Introduction
The prognosis of patients with advanced pancreatic
carcinoma is extremely poor despite numerous trials with palliative
chemotherapy or radiotherapy. Systemic chemotherapy with
gemcitabine has been the standard chemotherapy for advanced
pancreatic cancer since the mid-1990s (1). However, there is no standard
second-line chemotherapeutic drug in cases refractory to or
recurring following gemcitabine therapy. The median survival rate
with best supportive care in patients who have failed gemcitabine
therapy is approximately two months (2,3).
Approximately half of patients with gemcitabine-pretreated disease
may be candidates for further treatment. Data supporting the use of
second-line therapy compared with best supportive care are lacking.
Although there have been reports of clinical trials of second-line
therapy in advanced pancreatic cancer, most of these have been
published in abstract form with a small number of patients.
Therefore, there is a continuing need for clinical trials with a
new agent for advanced pancreatic cancer in cases of gemcitabine
failure.
S-1 is a fourth-generation oral fluoropyrimidine
that has been reported to be active with tolerable toxicity against
gemcitabine-refractory pancreatic cancer (4–6) and
chemotherapy-naïve pancreatic cancer (7,8),
although most of the studies have been case reports or
retrospective studies. The superior effect of combination therapy
with cisplatin compared with 5-fluorouracil (5-FU) monotherapy has
been demonstrated in advanced pancreatic cancer (9,10).
Thus, we conducted the present phase II study to investigate the
feasibility and efficacy of S-1 in combination with cisplatin as
palliative chemotherapy for gemcitabine-refractory advanced
pancreatic cancer patients.
Patients and methods
Ethics
This was a prospective multicenter study. All
patients provided written informed consent. In total, three centers
participated. This study was approved by the Institutional Review
Board (IRB) of each center and was conducted in accordance with the
Declaration of Helsinki.
Patients
The inclusion criteria for this study were: i)
histologically or cytologically proven pancreatic adenocarcinoma
and unresectable locally advanced or metastatic disease; ii) at
least one measurable lesion according to Response Evaluation
Criteria in Solid Tumors (RECIST) (11); iii) prior chemotherapy with
gemcitabine-based palliative chemotherapy; iv) the ability to take
oral medications; v) age, >18 years; vi) an Eastern Cooperative
Oncology Group (ECOG) performance status (PS) of 0–2; vii) adequate
bone marrow function (white blood cell count ≥4,000/mm3,
neutrophil count ≥2,000/mm3 and platelet count
≥100,000/mm3); viii) adequate renal function [serum
creatinine level ≤1.5 mg/dl or creatinine clearance level (Ccr) ≥50
ml/min]; ix) adequate liver function [total bilirubin ≤3× UNL (if
due to underlying liver metastasis, then total bilirubin may be ≤5×
UNL); aspartate transaminase (AST) and/or alanine transaminase
(ALT) ≤2.5× UNL (if liver function abnormalities were due to
underlying liver metastasis, then AST and/or ALT may be ≤5×
UNL)].
The exclusion criteria for this study were patients
who: i) had received chemotherapy or radiotherapy within 3 weeks;
ii) had previously received an oral fluoropyrimidine; iii) had
central nervous system metastases; iv) had an active infection or
uncontrolled concurrent medical illness; v) had a history of other
malignancies; vi) were pregnant or lactating; vii) had severe
neurological impairment, a mental disorder or any severe
drug-induced allergy.
Treatment protocol
S-1 [body surface area (BSA) <1.25 m2,
40 mg; BSA ≤1.25 to <1.5 m2, 50 mg; BSA ≥1.5
m2, 60 mg] was administered orally twice daily,
following breakfast and dinner, for 14 consecutive days, followed
by seven days of rest. Cisplatin (60 mg/m2) was
administered as a 60-min intravenous infusion on day 1 with
adequate hydration. The treatment courses were repeated every three
weeks. Antiemetic prophylaxis, including aprepitant, a 5-HT3
antagonist and dexamethasone, was used. Prophylactic myeloid growth
factors were not administered prior to the first cycle.
