Introduction
Ovarian cancer is the most common cause of
cancer-associated mortalities arising from gynecological tumors
(1,2). The most common treatment approach for
ovarian cancer consists of a combination of surgery and
chemotherapy. Over the past three decades, surgical tumor debulking
followed by platinum-based chemotherapy has been the standard
treatment for advanced ovarian cancer. Although response rates and
complete responses in advanced disease after first-line treatment
with carboplatin and paclitaxel are >80% and 40–60%,
respectively, the majority of patients eventually relapse, with a
median progression-free survival of 18 months (3). Therefore, there is an urgent demand to
test novel drugs for the prevention and treatment of ovarian
cancer.
Casticin is one of the main components of the fruit
of Vitex rotundifolia L. Casticin has been shown to exert an
anti-inflammatory effect in vivo(4) and has been widely used in traditional
Chinese medicine as an anti-inflammatory drug for thousands of
years. Increasing numbers of studies have shown that casticin
exhibits anticarcinogenic activity in breast (5), cervical (6,7), lung
and colon cancer (8–10) and hepatocellular carcinoma (11), in addition to ovarian cancer
(12). It has been proposed that
cell cycle arrest and casticin-induced apoptosis may be the
possible mechanisms of its anticancer effects. However, the precise
underlying mechanisms are not fully elucidated.
The forkhead box protein M1 (FoxM1) belongs to a
family of evolutionarily conserved transcriptional regulators that
are characterized by the presence of a DNA-binding domain called
the forkhead box or winged-helix domain (13,14).
Numerous studies have demonstrated the biological significance of
FoxM1 in controlling tumor aggressiveness. FoxM1 has been shown to
be involved in cell proliferation and apoptosis, which affects the
developmental function of several organs (14,15). A
number of studies have revealed FoxM1 to be a key cell cycle
regulator in the transition from G1 to S phase and in the
progression to mitosis (16,17).
The loss of FoxM1 expression leads to mitotic spindle defects, the
mitotic delay of cells and induction of mitotic catastrophe or
apoptotic cell death (18–20). FoxM1 has also been shown to regulate
the transcription of cell cycle genes essential for G1-S and G2-M
progression, including survivin, Cdc25B, cyclin B, cyclin D1,
p21CIP1 and p27KIP1(21–23).
FoxM1 has been shown to bind to the mammalian mitotic kinase
polo-like kinase 1 (PLK1), thus acting as a mediator of the
PLK1-dependent regulation of cell cycle progression (24,25).
FOXO3a is a member of the FOXO subfamily of
fork-head transcription factors. The phosphorylation of FOXO3a
results in the impairment of its DNA-binding ability and an
increased binding affinity for the 14-3-3 proteins. This causes
FoxM1 upregulation, which in turn promotes cell proliferation and
survival (26–28). By contrast, the dephosphorylation of
activated FOXO3a induces cell cycle arrest and apoptosis (29–31).
Since casticin promotes cell cycle arrest and apoptosis, it is
possible that it exerts these antitumor effects by regulating
FOXO3a/FoxM1 expression. However, the intracellular mechanisms by
which casticin induces apoptosis in ovarian cancer cells via the
regulation of FOXO3a/FoxM1 signaling have never been examined.
Hence, the aim of the present study was to examine the molecular
mechanisms by which casticin-induced activation of the
transcription factor FOXO3a induces apoptosis in ovarian cancer
cells. Our results demonstrated that casticin promotes the
dephosphorylation of FOXO3a, leading to the inhibition of FoxM1,
which ultimately causes ovarian cancer cell apoptosis.
Materials and methods
Chemicals and antibodies
Casticin was purchased from Chengdu Biopurify
Phytochemicals Ltd. (Chengdu, Sichuan, China). It has a molecular
weight of 374.3, appears as yellow crystals and has a purity of
98.0%. Casticin was prepared in dimethyl sulfoxide (DMSO) as a 10
mmol/l stock solution and diluted in Dulbecco’s minimum essential
medium (DMEM; Invitrogen, Carlsbad, CA, USA) to the indicated
concentration before use. Primary antibodies FoxM1, PLK1,
p27KIP1, survivin, β-actin, anti-caspase-3 and anti-poly
(ADP-ribose) polymerase (PARP) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Horseradish
peroxidase-conjugated rabbit anti-mouse secondary antibody was also
purchased from Santa Cruz Biotechnology, Inc. Mouse monoclonal
antibodies against FOXO3a and phospho-FOXO3a-Thr32 were
purchased from Millipore (Bedford, MA, USA). Lipofectamine™ 2000
was purchased from Invitrogen. Protease inhibitor cocktail and all
other chemicals were obtained from Sigma (St. Louis, MO, USA).
Cells and cell culture
The human ovarian cancer cell lines SKOV3 and A2780
were purchased from the China Center for Type Culture Collection
(CCTCC; Wuhan, China). The cells were maintained in DMEM
supplemented with 10% fetal bovine serum (FBS), 4 mM glutamine, 100
U/ml penicillin and 100 μg/ml streptomycin and incubated at
37°C in a humidified atmosphere of 5% CO2.
