Introduction
Cardiovascular disease is a challenging disease with
high morbidity and mortality worldwide (1). Myocardial ischemia/reperfusion (MI/R)
injury is the primary risk factor in the pathological process of
heart disease. Although reperfusion is the most efficient way to
mimic tissue damage, the re-establishment of the blood supply is
known to induce various adverse events (2). In addition, MI/R also induces
endoplasmic reticulum (ER) stress and activates apoptosis-related
signaling pathways (3).
The ER serves a number of roles in protein
synthesis, lipid biosynthesis and detoxification. When normal ER
function is disrupted by various cellular disturbances, unfolded
and misfolded proteins accumulate, resulting in a condition known
as ER stress (4). The alteration of
redox homeostasis results in excessive generation of reactive
oxygen species (ROS), which subsequently causes ER stress (5). ER stress promotes the dissociation of
the ER chaperone glucose-regulated protein 78 (GRP78) from
transmembrane proteins and triggers their activation (6). Protein kinase R-like endoplasmic
reticulum kinase (PERK) is one of transmembrane proteins and
dimerizes under ER stress, which phosphorylates eukaryotic
translation initiation factor 2 subunit α (eIF2α) (7). eIF2α mediates the transcription of
activating transcription factor-4 (ATF4), which interacts with the
pro-apoptotic factor C/EBP homologous protein (CHOP) (8). Additionally, ER stress induces cell
apoptosis by activating the caspase family cascade (9).
NAD-dependent protein deacetylase sirtuin-1 (SIRT1),
known as one of the mammalian homologues of yeast Sir2, is a
histone deacetylase and regulates various biological processes,
such as the inflammatory reaction, oxidative stress and apoptosis
(10,11). A previous study demonstrated that
SIRT1 overexpression protects cardiomyocytes against MI/R injury
from ER stress (12). The
protective effect is due to the activation of the PERK/eIF2α
pathway, which is associated with cardiomyocyte apoptosis. Hence,
SIRT1 may be a potential target to treat cardiac pathologies by
attenuating ER stress.
Inonotus obliquus (IO) is a fungus belonging
to the Hymenochaetaceae family of Basidiomycetes, which grows on
birch trees (13). The main
bioactive compounds of IO are polysaccharides, polyphenols, melanin
and triterpenes (14). IO exerts
various biological functions, including anti-inflammatory,
antitumor and immunomodulatory effects (15,16).
However, its effects on cardiovascular diseases has been rarely
studied. The current study was designed to investigate the role of
IO extract (IOE) in MI/R injury and determine the exact molecular
mechanisms.
Materials and methods
Ethanol extraction of IO
IO was collected from Jilin Province, Northeastern
China and identified by Professor GuangShu Wang (Jilin University).
A total of 2,500 g IO powder was weighed, soaked in 12.5 l
distilled water overnight at room temperature and extracted at 80°C
for 2.5 h. The supernatant was collected after filtration using
three layers of gauze, and the extraction was repeated twice in the
same manner. The filtrate was concentrated by rotary evaporation,
dried at 60°C, crushed and passed through an 80-mesh sieve to
obtain the preliminary extract. Next, 12.5 l 70% ethanol was added
to the preliminary extract obtained from water extraction
(extracted at 75°C for 2.5 h). The supernatant was gathered by
filtering through three layers of gauze, and the extraction was
repeated twice in the same manner. The filtrates were collected and
concentrated by rotary evaporation, then dried at 60°C, crushed and
passed through an 80-mesh sieve, and 127.9 g IOE was obtained. The
extraction rate of IOE was 5.12% according to the following
formula: Extraction rate = weight of IOE/weight of IO powder.
Meanwhile, the main composition of IOE was assessed using a T-6
Series UV–Vis spectrophotometer (Beijing Purkinje General
Instrument Co., Ltd.). The contents of total flavonoid, total
saponin, polysaccharide and polyphenol were calculated using
respective standard curves (y=0.0117×+0.0554, R2=0.9998;
y=0.0301×+0.0871, R2=0.9992; y=16.253×+0.1019,
R2=0.9999 and y=97.266×+0.1984, R2=0.9996)
with the percentage of 1.76, 1.06, 0.57 and 1.07%.
Animals
Wistar rats (female, 250–280 g) were purchased from
Changchun Yisi Experimental Animal Co., Ltd., and raised in a
controlled environment (22–24°C; 55–60% humidity and 12/12-h
light/dark cycle) with free access to food and water. Animals were
treated according to the Guide for the Care and Use of Laboratory
Animals (US National Institutes of Health) (17) and the Committee for the Care and Use
of Laboratory Animals of Jilin University (Changchun, China). The
animal experiments of the present study were approved by the
Ethical Committee for Experimental Animals, School of
Pharmaceutical Sciences, Jilin University (approval no.
20190049).
Experimental design
A total of 150 female rats were randomly assigned
into five groups (n=30): i) Non-MI/R sham group (Sham); ii) MI/R
group (MI/R); iii) MI/R+IOE 150 mg/kg group (MI/R-IOE150); iv)
MI/R+IOE 300 mg/kg group (MI/R-IOE300) and v) MI/R+IOE 600 mg/kg
group (MI/R-IOE600). The animals in the treatment groups were
intragastrically administered with IOE (150, 300 or 600 mg/kg) for
7 consecutive days. The rats of the Sham and MI/R groups were
intragastrically administered with 0.5% carboxymethylcellulose
sodium for 7 consecutive days. Surgical procedures were performed
on rats in the Sham group, but without left anterior descending
(LAD) coronary artery ligation. In other groups, LAD ligation was
performed on the hearts for 2 h and then reperfused for 2 h.
Experimental model establishment
At day 7, 3% sodium pentobarbital (30 mg/kg body
weight, intraperitoneal injection) was used to anesthetize the rats
after 1 h of administration. Rat hearts were exteriorized via
open-chest surgery. A 6/0 nylon suture was put to make a slip knot
around the LAD coronary artery and induce myocardial ischemia.
After 2 h of ligation, the ligature was loosened to restore
myocardial blood supply for myocardial reperfusion. Rats in the
Sham group were subjected to the same procedure, but without
ligation. Reperfusion was performed for 2 h. The rats were
anesthetized by intraperitoneal injection with 3% sodium
pentobarbital (30 mg/kg body weight). Then, ~8 ml volume of blood
was taken from the abdominal aorta of the unconscious rats for
painless euthanasia. Death was confirmed when respiratory and
cardiac arrest was observed, following which, cardiac tissues were
harvested for further experiments.
Cardiac function assessment
After 2 h of reperfusion, 3% sodium pentobarbital
(30 mg/kg body weight, intraperitoneal injection) was used to
anesthetize the rats. Once anesthetized, the thoracic area was
shaved and covered with ultrasonic transmission gel. A portable
echocardiography machine (GE Vivid I; GE Healthcare) was used to
evaluate the cardiac function of rats. The left ventricular (LV)
ejection fraction (EF), LV fractional shortening (FS), LV internal
dimensions at diastole (LVIDd) and LV internal diameter systole
(LVIDs) were evaluated. An observer who was blinded to the
experimental design measured these parameters in three consecutive
cardiac cycles.