Dose modification
Modifications to the S-1 or cisplatin dose were made
in patients who had any of the following: a leukocyte count
<1.0×103/μl, a neutrophil count <500/μl, a
platelet count <2.5×104/μl, grade 3–4 febrile
neutropenia or grade 3–4 non-hematological toxicity, based on the
most severe grade of toxicity that had occurred during the previous
cycle. Treatment was delayed for up to three weeks in patients with
persistent symptomatic toxicity, absolute neutrophil counts
<1,500/μl or platelet counts <100,000/μl. The dose of S-1 was
decreased in a stepwise manner by up to two levels as follows: BSA
<1.25 m2, from 40 to 25 and 20 mg/dose; BSA ≥1.25 to
<1.5 m2, from 50 to 40 and 25 mg/dose; BSA ≥1.5
m2, from 60 to 50 and 40 mg/dose. Additionally, the dose
of cisplatin was decreased according to the serum Ccr as follows:
Ccr ≥60 ml/min, no reduction; Ccr >40 ml/min to <60 ml/min,
reduced to 30 mg/m2; Ccr <40 ml/min, administration
of cisplatin was stopped. Treatment was continued until signs of
disease progression or unacceptable toxic effects developed or
until a patient refused further treatment.
Pretreatment evaluation
Baseline laboratory analyses [blood cell count,
serum creatinine, bilirubin, AST, ALT, alkaline phosphatase, lactic
dehydrogenase and carbohydrate antigen (CA) 19-9] were performed
within one week of starting the first cycle of therapy and tumor
status was assessed using computed tomography (CT) within 4
weeks.
Assessment of efficacy and toxicity
Tumor assessments, using CT of the lesions, abdomen,
pelvis and/or chest, were performed at baseline and repeated every
3 cycles using RECIST (11). The
tumor marker CA 19-9 was checked every three cycles. A physical
examination, including weight and toxicity assessments, ECOG
performance status, complete blood count and blood chemistry, was
performed prior to each cycle. Toxicity was graded according to
National Cancer Institute Common Toxicity Criteria (NCI-CTC),
version 3.0. Treatment-related mortality (TRM) was defined as
mortality that occurred within 30 days of treatment initiation.
Statistical analysis
The primary endpoint was response rate and the
secondary endpoints were safety, time to progression (TTP), disease
control rate and overall survival (OS). The sample size in this
trial was calculated to reject a 5% response rate in favor of a
target response rate of 20% with a significance level of 0.05 and a
power of 80%, using Simon’s optimal two-stage design. In the
initial stage, in total, 10 evaluable patients were to be entered
and evaluated for a response. If there was no response, accrual was
to be terminated. If any response was observed in the first stage,
then 19 additional patients were to be entered in the second stage,
to achieve a target sample size of 29 evaluable patients. Further
assessment of the regimen was thought to be necessary if more than
three responses were observed in the 29 patients. Considering a
withdrawal rate of 10%, the total target number was calculated to
be 32 patients.
Assessment of the response rate was performed using
the intention to treat (ITT) and per-protocol (PP) analyses. TTP
and OS were calculated using the Kaplan-Meier method. Survival
curves were compared using the log-rank test. Tests were two sided
and p<0.05 was considered to indicate a statistically
significant result. TTP was calculated from the date therapy was
initiated to the date of disease progression, mortality or final
follow-up. OS was calculated from the date therapy was initiated to
the date of mortality or final follow-up.