Histone/DNA enzyme-linked immunosorbent
assay (ELISA) for detecting apoptosis
The cell apoptosis ELISA detection kit (Roche
Applied Sciences, Penzberg, Germany) was used to detect apoptosis
in casticin-treated cells, according to the manufacturer’s
instructions. Briefly, cells were seeded in 96-well plates at a
density of 1×104 cells/well for 24 h and the test agents
were added to the culture medium containing 10% FBS. After 24 h,
the cytoplasm of cells in the control (untreated) and treatment
(casticin-treated) groups was transferred to streptavidin precoated
96-well plates, which had been previously incubated with a
biotinylated histone antibody and peroxidase-tagged mouse
anti-human DNA for 2 h at room temperature. The absorbance was
measured at 405 nm using an ELx800 ELISA plate reader (Bio-Tek,
Winchester, VA, USA).
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted using TRIzol®
reagent (Life Technologies, Gaithersburg, MD, USA). The integrity
of the RNA was assessed using 2% agarose gel electrophoresis. RNA
(∼2 μg) was reverse transcribed using the SuperScript™
First-Strand Synthesis System kit (Invitrogen). cDNAs encoding the
FoxM1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes
were amplified by PCR as follows: denaturation at 94°C for 30 sec,
annealing at 63°C for 30 sec and elongation at 72°C for 45 sec. The
primer sequences were designed as follows: for FoxM1, the forward
primer was 5′-AAC CGCTACTTGACATTGG-3′ and the reverse primer was
5′-GCAGTGGCTTCATCTTCC-3′. A GAPDH housekeeping gene was used as the
internal control, for which the forward primer was
5′-ACCCAGAAGACTGTGGATGG-3′ and the reverse primer was
5′-TGCTGTAGCCAAATTCGTTG-3′. PCR products were analyzed by 2%
agarose gel electrophoresis.
RNA interference
FoxM1, FOXO3a and control small interfering RNA
(siRNA) were purchased from Santa Cruz Biotechnology, Inc. Human