Determination of myocardial infarction
size (MIS)
After 2 h of reperfusion, the heart tissues were
excised to perform MIS measurements. Five pieces of the left
ventricle parallel to the atrioventricular groove were transected,
immersed in 0.5% nitro blue tetrazolium chloride (NBT) phosphate
buffer and stained for 1 min in a 37°C water bath. Normal
myocardial tissues were stained dark red, while ischemic myocardial
tissues were pale. The ischemic myocardial tissue was excised and
weighed. The MIS was calculated as follows: Ischemic myocardial
weight/wet weight of the left ventricle ×100%.
Determination of cardiac enzymes
A total of 2 ml blood was collected after 2 h of
reperfusion. Serum was separated from the blood samples by
centrifugation (2,000 × g, 10 min, 4°C) and used for the evaluation
of creatine kinase-MB (CK-MB; cat. no. H197; Nanjing Jiancheng
Bioengineering Institute), aminotransferase (AST; cat. no.
C010-1-1; Nanjing Jiancheng Bioengineering Institute) and lactate
dehydrogenase (LDH; cat. no. A020-2-2; Nanjing Jiancheng
Bioengineering Institute) activities using respective diagnostic
kits in accordance with the manufacturer's instructions.
Determination of oxidative stress
levels in myocardial tissue
After 2 h of reperfusion, rat hearts were excised to
prepare heart homogenates. Prepared myocardial tissues were excised
and homogenized (10%) in ice-cold physiological saline solution
using a Tissue-Tearor (Bio Spec Products, Inc.). The supernatant
was separated from heart homogenates by centrifugation (3,500 × g,
15 min, 4°C) and used for the evaluation of superoxide dismutase
(SOD; cat. no. A001-1-2; Nanjing Jiancheng Bioengineering
Institute), malondialdehyde (MDA; cat. no. A003-1-1; Nanjing
Jiancheng Bioengineering Institute), glutathione peroxidase
(GSH-px; cat. no. A005-1-2; Nanjing Jiancheng Bioengineering
Institute) and catalase (CAT; cat. no. A007-2-1; Nanjing Jiancheng
Bioengineering Institute) levels using respective diagnostic kits
in accordance with the manufacturer's instructions.
Histological examination
After 2 h of reperfusion, rat hearts were quickly
harvested. and the blood was expelled. Hearts were fixed in 4%
paraformaldehyde solution for 24 h at room temperature. After
rinsing with water, heart tissues were dehydrated with gradient
ethanol solution and embedded in paraffin. Subsequently, heart
tissues were cut into 3–5 µm-thick slices and stained using a
hematoxylin and eosin (H&E) kit for 3 min at room temperature.
An optical microscope was used to observe morphological changes.
The degree of cardiac damage was assessed by an independent
researcher blind to the experimental design.
TUNEL assay
Myocardial apoptosis was evaluated using a TUNEL
assay kit (cat. no. 11684817910; Sigma-Aldrich; Merck KGaA). Heart
tissue was fixed in 4% paraformaldehyde solution for 24 h at room
temperature, embedded in paraffin, then sectioned on slides at 3–5
µm-thickness. TUNEL (1:9 mixed in equilibration buffer) staining of
apoptotic cells (green fluorescence). DAPI (5 µg/ml) was used to
dye the nuclei for 5 min (blue fluorescence). The slides were then
sealed with 50% glycerin. Five randomly selected fields of view
were observed and apoptotic cells of each section were counted
under an ECLIPSE 80i fluorescence microscope (Nikon Corporation).
The cardiomyocyte apoptotic index (AI) was counted as follows:
Number of TUNEL-positive nuclei/the number of total nuclei
×100%.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from cardiac tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), quantified with the T-6 Series UV–VIS spectrophotometer
(Beijing Purkinje General Instrument Co., Ltd.) and reverse
transcribed into cDNA using a Hifair® II First Strand
cDNA Synthesis Kit (cat. no. 11120ES60; Shanghai Yeasen
Biotechnology Co., Ltd.). RT was carried out at 42°C for 15 min,
then 85°C for 15 sec. Subsequently, qPCR was performed with
sequence-specific primers using the SYBR Green Master Mix kit (cat.
no. 11202ES03; Shanghai Yeasen Biotechnology Co., Ltd.). The
thermocycling conditions consisted of 40–50 cycles at 95°C for 10
sec, 55°C for 20 sec and 72°C, 20 sec. The following primer pairs
were used for qPCR: Caspase-12 forward,
5′-CAGCACATTCCTGGTGTTTAT-3′ and reverse,
5′-GACTCTGGCAGTTACGGTTGTT-3′ and β-actin forward,
5′-GCACCGCAAATGCTTCTAGG-3′ and reverse,
5′-AAAGGGTGTAAAACGCAGCTC-3′. Data were calculated using the
2−∆∆Cq method (18) with
the mRNA levels of β-actin as an internal control.
Western blot assay
Myocardial tissues were homogenized in lysis buffer
(cat. no. P0013; Beyotime Institute of Biotechnology) and protease
inhibitor cocktail on ice and purified by centrifugation at 14,000
× g for 15 min at 4°C. The protein concentration was determined
using the BCA method. A total of 40 µg protein was loaded onto
10–12% gels and resolved via SDS-PAGE. Separated proteins were then
transferred to a PVDF membrane for 2 h. PVDF membranes were blocked
for 1.5 h in 5% bovine serum albumin (cat. no. P0252; Beyotime
Institute of Biotechnology) at room temperature and washed three
times with TBS+0.2% Tween-20. Membranes were incubated overnight at
4°C with the following primary antibodies (all from Affinity
Biosciences): SIRT1 (1:2,000; cat. no. DF6033), GRP78 (1:800; cat.
no. AF5366), eIF2α (1:1,000; cat. no. AF6087; Affinity
Biosciences), phosphorylated (p)-eIF2α (Ser52) (1:1,500; cat. no.
AF3087), PERK (1:1,000; cat. no. AF5304), p-PERK (Thr982) (1:1,500;
cat. no. DF7576), CHOP (1:1,500; cat. no. DF6025), caspase-12
(1:1,500; cat. no. AF5199), pro-caspase-3 (1:1,500; cat. no.
AF6311), pro-caspase-9 (1:1,500; cat. no. AF6348) or anti-β-actin
(1:5,000; cat. no. AF7018). A goat anti-rabbit secondary antibody
(1:5,000; cat. no. IH-0011; Beijing Dingguo Changsheng
Biotechnology Co., Ltd.) was used to observe primary antibody
binding, and then bands were visualized using an ECL western
blotting substrate (cat. no. KF001; Affinity Biosciences).
Densitometric analysis of the bands was conducted using ImageJ
software (version 1.5.1k; National Institutes of Health).
