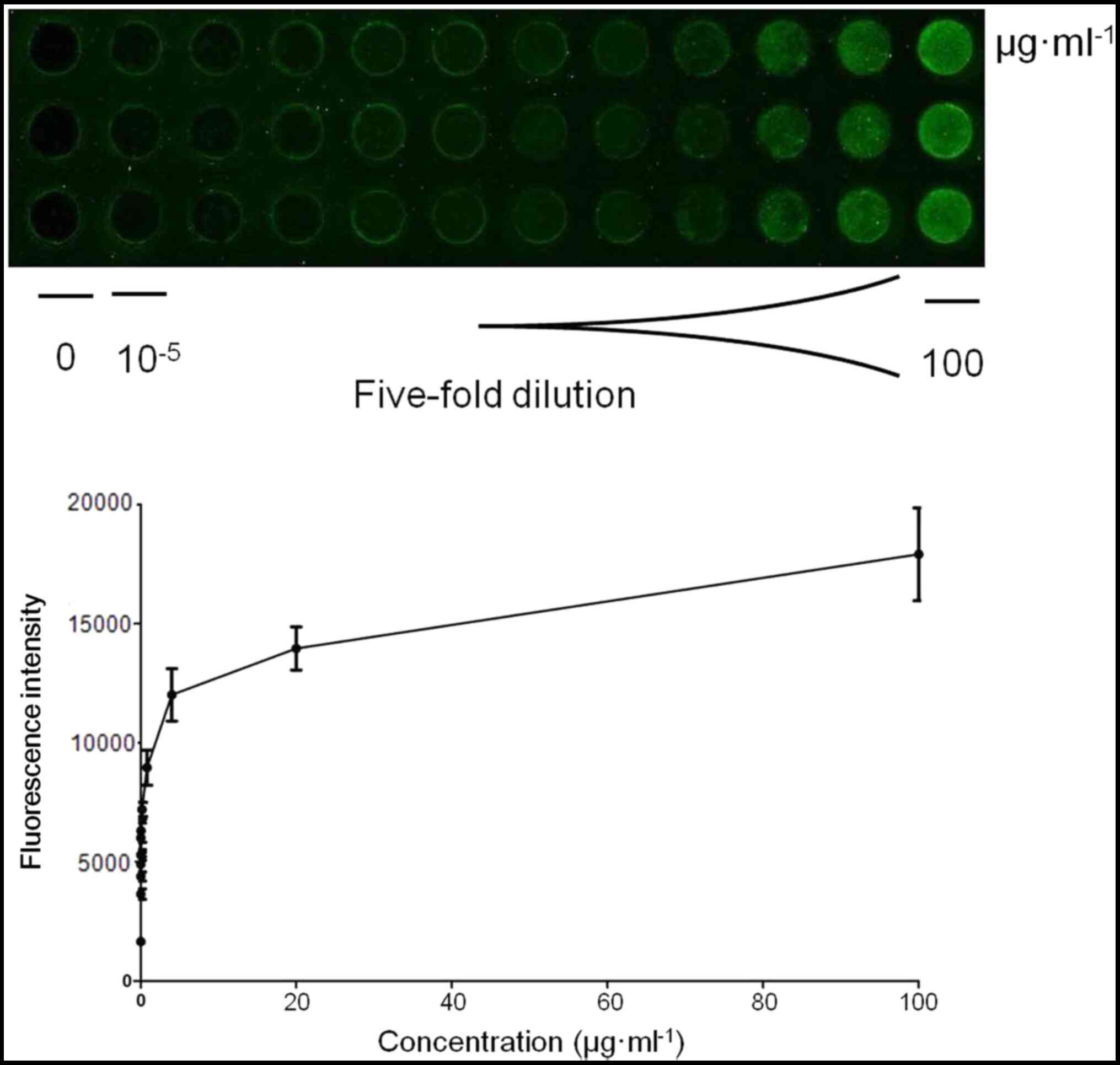
The LOD of IFN-γ protein was subsequently evaluated
via the PDITC-activated PAMAM-modified biochip; the cut-off for a
positive signal was a fluorescence intensity >2 times the mean
value and 2 standard deviations above the background value. The
defining method used to determine the cutoff value was that which
is usually clinically applied. According to these criteria, the LOD
of IFN-γ protein by the biochip was determined as 50 pg/ml
(Fig. 4).