Results
Patient characteristics
Between October 2009 and June 2010, in the stage I
analysis, 11 patients were enrolled in this prospective study. The
median age of the patients was 56 (range, 42–74) years. The
male:female ratio was 7:4. Of the 11 patients, nine had a PS of one
and two had a PS of two when enrolled. There were six (54.5%) cases
of primary tumors in the head, two (18.2%) cases in the body and
three (27.3%) in the tail portion of the pancreas. Of the 11
patients, nine (81.8%) had distant metastases, while the remaining
two (18.2%) had locally advanced disease. Six (54.5%) patients had
elevated CA 19-9 levels when enrolled (Table I).
 | Table IPatient characteristics (n=11). |
Table I
Patient characteristics (n=11).
| Characteristics | No. | % |
|---|
| Gender |
| Male | 7 | 63.6 |
| Female | 4 | 36.4 |
| Age, years |
| Median (range) | 56 (42–74) | |
| Performance status
(ECOG) |
| 0–1 | 9 | 81.8 |
| 2 | 2 | 18.2 |
| CA 19-9 level |
| Within normal
range | 5 | 45.5 |
| >Normal | 6 | 54.5 |
| Location of primary
tumor |
| Head | 6 | 54.5 |
| Body | 2 | 18.2 |
| Tail | 3 | 27.3 |
| Disease status |
| Locally
advanced | 2 | 18.2 |
| Distant
metastases | 9 | 81.8 |
| Sites of distant
metastases |
| Liver | 7 | - |
| Lymph node | 3 | - |
| Lung | 5 | - |
Delivery of drugs
In total, 21 cycles of therapy were administered,
with a median of 1.5 (range, 1–5) cycles per patient and a median
treatment duration of 21 (range, 7–96) days. The average relative
dose intensities of S-1 and cisplatin were 0.98 and 0.91,
respectively. Dose reduction for S-1 was required in one patient
(two cycles) due to non-hematological toxicity, including diarrhea
and fatigue. Dose reduction for cisplatin was required in two
patients (three cycles) due to nausea and vomiting in one case (two
cycles) and peripheral neuropathy in the other case (one
cycle).
Tumor responses
Of the 11 patients enrolled in this study, six were
evaluable in terms of treatment response. Five patients could not
be evaluated for the following reasons: three withdrew consent due
to therapy-related toxicities and worsening of their general
condition and two died prior to the response evaluation. None of
the six evaluable patients achieved a complete or partial response.
Only one patient (9.1% by ITT analysis and 16.7% by PP analysis)
achieved stable disease and five had progressive disease (Table II).
 | Table IIResponse rate of S-1 and cisplatin for
gemcitabine-refractory pancreatic cancer. |
Table II
Response rate of S-1 and cisplatin for
gemcitabine-refractory pancreatic cancer.
| No. of patients
(n=11) | ITT analysis (%) | PP analysis (%) |