Statistical analysis
All data are presented as the mean ± SD and were
analyzed using SPSS 22.0 (IBM Corp.). Data were analyzed using
one-way ANOVA followed by Tukey's Honest Significant Difference
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
IOE improves cardiac function
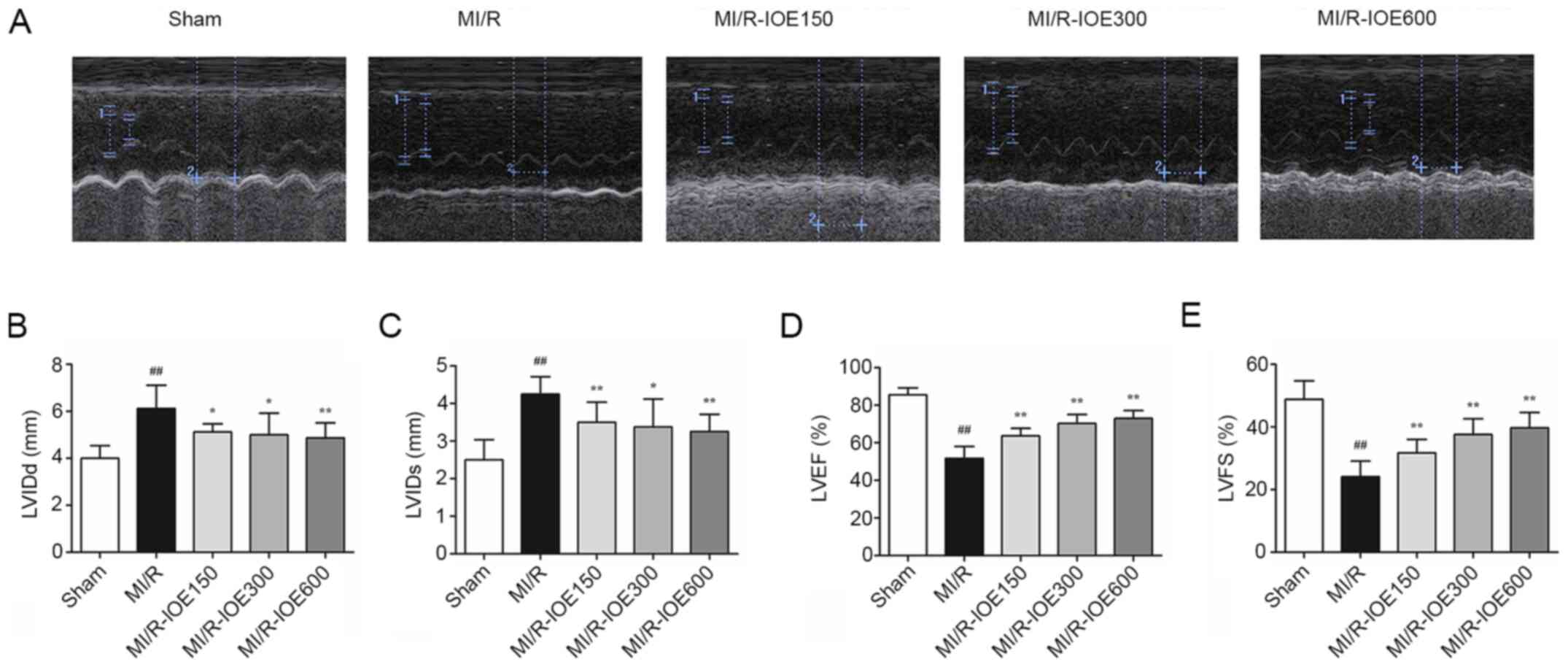
The cardiac function of post-surgical hearts was
evaluated by echocardiography. As shown in Fig. 1A-E, deterioration of cardiac
function was induced in the MI/R group, LVIDd and LVIDs increased
to 6.13±0.99 and 4.25±0.46 mm, respectively, whereas LVEF and LVFS
decreased to 51.75±6.39 and 24.13±5.00%, respectively, compared
with the Sham group (P<0.01). IOE pretreatment increased the
recovery of cardiac function in the impaired hearts, which was
demonstrated by increased LVEF and LVFS, as well as decreased LVIDd
and LVIDs, compared with the M/R group (P<0.05 or
P<0.01).
IOE decreases infarct size and
attenuates MI/R Injury
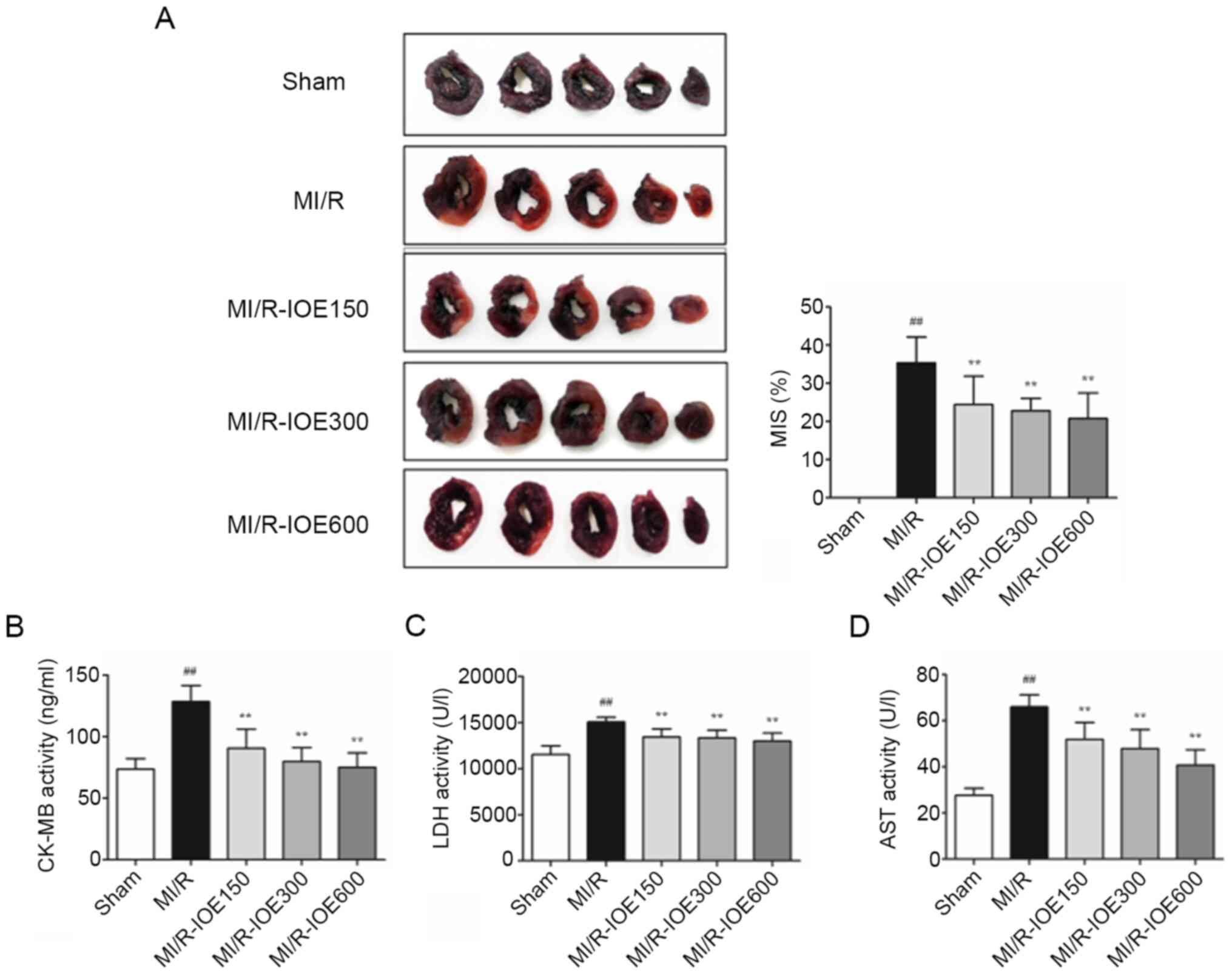
NBT staining was used to observe the effects of IOE
on the infarction scale. As shown in Fig. 2A, the infarct size reached up to
36.42±6.67% in the MI/R group. IOE (150, 300 and 600 mg/kg)
significantly decreased the infarct size compared with the MI/R
group (P<0.01). Furthermore, the activities of myocardial
enzymes (LDH, CK-MB and AST) were significantly increased following
MI/R injury compared with the Sham group, which further caused
myocardial injury (P<0.01; Fig.