Detection of IFN-γ and anti-human
IFN-γ Ab in HLH serum samples by the PDITC-activated PAMAM-modified
biochip and immunological blocking assays
The clinical applicability of the PDITC-activated
PAMAM-modified biochip was evaluated using serum samples from 77
patients with HLH (Fig. 5). The
positive detection rates for IFN-γ and anti-IFN-γ IgG Ab were 63.6%
(49/77) and 61.0% (47/77), respectively. Simultaneous detection of
IFN-γ and anti-human IFN-γ IgG Ab expression levels in serum
samples using the biochip revealed no correlation
(R2=−0.218; P>0.05, Pearson's analysis; data not
shown).
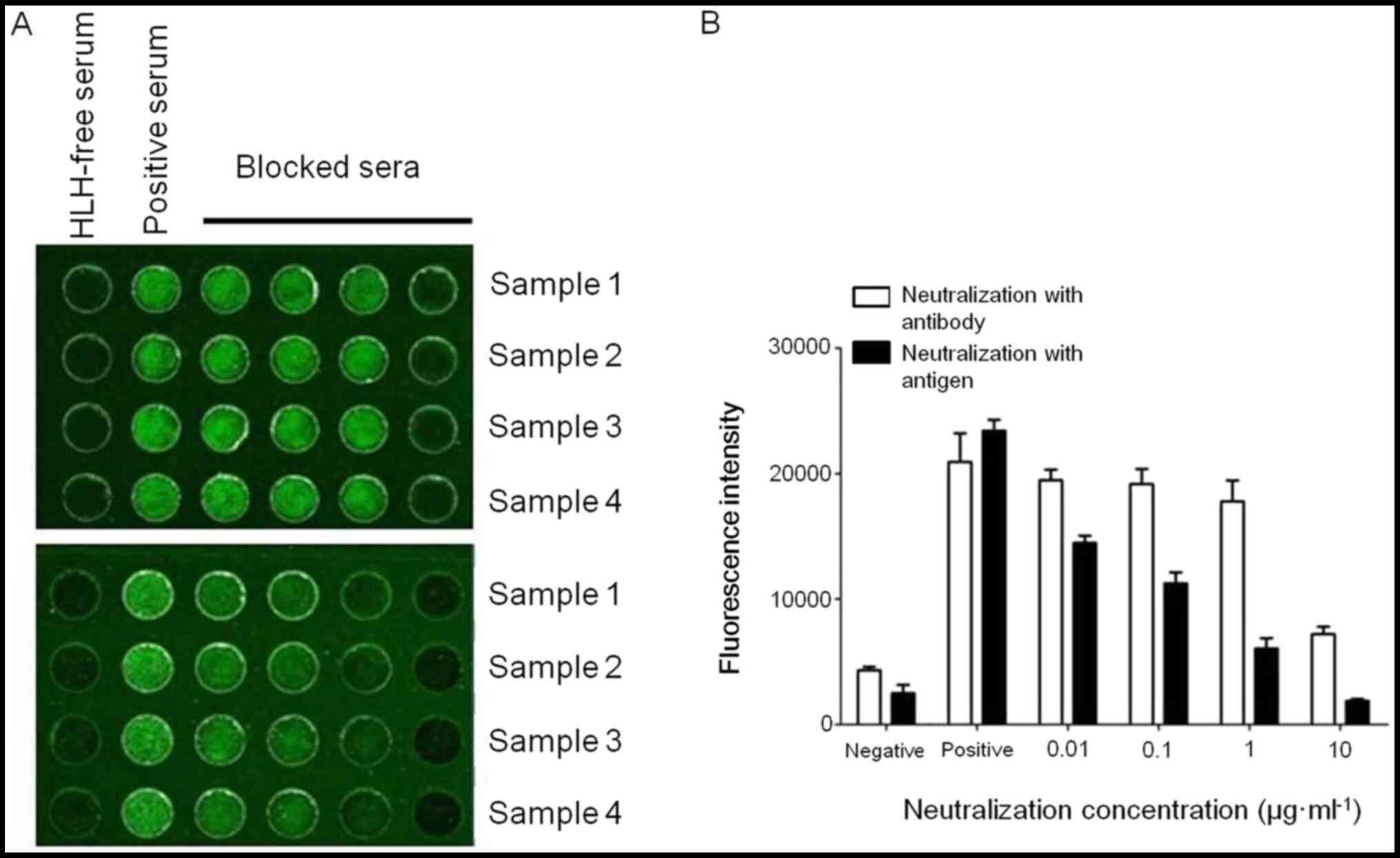
Immunological blocking assays were performed to
measure the specificity of the biochip. IFN-γ and anti-human IFN-γ
IgG Ab in serum samples were completely neutralized by adding
blocking molecules at increasing concentrations ranging from
0.00005 to 0.05 µg/ml, as evidenced by the dose-dependent decrease
in the fluorescent signal (Fig.
6). The present results indicated that the biochip did not
detect any other serum factors besides IFN-γ protein or anti-human
IFN-γ Ab.
Discussion
The specificity and sensitivity of target molecule
detection are critical considerations in biochip fabrication. PAMAM
is a type of cationic and branched dendrimer that has numerous
applications; the abundance of amino groups at the outer ends of
the PAMAM molecule provides multiple potential sites for protein
loading and signal amplification in protein detection (22,23).
The present study used three strategies to
chemically modify the gold surface of a biochip, namely PDITC
modification, PAMAM modification and PDITC-activated PAMAM
modification. Although PAMAM has multiple surface amino moieties,
these cannot be directly conjugated with proteins, and PDITC was
therefore used for their complete activation. The present results