ovarian cancer SKOV3 and A2780 cells were transfected with FoxM1,
FOXO3a and control siRNA using Lipofectamine™ 2000, as described by
Wang et al(32). The cells
were then collected and processed for western blot analysis and
histone/DNA ELISA.
Western blot analysis
Total cell extracts for western blot analysis were
obtained, as previously described (33). Cell lysates containing 50 μg
protein were electrophoresed on a 7.5–12.5% sodium dodecyl sulfate
polyacrylamide gel and blotted onto polyvinylidene difluoride
membranes (Millipore). Anti-FoxM1, anti-p27KIP1,
anti-survivin, anti-FOXO3a, anti-phospho-FOXO3a-Thr32,
anti-caspase-3 and anti-PARP were used as primary antibodies. The
blots were stripped and reprobed with an anti-actin antibody to
normalize for differences in protein loading. Changes in the levels
of the desired proteins were determined by densitometric scanning
of the immunoreactive bands and corrected for β-actin loading
control. Immunoblotting for each protein was performed at least
twice using independently prepared lysates to ensure
reproducibility of the results.
Statistical analysis
The database was set up with the SPSS 15.0 software
package (SPSS Inc., Chicago, IL, USA) for statistical analysis.
Data are presented as mean ± standard deviation (SD). The means of
multiple groups were compared using one-way analysis of variance
(ANOVA) after the equal check of variance and comparisons between
the means were performed using the least significant difference
(LSD) method. Statistical comparison was also performed using a
two-tailed t-test when appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of casticin on apoptosis in
ovarian cancer cells
Based on results from studies that demonstrated the
casticin-induced growth inhibition of ovarian cancer cells
(12) and apoptosis in human
cervical cancer (6,7) and hepatocellular carcinoma (11) cells, we first investigated whether