2B-D). IOE attenuated MI/R injury through inhibiting the
activities of myocardial enzymes in MI/R-IOE groups
(P<0.01).
IOE relieves myocardial tissue
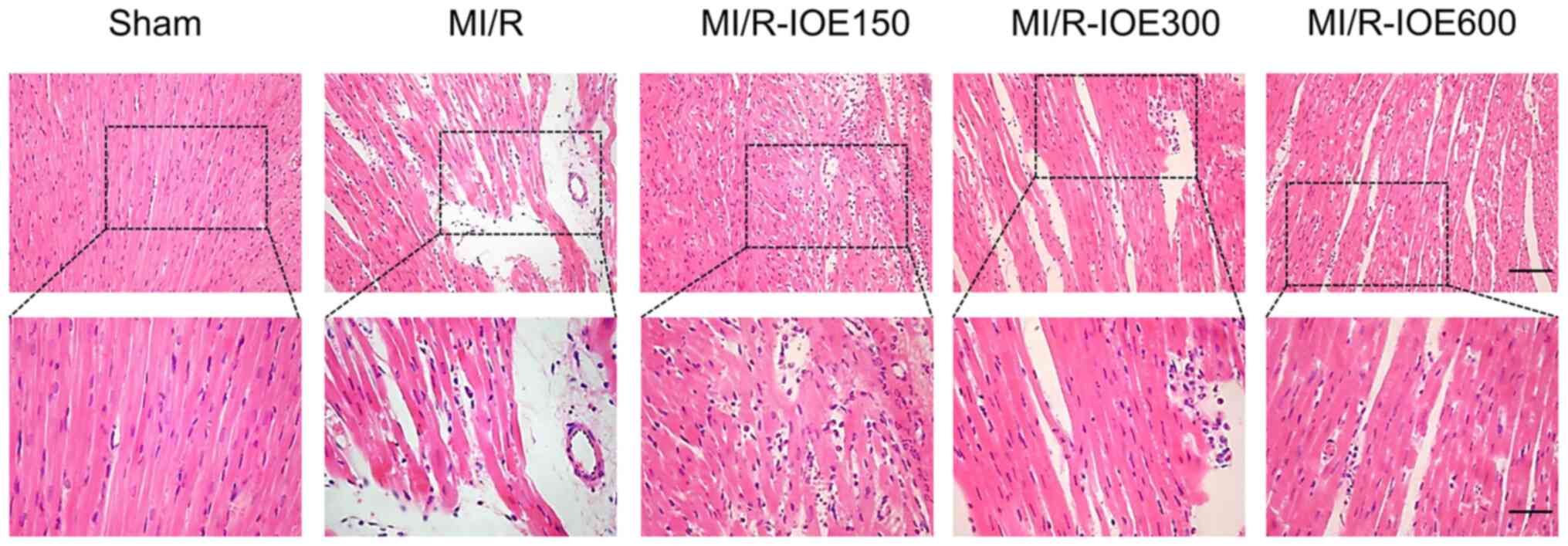
injury
Myocardial tissues showed partially disordered and
disorderly arrangement of myocardial fiber stripes, and partial
lysis of cardiomyocytes accompanied by interstitial edema in the
MI/R group (Fig. 3). Following
treatment with IOE, myocardial fibers and cell morphology notably
improved to varying degrees.
