Introduction
Over the last several decades, numerous studies have
demonstrated that a number of naturally occurring compounds exhibit
beneficial effects for human health, including cancer
chemopreventive properties (1–3).
Despite the improvement of oral cancer treatment with conventional
strategies, including surgery, radiotherapy and chemotherapy in
recent years, the 5-year mortality rate remains low at
approximately 50% (4,5). For this reason, cancer biologists have
focused on the use of natural products as an alternative
therapeutic tool to improve the survival rate of oral cancer
patients.
Codonopsis lanceolata is a perennial herb
that grows naturally in moist places in woodlands and hills and is
commonly found in East Asia (6).
Codonopsis lanceolata has been used as a traditional
medicine in Korea, Japan and China (7,8). The
plant has been demonstrated to have anti-inflammatory properties
and a protective effect against ischemic damage and alcoholic fatty
liver (9,10). Recently, Codonopsis
lanceolata has been demonstrated to affect apoptosis and cell
cycle arrest in colon cancer and leukemia cells, therefore this
herb is likely to also have therapeutic potential against oral
cancer (11,12). Tricholoma matsutake is an
ectomycorrhizal fungus that is economically significant in Japan
(13). As a traditional edible
fungus in oriental countries, it has been used as a vegetable and a
traditional Chinese medicine for the prevention and treatment of
disease for several thousand years (14). It has been shown that a novel
polysaccharide isolated from Tricholoma matsutake has
antioxidant (14) and
immunostimulatory properties (15).
However, the anticancer activities of these two natural products
require further investigation.
The mitochondrial apoptotic signaling pathway is
mainly governed by Bcl-2 protein family members. The Bcl-2 family
consists of anti- and pro-apoptotic proteins. The anti-apoptotic
proteins, including Bcl-2, Bcl-XL and Mcl-1, contain four Bcl-2
homology (BH) domains, whereas the pro-apoptotic members are
divided into proteins with three BH domains (e.g., Bax, Bak and
Bok) and BH3-only proteins (e.g., Bim, Bad and Bik). Bak, one of
the multi-domain proapoptotic proteins, is essential for apoptotic
cell death (16). Bak-deficient
Jurkat T leukemia cells were resistant to apoptosis triggered by
ultraviolet or anticancer drugs and the expression of antisense Bak
in breast cancer cells increased the resistance to
cisplatin-induced apoptosis (17,18).
Thus, Bak is markedly implicated in apoptotic cell death in various
types of cancer cells.
The present study aimed to determine whether
Codonopsis lanceolata and Tricholoma matsutake
possess anticancer properties in HSC-2 human oral cancer cells. The
results of this study reveal that two natural products induce
apoptosis to inhibit the growth of oral cancer cells by regulating
the Bak protein.
The study was approved by the ethics committee of
Chonbuk National University (Chonbuk National University, Jeon-ju,
Korea).
Materials and methods
Chemicals and antibodies
MECI and METM were provided by Professor Ki-Han Kwon
(Kwangju University, Kwangju, Korea). Poly (ADP-ribose) polymerase
(PARP) antibody was purchased from BD Pharmingen™ (San Jose, CA,
USA). Antibodies against Bak, Bax, Bcl-xL and Mcl-1 were obtained
from Cell Signaling Technology (Charlottesville, VA, USA). Actin
antibody was supplied from Santa Cruz Biotechnology Inc. (Santa
Cruz, CA, USA).
Cell culture and treatments
HSC-2 oral squamous carcinoma cells were provided by
Hokkaido University (Hokkaido, Japan). Cells were cultured in DMEM
supplemented with 10% FBS and antibiotics at 37°C in a 5%
CO2 incubator. Cells were treated with DMSO or various
doses of MECI and METM (300, 600 and 900 μg/ml) for 24 or 48
h.