casticin was able to induce apoptosis in ovarian cancer cells.
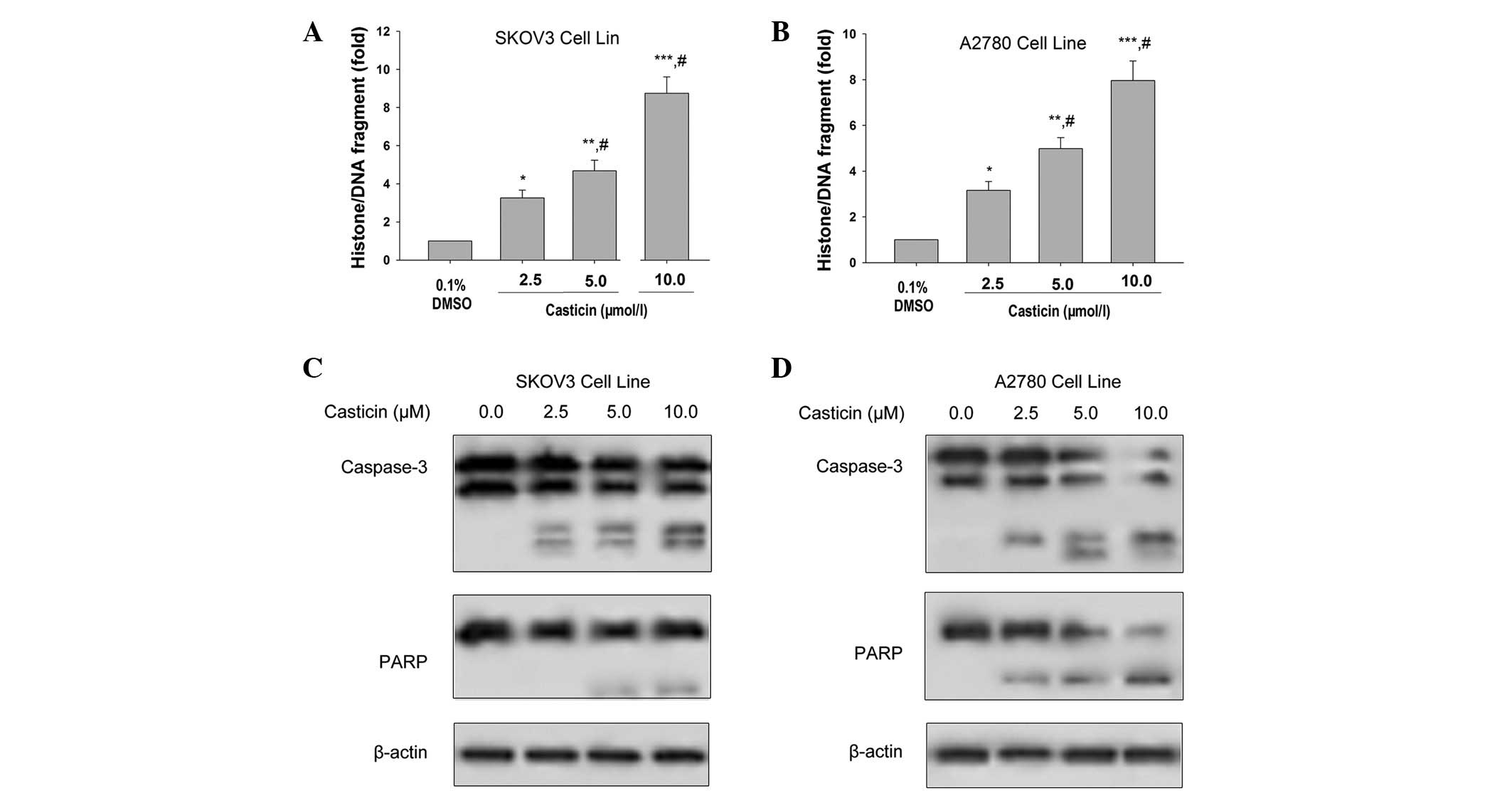
Exposing SKOV3 and A2780 cells to 2.5, 5.0 and 10.0 μmol/l
casticin for 24 h significantly induced histone/DNA fragmentation
in a concentration-dependent manner (Fig. 1A and B). Additionally, casticin
(2.5, 5.0 and 10.0 μmol/l) activated caspase-3 and induced
the cleavage of its substrate, PARP, in SKOV3 and A2780 cells.
Substrate cleavage was indicated by a reduction in the uncleaved
forms of caspases and their substrates and/or the appearance of
their cleaved forms (Fig. 1C and
D). Taken together, these results demonstrated that casticin
induces apoptosis in ovarian cancer cells.
Effects of casticin on FoxM1 expression
in ovarian cancer cells
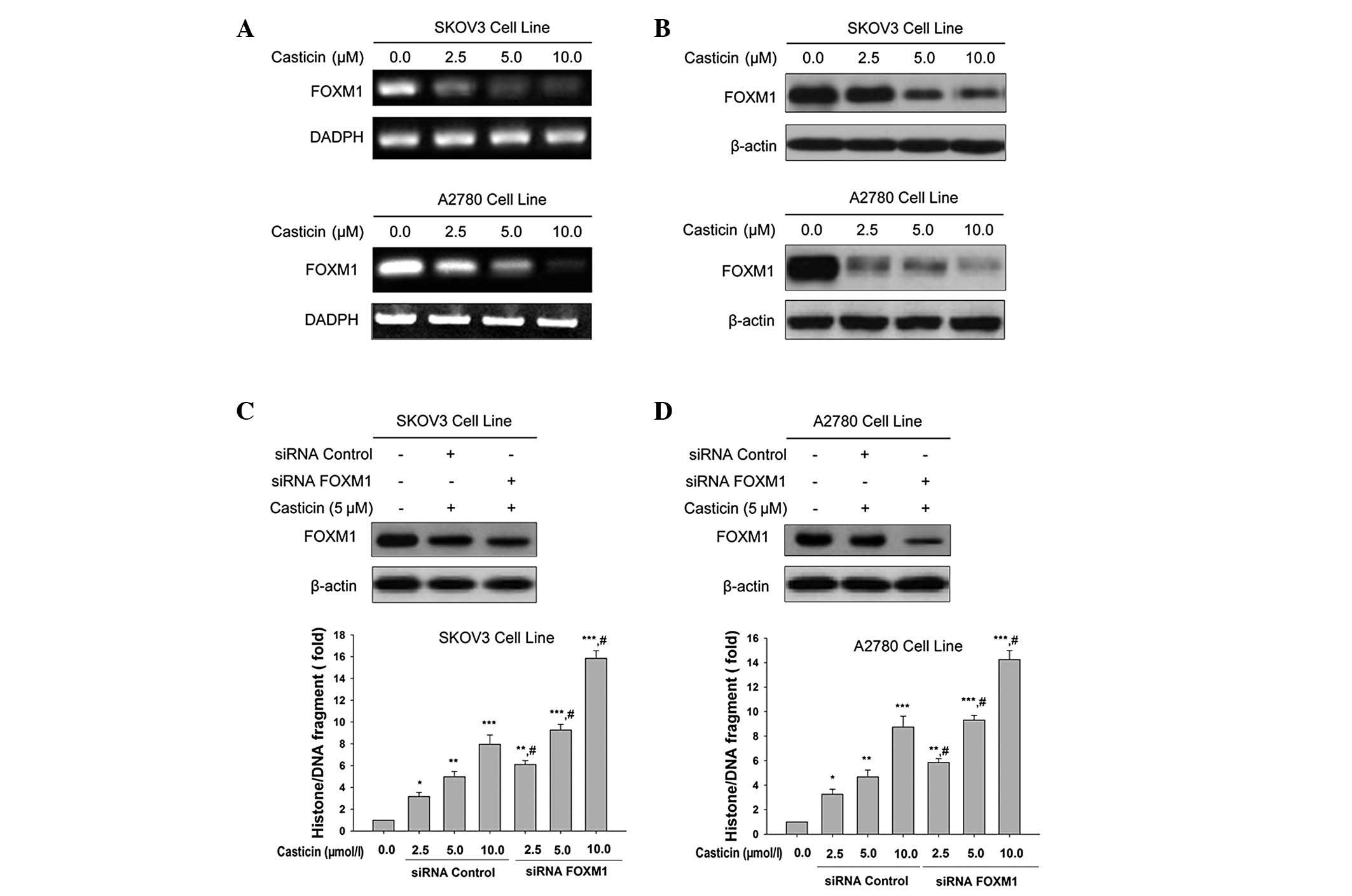
Studies have shown that the loss of FoxM1 expression
induces apoptosis (19). We
investigated whether casticin was able to regulate FoxM1 expression
during casticin-induced apoptosis in ovarian cancer cells. The
expression of FoxM1 was determined using RT-PCR and western blot
analysis. We showed that FoxM1 was overexpressed in SKOV3 (Fig. 2A) and A2780 (Fig. 2B) cell lines. Exposing SKOV3 and
A2780 cells to 2.5, 5.0 and 10.0 μmol/l casticin for 24 h
significantly reduced the expression of FoxM1 at the protein level
(Fig. 2A and B). Silencing FoxM1
expression using siRNA resulted in the enhanced induction of
apoptosis with casticin treatment in SKOV3 (Fig. 2C) and A2780 (Fig. 2D) cell lines.
Effects of casticin on the expression of
downstream targets of FoxM1 in ovarian cancer cells
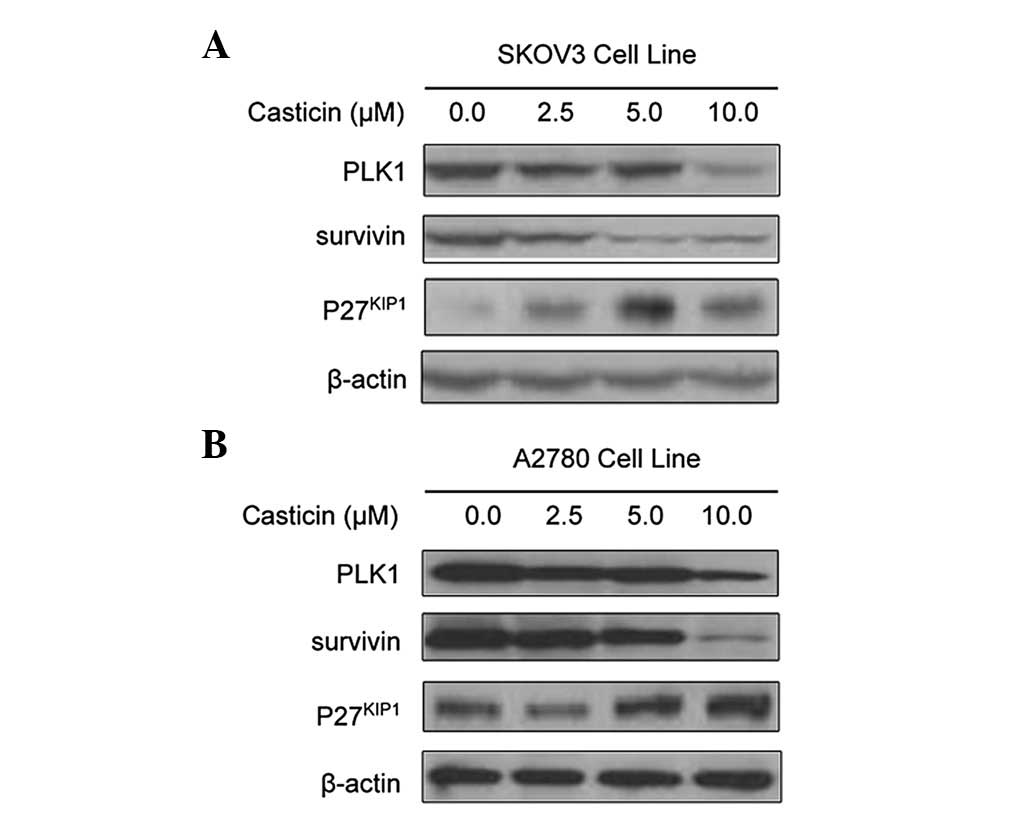
To further confirm the effects of casticin on the
functional regulation of FoxM1, we assessed the expression of FoxM1
downstream target genes in SKOV3 and A2780 cells following casticin
treatment. FoxM1 is known to have several downstream target genes,
including PLK1, survivin and p27KIP1(21,34–36).