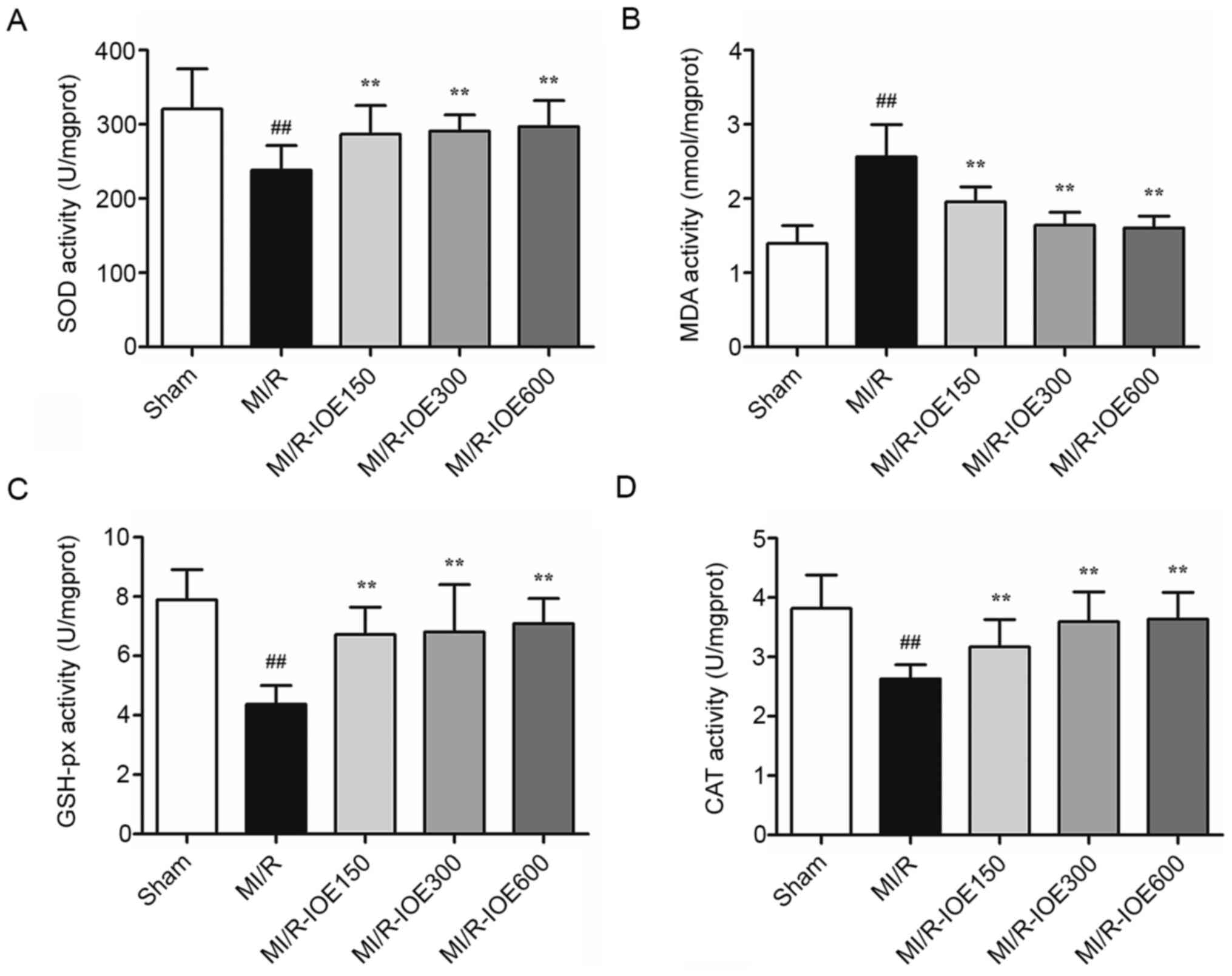
IOE reduces oxidative stress
MDA content and antioxidant enzymes (SOD, GSH-px and
CAT) are key molecules for evaluating the degree of oxidative
stress. After MI/R, the activities of these antioxidant enzymes
were significantly reduced (Fig. 4A, C
and D). Meanwhile, the content of MDA was significantly higher
compared with the Sham group (P<0.01; Fig. 4B). IOE pretreatment (150, 300 and
600 mg/kg) increased the activities of antioxidant enzymes and
decreased MDA content compared with the MI/R group (P<0.01).
These values indicated that IOE pretreatment protected
cardiomyocytes against oxidative stress.
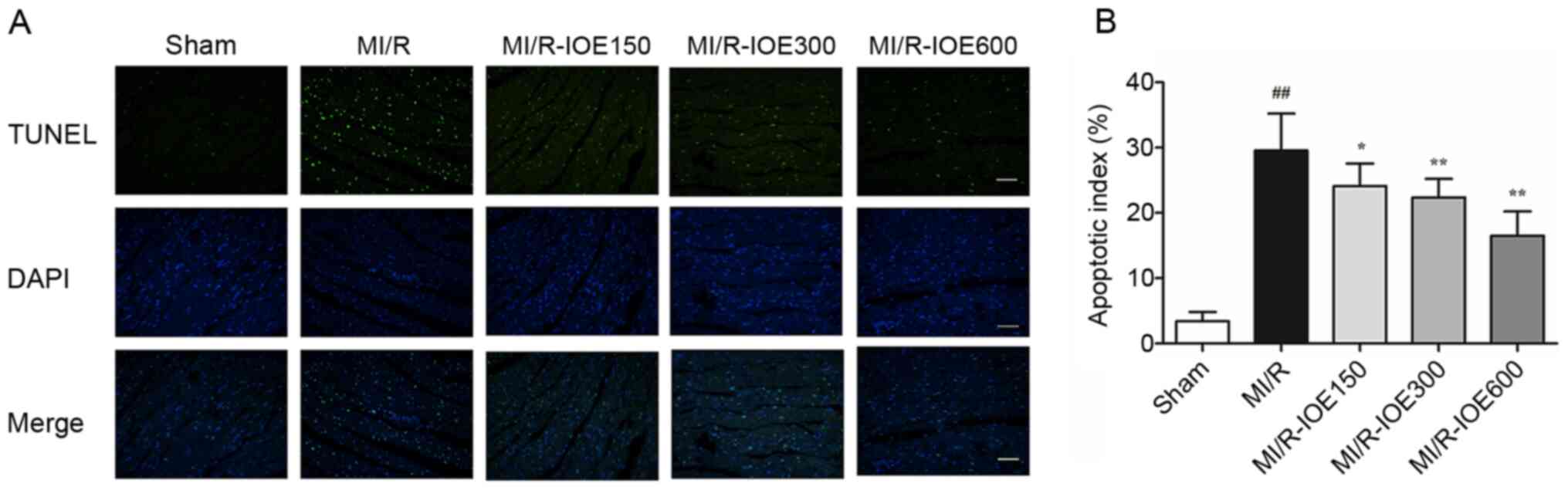
IOE decreases cardiomyocyte
apoptosis
The present study aimed to detect the role of IOE in
cell death by TUNEL staining. MI/R injury induced a certain degree
of cardiomyocyte apoptosis by increasing the percentage of
apoptotic cells in the MI/R group compared with the Sham group
(P<0.01; Fig. 5A and B). As
expected, pretreatment with IOE (150, 300 and 600 mg/kg)
significantly decreased the percentage of apoptotic cells
(P<0.05 or P<0.01). Therefore, the data suggested that IOE
could alleviate cardiomyocyte apoptosis after MI/R injury.
IOE activates SIRT1 and downregulates
the PERK/eIF2α/CHOP pathway
Considering the role of SIRT1 in cardioprotection,
SIRT1 expression in heart tissue was examined. MI/R injury could
suppress SIRT1 expression compared with the Sham group, which was
then activated by IOE (P<0.01; Fig.
6A and B). Furthermore, upregulation of ER chaperone GRP78,
p-PERK, p-eIF2α, CHOP and caspase-12 was observed after MI/R injury
in the heart (P<0.01; Fig. 6A and
C-G). Moreover, the changes in mRNA expression of
caspase-12 were investigated. As shown in Fig. 6H, the mRNA levels of
caspase-12 in the ischemic myocardium were also notably
increased in the MI/R group compared with the Sham group
(P<0.01). As expected, IOE inhibited ER stress by downregulating
the expression of ER stress-related markers (GRP78, p-PERK,
p-eIF2α, CHOP and caspase-12) compared with the MI/R group
(P<0.05 or P<0.01). IOE decreased the mRNA levels of
caspase-12 in the MI/R-IOE groups (P<0.01). Collectively,
the aforementioned values suggested that IOE attenuated MI/R injury
by suppressing ER stress, which may be associated with SIRT1
activation.