demonstrated that the PDITC-activated PAMAM-modified biochip
revealed stronger protein immobilization than those modified by
either PIDTC or PAMAM alone, confirming that activation of amino
groups enhances protein conjugation capacity on the PDITC-activated
PAMAM-modified biochip (24).
PDITC is a homobifunctional chemical that can engage in
interactions via amino moieties at both of its termini, leaving few
active isothiocyanate terminals on PDITC biochips (25). The present study revealed that
PDITC-activated PAMAM biochips exhibited a signal amplification
effect in interactions with proteins. In the present study, the
total bond length of PDITC ranged from 1,012/10−12 to
1,032/10−12 m [C-N, 148/10−12 m; C=N,
135/10−12 m; C=S, 156/10−12 m;
C6H6 (diameter of benzene ring),
134–154/10−12 m], which are much shorter than the
distance between the amino moieties at its termini, enabling the
PDITC-activated PAMAM biochip to bind proteins more efficiently.
Thus, it was inferred that bioconjugation may occur between the
isothiocyanate terminal of PDITC and the amino terminals of
proteins. In the present study, the LOD of IFN-γ protein by the
biochip was determined as 50 pg/ml. The optimal immobilization
concentration for the capture Ab was extremely low (6.25 µg/ml),
which indicated that this surface may hold a higher sensitivity in
measuring proteins. A previous study reported serum IFN-γ
concentration in the order of ng/ml in patients with HLH (6). In a previous study, the LOD for a
target protein using the biochip with a single-branched chemical
modification was 0.78 µg/ml (26).
Notably, the novel multi-branched PAMAM biochip in the present
study seems to have greater protein-loading and signal
amplification capacities, which increases the sensitivity of
protein detection.