Western blot analysis revealed that casticin reduced the expression
levels of PLK1 and survivin and increased expression of
p27KIP1 at the protein level in SKOV3 (Fig. 3A) and A2780 (Fig. 3B) cells. These results provide
molecular evidence suggesting that casticin-induced apoptosis in
ovarian cancer cells may be mediated via the inactivation of
FoxM1.
Effects of casticin on the
phosphorylation level of FOXO3a protein in ovarian cancer
cells
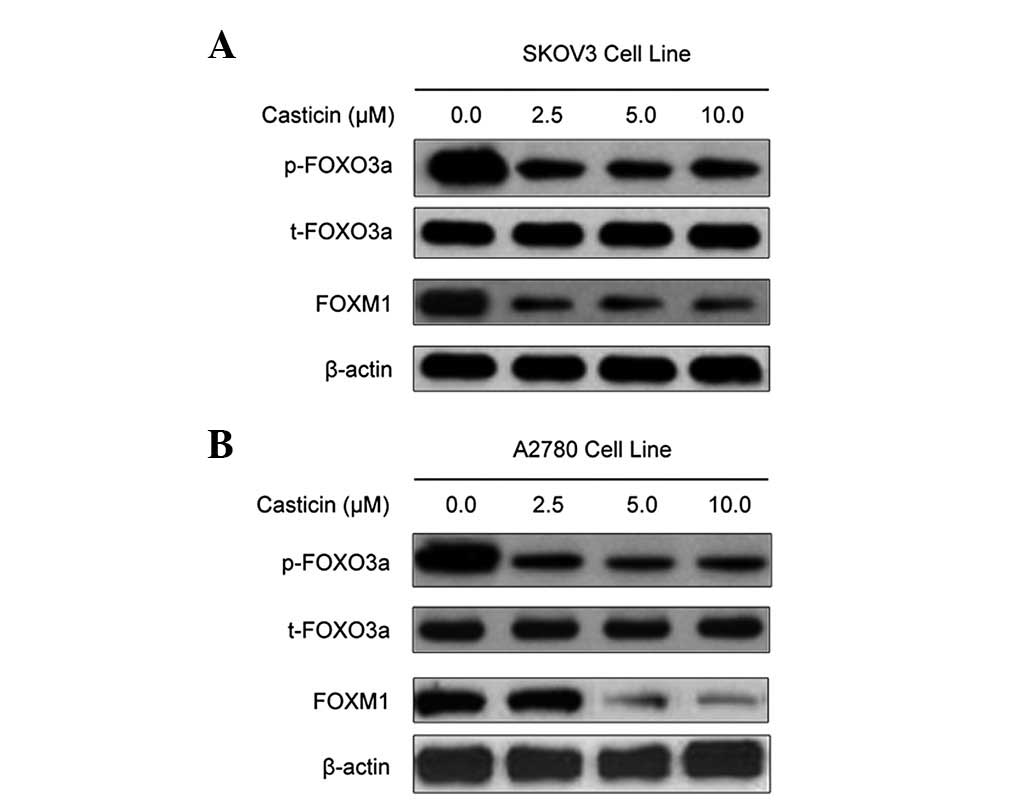
Since FOXO3a is considered to be an upstream
regulator of the FoxM1 transcription factor (29), we investigated the expression of
phosphorylated FOXO3a protein in order to explain the mechanism of
casticin-dependent FoxM1 inhibition. Western blot analysis showed
that treatment with casticin led to a decrease in the FOXO3a
phosphorylation level, with a corresponding decrease in FoxM1
expression levels (Fig. 4). These
results indicate that casticin-mediated inhibition of FoxM1
expression may be associated with the inhibition of FOXO3a
phosphorylation.
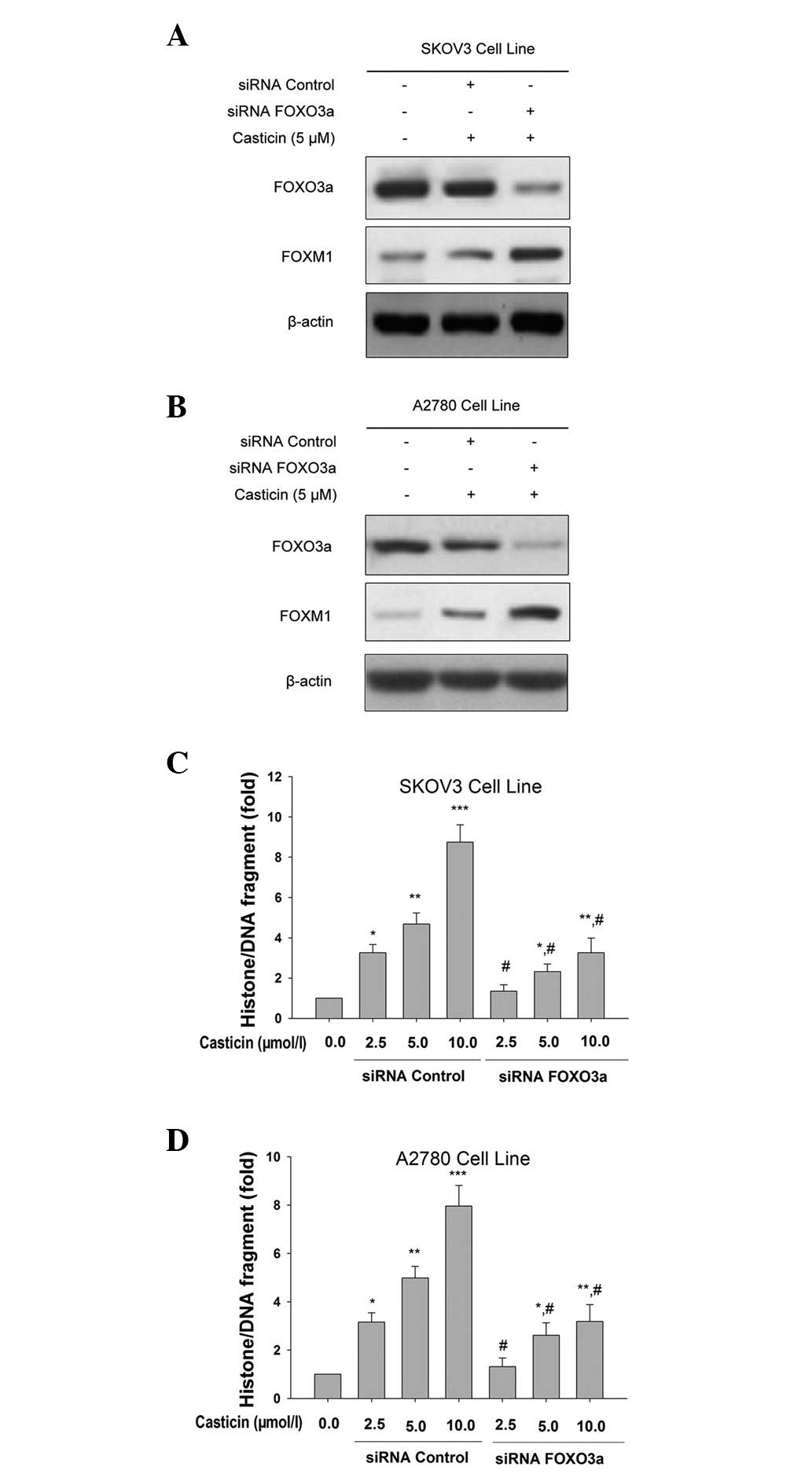
Effects of silencing the FOXO3a gene on
casticin-mediated apoptosis in ovarian cancer cells
In order to confirm the role of the FOXO3a
transcription factor in the cellular response to casticin, we
performed gene silencing experiments. SKOV3 and A2780 cells were
generated and expression of the FOXO3a protein was attenuated using
siRNA technology. In FOXO3a siRNA-transfected cells, the expression
levels of FoxM1 clearly increased (Fig.
5A and B). Additionally, we identified that the knockdown of
FOXO3a significantly attenuated casticin-induced apoptosis in
ovarian cancer cells (Fig. 5C and
D). These findings are consistent with our hypothesis that
casticin induces ovarian cancer cell apoptosis by repressing FoxM1
expression through the induction of FOXO3a activity.
Discussion
In the present study, we showed that casticin
induced apoptosis in ovarian cancer cells occurs due to a decrease
in the expression levels of FoxM1 and its downstream targets PLK1
and survivin and an increase in p27KIP1, all of which
are associated with activation of the FOXO3a transcription factor
via dephosphorylation. Additionally, silencing the FOXO3a
transcription factor using an siRNA approach blocked
casticin-induced downregulation of FoxM1 and inhibited apoptosis.
Our results suggest that casticin induces apoptosis in ovarian