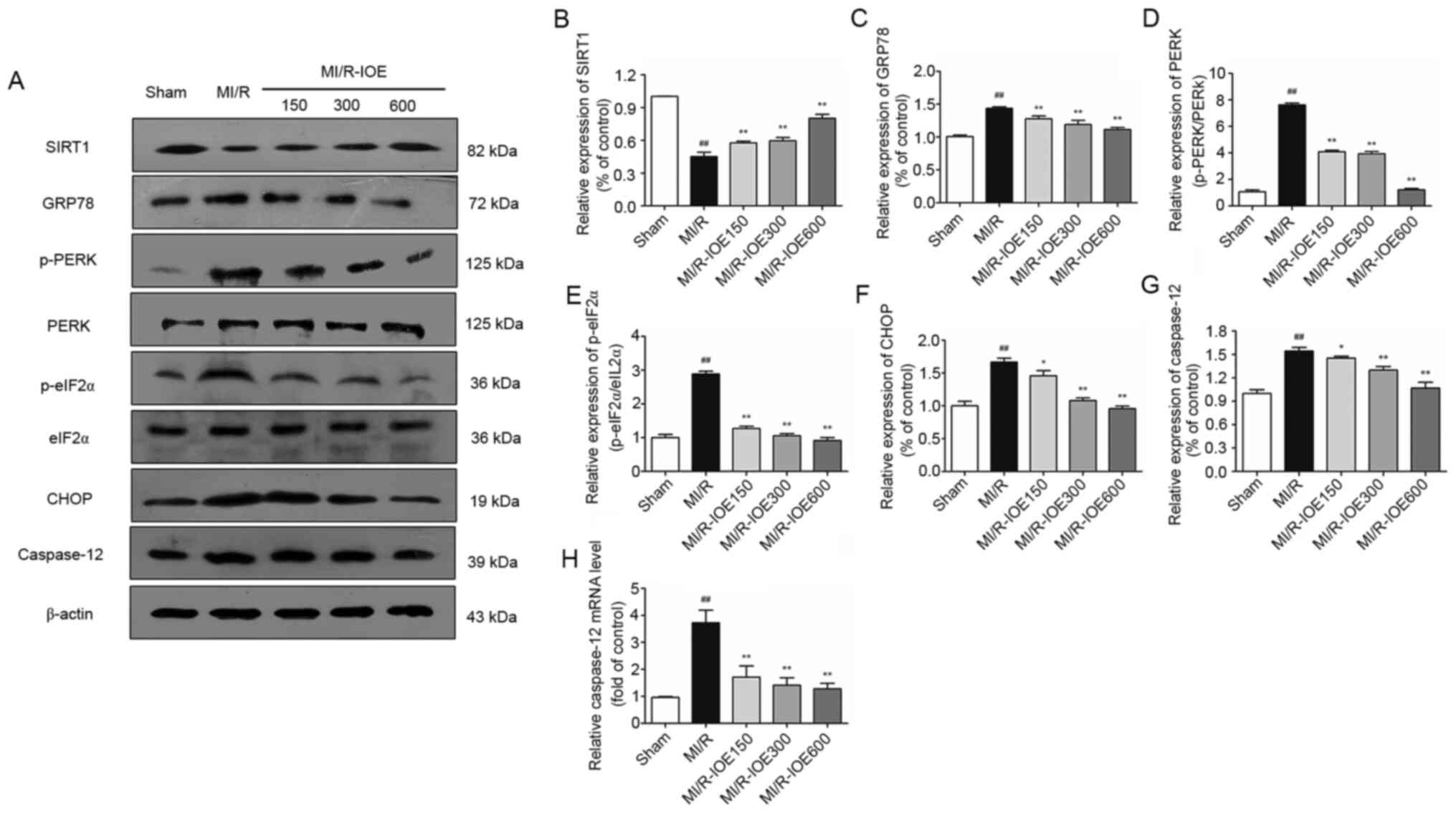 | Figure 6.Expression of SIRT1, GRP78, p-PERK,
p-eIF2α, CHOP, caspase-12 and the mRNA levels of caspase-12
in the myocardium. (A) Representative blots of SIRT1, GRP78,
p-PERK, p-eIF2α, CHOP and caspase-12. Semiquantitative analysis of
(B) SIRT1, (C) GRP78, (D) p-PERK, (E) p-eIF2α, (F) CHOP and (G)
caspase-12. (H) The mRNA levels of caspase-12 in the
myocardium. Data are presented as the mean ± standard deviation.
n=3. ##P<0.01 vs. Sham. *P<0.05 and **P<0.01
vs. MI/R. IOE, Inonotus obliquus extract; SIRT1,
NAD-dependent protein deacetylase sirtuin-1; GRP78,
glucose-regulated protein 78; PERK, protein kinase R-like
endoplasmic reticulum kinase; eIF2α, eukaryotic translation
initiation factor 2 subunit α; CHOP, C/EBP homologous protein; p,
phosphorylated; MI/R, myocardial ischemia/reperfusion. |
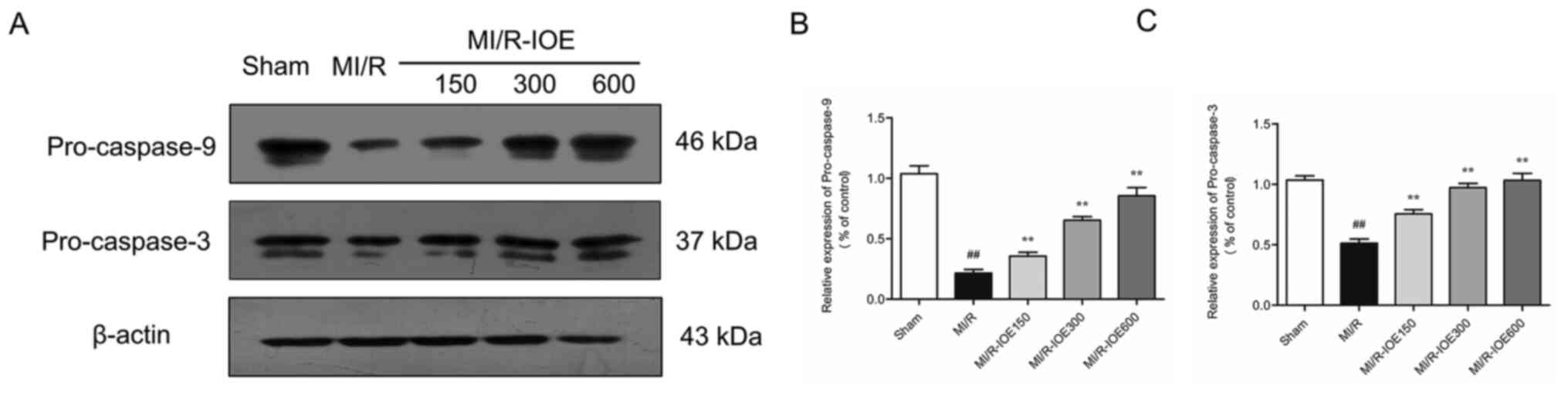
IOE suppresses ER stress-induced
apoptosis
To evaluate the condition of cardiomyocyte
apoptosis, the expression of pro-caspase-9 and pro-caspase-3 in
ischemic hearts were measured. MI/R injury significantly decreased
the expression of pro-caspase-9 and pro-caspase-3 compared with the
Sham group (P<0.01; Fig. 7A-C).