3-(4,5-dimethylthiazol-20yl)-(3-carboxymethoxyphenyl)-2-(4-
sulphophenyl)-2H-tetrazolium (MTS) assay
The effects of MECI and METM on cell growth were
examined by the CellTiter 96R Aqueous One Solution Cell
Proliferation Assay kit (Promega, Madison, WI, USA). HSC-2 cells
were seeded in 96-well plates and then treated with DMSO or various
doses of MECI and METM (300, 600 and 900 μg/ml) for 24 and
48 h. MTS solution was added to each well and incubated for 2 h at
37°C. The absorbance was analyzed using a ELISA microplate reader
(Bio-Tek Instruments, Inc., Madison, WI, USA) at 490 and 690 nm
(background).
Western blot analysis
HSC-2 cells were seeded and treated with DMSO or
various doses of MECI and METM (300, 600 and 900 μg/ml) for
48 h. Cells were harvested and then whole cell lysates were
extracted with lysis buffer. The protein concentrations were
analyzed by DC Protein Assay (Bio-Rad Laboratories, Hercules, CA,
USA). Samples containing equal amounts of protein were separated by
SDS-PAGE and then transferred to Immun-Blot™ PVDF membranes
(Bio-Rad Laboratories). The membranes were blocked with 5% skimmed
milk in TBST at room temperature for 1 h 30 min and incubated
overnight at 4°C with primary antibodies against PARP, Bak, Bax,
Bcl-xL, Mcl-1 and actin, followed by incubation with HRP-conjugated
secondary antibodies at room temperature for 1 h 30 min. The
membranes were detected by ECL Western Blotting Luminol reagent
(Santa Cruz Biotechnology, Inc.).
DAPI staining
The effects of MECI and METM on chromatin
condensation and nuclear fragmentation were detected using a
fluorescent nuclear dye, DAPI (4′-6-diamidino-2-phenylindole;
Sigma, St. Louis, MO, USA). HSC-2 cells were seeded and treated
with DMSO or various doses (300, 600 and 900 μg/ml) of MECI
and METM. After 48 h, cells were harvested by trypsinization and
resuspended in PBS. The cells were fixed in 100% methanol at room
temperature (RT) for 10 min and then deposited on slides and
stained with DAPI solution (2 μg/ml). DAPI-stained cells
were observed under a fluorescence microscope equipped with a
suitable filter for the DAPI fluorescent dye.
Results
MECI and METM significantly decrease the
growth of HSC-2 cells
The effects of MECI and METM on cell viability were
first investigated. The morphological changes of HSC-2 cells were
observed with a microscope following treatment with various doses
of MECI and METM (300, 600 and 900 μg/ml) for 48 h. The
results revealed that cells treated with MECI and METM were
detached from plates and floated with rounded shapes. Marked
morphological changes of HSC-2 cells were observed following higher
doses of MECI and METM (Fig. 1).
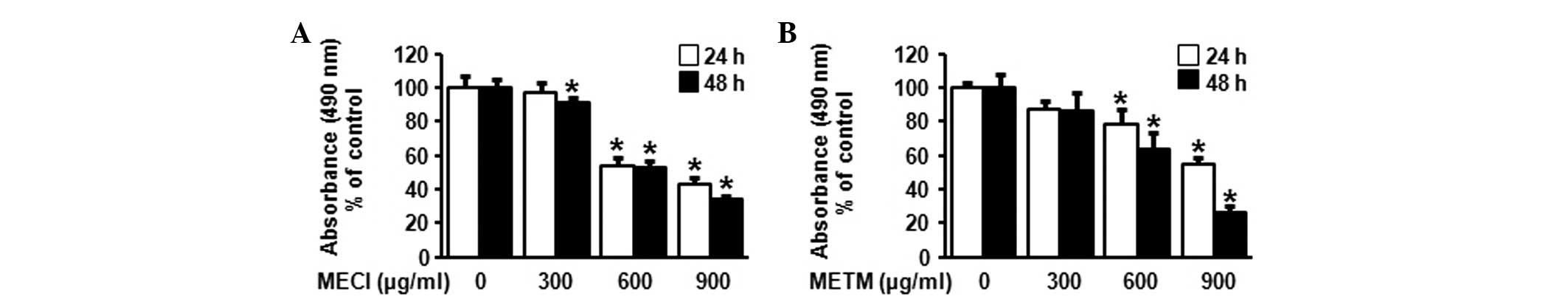
The effect of MECI and METM on cell viability was investigated
using an MTS assay. As shown in Fig. 2A
and B, MECI and METM significantly decreased cell viability in
a dose- and time-dependent manner. The calculated IC50
values of MECI and METM were 692.57 and 685.87 μg/ml at 48
h, respectively. These results indicate that the treatment of HSC-2
cells with MECI and METM inhibits tumor cell growth.