|---|
| Complete
response | 0 | 0 | 0 |
| Partial response | 0 | 0 | 0 |
| Stable disease | 1 | 9.1 | 16.7 |
| Not evaluated | 5 | - | - |
| Response rate | 0 | 0 | 0 |
| Disease control
rate | 1 | 9.1 | 16.7 |
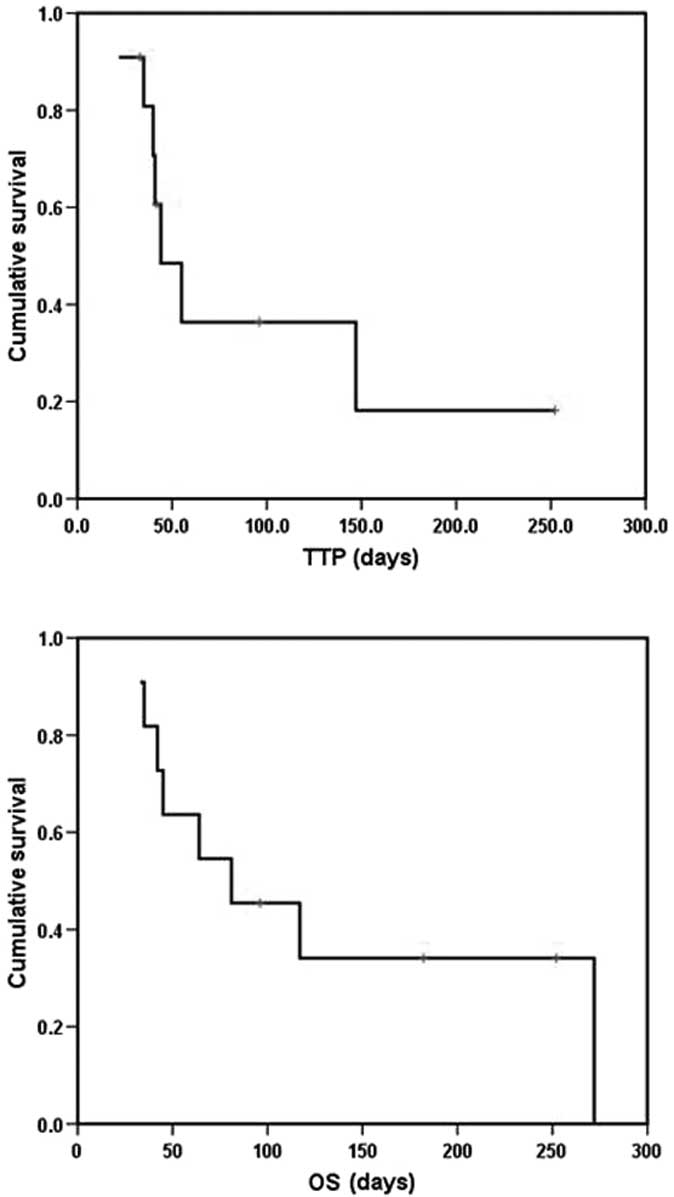
Survival (TTP and OS)
The patients were evaluable for the survival
analysis. With a median follow-up of 8.9 (range, 3.2–11.3) months,
the median TTP was 44 days [95% confidence interval (CI) 25.4–62.6]
and the median OS was 81 days (95% CI 9.3–152.7). Kaplan-Meier
curves for TTP and OS are shown in Fig.
1.
Toxicities
Grade 3–4 hematological toxicities included anemia
in one cycle, neutropenia in one cycle and thrombocytopenia in one
cycle. Grade 3–4 non-hematological toxicities included fatigue in
three cycles, nausea in four cycles, anorexia in two cycles,
diarrhea in one cycle and peripheral neuropathy in two cycles (one
patient). There were two TRMs. The cause of mortality in the first
case was septic shock associated with grade 3 neutropenia following
the first cycle; in the second case, the patient died suddenly
without documented cause following the second cycle. These
toxicities are shown in Table
III. This study was terminated early, prior to the first stage,
without reaching 10 response-evaluable patients due to severe
toxicity, including TRM, and poor compliance, by agreement of the
investigators and the IRB.
 | Table IIIAdverse effects of S-1 and cisplatin
for gemcitabine-refractory pancreatic cancer. |
Table III
Adverse effects of S-1 and cisplatin
for gemcitabine-refractory pancreatic cancer.
| NCI-CTC Grade 3–4
toxicities | Per cycle no. (%)
n=21 | Per patient no. (%)
n=21 |
|---|
| Hematologic
toxicity |
| Neutropenia | 1 (4.8) | 1 (9.1) |
| Anemia | 1 (4.8) | 1 (9.1) |
|
Thrombocytopenia | 1 (4.8) | 1 (9.1) |
| Non-hematologic
toxicity |
| Nausea | 4 (19.0) | 3 (27.3) |
| Vomiting | 1 (4.8) | 1 (9.1) |
| Diarrhea | 1 (4.8) | 1 (9.1) |
| Fatigue | 3 (14.3) | 3 (27.3) |
| Anorexia | 2 (9.5) | 2 (18.2) |
| Peripheral
neuropathy | 2 (9.5) | 1 (9.1) |
Discussion
Pancreatic cancer is the fourth most common cause of
cancer-related mortality in the US (12). In Korea, the incidence of this
disease has increased. In 2009, the disease ranked 9th in incidence
in Korea according to an annual report of cancer statistics; for
cancer-related mortality, the disease ranked 5th (5.8% of the
total). The prognosis of locally unresectable or metastatic
pancreatic cancer remains extremely poor. Gemcitabine monotherapy
or gemcitabine-based combination therapy, according to PS, has been
the standard systemic therapy for advanced pancreatic cancer.