By contrast, IOE increased the expression of pro-caspase-9 and
pro-caspase-3 in ischemic heart tissue compared with the MI/R group
(P<0.05 or P<0.01). The data indicated that IOE protected the
heart from MI/R injury via suppressing ER stress-induced
cardiomyocyte apoptosis.
Discussion
IO possesses various pharmacological and biological
properties, including antitumor, antimicrobial and antioxidant
activities (19). It was previously
reported that IO inhibited lipid peroxidation in hyperlipidemic
rats (20). In the present study,
IOE exerted cardioprotective effects by attenuating oxidative
damage and suppressing ER stress-induced apoptosis.
Redox signaling is crucial in the regulation of cell
function, including defense against invading microorganisms and
gene expression for physiological activity. MI/R induces excessive
generation of redox signaling of ROS to break redox homeostasis,
which results in oxidative stress (21). The severity of reversible and
irreversible cellular injury within cardiomyocytes is proportional
to the level of ROS activation (22). ROS can induce lipid peroxidation,
protein oxidation and nitration, mitochondrial permeability
transition and DNA damage, causing cardiomyocyte death following
MI/R (23). To keep the redox state
in balance, redundant ROS is typically eliminated by several
enzymes, such as SOD, GSH-px and CAT (24). Several therapeutic strategies can
protect cardiomyocytes by inhibiting oxidative stress, for example,
pre-conditioning, pro-conditioning and antioxidants (23). Our previous study revealed that
ginsenoside Rg2 protected cardiomyocytes from MI/R injury by
inhibiting oxidative stress and inflammation (25). In the present study, MI/R injury
induced downregulation of GSH-px, SOD and CAT activities and
upregulation of MDA content, which were counteracted by IOE. These
results indicated that IOE pretreatment protected cardiomyocytes
against oxidative stress.
Ischemia and reperfusion activate a variety of cell
death programs, categorized as apoptosis, necrosis or
autophagy-associated cell death (26). ER stress plays a vital role in the
pathogenesis of cardiac diseases and may be a therapeutic target to
protect cardiomyocytes. In the MI/R process, ER stress occurs due
to diverse stimuli, and then triggers the unfolded protein response
(UPR). Overproduction of ROS disrupts protein folding and results
in amplified UPR signaling (27).
Previous evidence has suggested that UPR is initially activated by
three endoplasmic reticulum stress sensors,
serine/threonine-protein kinase/endoribonuclease IRE1, PERK and
ATF6 (28). GRP78, an
immunoglobulin binding protein, physically binds to these proteins
and inhibits their activities under a steady state (29). PERK, also known as eukaryotic
initiation factor 2α protein kinase 3, interacts with and
phosphorylates eIF2α (30). Under
ER stress, PERK dissociates from GRP78 and induces the
phosphorylation of eIF2α. The PERK/eIF2α signaling pathway can
limit protein translation and induce apoptosis by inducing CHOP
expression (31). Caspase-12
localizes in the ER and is a critical mediator in ER stress-induced
apoptosis by activating other caspases (32). Caspase-12 specifically clears
pro-caspase-9 for activation and caspase-9 is active. Subsequently,
caspase-9 ultimately activates caspase-3, the major effector
caspase for apoptosis (33). In the
present study, MI/R injury induced ER stress in rat cardiomyocytes.
It was found that IOE pretreatment significantly decreased the
expression of GRP78, p-PERK, p-eIF2α, CHOP and caspase-12 and the
mRNA levels of Caspase-12. The present study also found that
ER stress-induced apoptosis by MI/R injury was alleviated by IOE.
Above all, the cardioprotective effects of IOE are based on the
suppression of ER stress-induced cardiomyocyte apoptosis.
SIRT1 is a highly conserved histone deacetylase,
which can deacetylate various transcription factors (34). It has been demonstrated that SIRT1
activation has protective effects in myocardial ischemia. SIRT1 and
ER stress are involved in various pathologies, including diabetes,
obesity, cancer and cardiovascular diseases (4,35,36).
Resveratrol, a SIRT1 activator, inhibits ER stress via SIRT1
activation in vitro (37).
Previous studies have indicated that SIRT1 may regulate eIF2α
phosphorylation via regulating its acetylation process, thereby
affecting its activity (38,39).
SIRT1 inhibition has been demonstrated to cause the hyperactivation
of the PERK/eIF2α pathway, which induces cardiomyocyte apoptosis by
sustaining CHOP expression (12).
The present study revealed that IOE notably increased SIRT1
expression in the ischemic myocardium. IOE also suppressed ER
stress-induced apoptosis by mediating the PERK/eIF2α/CHOP signaling
pathway. The present results demonstrated that IOE-induced SIRT1
activation may suppress ER stress-induced cardiomyocyte apoptosis,
thereby reducing the infarct size in vivo. However, there is
a complex interaction between SIRT1 and ER stress. SIRT1 activation
has been reported to inhibit ER stress and apoptosis of
cardiomyocytes by promoting autophagic clearance and decreasing the
expression of the protein disulfide isomerase and S-nitrosylation
(40,41). The role of IOE in these mechanisms
need to be further studied.
In conclusion, the present findings showed that IOE
pretreatment protected cardiomyocytes from MI/R injury by
attenuating oxidative damage and suppressing ER stress-induced
apoptosis. IOE may suppress ER stress-induced cardiomyocyte
apoptosis via the PERK/eIF2α/CHOP pathway by the activation of
SIRT1. Therefore, IO may be a potential therapeutic candidate in
cardiovascular diseases.
Acknowledgements
Not applicable.
Funding
This project was supported by the Jilin Scientific
and Technological Development Program (grant no.
20190701056GH).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX and WF conceived the study. YW, HC, YZ, PY, YL,
DW, YX and WF performed the experiments. HC, YZ, PY, YL and DW
analyzed the data. YW, YX and WF wrote the manuscript. YX and WF
improved the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experiments of this study were approved
by the Ethical Committee for Experimental Animals, School of
Pharmaceutical Sciences Jilin University (approval no. 20190049;
Changchun, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu D and Thum T: RNA-based diagnostic and
therapeutic strategies for cardiovascular disease. Nat Rev Cardiol.