MECI and METM markedly induce apoptosis
in HSC-2 cells
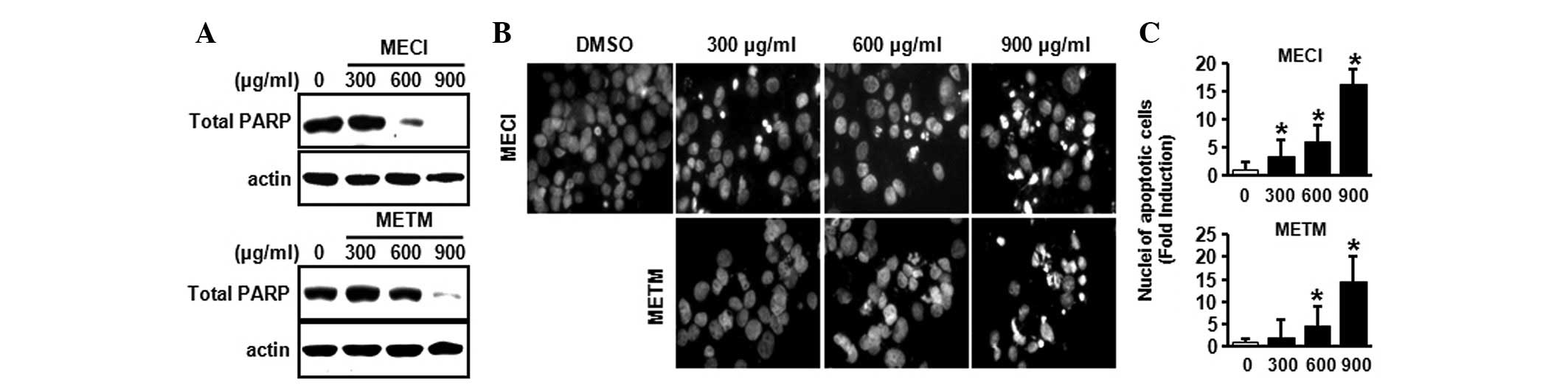
To demonstrate whether the growth-inhibitory effects
of MECI and METM were due to apoptosis, a western blot analysis was
performed using the PARP antibody and DAPI staining. As shown in
Fig. 3A, MECI and METM markedly
decreased total PARP expression, indicating that MECI and METM may
increase cleaved PARP. Furthermore, the effects of MECI and METM on
apoptosis were also confirmed by DAPI staining. Fig. 3B and C indicate that MECI and METM
significantly increased the number of DAPI-stained apoptotic cells,
as demonstrated by nuclear condensation and fragmentation. These
results suggest that MECI and METM induce apoptosis in HSC-2 oral
cancer cells.
MECI and METM increase Bak expression in
HSC-2 cells
In order to further explore the molecular mechanisms
of MECI- and METM-induced apoptosis, the apoptotic effects of MECI
and METM in HSC-2 cells with regard to pro- or anti-apoptotic
proteins in mitochondria were studied. As shown in Fig. 4, MECI and METM increased the
expression of the pro-apoptotic protein Bak in a dose-dependent
manner. However, the pro-apoptotic protein Bax was changed only by
MECI. By contrast, MECI and METM had no significant effect on the
anti-apoptotic proteins Bcl-xL and Mcl-1. These results reveal that
the induction of Bak protein by MECI and METM may be commonly
associated with MECI- and METM-induced apoptosis.