FOLFIRINOX has been recommended as a first-line therapy with
gemcitabine monotherapy or a gemcitabine-containing double regimen,
based on a published phase III trial in which patients with
metastatic pancreatic cancer showed marked improvements in median
progression-free survival (PFS) (6.4 vs. 3.4 months; p<0.0001)
and median OS (10.5 vs. 6.9 months; p<0.001) (13).
While first-line therapy has been established in
advanced pancreatic cancer, there is no consensus with regards to a
second-line therapy for advanced pancreatic cancer, particularly in
gemcitabine-refractory cancer. It is difficult to conduct a
clinical trial for second-line chemotherapy in advanced pancreatic
cancer due to the rapidly progressive nature of the general
condition and the lack of agents active in pancreatic cancer.
However, it has been reported that 55–60% of patients had a
relatively good PS following the failure of first-line therapy;
thus, physicians should consider second-line therapy in such
patients (14).
The results of previous studies concerning
oxaliplatin (15), ralitrexed
(16), paclitaxel (17) and pemetrexed (18) monotherapies in the second-line
treatment of pancreatic cancer have revealed modest antitumor
effects with no survival benefit. Studies have also reported
combination chemotherapeutic regimens as second-line therapies for
advanced pancreatic cancer. A representative study of second-line
chemotherapy in pancreatic cancer is the CONKO-003 trial. In a
preliminary report from the CONKO-003 trial, the use of second-line
chemotherapy was compared with best supportive care (2). The study revealed the benefit of
combination therapy with oxaliplatin, 5-FU and leucovorin as a
second-line therapy compared with 5-FU and leucovorin (19). There have been other studies
concerning combination chemotherapy for second-line therapy in
pancreatic cancer with biological agents. However, the results are
generally modest and preliminary (20,21).
S-1, an oral agent, consists of a mixture of
tegafur, 5-chloro-2,4-dihydroxypyridine and potassium oxonate at a
molar ratio of 1:0.4:1. The antitumor effect of S-1 in advanced
pancreatic cancer as a first- or second-line therapy has been
reported in Japan (8,22–24).
Generally, the antitumor effect was promising and the toxicity was
tolerable in these studies. In view of the favorable toxicity
profile of S-1 monotherapy, its combination with other agents may
improve therapeutic results.
The addition of cisplatin offers the possibility of
a synergistic antitumor effect, beyond that observed with S-1
monotherapy. Cisplatin combined with 5-FU appears to be promising
in metastatic pancreatic carcinoma, with a 26% response rate and a
median survival rate of 7 months in a phase II trial (10). In a randomized trial comparing 5-FU
with 5-FU plus cisplatin, FU-cisplatin was found to be superior to
FU in terms of response and PFS, but not OS (9,10). The
combination of S-1 and cisplatin has also been adopted in advanced
gastric cancer, based on previous studies which revealed that
combination therapy with S-1 and cisplatin had promising effects
with tolerable toxicity (25,26).
Although this study was conducted based on published
data similar to those above, the results were disappointing
compared with those of previous studies concerning monotherapy in
advanced pancreatic cancer as a second-line therapy. The cause of
the poor response and poor compliance may have been the rapid
worsening of the general condition of the patients. In contrast to
our results, Togawa et al (27) revealed that S-1 with cisplatin had
promising effects in patients who failed postoperative gemcitabine
treatment for pancreatic cancer. In that study, the dose of
cisplatin was 40 mg/m2 and it was administered on day 8
every five weeks, to avoid the severe toxicity of cisplatin.
Additionally, the group of patients had relapsed following
postoperative gemcitabine treatment, unlike our patients who failed
first-line palliative chemotherapy containing gemcitabine. These
patients may have had a relatively good PS and maintained a good
general condition relatively longer than those in our group.
Additionally, in their group, there was no patient with previous
exposure to cisplatin, unlike our group. These factors may explain
the differences between our results and those of Togawa et
al (27).
In this study, one patient achieved stable disease.