16:661–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robin E, Guzy RD, Loor G, Iwase H, Waypa
GB, Marks JD, Hoek TL and Schumacker PT: Oxidant stress during
simulated ischemia primes cardiomyocytes for cell death during
reperfusion. J Biol Chem. 282:19133–19143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Hu X and Jiang H: ER
stress-induced apoptosis: A novel therapeutic target in myocardial
ischemia and reperfusion injury. Int J Cardiol. 214:233–234. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao SS and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress in cell fate decision and
human disease. Antioxid Redox Signal. 21:396–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee AS: GRP78 induction in cancer:
Therapeutic and prognostic implications. Cancer Res. 67:3496–3499.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harding HP, Zhang Y and Ron D: Protein
translation and folding are coupled by an
endoplasmic-reticulum-resident kinase. Nature. 397:271–274. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harding HP, Novoa I, Zhang Y, Zeng H, Wek
R, Schapira M and Ron D: Regulated translation initiation controls
stress-induced gene expression in mammalian cells. Mol Cell.
6:1099–1108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Wang Y, Wang H, Huang C, Huang Y and
Li J: Endoplasmic reticulum stress is the crossroads of autophagy,
inflammation, and apoptosis signaling pathways and participates in
liver fibrosis. Inflamm Res. 64:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh V and Ubaid S: Role of Silent
Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and
Inflammation. Inflammation. 43:1589–1598. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo G, Jian Z, Zhu Y, Zhu Y, Chen B, Ma R,
Tang F and Xiao Y: Sirt1 promotes autophagy and inhibits apoptosis
to protect cardiomyocytes from hypoxic stress. Int J Mol Med.
43:2033–2043. 2019.PubMed/NCBI
|
|
12
|
Prola A, Pires Da Silva J, Guilbert A,
Lecru L, Piquereau J, Ribeiro M, Mateo P, Gressette M, Fortin D,
Boursier C, et al: SIRT1 protects the heart from ER stress-induced
cell death through eIF2α deacetylation. Cell Death Differ.
24:343–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wasser SP: Medicinal Mushroom Science:
History, Current Status, Future Trends, and Unsolved Problems. Int
J Med Mushrooms. 12:1–16. 2010. View Article : Google Scholar
|
|
14
|
Lee I-K and Yun B-S: Styrylpyrone-class
compounds from medicinal fungi Phellinus and Inonotus
spp., and their medicinal importance. J Antibiot (Tokyo).
64:349–359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Javed S, Mitchell K, Sidsworth D, Sellers
SL, Reutens-Hernandez J, Massicotte HB, Egger KN, Lee CH and Payne
GW: Inonotus obliquus attenuates histamine-induced
microvascular inflammation. PLoS One. 14:e02207762019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan L, Ding S, Ai L and Deng K: Antitumor
and immunomodulatory activity of water-soluble polysaccharide from
Inonotus obliquus. Carbohydr Polym. 90:870–874. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington, DC: 2011, PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(T)(-Delta Delta C) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Balandaykin ME and Zmitrovich IV: Review
on Chaga Medicinal Mushroom, Inonotus obliquus (Higher
Basidiomycetes): Realm of Medicinal Applications and Approaches on
Estimating its Resource Potential. Int J Med Mushrooms. 17:95–104.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang L, Zhang Z, Sun W and Wang Y: Effect
of the Inonotus obliquus polysaccharides on blood lipid
metabolism and oxidative stress of rats fed high-fat diet in vivo.
Proceedings of the 2009 2nd International Conference on Biomedical
Engineering and Informatics. IEEE; New York, NY: pp. 1114–1117.
2009
|
|
21
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Ischemia/Reperfusion. Compr Physiol. 7:113–170. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Horwitz LD, Wallner JS, Decker DE and
Buxser SE: Efficacy of lipid soluble, membrane-protective agents
against hydrogen peroxide cytotoxicity in cardiac myocytes. Free
Radic Biol Med. 21:743–753. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raedschelders K, Ansley DM and Chen DD:
The cellular and molecular origin of reactive oxygen species
generation during myocardial ischemia and reperfusion. Pharmacol
Ther. 133:230–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He L, He T, Farrar S, Ji L, Liu T and Ma
X: Antioxidants Maintain Cellular Redox Homeostasis by Elimination
of Reactive Oxygen Species. Cell Physiol Biochem. 44:532–553. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu WW, Xu HL, Yu XF, Lyu C, Tian Y, Guo M,
Sun J and Sui D: 20(S)-Ginsenoside Rg2 attenuates myocardial
ischemia/reperfusion injury by reducing oxidative stress and
inflammation: Role of SIRT1. Rsc Adv. 8:23947–23962. 2018.
View Article : Google Scholar
|
|
26
|
Hotchkiss RS, Strasser A, McDunn JE and
Swanson PE: Cell death. N Engl J Med. 361:1570–1583. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malhotra JD, Miao H, Zhang K, Wolfson A,
Pennathur S, Pipe SW and Kaufman RJ: Antioxidants reduce
endoplasmic reticulum stress and improve protein secretion. Proc
Natl Acad Sci USA. 105:18525–18530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Louessard M, Bardou I, Lemarchand E,
Thiebaut AM, Parcq J, Leprince J, Terrisse A, Carraro V, Fafournoux
P, Bruhat A, et al: Activation of cell surface GRP78 decreases
endoplasmic reticulum stress and neuronal death. Cell Death Differ.
24:1518–1529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taniuchi S, Miyake M, Tsugawa K, Oyadomari
M and Oyadomari S: Integrated stress response of vertebrates is
regulated by four eIF2α kinases. Sci Rep. 6:328862016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Duan W, Li Y, Jin Z, Yan J, Yu S
and Yi D: Novel role of silent information regulator 1 in
myocardial ischemia. Circulation. 128:2232–2240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hetz C, Chevet E and Harding HP: Targeting
the unfolded protein response in disease. Nat Rev Drug Discov.
12:703–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Winnik S, Auwerx J, Sinclair DA and Matter
CM: Protective effects of sirtuins in cardiovascular diseases: From
bench to bedside. Eur Heart J. 36:3404–3412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu LQ, Fan ZQ, Tang YF and Ke ZJ: The
resveratrol attenuates ethanol-induced hepatocyte apoptosis via
inhibiting ER-related caspase-12 activation and PDE activity in
vitro. Alcohol Clin Exp Res. 38:683–693. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hubbard BP, Gomes AP, Dai H, Li J, Case
AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, et al:
Evidence for a common mechanism of SIRT1 regulation by allosteric
activators. Science. 339:1216–1219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pires Da Silva J, Monceaux K, Guilbert A,
Gressette M, Piquereau J, Novotova M, Ventura-Clapier R, Garnier A
and Lemaire C: SIRT1 Protects the Heart from ER Stress-Induced
Injury by Promoting eEF2K/eEF2-Dependent Autophagy. Cells.
9:4262020. View Article : Google Scholar
|
|
41
|
Hsu YJ, Hsu SC, Hsu CP, Chen YH, Chang YL,
Sadoshima J, Huang SM, Tsai CS and Lin CY: Sirtuin 1 protects the
aging heart from contractile dysfunction mediated through the
inhibition of endoplasmic reticulum stress-mediated apoptosis in
cardiac-specific Sirtuin 1 knockout mouse model. Int J Cardiol.
228:543–552. 2017. View Article : Google Scholar : PubMed/NCBI
|