The diagnostic criteria for HLH are mainly based on
clinical signs and laboratory findings. However, due to the
relatively non-specific nature of symptoms and their extensive
overlap with those of other illnesses, HLH is often misdiagnosed
(4). Conventional diagnostic tests
have certain disadvantages, for example, the diagnostic accuracy of
serum ferritin concentration is limited to the range of 500–10,000
µg/l, whereas ferritin expression levels can reach 10,000 µg/l at
the early stage of HLH. Furthermore, serum ferritin expression
levels decrease slowly in response to treatment, undermining its
utility as a biomarker for evaluating therapeutic efficacy
(27). By contrast, the expression
levels of IFN-γ produced by CD8+ T-cells increase soon
after HLH onset and rapidly decrease from >5,000 pg/ml to normal
levels within 48 h after treatment (8). In addition, overproduction of
endogenous IFN-γ may contribute to liver impairment and coagulation
disease (28), making IFN-γ a
potential biomarker for monitoring HLH progression. According to
the manufacturer's information, the detectable concentration limit
of the biochips is comparable with that of traditional ELISA [e.g.
50 pg/ml vs. 15 pg/ml with the Human Interferon-γ ELISA Kit (cat.
no. ab100573; Abcam)]. Binding affinity of IFN-γ protein was
evaluated by the biochip in comparison with conventional assay
techniques (19,20) (Table
I). However, biochips have the advantages of high throughput,
concurrent measurement of multiple serum factors and requirement of
a small volume of biological sample.
 | Table I.Comparison of the biochip and
conventional assay techniques in binding affinity of interferon-γ
protein. |
Table I.
Comparison of the biochip and
conventional assay techniques in binding affinity of interferon-γ
protein.
| First author,
year | Signal
transduction | Substrate | Surface
modification | Limit of detection in
protein determination | Advantage | (Ref.) or
supplier |
|---|
| Du et al,
2020 | Fluorescence | Gold platform |
PDITC/PAMAM/PDITC+PAMAM | 50 pg/ml | High-throughput; less
assay volume requirement; detection of multiplex protein
targets | (Present study) |
| Jin et al,
2019 | Electrochemistry | Gold
nanoparticles |
PAMAM/MoS2/MB/GCE | 2 fg/ml | Extreme
sensitivity | (19) |
| Ma et al,
2018 | Fluorescence | – | TPE-aptamer | 2 pg/ml | Sensitivity | (20) |
|
| ELISA | Polystyrene | – | 15 pg/ml | Easy to operate | Abcam (cat. no.
ab100573) |
Autoantibodies, antigens and inflammatory mediators
can trigger inflammation (29,30).
Although circulating autoantibodies have been detected in patients
with HLH (29,31), to the best of our knowledge, there
have been no reports of endogenous anti-IFN-γ IgG production in the
context of HLH pathogenesis. In preclinical trials, neutralizing
anti-IFN-γ Ab was revealed to be a promising treatment for HLH
(10,32). Therapeutic IFN-γ neutralization in
an animal model of macrophage activation syndrome improved survival
rate and body weight recovery and decreased downstream
proinflammatory cytokine and chemokine levels, which suggested that
this strategy can be used to control the cytokine storm in patients
with HLH (33). In the present
work, an imbalance between IFN-γ and endogenous anti-IFN-γ IgG Ab
may have interfered with cytokine production, which induced an
autoimmune response that contributed to HLH pathogenesis.
There were limitations in the present study.
Firstly, the sample size was relatively small, as HLH is a rare
hematological disease with an extremely low incidence rate; a
larger panel of HLH samples would increase the statistical power of
the analysis. Secondly, only a description of the clinical test
results were provided, without extensive quantification. And
finally, HLH diagnosis based on a single factor (IFN-γ) may not
meet clinical needs. Therefore, future studies should investigate
the feasibility of using the biochip for combined detection of
multiplex HLH biomarkers, such as IL-10, IL1β, IL-6, IL-8, TNFα and
sCD25, in a larger panel of samples.