However, this patient experienced severe adverse events, including
anorexia and nausea, and ultimately did not undergo more than 5
cycles. In the present study, we observed several types of
hematological and non-hematological severe adverse events,
including anorexia, nausea, fatigue, peripheral neuropathy and
cytopenia. These adverse events resulted in poor compliance. Two
patients had moderate toxicities, including nausea and anorexia;
however, even these patients were reluctant to undergo further
chemotherapy. The general fragility of the patients may have
contributed to their poor compliance.
We also observed TRM in two patients. One
experienced sepsis with severe neutropenia following the first
cycle. Another patient succumbed to the disease suddenly following
the second cycle, complaining of abdominal pain. The cause of
mortality was not certain, but a thromboembolic event may have been
the cause. These adverse events resulted in the discontinuation of
the second-line therapy in these vulnerable patients. This issue
should be considered in designing future clinical studies of
advanced pancreatic cancer patients.
In the present study, we observed that the advanced
pancreatic cancer patients progressed rapidly and that the general
condition of the patients often deteriorated rapidly to perform
additional chemotherapy cycles. Thus, prospective studies of
palliative second-line therapy in patients with pancreatic cancer
using combinations of novel or biological agents should consider
the expectation for the worsening of the PS of the patients.
Additionally, patients with good prognostic factors, as suggested
by Nakachi et al (3),
including a good PS, lower serum C-reactive protein levels and no
peritoneal dissemination, should be considered as initial
candidates for second-line chemotherapy in advanced pancreatic
cancer.
In conclusion, this prospective combination
chemotherapy study of S-1 and cisplatin did not demonstrate
promising antitumor activity. Additionally, moderate toxicity
profiles, with two cases of TRM, and poor compliance were observed
in patients with advanced pancreatic cancer. In future studies,
dose and schedule modification, as well as patient selection, are
necessary for the precise evaluation of the effects of S-1 plus
cisplatin on pancreatic cancer.
References
|
1
|
Burris HA III, Moore MJ, Andersen J, et
al: Improvements in survival and clinical benefit with gemcitabine
as first line therapy for patients with advanced pancreas cancer: a
randomized trial. J Clin Oncol. 15:2403–2413. 1997.PubMed/NCBI
|
|
2
|
Oettle H, Pelzer U, Stieler J, Hilbig A,
et al: Oxaliplatin/folinic acid/5-fluorouracil [24h] (OFF) plus
best supportive care versus best supportive care alone in
second-line therapy of gemcitabine-refractory advanced pancreatic
cancer (CONKO 003). J Clin Oncol. 23(Suppl): 40312005.PubMed/NCBI
|
|
3
|
Nakachi K, Furuse J, Ishii H, Suzuki E and
Yoshino M: Prognostic factors in patients with
gemcitabine-refractory pancreatic cancer. Jpn J Clin Oncol.
37:114–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morizane C, Okusaka T, Furuse J, et al: A
phase II study of S-1 in gemcitabine-refractory metastatic
pancreatic cancer. Cancer Chemother Pharmacol. 63:313–319. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morizane C: A case of
gemcitabine-refractory pancreatic cancer responsive to second-line
chemotherapy using S-1. Jpn J Clin Oncol. 37:9732007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Todaka A, Fukutomi A, Boku N, et al: S-1
monotherapy as second-line treatment for advanced pancreatic cancer
after gemcitabine failure. Jpn J Clin Oncol. 40:567–572. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueno H, Okusaka T, Ikeda M, Takezako Y and
Morizane C: An early phase II study of S-1 in patients with
metastatic pancreatic cancer. Oncology. 68:171–178. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Funakoshi A, Senju T and Sumii T: Two
cases of advanced pancreatic cancer with cervical lymph node or
liver metastasis responding well to S-1 monotherapy. Gan To Kagaku
Ryoho. 33:1505–1509. 2006.(In Japanese).
|
|
9
|
Ducreux M, Rougier P, Pignon JP, et al: A
randomised trial comparing 5-FU with 5-FU plus cisplatin in
advanced pancreatic carcinoma. Ann Oncol. 13:1185–1191. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rougier P, Zarba J, Ducreux M, et al:
Phase II study of cisplatin and 120-hour continuous infusion of
5-fluorouracil in patients with advanced pancreatic adenocarcinoma.