cancer cells (SKOV3 and A2780) through the regulation of
FOXO3a/FoxM1 signaling.
A number of studies have demonstrated overexpression
of the FoxM1 gene in human cancer cells and tissues, including in
ovarian cancer (32,33,37,38),
and emerging evidence suggests that the inactivation of FoxM1 may
have important implications in cancer therapy. For example, it may
be possible to downregulate FoxM1 expression using specific drugs,
such as siomycin A, thiostrepton and the epidermal growth factor
receptor (EGFR) inhibitor gefitinib (27,39,40).
Wang et al demonstrated that genistein is capable of
inhibiting FoxM1 activation in pancreatic cancer cells, leading to
cell growth inhibition and the induction of apoptosis (32). In the present study, we investigated
the possibility that casticin induces apoptotic cell death and
aimed to determine the role of FoxM1 in casticin-dependent ovarian
cancer cell apoptosis. Our data showed that casticin elicited a
marked effect on apoptosis in ovarian cancer cells, as demonstrated
by histone/DNA ELISA results which showed the activation of
caspase-3 and cleavage of PARP, accompanied by the downregulation
of FoxM1 expression. Our results suggest that FoxM1 is a target of
casticin in ovarian cancer cells, as FoxM1 is known to induce
oncogenesis and its downregulation causes the inhibition of cell
growth (40).
The FOXO3a transcription factor is important in the
regulation of the cell cycle and apoptosis (41,42).
FOXO3a is regulated by phosphorylation (43). Upon activation of the PI3K/AKT
signaling pathway, FOXO proteins undergo AKT-mediated
phosphorylation, which promotes their binding to 14-3-3 proteins
and facilitates their nuclear export through chromosome region
maintenance 1 (CRM1) and cytoplasmic sequestration. Under
conditions of stress or in the absence of growth or survival
factors, the PI3K/AKT pathway is inhibited and FOXO3a proteins
translocate to the cell nucleus, where they execute their
transcriptional functions (40). In
the present study, we demonstrated that casticin inhibited FOXO3a
phosphorylation. Furthermore, knockdown of FOXO3a by transfection
with siRNA blocked the casticin-induced down-regulation of FoxM1
expression and inhibited ovarian cancer cell apoptosis. These
findings indicate that FOXO3a is a key regulator of
casticin-induced apoptosis and FoxM1 expression in ovarian cancer
cells.