In conclusion, a PDITC-activated PAMAM modification
strategy was developed to enhance the protein detection sensitivity
and specificity of a gold biochip. The LOD of IFN-γ in serum by the
modified biochip was 50 pg/ml. In addition, the biochip was able to
simultaneously detect IFN-γ and antihuman IFN-γ IgG Ab in clinical
samples from patients with HLH. The present results demonstrated
that the PDITC-activated PAMAM-modified biochip may be a promising
tool for the early and accurate diagnosis of HLH.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was in part supported by the
Translational Medicine Research Program of Anhui Province of China
(grant no. 2017zhyx37) and the National Natural Science Foundation
of China (grant no. 81901238).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WDD, MES, YXD and LY designed the research. ZJS, LY,
HL, SSL and JH performed the experiments and contributed to the
data acquisition. ZJS, QL and SGL interpreted and analyzed the
data. JH, YG, YXD and MES performed the sample collection and
clinical tests. YXD, LY and ZJS drafted the manuscript, and LY, MES
and WDD reviewed the manuscript. SSL and LY revised the manuscript
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethical Committee of Anhui Medical University. All procedures
performed in studies involving human participants were in
accordance with the ethical standards of the institutional and/or
national research committee (include name of committee + reference
number) and with the 1964 Helsinki declaration and its later
amendments or comparable ethical standards. Written informed
consent was provided by all individual participants included in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gupta S and Weitzman S: Primary and
secondary hemophagocytic lymphohistiocytosis: Clinical features,
pathogenesis and therapy. Expert Rev Clin Immunol. 6:137–154.
2010.PubMed/NCBI
|
|
2
|
Tang YM and Xu XJ: Advances in
hemophagocytic lymphohistiocytosis: Pathogenesis, early
diagnosis/differential diagnosis, and treatment.
ScientificWorldJournal. 11:697–708. 2011.PubMed/NCBI
|
|
3
|
Trottestam H, Horne A, Aricò M, Egeler RM,
Filipovich AH, Gadner H, Imashuku S, Ladisch S, Webb D, Janka G, et
al: Chemoimmunotherapy for hemophagocytic lymphohistiocytosis:
Long-term results of the HLH-94 treatment protocol. Blood.
118:4577–4584. 2011.PubMed/NCBI
|
|
4
|
Henter JI, Horne A, Aricó M, Egeler RM,
Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski
J and Janka G: HLH-2004: Diagnostic and therapeutic guidelines for
hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer.
48:124–131. 2007.PubMed/NCBI
|
|
5
|
Chandrakasan S and Filipovich AH:
Hemophagocytic lymphohistiocytosis: Advances in pathophysiology,
diagnosis, and treatment. J Pediatr. 163:1253–1259. 2013.PubMed/NCBI
|
|
6
|
Xu XJ, Tang YM, Song H, Yang SL, Xu WQ,
Zhao N, Shi SW, Shen HP, Mao JQ, Zhang LY and Pan BH: Diagnostic
accuracy of a specific cytokine pattern in hemophagocytic
lymphohistiocytosis in children. J Pediatr. 160:984–990.e1.
2012.PubMed/NCBI
|
|
7
|
Tsuji T, Hirano T, Yamasaki H, Tsuji M and
Tsuda H: A high sIL-2R/ferritin ratio is a useful marker for the
diagnosis of lymphoma-associated hemophagocytic syndrome. Ann
Hematol. 93:821–826. 2014.PubMed/NCBI
|
|
8
|
Allen CE, Yu X, Kozinetz CA and McClain
KL: Highly elevated ferritin levels and the diagnosis of
hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer.