Ann Oncol. 4:333–336. 1993.PubMed/NCBI
|
|
11
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
12
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
13
|
Conroy T, Desseigne F, Ychou M, et al:
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dahan L, Bonnetain F, Ychou M, et al:
Combination 5-fluorouracil, folinic acid and cisplatin
(LV5FU2-CDDP) followed by gemcitabine or the reverse sequence in
metastatic pancreatic cancer: final results of a randomised
strategic phase III trial (FFCD 0301). Gut. 59:1527–1534. 2010.
View Article : Google Scholar
|
|
15
|
Androulakis N, Syrigos K, Polyzos A, et
al: Oxaliplatin for pretreated patients with advanced or metastatic
pancreatic cancer: a multicenter phase II study. Cancer Invest.
23:9–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ulrich-Pur H, Raderer M, Verena Kornek G,
et al: Irinotecan plus raltitrexed vs raltitrexed alone in patients
with gemcitabine-pretreated advanced pancreatic adenocarcinoma. Br
J Cancer. 88:1180–1184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oettle H, Arnold D, Esser M, Huhn D and
Riess H: Paclitaxel as weekly second-line therapy in patients with
advanced pancreatic carcinoma. Anticancer Drugs. 11:635–658. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boeck S, Weigang-Köhler K, Fuchs M, et al:
Second-line chemotherapy with pemetrexed after gemcitabine failure
in patients with advanced pancreatic cancer: a multicenter phase II
trial. Ann Oncol. 18:745–751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pelzer U, Kubica K, Stieler I, et al: A
randomized trial in patients with gemcitabine refractory pancreatic
cancer. Final results of the CONKO 003 study. J Clin Oncol.
26(Suppl 15): 45082008.
|
|
20
|
Lubner SJ, Schelman WR, Mulkerin D, Holen
KD, Seo S and LoConte NK: Phase II study of oxaliplatin, high-dose
capecitabine, and sorafenib in patients with advanced pancreatic
cancer. J Clin Oncol. 28(Suppl 15): 41432010.
|
|
21
|
Starling N, Hawkes EA, Chau I, et al: A
dose-escalation study of gemcitabine plus oxaliplatin in
combination with imatinib in patients with gemcitabine-refractory
advanced pancreatic adenocarcinoma. Ann Oncol. Jul 12–2011.(E-pub
ahead of print).
|
|
22
|
Nakai Y, Isayama H, Sasaki T, et al:
Impact of S-1 in patients with gemcitabine-refractory pancreatic
cancer in Japan. Jpn J Clin Oncol. 40:774–780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alsamarai S, Zergebel C, Zhang J, Furuie
T, Urrea PD and Saif MW: Long term survival on S-1 monotherapy in a
patient with recurrent stage IV pancreatic cancer. JOP. 9:185–191.
2008.PubMed/NCBI
|
|
24
|
Yoshino T, Fukutomi A and Boku N:
Chemotherapy-naïve advanced pancreatic cancer with multiple liver
metastases successfully treated by S-1 monotherapy. A case report.
Gan To Kagaku Ryoho. 33:1521–1523. 2006.(In Japanese).
|
|
25
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ajani JA, Rodriguez W, Bodoky G, et al:
Multicenter phase III comparison of cisplatin/S-1 with
cisplatin/infusional fluorouracil in advanced gastric or
gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin
Oncol. 28:1547–1553. 2010. View Article : Google Scholar
|
|
27
|
Togawa A, Yoshitomi H, Ito H, et al:
Treatment with an oral fluoropyrimidine, S-1, plus cisplatin in
patients who failed postoperative gemcitabine treatment for
pancreatic cancer: a pilot study. Int J Clin Oncol. 12:268–273.
2007. View Article : Google Scholar : PubMed/NCBI
|