Discussion
Cancer chemoprevention includes the use of natural
synthetic or biological compounds to prevent cancer development.
Numerous studies have demonstrated that natural products play
critical roles against oral cancer. For example, green tea extracts
(GTEs) reduce the incidence of oral cancer development by
inhibiting invasion and metastasis (19,20).
Withania somnifera extract also reduces the overall
occurrence of oral tumors by increasing antioxidant activity
(21,22). Recently, it has been demonstrated
that Polygonum cuspidatum induces apoptotic cell death in
oral cancer cells through the regulation of specificity protein 1
(23). This clearly suggests that
the extracts of natural products have anticarcinogenic activities
against oral cancer. Codonopsis lanceolata extract induces
apoptosis in human colon tumor HT-29 cells and human acute
promyelocytic leukemia HL-60 cells, while Tricholoma
matsutake has also exhibited anti-tumor effects (11,12,24),
indicating that these two natural products may have
anticarcinogenic properties. However, no study concerning their
anticancer effects against oral cancer has been published. Thus,
the present study investigated the anticancer effects of MECI and
METM. The results demonstrated that MECI and METM significantly
decreased cell viability and induced apoptosis, consistent with the
results of previous studies.
Permeabilization of the mitochondria during
apoptosis is a critical control point for the regulation of
programmed cell death (25). Thus,
it is important to investigate the Bcl-2 family protein that
regulates the integrity of the mitochondria. Numerous studies have
shown that natural products regulate the mitochondrial membrane
potential to induce apoptosis in cancer cells. Anonaine, an
alkaloid compound extracted from the leaves of Michelia
alba, induced apoptosis through Bax- and caspase-dependent
pathways in human cervical cancer (HeLa) cells (26). Verticinone, a major alkaloid
isolated from the bulbus of Fritillaria ussuriensis, also
induced apoptosis by damaging mitochondrial membrane potential
(increase of Bax/decrease of Bcl-2) in immortalized and malignant
human oral keratinocytes (27).
Conversely, certain natural products have protective effects
against apoptosis. Ginkgo biloba extract protected rat
pheochromocytoma (PC12) cells from possible oxidative damage
induced by trophic factors and garlic extracts are also protective
against the apoptosis of intestinal epithelial cells caused by
methotrexate (28,29). Therefore, the effect of natural
products on mitochondrial function remains controversial. The
current study also investigated whether MECI and METM affect Bcl-2
family proteins, including Bak, Bax, Bcl-XL and Mcl-1. The results
demonstrated that only Bax was affected by MECI and METM in HSC-2
cells. This suggests that MECI- and METM-induced apoptosis is
associated with the regulation of Bak protein and that the extracts
damage mitochondrial membrane potential instead of protecting it.
Neise et al (30) reported
that Mcl-7 human breast cancer cells expressing Bak protein were
more sensitive to apoptosis induced by staurosporine and TRAIL.
Activation of Bak also increased the sensitivity of
cisplatin-induced apoptosis in melanoma cell lines (31). These results support the theory that
Bak is a significant molecule in MECI- and METM-induced apoptosis.
The homo-oligomerization of Bak is thought to be responsible for
mitochondrial outer membrane permeabilization, therefore it is
likely to be investigated in future studies.
In conclusion, our results suggest that MECI and
METM inhibit tumor growth of HSC-2 cell lines and induce apoptosis.
This effect is due to the induction of Bak protein. Therefore, this
study presents Codonopsis lanceolata and Tricholoma
matsutake as potential anticancer drug candidates targeting Bak
in oral cancer.
Acknowledgements
This study was supported by the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (2012002481 and 2012003731).