In summary, our study demonstrated that
casticin-induced dephosphorylation of FOXO3a regulates the
expression of FoxM1 and its target genes, including survivin, PLK1
and p27KIP1, and this causes apoptosis in ovarian cancer
cells. Further studies are required to assess the upstream events
leading to FOXO3a phosphorylation and casticin-dependent anticancer
effects in ovarian cancer cells. A thorough understanding of the
mechanisms and effects of casticin on the cell cycle and apoptosis
may lead to the identification and development of novel therapeutic
molecules for the treatment and prevention of ovarian cancer and
other malignant diseases.
Acknowledgements
This study was supported by the Hunan
province Science and Technology Project (No. 2011FJ4144).
References
|
1
|
Lan C, Chenggang W, Yulan B, Xiaohui D,
Junhui Z and Xiao W: Aberrant expression of WWOX protein in
epithelial ovarian cancer: a clinicopathologic and
immunohistochemical study. Int J Gynecol Pathol. 31:125–132. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vlahovic G, Meadows KL, Uronis HE, et al:
A phase I study of bevacizumab, everolimus and panitumumab in
advanced solid tumors. Cancer Chemother Pharmacol. 70:95–102. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hennessy BT, Coleman RL and Markman M:
Ovarian cancer. Lancet. 374:1371–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin S, Zhang H, Han T, Wu JZ, Rahman K and
Qin LP: In vivo effect of casticin on acute inflammation. Zhong Xi
Yi Jie He Xue Bao. 5:573–576. 2007. View Article : Google Scholar
|
|
5
|
Li WX, Cui CB, Cai B, Wang HY and Yao XS:
Flavonoids from Vitex trifolia Linhibit cell cycle
progression at G2/M phase and induce apoptosis in mammalian cancer
cells. J Asian Nat Prod Res. 7:615–626. 2005.
|
|
6
|
Chen D, Cao J, Tian L, Liu F and Sheng X:
Induction of apoptosis by casticin in cervical cancer cells through
reactive oxygen species-mediated mitochondrial signaling pathways.
Oncol Rep. 26:1287–1294. 2011.PubMed/NCBI
|
|
7
|
Zeng F, Tian L, Liu F, Cao J, Quan M and
Sheng X: Induction of apoptosis by casticin in cervical cancer
cells: reactive oxygen species-dependent sustained activation of
Jun N-terminal kinase. Acta Biochim Biophys Sin (Shanghai).
44:442–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Díaz F, Chávez D, Lee D, et al: Cytotoxic
flavone analogues of vitexicarpin, a constituent of the leaves of
Vitex negundo. J Nat Prod. 66:865–867. 2003.PubMed/NCBI
|
|
9
|
Haïdara K, Zamir L, Shi QW and Batist G:
The flavonoid Casticin has multiple mechanisms of tumor
cytotoxicity action. Cancer Lett. 242:180–190. 2006.PubMed/NCBI
|
|
10
|
Imai M, Kikuchi H, Denda T, Ohyama K,
Hirobe C and Toyoda H: Cytotoxic effects of flavonoids against a
human colon cancer derived cell line, COLO 201: a potential natural
anti-cancer substance. Cancer Lett. 276:74–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang J, Yang Y, Tian L, Sheng XF, Liu F
and Cao JG: Casticin-induced apoptosis involves death receptor 5
upregulation in hepatocellular carcinoma cells. World J
Gastroenterol. 17:4298–4307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hernández MM, Heraso C, Villarreal ML,
Vargas-Arispuro I and Aranda E: Biological activities of crude
plant extracts from Vitex trifolia L. (Verbenaceae). J
Ethnopharmacol. 67:37–44. 1999.
|
|
13
|
Hannenhalli S and Kaestner KH: The
evolution of Fox genes and their role in development and disease.
Nat Rev Genet. 10:233–240. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Korver W, Roose J and Clevers H: The
winged-helix transcription factor Trident is expressed in cycling
cells. Nucleic Acids Res. 25:1715–1719. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kwok JM, Peck B, Monteiro LJ, et al: FOXM1
confers acquired cisplatin resistance in breast cancer cells. Mol
Cancer Res. 8:24–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bergamaschi A, Christensen BL and
Katzenellenbogen BS: Reversal of endocrine resistance in breast
cancer: interrelationships among 14-3-3ζ, FOXM1, and a gene
signature associated with mitosis. Breast Cancer Res.
13:R702011.PubMed/NCBI
|
|
17
|
Feo F, Frau M and Pascale RM: Interaction
of major genes predisposing to hepatocellular carcinoma with genes
encoding signal transduction pathways influences tumor phenotype
and prognosis. World J Gastroenterol. 14:6601–6615. 2008.
View Article : Google Scholar
|
|
18
|
Chen W, Yuan K, Tao ZZ and Xiao BK:
Deletion of Forkhead Box M1 transcription factor reduces malignancy
in laryngeal squamous carcinoma cells. Asian Pac J Cancer Prev.