50:1227–1235. 2008.PubMed/NCBI
|
|
9
|
Tang Y, Liao C, Xu X, Song H, Shi S, Yang
S, Zhao F, Xu W, Chen X, Mao J, et al: Evaluation of Th1/Th2
cytokines as a rapid diagnostic tool for severe infection in
paediatric haematology/oncology patients by the use of cytometric
bead array technology. Clin Microbiol Infect. 17:1666–1673.
2011.PubMed/NCBI
|
|
10
|
Jordan MB, Hildeman D, Kappler J and
Marrack P: An animal model of hemophagocytic lymphohistiocytosis
(HLH): CD8+ T cells and interferon gamma are essential for the
disorder. Blood. 104:735–743. 2004.PubMed/NCBI
|
|
11
|
Pachlopnik Schmid J, Ho CH, Chrétien F,
Lefebvre JM, Pivert G, Kosco-Vilbois M, Ferlin W, Geissmann F,
Fischer A and de Saint Basile G: Neutralization of IFNgamma defeats
haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient
mice. EMBO Mol Med. 1:112–124. 2009.PubMed/NCBI
|
|
12
|
Weidong D, Xueling M and Schneider EM: A
direct immunoassay assessment of streptavidin- and
N-hydroxysuccinimide-modified biochips in validation of serological
TNFalpha responses in hemophagocytic lymphohistiocytosis. J Biomol
Screen. 13:515–526. 2008.PubMed/NCBI
|
|
13
|
Liu Q, Liu SS, Li SG, Gao Y, Ye L, Johnson
GOR, Song ZJ and Du WD: Establishment of a protein biochip to
detect serum IgG antibodies against IL-2 and soluble CD25 in
hemophagocytic lymphohistiocytosis. Clin Chim Acta. 487:256–263.
2018.PubMed/NCBI
|
|
14
|
Dupuy AM, Lehmann S and Cristol JP:
Protein biochip systems for the clinical laboratory. Clin Chem Lab
Med. 43:1291–1302. 2005.PubMed/NCBI
|
|
15
|
Moore CD, Ajala OZ and Zhu H: Applications
in high-content functional protein microarrays. Curr Opin Chem
Biol. 30:21–27. 2016.PubMed/NCBI
|
|
16
|
Liu RH, Dill K, Fuji HS and McShea A:
Integrated microfluidic biochips for DNA microarray analysis.
Expert Rev Mol Diagn. 6:253–261. 2006.PubMed/NCBI
|
|
17
|
Campos BB, Oliva MM, Contreras-Cáceres R,
Rodriguez-Castellón E, Jiménez-Jiménez J, da Silva JC and Algarra
M: Carbon dots on based folic acid coated with PAMAM dendrimer as
platform for Pt(IV) detection. J Colloid Interface Sci.
465:165–173. 2016.PubMed/NCBI
|
|
18
|
Müller M, Agarwal N and Kim J: A
cytochrome P450 3A4 biosensor based on generation 4.0 PAMAM
dendrimers for the detection of caffeine. Biosensors. 6:442016.
|
|
19
|
Jin H, Gui R, Gao X and Sun Y: An
amplified label-free electrochemical aptasensor of γ-interferon
based on target-induced DNA strand transform of hairpin-to-linear
conformation enabling simultaneous capture of redox probe and
target. Biosens Bioelectron. 145:1117322019.PubMed/NCBI
|
|
20
|
Ma K, Zhang F, Sayyadi N, Chen W, Anwer
AG, Care A, Xu B, Tian W, Goldys EM and Liu G: ‘Turn-on’
fluorescent aptasensor based on AIEgen labeling for the
localization of IFN-γ in live cells. ACS Sens. 3:320–326.
2018.PubMed/NCBI
|
|
21
|
Pavlickova P, Jensen NM, Paul H,
Schaeferling M, Giammasi C, Kruschina M, Du WD, Theisen M, Ibba M,
Ortigao F and Kambhampati D: Antibody detection in human serum
using a versatile protein chip platform constructed by applying
nanoscale self-assembled architectures on gold. J Proteome Res.