References
|
1.
|
H TsudaY OhshimaH NomotoCancer prevention
by natural compoundsDrug Metab
Pharmacokinet19245263200410.2133/dmpk.19.245
|
|
2.
|
S NobiliD LippiE WitortNatural compounds
for cancer treatment and preventionPharmacol
Res59365378200910.1016/j.phrs.2009.01.01719429468
|
|
3.
|
T KunoT TsukamotoA HaraT TanakaCancer
chemoprevention through the induction of apoptosis by natural
compoundsBiophys Chem3156173201210.4236/jbpc.2012.32018
|
|
4.
|
RM NaglerSaliva as a tool for oral cancer
diagnosis and prognosisOral
Oncol4510061010200910.1016/j.oraloncology.2009.07.00519828359
|
|
5.
|
MA RahmanAR AminDM ShinChemopreventive
potential of natural compounds in head and neck cancerNutr
Cancer62973987201010.1080/01635581.2010.50953820924973
|
|
6.
|
WL GuoL GongZF DingGenomic instability in
phenotypically normal regenerants of medicinal plant Codonopsis
lanceolata Benth. et Hook. f., as revealed by ISSR and RAPD
markersPlant Cell
Rep25896906200610.1007/s00299-006-0131-816575572
|
|
7.
|
KT LeeJ ChoiWT JungJH NamHJ JungHJ
ParkStructure of a new echinocystic acid bisdesmoside isolated from
Codonopsis lanceolata roots and the cytotoxic activity of
prosapogeninsJ Agric Food
Chem5041904193200210.1021/jf011647l12105944
|
|
8.
|
YG LeeJY KimJY LeeRegulatory effects of
Codonopsis lanceolata on macrophage-mediated immune
responsesJ Ethnopharmacol1121801882007
|
|
9.
|
K ChoSJ KimSH ParkS KimT ParkProtective
effect of Codonopsis lanceolata root extract against
alcoholic fatty liver in the ratJ Med Food12129313012009
|
|
10.
|
SE ByeonWS ChoiEK HongInhibitory effect of
saponin fraction from Codonopsis lanceolata on immune
cell-mediated inflammatory responsesArch Pharm
Res32813822200910.1007/s12272-009-1601-719557357
|
|
11.
|
L WangML XuJH HuSK RasmussenMH
WangCodonopsis lanceolata extract induces G0/G1 arrest and
apoptosis in human colon tumor HT-29 cells - involvement of ROS
generation and polyamine depletionFood Chem
Toxicol49149154201110.1016/j.fct.2010.10.010
|
|
12.
|
KW LeeHJ JungHJ ParkDG KimJY LeeKT
LeeBeta-D-xylopyranosyl-(1-->3)-beta-D-glucuronopyranosyl
echino- cystic acid isolated from the roots of Codonopsis
lanceolata induces caspase-dependent apoptosis in human acute
promyelocytic leukemia HL-60 cellsBiol Pharm Bull288548592005
|
|
13.
|
C LianM NarimatsuK NaraT
HogetsuTricholoma matsutake in a natural Pinus
densiflora forest: correspondence between above- and
below-ground genets, association with multiple host trees and
alteration of existing ectomycorrhizal communitiesNew
Phytol171825836200610.1111/j.1469-8137.2006.01801.x
|
|
14.
|
X DingJ TangM CaoStructure elucidation and
antioxidant activity of a novel polysaccharide isolated from
Tricholoma matsutakeInt J Biol
Macromol47271275201010.1016/j.ijbiomac.2010.04.01020430053
|
|
15.
|
JY KimSE ByeonYG LeeImmunostimulatory
activities of polysaccharides from liquid culture of pine-mushroom
Tricholoma matsutakeJ Microbiol
Biotechnol1895103200818239423
|
|
16.
|
MC WeiWX ZongEH ChengProapoptotic BAX and
BAK: a requisite gateway to mitochondrial dysfunction and
deathScience292727730200110.1126/science.105910811326099
|
|
17.