12:1785–1788. 2011.PubMed/NCBI
|
|
19
|
Halasi M and Gartel AL: Suppression of
FOXM1 sensitizes human cancer cells to cell death induced by
DNA-damage. PLoS One. 7:e317612012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wonsey DR and Follettie MT: Loss of the
forkhead transcription factor FoxM1 causes centrosome amplification
and mitotic catastrophe. Cancer Res. 65:5181–5189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Down CF, Millour J, Lam EW and Watson RJ:
Binding of FoxM1 to G2/M gene promoters is dependent upon B-Myb.
Biochim Biophys Acta. 1819:855–862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Millour J, de Olano N, Horimoto Y, et al:
ATM and p53 regulate FOXM1 expression via E2F in breast cancer
epirubicin treatment and resistance. Mol Cancer Ther. 10:1046–1058.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schüller U, Zhao Q, Godinho SA, et al:
Forkhead transcription factor FoxM1 regulates mitotic entry and
prevents spindle defects in cerebellar granule neuron precursors.
Mol Cell Biol. 27:8259–8270. 2007.PubMed/NCBI
|
|
24
|
Fu Z, Malureanu L, Huang J, et al:
PLK1-dependent phosphorylation of FoxM1 regulates a transcriptional
programme required for mitotic progression. Nat Cell Biol.
10:1076–1082. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laoukili J, Alvarez-Fernandez M, Stahl M
and Medema RH: FoxM1 is degraded at mitotic exit in a
Cdh1-dependent manner. Cell Cycle. 7:2720–2726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delpuech O, Griffiths B, East P, et al:
Induction of Mxi1-SR alpha by FOXO3a contributes to repression of
Myc-dependent gene expression. Mol Cell Biol. 27:4917–4930. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McGovern UB, Francis RE, Peck B, et al:
Gefitinib (Iressa) represses FOXM1 expression via FOXO3a in breast
cancer. Mol Cancer Ther. 8:582–591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wilson MS, Brosens JJ, Schwenen HD and Lam
EW: FOXO and FOXM1 in cancer: the FOXO-FOXM1 axis shapes the
outcome of cancer chemotherapy. Curr Drug Targets. 12:1256–1266.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang L, Wang H, Zhou L and Yu D:
Akt-FOXO3a signaling axis dysregulation in human oral squamous cell
carcinoma and potent efficacy of FOXO3a-targeted gene therapy. Oral
Oncol. 47:16–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lam M, Carmichael AR and Griffiths HR: An
aqueous extract of Fagonia cretica induces DNA damage, cell
cycle arrest and apoptosis in breast cancer cells via FOXO3a and
p53 expression. PLoS One. 7:e401522012.PubMed/NCBI
|
|
31
|
Yusuf I, Zhu X, Kharas MG, Chen J and
Fruman DA: Optimal B-cell proliferation requires phosphoinositide
3-kinase-dependent inactivation of FOXO transcription factors.
Blood. 104:784–787. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Ahmad A, Banerjee S, et al: FoxM1
is a novel target of a natural agent in pancreatic cancer. Pharm
Res. 27:1159–1168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ning Y, Li Q, Xiang H, Liu F and Cao J:
Apoptosis induced by 7-difluoromethoxyl-5,4’-di-n-octyl genistein
via the inactivation of FoxM1 in ovarian cancer cells. Oncol Rep.
27:1857–1864. 2012.PubMed/NCBI
|
|
34
|
Bellelli R, Castellone MD, Garcia-Rostan
G, et al: FOXM1 is a molecular determinant of the mitogenic and
invasive phenotype of anaplastic thyroid carcinoma. Endocr Relat
Cancer. 19:695–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dibb M, Han N, Choudhury J, et al: The
FOXM1-PLK1 axis is commonly upregulated in oesophageal
adenocarcinoma. Br J Cancer. 107:1766–1775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ha SY, Lee CH, Chang HK, et al:
Differential expression of forkhead box M1 and its downstream
cyclin-dependent kinase inhibitors p27(kip1) and p21(waf1/cip1) in
the diagnosis of pulmonary neuroendocrine tumours. Histopathology.
60:731–739. 2012. View Article : Google Scholar
|
|
37
|
Cancer Genome Atlas Research Network:
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar
|
|
38
|
Petrovic V, Costa RH, Lau LF, Raychaudhuri
P and Tyner AL: FoxM1 regulates growth factor-induced expression of
kinase-interacting stathmin (KIS) to promote cell cycle
progression. J Biol Chem. 283:453–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kwok JM, Myatt SS, Marson CM, Coombes RC,
Constantinidou D and Lam EW: Thiostrepton selectively targets
breast cancer cells through inhibition of forkhead box M1
expression. Mol Cancer Ther. 7:2022–2032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Petrovic V, Costa RH, Lau LF, Raychaudhuri
P and Tyner AL: Negative regulation of the oncogenic transcription
factor FoxM1 by thiazolidinediones and mithramycin. Cancer Biol
Ther. 9:1008–1016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Roy SK, Srivastava RK and Shankar S:
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of
FOXO transcription factor, leading to cell cycle arrest and
apoptosis in pancreatic cancer. J Mol Signal. 5:102010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zanella F, Link W and Carnero A:
Understanding FOXO, new views on old transcription factors. Curr
Cancer Drug Targets. 10:135–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Myatt SS and Lam EW: The emerging roles of
forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 7:847–859.
2007. View Article : Google Scholar : PubMed/NCBI
|