1:227–231. 2002.PubMed/NCBI
|
|
22
|
Chanphai P, Froehlich E, Mandeville JS and
Tajmir-Riahi HA: Protein conjugation with PAMAM nanoparticles:
Microscopic and thermodynamic analysis. Colloids Surf B
Biointerfaces. 150:168–174. 2017.PubMed/NCBI
|
|
23
|
Zhang Y, Wang F, Zhang H, Wang H and Liu
Y: Multivalency interface and g-C3N4 coated
liquid metal nanoprobe signal amplification for sensitive
electrogenerated chemiluminescence detection of exosomes and their
surface proteins. Anal Chem. 91:12100–12107. 2019.PubMed/NCBI
|
|
24
|
Zhang M, Ren Y, Wang Y, Wang R, Zhou Q,
Peng Y, Li Q, Yu M and Jiang Y: Regulation of smooth muscle
contractility by competing endogenous mRNAs in intracranial
aneurysms. J Neuropathol Exp Neurol. 74:411–424. 2015.PubMed/NCBI
|
|
25
|
Gandhiraman RP, Gubala V, Le NC, Volcke C,
Doyle C, James B, Daniels S and Williams DE: Deposition of
chemically reactive and repellent sites on biosensor chips for
reduced non-specific binding. Colloids Surf B Biointerfaces.
79:270–275. 2010.PubMed/NCBI
|
|
26
|
Ye L, Huang NL, Ma XL, Schneider M, Huang
XJ and Du WD: Establishment of N-succinimidyl 4-(maleimidomethyl)
cyclohexanecarboxylate (SMCC) modified biochip enabling concurrent
detection of serum infectious antibodies in neuroborreliosis.
Biosens Bioelectron. 78:404–410. 2016.PubMed/NCBI
|
|
27
|
Otrock ZK, Hock KG, Riley SB, de Witte T,
Eby CS and Scott MG: Elevated serum ferritin is not specific for
hemophagocytic lymphohistiocytosis. Ann Hematol. 96:1667–1672.
2017.PubMed/NCBI
|
|
28
|
Kurzrock R, Rohde MF, Quesada JR,
Gianturco SH, Bradley WA, Sherwin SA and Gutterman JU: Recombinant
gamma interferon induces hypertriglyceridemia and inhibits
post-heparin lipase activity in cancer patients. J Exp Med.
164:1093–1101. 1986.PubMed/NCBI
|
|
29
|
Lin A, Ma TP, Cheng FW and Ng PC: Neonatal
haemophagocytic lymphohistiocytosis associated with maternal
adult-onset still's disease. Neonatology. 110:267–269.
2016.PubMed/NCBI
|
|
30
|
Oaks M, Taylor S and Shaffer J:
Autoantibodies targeting tumor-associated antigens in metastatic
cancer: Sialylated IgGs as candidate anti-inflammatory antibodies.
Oncoimmunology. 2:e248412013.PubMed/NCBI
|
|
31
|
Lovisari F, Terzi V, Lippi MG, Brioschi PR
and Fumagalli R: Hemophagocytic lymphohistiocytosis complicated by
multiorgan failure: A case report. Medicine (Baltimore).
96:e91982017.PubMed/NCBI
|
|
32
|
Avau A and Matthys P: Therapeutic
potential of interferon-γ and its antagonists in autoinflammation:
Lessons from murine models of systemic juvenile idiopathic
arthritis and macrophage activation syndrome. Pharmaceuticals
(Basel). 8:793–815. 2015.PubMed/NCBI
|
|
33
|
Prencipe G, Caiello I, Pascarella A, Grom
AA, Bracaglia C, Chatel L, Ferlin WG, Marasco E, Strippoli R, de
Min C and De Benedetti F: Neutralization of IFN-γ reverts clinical
and laboratory features in a mouse model of macrophage activation
syndrome. J Allergy Clin Immunol. 141:1439–1449. 2018.PubMed/NCBI
|