|
PG HemmatiB GillissenC von
HaefenAdenovirus-mediated overexpression of p14(ARF) induces p53
and Bax-independent
apoptosisOncogene2131493161200210.1038/sj.onc.120545812082630
|
|
18.
|
GQ WangBR GastmanE WieckowskiA role for
mitochondrial Bak in apoptotic response to anticancer drugsJ Biol
Chem2763430734317200110.1074/jbc.M10352620011447222
|
|
19.
|
YC HoSF YangCY PengMY ChouYC
ChangEpigallocatechin-3-gallate inhibits the invasion of human oral
cancer cells and decreases the productions of matrix
metalloproteinases and urokinase-plasminogen activatorJ Oral Pathol
Med36588593200710.1111/j.1600-0714.2007.00588.x17944751
|
|
20.
|
WC ChiangYK WongSC LinKW ChangCJ
LiuIncrease of MMP-13 expression in multi-stage oral carcinogenesis
and epigallocatechin-3-gallate suppress MMP-13 expressionOral
Dis122733200610.1111/j.1601-0825.2005.01151.x16390465
|
|
21.
|
S ManoharanK PanjamurthyS BalakrishnanK
VasudevanL VellaichamyCircadian time-dependent chemopreventive
potential of withaferin-A in 7,12-dimethylbenz[a]
anthracene-induced oral carcinogenesisPharmacol
Rep61719726200919815955
|
|
22.
|
K PanjamurthyS ManoharanMR NirmalL
VellaichamyProtective role of Withaferin-A on immunoexpression of
p53 and bcl-2 in 7,12-dimethylbenz(a)anthracene-induced
experimental oral carcinogenesisInvest New
Drugs27447452200910.1007/s10637-008-9199-z19009234
|
|
23.
|
JA ShinJH ShimJG JeonApoptotic effect of
Polygonum cuspidatum in oral cancer cells through the
regulation of specificity protein 1Oral Dis171621702011
|
|
24.
|
T EbinaAntitumor effects of intratumoral
injection of Basidiomycetes preparationsGan To Kagaku
Ryoho32165416562005(In Japanese)
|
|
25.
|
NN DanialSJ KorsmeyerCell death: critical
control
pointsCell116205219200410.1016/S0092-8674(04)00046-714744432
|
|
26.
|
CY ChenTZ LiuWC TsengFJ LuRP HungCH
Chen(-)-Anonaine induces apoptosis through Bax- and
caspase-dependent pathways in human cervical cancer (HeLa)
cellsFood Chem
Toxicol4626942702200810.1016/j.fct.2008.04.02418524447
|
|
27.
|
YG YunBH JeonJH LeeVerticinone induces
cell cycle arrest and apoptosis in immortalized and malignant human
oral keratinocytesPhytother
Res22416423200810.1002/ptr.234518058993
|
|
28.
|
T LiK ItoS SumiT FuwaT HorieProtective
effect of aged garlic extract (AGE) on the apoptosis of intestinal
epithelial cells caused by methotrexateCancer Chemother
Pharmacol63873880200910.1007/s00280-008-0809-418677483
|
|
29.
|
C ShiZ YaoJ XuDT YewEffects of Gingko
Extract (EGb761) on oxidative damage under different conditions of
serum supplyJ Bioenerg
Biomembr416169200910.1007/s10863-009-9197-719205855
|
|
30.
|
D NeiseV GraupnerBF GillissenActivation of
the mitochondrial death pathway is commonly mediated by a
preferential engagement of
BakOncogene2713871396200810.1038/sj.onc.121077317724463
|
|
31.
|
A MandicK ViktorssonM MolinCisplatin
induces the proapoptotic conformation of Bak in a
deltaMEKK1-dependent mannerMol Cell
Biol2136843691200110.1128/MCB.21.11.3684-3691.200111340162
|